Abstract
Caloric restriction has been known to extend lifespan and delay age-associated pathology in laboratory animals for nearly a century. More recently, alternative “anti-aging” diet modalities have been described that provide new mechanistic insights and potential clinical applications. These include intermittent fasting, fasting-mimicking diets, ketogenic diets, time-restricted feeding, protein restriction, and dietary restriction of specific amino acids. Despite mainstream popularization of some of these diets, many questions remain about their efficacy outside of a laboratory setting. Studies of these interventions support at least partially overlapping mechanisms of action and provide important insights into what appear to be highly conserved mechanisms of biological aging.
Modern aging research can trace its roots back to studies from the early 1900s examining the effects of reduced food intake on lifespan in rats (1, 2). These pioneering experiments showed that reducing caloric intake in lab-reared animals delays development and results in a substantial increase in adult lifespan. Work by Weindruch, Masoro, Walford and others in the 1970s, 80s and 90s expanded and popularized this area of research in both rats and mice and established caloric restriction (CR) as the dominant paradigm for anti-aging intervention (see Text Box 1) for the rest of the 20th century (3). Importantly, these foundational studies provided strong evidence that CR not only increases lifespan in rodents but also reduces disease burden and delays many functional declines of old age (4).
Text Box 1. Reclaiming the term “anti-aging”.
The phrase “anti-aging” is greatly abused in popular culture, often for the purpose of marketing cosmetic procedures or unproven nutritional supplements purported to slow or reverse aging. This has the unfortunate consequence of creating confusion among the general public and diminishing the impact of legitimate scientific discovery. Here, we define “anti-aging” as delaying or reversing biological aging by targeting the established molecular mechanisms of aging, which have been formalized as “hallmarks” or “pillars” of aging (92, 93). Effective anti-aging interventions in laboratory animals increase both median and maximum population lifespan and broadly delay the onset and progression of many age-related functional declines and diseases. The latter effect is often referred to as “extending healthspan”, which is a qualitative term referring to the period of life free from chronic disease and disability (94). Recent studies show that at least some anti-aging interventions, such as the drug rapamycin, can reverse functional declines across multiple tissues in aged animals (95). Based on this definition, there are as yet no clinically validated anti-aging interventions in humans. However, there is some evidence consistent with anti-aging effects for CR and related diets in humans (see below) as well as a small number of putative geroprotective compounds including metformin and rapamycin (96).
A common definition for CR in these early studies is “reduced caloric intake in the absence of malnutrition” (5). Typically, this was accomplished by limiting chow by a fixed amount while supplementing with vitamins and micronutrients. The precise method of food limitation varied (e.g. intermittent vs. continuous feeding) as did the timing of initiation (pre- or post-weaning) and degree of restriction. The data from these studies support the idea that, at least in some common laboratory strains of both mice and rats, total caloric intake correlates inversely with lifespan up to about 50-60% restriction (so long as essential nutrition is maintained), and starting earlier in life gives larger effects on lifespan than starting later in life (5).
With popularization of invertebrate models in aging research near the end of the 20th century, it was natural for scientists to test whether CR could similarly affect aging in these organisms. Because culture conditions differ across species, multiple alternative methods of nutritional intervention were tested and found to increase lifespan. We will refer to these interventions collectively using the term dietary restriction (DR) which includes both sugar and amino acid limitation in budding yeast, reduced bacterial food availability in nematode worms, and lower levels of protein (yeast extract) or sugar in fruit flies (6). These important foundational studies allowed for mechanistic analyses not previously feasible in rodents and identified a highly conserved nutrient-sensing/growth-promoting network that appears to regulate biological aging in many different organisms. Among the key proteins in this network are the mechanistic target of rapamycin (mTOR, discussed further below), AMP-activated protein kinase (AMPK), insulin/IGF-1-like receptors, FOXO-family transcription factors, and NAD-dependent sirtuin deacetylases (7, 8).
As the molecular underpinnings of CR, and DR more generally, became established, attention shifted toward identifying small molecules that mirror the effects of CR on lifespan and health without requiring reduced food consumption. Such “CR mimetics” include the mTOR inhibitor rapamycin, the anti-diabetes drug metformin, the glycolytic inhibitor 2-deoxyglucose, the intestinal alpha-glucosidase inhibitor acarbose, and sirtuin-activating compounds (9, 10). It is worth noting that most putative CR mimetic compounds, with the possible exception of rapamycin, have thus far failed to match the magnitude of lifespan extension and healthspan benefits of CR. For example, metformin (a non-specific AMPK activator) and resveratrol (a non-specific sirtuin activator) primarily improve measures of metabolic health during aging in mice, including increased insulin sensitivity and reduced cancer incidence, without reproducibly extending lifespan (11, 12). These discrepancies likely reflect a still incomplete understanding of the varied and diverse effects of CR which have yet to be fully recapitulated pharmacologically.
The rise of anti-aging diets
The goal of this critical review is to summarize the current state of the field with respect to the most commonly studied anti-aging dietary interventions, with a focus on potential shared mechanisms of action, important unanswered questions and areas for future inquiry, and addressing common misconceptions in the literature. We will largely restrict our considerations to preclinical studies in rodents and, where applicable, relevant human data. For detailed reviews of CR in rodents we refer the interested reader to these resources (4, 5, 13-15). Here, we will only briefly discuss the evidence for anti-aging effects of classic CR and instead focus on placing alternative dietary interventions into context with what is known about CR and potential impacts on human health and longevity. Among the interventions we consider are ketogenic diets (KD), intermittent fasting (IF), fasting-mimicking diets (FMD), time-restricted feeding (TRF), protein restriction (PR), and diets restricted for specific amino acids including methionine restriction, tryptophan restriction, and branched chain amino acid (BCAA) restriction.
One critical, but often overlooked (and therefore quite confusing) consideration when evaluating different anti-aging diets is the relative caloric intake of control and experimental cohorts. Anti-aging diets can be considered in two broad groups: caloric restriction and iso-caloric nutrient restriction (Table 1). Several of the most prominent anti-aging diets such as intermittent fasting, fasting-mimicking diets, and ketogenic diets generally fall under the CR umbrella because the experimental group typically consumes 20-40% fewer calories than the control group. This makes evaluating the effects of dietary composition challenging to differentiate from the effects of reduced caloric intake. Other interventions, such as time-restricted feeding and protein or amino acid restriction are somewhat better characterized under iso-caloric experimental conditions. In all cases, however, it is important to carefully evaluate relative caloric intake when considering studies in this area.
Table 1. Summary of anti-aging diets.
Lifespan effect refers to studies in rodents. Number of arrows are intended to represent relative robustness and consistency of reported effects.
| Dietary Intervention |
Description | Lifespan Effect |
|---|---|---|
| Low calorie interventions | ||
| “Classic” CR | Daily reduction in calories, typically by 20 to 50%, without malnutrition. Macronutrient ratios are unchanged. | ↑ ↑ ↑ |
| CR without protein restriction | CR where protein content is modified so that only calories are reduced and protein intake is not changed. | ↑ ↑ ↑ |
| Intermittent Fasting (IF) | CR variant with at least one day of fasting between feedings. Many classic CR studies used intermittent fasting protocols where mice were fed 3 times per week. | ↑ ↑ ↑ |
| Fasting-Mimicking (FMD) | Cyclic CR where a low-calorie, ketogenic diet is provided during the restricted phase. In mice, FMD cycles are typically 3–4 days followed by 3 days of refeeding. | ↑ ↑ |
| Ketogenic diet (KD) | Restriction of carbohydrates in order to induce ketosis. In mice, carbohydrates are limited to less than 1% of total calories. Note that KDs do not have to be low calorie, but the variations studies in mice resulted in reduced caloric consumption. | ↑ |
| Iso-caloric Diets | ||
| Protein restriction (PR) | In mice and rats, isocaloric protein restriction has been reported to extend lifespan, but the effects appear to be much smaller than CR and may be sex-specific in mice*. | ↑ |
| Essential Amino Acid Restriction | Restriction of methionine, tryptophan, or branched chain amino acid content in the diet. Essential amino acid restriction in mice typically involves reducing methionine by about 80%, tryptophan by about 40%, or branched chain amino acids by about 67%. It remains unclear what extent these interventions share similar mechanisms. | ↑ |
| Time Restricted Feeding (TRF) | Ad libitum feeding restricted to a specific period of the day. In people, a common TRF protocol is 16:8 (hours fasting: hours feeding). In mice 12:12 has been tested**. | ↑ |
| Isocaloric Intermittent Fasting | Intermittent fasting where the IF group consumes an equal number of calories as the control group by overfeeding during the ad libitum phase.*** | ↑ |
A recent report found that protein restriction increased lifespan in male mice but not female mice (55).
One study of only male mice reported 11% lifespan extension in “isocaloric” TRF mice, but the experimental mice consumed slightly but significantly less kcal/day than the control mice (40).
Every-other-day feeding increased lifespan in one study by 13% under roughly iso-caloric conditions (99).
Ketogenic diets
Ketogenic diets (KDs) refer to dietary compositions designed to maintain a constant state of ketogenesis, the metabolic production of ketone bodies (acetoacetate, beta-hydroxybutyrate, and acetone) as a byproduct of fat metabolism in the liver. This results in ketosis, a state of elevated ketone bodies in the blood that can then be taken up and metabolized by other tissues. KDs have been studied in humans for many decades as a treatment for epilepsy and have achieved mainstream popularity due to their palatability and effectiveness at inducing weight loss (16). In humans, the most common KD is typically very low in carbohydrates (less than 30-50 grams per day) with approximately 75% of calories derived from fats (17). Many other KD variations are possible, so long as carbohydrate levels remain low enough to induce ketogenesis, such as the popular high-protein Atkins Diet (18). Currently, the long-term health consequences of KDs in humans and the relative merits of low versus high protein KD diets are vigorously debated within the nutrition community, with little consensus beyond clear efficacy for epilepsy and weight loss.
KDs recently gained recognition for potential effects on biological aging with two 2017 papers reporting that a low carbohydrate, low protein KD is sufficient to increase mean lifespan as well as healthspan measures in mice (19, 20). In one study, a zero percent carbohydrate KD that achieved high levels of beta-hydroxybutyrate in blood was initiated at 12 months of age and given to the mice either continuously or in a cyclic fashion interspersed with control chow on a weekly basis (19). Interestingly, the continuous KD failed to increase lifespan while the cyclic KD increased mean, but not maximum, lifespan and improved both memory function and metabolic parameters late in life. In the other study, a low carbohydrate diet (12% carbohydrates) or a KD (less than 1% carbohydrates) were initiated at 12 months of age (20). Both diets appeared to increase median lifespan relative to control fed animals, with the KD resulting in a 13% increase in median lifespan and trend toward a smaller increase in maximum lifespan which did not reach statistical significance. Improvements in memory, motor function, and reduced cancer incidence were observed in the mice fed a KD in this study. Both studies observed reduced mTOR activity in the longer-lived mice eating a KD.
One important question is whether KD effects are mediated by ketone bodies directly. Ketone bodies produced by the liver enter circulation and are taken up by other tissues where they can directly enter the tricarboxylic acid cycle through acetyl-CoA, bypassing the need for glycolytic breakdown of glucose. The ketone body beta-hydroxybutyrate has also been implicated as a signaling molecule that can act via extracellular receptors to regulate histone acetylation and thereby impact gene expression (21). One study reported that beta-hydroxybutyrate supplementation could extend lifespan in C. elegans through a mechanism linked to reduced mTOR signaling (22), though there is not yet direct evidence that ketone body metabolism is sufficient to confer increased lifespan in mice. Ketone esters are reported to reduce anxiety-like behaviors and decrease amyloid beta and tau deposits in a mouse Alzheimer’s disease model (23), and have also been reported to reduce blood insulin and glucose levels and to inhibit mTOR signaling (24). Taken together, these findings are highly suggestive that ketone esters themselves could have anti-aging properties and highlight the importance of additional research in this area.
Intermittent Fasting and Fasting Mimicking Diets
Fasting has long been touted for its putative health benefits in different cultures, and recent years have seen a resurgence of research on possible anti-aging effects of diets that incorporate fasting or “fasting-mimicking” components. Reviews of this topic will often make blanket statements about the health benefits of intermittent fasting for numerous age-related conditions in mice, and while there is certainly evidence to support such assertions, the experimental protocols employed generally amount to CR (25, 26). Indeed, it appears that modern IF protocols are largely a rebranding of classic CR methods (e.g. see ref (3) where the CR mice were fed three times per week). Hungry laboratory mice (and any CR researcher will tell you that their mice are hungry) will typically eat all of the available food in their cage quickly compared to control-fed mice. Thus, even in a CR study where mice are fed daily, the restricted mice will typically be fasting for at least 18 hours between meals. This is not intended to downplay the potential importance of the physiological changes associated with fasting, such as ketogenesis, but is simply an observation that the majority of preclinical studies referring to fasting-based experimental protocols cannot be differentiated from CR because researchers rarely ensure isocaloric conditions (Table 1).
Over the past decade, “fasting-mimicking diets” (FMDs) have emerged as a highly studied cyclic variant of CR designed to induce metabolic responses akin to fasting through a nutrient dense, low-calorie diet. FMDs induce ketogenesis by restricting protein and simple carbohydrates while maintaining high fat levels (27, 28), and could therefore be considered as an intermittent KD. Initial studies in rodents showed that bi-monthly four-day FMD reduces both body and organ size and improves a wide range of age-related parameters, including adiposity, tumor burden, motor and cognitive function, neurogenesis, and median (but not maximum) lifespan (27). One interesting effect of cyclic FMD was the induction of atrophy/quiescence followed by vigorous regeneration and stem cell activation in several tissues. Similarly, metabolic parameters such as blood glucose, insulin, IGF-1, and IGF-1BP were reduced during the FMD phase and returned to control levels during a regular feeding regimen in both mice and human subjects (27, 28).
There is significant clinical interest in FMDs based on the rationale that their cyclic nature will increase compliance relative to traditional diets. In one randomized controlled cross-over study, tri-monthly FMD cycles of five days each reduced BMI, fasting blood glucose, and blood pressure in obese, prediabetic subjects, and subjects with hypertension, respectively (28). Cycles of FMD appear potentially beneficial in other clinical contexts such as multiple sclerosis, autoimmune diseases, and cancer (29-31). Use of FMDs to improve outcomes in combination with chemotherapy is a particularly active area of research, with initial studies indicating that FMDs increase tumor sensitivity to chemotherapy in mouse models of breast cancer and melanoma while reducing collateral toxicity to healthy cells (30, 31). However, a recent clinical trial failed to detect any difference in the specificity of chemotherapy in breast cancer patients undergoing FMD cycles (32), possibly due to low compliance (33).
Like IF, most FMD studies have been performed under conditions where the experimental group consumed fewer calories than the control group, and the extent to which isocaloric IF or FMDs have a significant impact on lifespan or age-related pathology remains unclear. Some FMD studies report that FMD and control mice consume energetically equivalent amounts of food when normalized to body weight (27), but because the FMD mice weigh less than control mice their total caloric intake is likely reduced. There is evidence that true isocaloric IF implemented as alternating feeding and fasting days (1:1 IF) is sufficient to induce ketogenesis during the fasting day and may improve metabolic homeostasis, stress resistance, and markers of inflammation compared to daily pair-fed mice (34). A 2-day feeding/1-day fasting isocaloric regimen (2:1 IF) also prevented weight and fat gain, maintained glucose and insulin homeostasis, and reduced adipocyte hypertrophy in mice fed an obesogenic diet, despite comparable energy intake. However, a recent 1:1 IF study over 3 weeks in lean, healthy people found that IF was less effective than isocaloric daily energy restriction and showed no benefits for measures of metabolic regulation or cardiovascular health (35). Because all of these studies were limited in scope and duration, future studies will need to assess whether isocaloric IF or FMDs have substantial long-term benefits on health and longevity in either rodents or people.
Time-restricted feeding (TRF)
Time restricted feeding can be considered as a variant of IF where subjects receive food every day, but only for a specified time window. Isocaloric TRF studies in rodents suggest improvements in several metabolic parameters, including glucose and insulin homeostasis, energy expenditure, liver pathology, and resistance to different obesogenic diets (36-38). Intriguingly, isocaloric TRF seems to promote and maintain intrinsic circadian rhythms in mice (36, 37), a phenotype also associated with CR (39). To the best of our knowledge, only one study has attempted to carefully examine the effect of isocaloric TRF on lifespan and age-related health outcomes in mice (40). In this report, which was limited to male mice, the TRF animals were trained to eat all of their food in a 12-hour window each day. The TRF mice were fed a diet intended to be isocaloric to the ad libitum group; however, the TRF animals still ended up eating less than the ad libitum fed animals. A 30% CR group in which the mice ate all of their food in a 3-hour window each day was also studied in parallel. The TRF group lived about 11% longer than the ad libitum group, on average, while the 30% CR group showed a 28% increase in mean lifespan. Circulating levels of beta-hydroxybutyrate were higher in the CR group but not in the TRF group.
Despite promising results of TRF in animal models, human studies are mixed. Some studies show only mild improvements (41) even when subjects naturally restricted themselves to 75-80% of their daily intake during feeding (42). Other studies indicate detrimental effects on glucose homeostasis (43). Notably, these studies designed their feeding window without regard for circadian variation. Larger, longer-term studies, carefully crafted around human circadian rhythms, are needed to determine whether TRF regimens can be beneficial to metabolic homeostasis and ultimately aging in humans.
Protein and amino acid restriction
The importance of protein as a dietary modulator of lifespan can be traced back to work in the 1920s demonstrating that trout raised on a protein deficient diet were both developmentally delayed and significantly longer-lived (44). A few years later, dietary protein restriction (PR) was found to delay development, sexual maturation, and signs of aging in rats (45). Since these early reports, numerous studies have described increased lifespan and reduced age-related pathology resulting from PR in rodents, which has been proposed to be mediated largely through reduced growth hormone, IGF, and mTOR signaling (46, 47).
As with IF and FMDs, a particularly challenging aspect of the literature related to PR is disentangling the effects of reduced caloric intake from specific effects of protein itself as a dietary macronutrient. Speakman and colleagues performed a detailed meta-analysis comparing published effects of dietary interventions on lifespan in mice and rats and concluded that, while there is evidence for lifespan extension from PR alone, the effects are substantially reduced compared to those reported associated with CR (13). This is consistent with work from Masoro and colleagues who compared isocaloric PR to classic CR in rats, and found that PR alone increased median lifespan by about 15% compared to about 50% increase from CR (48).
Nutritional geometry studies, carried out under ad libitum conditions where ratios of different macronutrients are varied across numerous diets, provide an independent line of evidence that dietary protein may have an outsized impact on longevity in insects and mice, relative to other macronutrients (49). In mice, for example, a comprehensive study of longevity effects across 25 different diets found that those with lower protein to carbohydrate ratios yield the longest maximum lifespans (50). The authors concluded that longevity and health are optimized in mice when protein is replaced with carbohydrate. It is important to note, however, that the absolute lifespans of the mice in this study were generally lower than those reported elsewhere for the same strain background (C57BL/6J), and the lowest energy diets failed to yield the longest lifespans (50). Further, out of all the diets studied, the one that resulted in the longest median lifespan (139 weeks) was quite high in protein (42% protein, 29% carbs, 29% fat). Thus, the relationship between dietary protein and longevity, at least under ad libitum conditions, appears to be quite complex and influenced both by other macronutrients and additional variables that are not yet understood.
In addition to reducing the availability of total dietary protein, there are several reports of lifespan extension from restriction of specific essential amino acids, which must come from the diet and cannot be synthesized endogenously. The earliest of these may be from Segal, who published in 1977 that restriction of dietary tryptophan delayed growth, reduced cancer, and increased lifespan in rats (51). Orentreich and colleagues later found that restriction of dietary methionine (or more properly sulfur amino acids which include both cysteine and methionine) similarly increased lifespan in rats (52). These seminal discoveries went largely unappreciated for many years but have gained attention as others reproduced and extended these findings to yeast, worms, fruit flies, and mice (53). More recently, restriction of the dietary branched chain amino acids (BCAAs) leucine, valine, and isoleucine has also been found to increase lifespan and delay age-related frailty in both fruit flies (54) and mice (55). BCAA restriction appears to increase lifespan via inhibition of mTOR signaling (discussed further below). Other studies show that restricting methionine and cysteine are key to promoting a protective DR-mediated stress response that is blocked with mTOR activation. Interestingly, this sulfur amino acid restriction appears to be mediated through a mechanism that involves increased production of hydrogen sulfide gas via the transsulfuration pathway (56). Unlike most of the other anti-aging diets including CR, mice restricted for particular amino acids appear to actually eat more food than control fed animals but fail to gain weight (57, 58).
Along with inhibition of mTOR, the hormone fibroblast growth factor 21 (FGF21) has emerged as an important mediator of longevity benefits from PR and perhaps also amino acid restriction. FGF21 is actively secreted in response to reduced dietary protein in both mice and humans and is required for metabolic improvements in response to PR (59). Remarkably, FGF21 overexpression in mice fed ad libitum is sufficient to robustly increase lifespan (60). There is also evidence that FGF21 can be similarly induced by methionine restriction (61) and KDs (62). It has been proposed that FGF21 modulates lifespan in mice primarily by reducing growth hormone and IGF-1 signaling in liver (60).
Do anti-aging diets work in the “real world”?
Fad diets spawned from legitimate scientific research are nothing new, and recent years have seen a notable infiltration of anti-aging diets into mainstream society. The renowned CR researcher Roy Walford attempted to popularize CR in the 1980s with his book “The 120 Year Diet: How to Double Your Vital Years”. Walford’s “CR Society” never grew beyond a small group of followers, likely because of the severe abstinence required to maintain a CR lifestyle, and Walford himself died at the age of 79, well short of the 120 years promised in the book title. Recently, however, several less stringent variations on CR have achieved greater popularity, including IF, TRF, PR, and KDs. Some researchers studying these nutritional interventions follow in Walford’s footsteps by practicing various forms of CR themselves and making dietary recommendations to the general public. Absent results from carefully designed clinical trials, this raises thorny questions around safety, efficacy, and scientific integrity.
When considering the evidence for anti-aging diets in humans, we address two primary considerations. First, how strong is the case that these interventions actually slow or reverse biological aging in people? Second, are there potential side effects which may offset any benefits for healthy longevity? Before delving into these topics, however, it is important to differentiate between health benefits from modulating biological aging versus effects which derive from being anti-obesogenic. CR, PR, and KDs are each used clinically to induce weight loss (63), and there is no question that weight loss in obese individuals can reduce disease risk. One rationale in favor of recommending these diets to the general public is the perception that they are all likely healthier, at least in the short-term, than the typical Western diet. These potential benefits in overweight individuals should not, however, be conflated with effects on biological aging.
Unfortunately, it is not currently possible to know whether CR-like diets impact biological aging in people (Text Box 2). Unlike mice, controlled studies would need to be performed over many years to assess long-term benefits for lifespan and healthspan in humans. The recent development of various “aging clocks” that accurately predict chronological age, and may soon be useful for predicting biological age, offer the possibility that this question may be addressable in the relatively near future (64). For now, however, the data remain correlative. One line of evidence often cited to support anti-aging effects of CR in natural human populations comes from studies of Okinawans, who inhabit a small Japanese island and smaller islands in the surrounding archipelago where the indigenous population historically consumed about 20% fewer calories than mainland Japan. Traditional Okinawan diets are very low protein (9% of total calories) and high carbohydrate (85% of total calories) (65). Historically, Okinawans enjoyed the longest life expectancy at birth and highest centenarian prevalence in the world, with remarkably low rates of age-associated diseases, like cancer, heart and cardiovascular disease, and diabetes (66). Another line of evidence for health benefits from CR comes from the Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) studies. These are a series of controlled clinical trials in normal and overweight adults subjected to a 25% reduction in caloric intake over periods ranging from a few months to two years. The results of these studies were generally consistent with improved clinical biomarkers of health such as decreased weight, enhanced insulin sensitivity and glucose tolerance, and improvements in major cardiometabolic risk factors (67). The CALERIE data are further complemented by uncontrolled studies of people self-practicing CR. Data from individuals self-practicing CR are also consistent with improved age-related health measures including reduced weight and fat mass, lower blood pressure and other markers of heart disease, and improved glucose tolerance and insulin action (68, 69).
Text Box 2. Common fictions about anti-aging diets.
FICTION: CR always “works”.
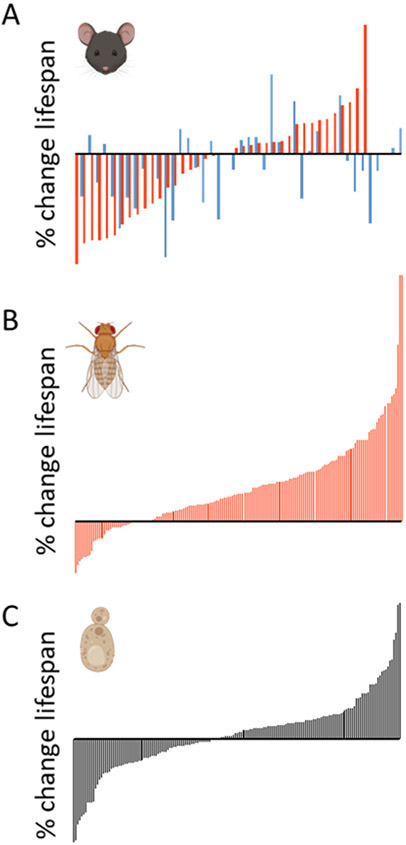
Although there are many reports of lifespan and healthspan extension from CR, there are also multiple published examples where CR failed to extend lifespan. Among these are studies of wild-derived mice (70) and studies of genetically inbred mouse strains (73). Of the two long-term studies in rhesus monkeys, one reported a substantial increase in lifespan, while the other failed to detect any significant change (97, 98). Although negative results can be challenging to interpret, the efficacy of CR even in laboratory animals appears to be highly dependent on sex, genetic background, the level of restriction employed, and other variables yet to be identified (Figure 1).
FICTION: CR extends lifespan only by preventing cancer.
While CR has been shown in many studies to have potent anti-cancer effects in rodents, it also delays age-related declines in immune, brain, heart, muscle, kidney, reproductive and other tissues (5). CR also extends lifespan in non-mammalian species that do not get cancer, such as budding yeast, fruit flies and nematode worms (6).
FICTION: Individual macronutrients are “good” or “bad” for aging.
Dietary composition, total caloric intake, and feeding interval all have the potential to impact longevity and healthspan. It is possible to extend lifespan in mice by limiting total caloric intake, limiting primarily carbohydrates, or limiting primarily protein or even specific amino acids (Table 1). The mechanisms underlying these effects are complex and still poorly understood, even in highly controlled environments.
FICTION: Anti-aging diets are known to slow aging in people.
Despite their recent popularization, there is not yet strong evidence that any of the anti-aging diets studied in laboratory animals have significant long-term health benefits in non-obese humans.
Despite these suggestive data, there are concerns that laboratory nutrition studies may introduce artifacts that limit translation to humans. One example of this is that laboratory strains of rodents have been subjected to strong selection for rapid growth and early reproduction, which may lead to sensitization to the lifespan extending effects of CR on growth-promoting signaling pathways such as growth hormone and mTOR (70). Another concern is that features of the laboratory environment may impact how animals respond to CR-like diets. Among these, mice and rats are typically housed in specific pathogen free conditions and receive daily supervision and veterinary care as needed. Due to small body size and low temperatures at which they are housed, mice expend upwards of half of the energy they consume just to maintain core body temperature (71). They experience consistent light/dark cycles and consume a refined, fixed composition chow for most of their adult life. None of these things are true in people, and it seems plausible that human environmental variation could have a large impact on health outcomes from dietary intervention. For example, severe CR can impair both immune function and wound healing (72), which could offset any potential lifespan-extending benefits under adverse environmental conditions where the immune system is challenged (for example a global viral pandemic) or in the absence of quality healthcare. Further complicating this interaction is the large variety in nutritional quality of human dietary composition, even among individuals who are consuming comparable total calories or macronutrient ratios.
The impact of genetic background may also limit translation from laboratory models to humans. Although not widely appreciated, genetic variation plays a large role in determining individual responses to CR in laboratory animals (Figure 1). In one important study, for example, a 40% reduction in caloric intake among recombinant inbred mouse lines significantly shortened lifespan in a greater number of genetic backgrounds than it extended (73). Sex-specific differences were also apparent, with CR having opposite effects on lifespan between sexes in some strains. While this particular study was underpowered at the individual genotype level (73), several others have reported large variation in the response to CR in individual mouse and rat strains (74), and strikingly similar genotype-dependent effects of DR by glucose restriction have been observed in budding yeast (75). The role of genetic variation in efficacy of other anti-aging diets is largely unexplored, but one recent study found that about one third of fruit fly strains experienced lifespan shortening in response to PR (76). It seems clear that a more detailed understanding of the mechanisms underlying variable response to anti-aging diets in laboratory and genetically diverse animals will be important for predicting both efficacy and risk in people.
Figure 1.
Percent lifespan change on restricted compared to unrestricted diet for genetically distinct strains of model organisms. In every case, each bar along the x-axis represents a unique genetic background and the percent change in lifespan of that strain in response to dietary restriction is shown on the y-axis. A) Change in mean lifespan for recombinant-inbred female (red) and male (blue) mice under ad libitum and 40% caloric restriction (CR) diets (67). B) Change in median lifespan in genetically variable female flies from a natural population under a 10-fold protein restriction (69, 70). C) Change in mean lifespan for single gene deletion mutant yeast under a 40-fold glucose restriction (68). Organism cartoons created using Biorender.com.
Differences in lifespan and age-associated nutritional requirements between humans and laboratory rodents present yet another layer of complexity when considering translation of anti-aging diets. While most animal studies examine the effects of lifelong nutritional intervention, very few people will maintain a CR (or PR or KD) lifestyle continuously over many decades of adulthood. Instead, repeated cycles of ad libitum and CR consumption is the norm. Detrimental effects of so-called “yo-yo dieting” are well documented and, taken to extremes, can result in a potentially fatal refeeding syndrome with severe consequences such as hypotension, kidney injury, and heart failure (77). Even moderate dietary interventions are not without some level of risk over the long-term. For example, one study found that low protein diets are associated with reduced mortality in young people but higher mortality in people over the age of 65 (78).
These correlative observations should provide a cautionary note. While many people tend to assume that dietary interventions are safe, the biological effects of these anti-aging diets are profound and generally less specific than pharmacological interventions. Like any drug, dietary interventions have a dose response profile and at high enough “doses” will lead to significant adverse effects and ultimately death. Among the potential side effects of CR-like diets are poor thermotolerance, loss of libido and sexual dysfunction, psychological problems, chronic fatigue, poor sleep, muscle weakness, susceptibility to infection, impaired wound healing, and social isolation (79). There is a very real likelihood that any given CR-like diet could enhance longevity in some people while shortening lifespan in others. The optimal nutritional strategy for longevity will certainly be different in different people, and there are few studies quantifying short-term or long-term side effects of anti-aging diets in adults.
mTOR inhibition: A common mechanism for anti-aging diets?
Although many important questions remain, research on anti-aging diets has had a large impact on the field by providing insight into fundamental mechanisms of aging. It goes without saying that the physiological consequences of dietary interventions are complex and multifactorial, even in relatively simple laboratory model organisms. Despite this, intriguing similarities across the spectrum of anti-aging diets in diverse model systems have emerged which suggest at least partially overlapping mechanisms of action. As alluded to above, these mechanisms appear to converge on key nodes in a highly conserved aging-regulatory network (7, 8). While several components of this network have been implicated in mediating aspects of CR (80), a case can be made that the mechanistic target of rapamycin (mTOR) is a particularly important and robust molecular transducer of diet-induced anti-aging signals (81).
The mTOR protein is a kinase which mediates nutrient response signaling by acting in two distinct complexes, mTORC1 and mTORC2. Both complexes are impacted by nutrient and growth cues, but mTORC1 has received the most scrutiny due to observations that its inhibition is sufficient to mimic the effects of CR on lifespan in yeast, worms, flies, and mice (82). This has been replicated both genetically and pharmacologically (rapamycin) in each of these model systems. Importantly, like CR, mTORC1 inhibition appears to broadly delay or reverse age-related phenotypes across multiple tissues in mice including brain, heart, liver, kidney, immune system, skeletal muscle, auditory system, adipose, ovaries, and the oral cavity (82-84).
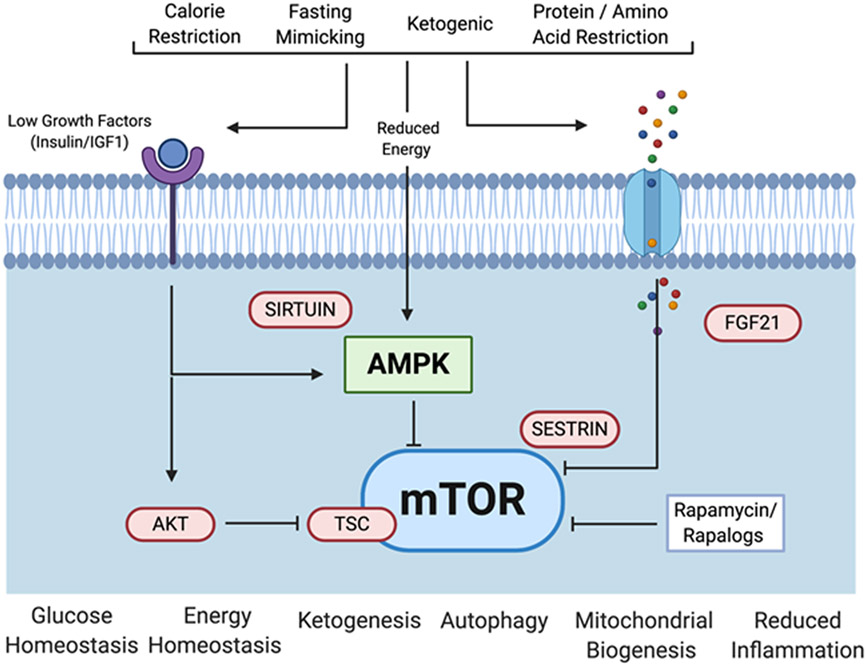
There is accumulating evidence that each of the anti-aging diets discussed here can inhibit mTORC1 signaling through both direct and indirect mechanisms (Figure 2). Specifically, mTORC1 is directly activated by leucine, an effect mediated in part by the sestrin family of proteins that inhibit mTORC1 only when not bound to leucine (85). Glucocorticoid signaling, which is induced by CR (86), also has an inhibitory effect on mTORC1 (87). mTORC1 is activated by the growth promoting hormone IGF-1 through Akt and the tuberous sclerosis complex (TSC), and reduced IGF-1 signaling is one of the classic hallmarks of CR and CR-like diets (5). AMPK becomes activated in response to CR and regulates mTORC1 through phosphorylation of TSC (81). Several processes are proposed to mediate the lifespan extending effects of CR downstream of mTORC1 inhibition. Covered in detail elsewhere, these include activation of autophagy, inhibition of mRNA translation, enhanced mitochondrial function, increased ketogenesis, improved stem cell function, and attenuation of senescence-associated inflammation (81, 88, 89).
Figure 2.
Diet modalities, molecular mechanisms, and downstream consequences of anti-aging diets. Dietary interventions that impact aging in mice limit one or more of the major dietary macromolecules and elicit cellular responses via a complex nutrient sensing network. Key components of this network which have been implicated in effects on lifespan and healthspan in various laboratory model organisms include mechanistic Target Of Rapamycin (mTOR), Fibroblast Growth Factor 21 (FGF21), Adenosine Monophosphate-activated Protein Kinase (AMPK), insulin/Insulin Growth Factor 1 (IGF-1) receptors, AK strain Transforming (AKT), sestrin, and sirtuins. Figure created using Biorender.com.
We recognize that many questions remain regarding the relative importance of mTORC1 in mediating effects of CR and other anti-aging interventions, and others have suggested that other factors such as sirtuins and AMPK are equally important (90). As described above, we favor the model that these factors all act together within a complex network that has yet to be fully characterized. Our focus here on mTORC1 reflects the fact that it appears to be a particularly useful node within this network for modulating aging, as evidenced by the robust and reproducible data supporting beneficial effects on lifespan and healthspan from pharmacological or genetic inhibition in yeast, worms, flies and mice (81, 82). We recognize that mTORC1 regulation is extremely complex, with tissue-dependent and circadian components that are still poorly understood. It is also clear that CR and mTOR inhibition have overlapping but distinct physiological effects such that treatment with rapamycin, for example, does not recapitulate all of the effects of CR and vice versa (91). We are not aware of genetic backgrounds that fail to show lifespan extension in response to mTORC1 inhibition in mice, and, unlike the case for CR, there have been no studies yet broadly assessing this question. High doses of rapamycin used to treat organ transplant patients are associated with multiple side effects and, although side effects are greatly reduced at lower doses in healthy people, it remains unclear whether mTORC1 inhibition is a useful therapeutic strategy to combat aging in humans. Undoubtedly, much remains to be understood regarding the various interactions between dietary nutrients, longevity pathways, and healthy aging.
Conclusion
Research on anti-aging dietary interventions that increase lifespan and healthspan in laboratory models has greatly facilitated our mechanistic understanding of biological aging. Evolutionarily conserved signaling pathways appear to mediate many of the overlapping effects of anti-aging diets, and their study has provided molecular targets for pharmacological interventions which may prove useful for increasing healthy longevity and reducing disease burden in humans. Although these diets have already achieved mainstream popularity in some cases, many questions remain about individual outcomes and relative risks associated with their long-term implementation. Future research should focus both on better understanding the cellular and molecular mediators of anti-aging diets under highly controlled laboratory conditions as well as the impact of genetic and environmental variation on health outcomes associated with these diets.
Acknowledgements
The authors would like to thank Daniel Promislow, Tammi Kaeberlein, and Christopher Morrison for careful review of the manuscript. All authors contributed to drafting the manuscript.
Funding
M.K. and A.B. are supported by the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (P30 AG013280). M.B.L. was supported by the National Institutes of Health (NIH) Alzheimer’s Disease Training Program (T32 AG052354). C.M.H. was supported by NRSA (F32 DK115137) and the Pennington Biomedical NORC-Nutrition and Metabolic Health Through the Lifespan (P30 DK072476).
Footnotes
Competing interests
We declare no competing interests.
Dedication
The authors would like to dedicate this work to the memory of James R. Mitchell, whose recent untimely passing could not overshadow his important contributions to the field of aging research.
References
- 1.McCay CM, Crowell MF, Maynard LA, The effect of retarded growth upon the length of life and upon ultimate size. The Journal of nutrition 10, 63–79 (1935). [PubMed] [Google Scholar]
- 2.Osborne TB, Mendel LB, Ferry EL, The Effect of Retardation of Growth Upon the Breeding Period and Duration of Life of Rats. Science 45, 294–295 (1917). [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Walford RL, Fligiel S, Guthrie D, The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition 116, 641–654 (1986). [DOI] [PubMed] [Google Scholar]
- 4.Speakman JR, Mitchell SE, Caloric restriction. Molecular aspects of medicine 32, 159–221 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Weindruch R, Walford RL, The retardation of aging and disease by dietary restriction. (Thomas CC, Springfield, Ill., U.S.A., 1988), pp. xvii, 436 p. [Google Scholar]
- 6.Kapahi P, Kaeberlein M, Hansen M, Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res Rev 39, 3–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu T et al. , Mechanisms of Calorie Restriction: A Review of Genes Required for the Life-Extending and Tumor-Inhibiting Effects of Calorie Restriction. Nutrients 11, 3068 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana L, Partridge L, Longo VD, Extending healthy life span--from yeast to humans. Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba T et al. , Development of calorie restriction mimetics as therapeutics for obesity, diabetes, inflammatory and neurodegenerative diseases. Current genomics 11, 562–567 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G, Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metabolism 29, 592–610 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Harrison DE et al. , Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strong R et al. , Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speakman JR, Mitchell SE, Mazidi M, Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp Gerontol 86, 28–38 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Anderson RM, Weindruch R, The caloric restriction paradigm: implications for healthy human aging. American journal of human biology : the official journal of the Human Biology Council 24, 101–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingram DK, de Cabo R, Calorie restriction in rodents: Caveats to consider. Ageing Res Rev 39, 15–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampaio LP, Ketogenic diet for epilepsy treatment. Arq Neuropsiquiatr 74, 842–848 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Hall KD et al. , Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. The American journal of clinical nutrition 104, 324–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins RC, Dr. Atkins' diet revolution; the high calorie way to stay thin forever. (D. McKay Co., New York,, 1972), pp. x, 310 p. [Google Scholar]
- 19.Newman JC et al. , Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab 26, 547–557 e548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts MN et al. , A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab 26, 539–546 e535 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman JC, Verdin E, Ketone bodies as signaling metabolites. Trends Endocrinol Metab 25, 42–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards C et al. , D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY) 6, 621–644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashiwaya Y et al. , A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging 34, 1530–1539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava S et al. , Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26, 2351–2362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson MP, Longo VD, Harvie M, Impact of intermittent fasting on health and disease processes. Ageing Res Rev 39, 46–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Cabo R, Mattson MP, Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 381, 2541–2551 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Brandhorst S et al. , A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei M et al. , Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi IY et al. , A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell reports 15, 2136–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caffa I et al. , Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Biase S et al. , Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 30, 136–146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Groot S et al. , Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat Commun 11, 3083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernieri C, Ligorio F, Zattarin E, Rivoltini L, de Braud F, Fasting-mimicking diet plus chemotherapy in breast cancer treatment. Nat Commun 11, 4274 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anson RM et al. , Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 100, 6216–6220 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templeman I et al. , A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med 13, (2021). [DOI] [PubMed] [Google Scholar]
- 36.Hatori M et al. , Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Lopez N et al. , System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metab 26, 856–871 e855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaix A, Zarrinpar A, Miu P, Panda S, Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Froy O, Miskin R, The interrelations among feeding, circadian rhythms and ageing. Prog Neurobiol 82, 142–150 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Mitchell SJ et al. , Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab 29, 221–228 e223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stote KS et al. , A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. The American journal of clinical nutrition 85, 981–988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cienfuegos S et al. , Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab 32, 366–378 e363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlson O et al. , Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism 56, 1729–1734 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCay CM, Bing FC, Dilley WE, Factor H in the Nutrition of Trout. Science 67, 249–250 (1928). [DOI] [PubMed] [Google Scholar]
- 45.Slonaker James Rollin, The effect of different per cents of protein in the diet VII. Life span and cause of death. Am J Physiol 2:266–275, (1931). [Google Scholar]
- 46.Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R, Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci 65, 695–703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirzaei H, Raynes R, Longo VD, The conserved role of protein restriction in aging and disease. Curr Opin Clin Nutr Metab Care 19, 74–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu BP, Masoro EJ, McMahan CA, Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. Journal of gerontology 40, 657–670 (1985). [DOI] [PubMed] [Google Scholar]
- 49.Simpson SJ et al. , Dietary protein, aging and nutritional geometry. Ageing Res Rev, (2017). [DOI] [PubMed] [Google Scholar]
- 50.Solon-Biet SM et al. , The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segall P, Long-term tryptophan restriction and aging in the rat. Aktuelle Gerontol 7, 535–538 (1977). [PubMed] [Google Scholar]
- 52.Orentreich N, Matias JR, DeFelice A, Zimmerman JA, Low methionine ingestion by rats extends life span. The Journal of nutrition 123, 269–274 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Hine C, Mitchell JR, Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp Gerontol 68, 26–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J et al. , Sestrin is a key regulator of stem cell function and lifespan in response to dietary amino acids. Nature Aging, (2020). [DOI] [PubMed] [Google Scholar]
- 55.Richardson NE et al. , Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nature Aging, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hine C et al. , Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasek BE et al. , Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol 299, R728–739 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fontana L et al. , Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell reports 16, 520–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laeger T et al. , FGF21 is an endocrine signal of protein restriction. The Journal of clinical investigation 124, 3913–3922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y et al. , The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lees EK et al. , Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13, 817–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badman MK et al. , Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5, 426–437 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Koliaki C et al. , Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare (Basel) 6, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhavoronkov A, Mamoshina P, Deep Aging Clocks: The Emergence of AI-Based Biomarkers of Aging and Longevity. Trends Pharmacol Sci 40, 546–549 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Willcox BJ et al. , Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Ann N Y Acad Sci 1114, 434–455 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Willcox BJ, Willcox DC, Suzuki M, Demographic, phenotypic, and genetic characteristics of centenarians in Okinawa and Japan: Part 1—centenarians in Okinawa. Mechanisms of Ageing and Development 165, 75–79 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Dorling JL et al. , Effects of caloric restriction on human physiological, psychological, and behavioral outcomes: highlights from CALERIE phase 2. Nutrition Reviews 79, 98–113 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fontana L, Meyer TE, Klein S, Holloszy JO, Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the National Academy of Sciences of the United States of America 101, 6659–6663 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana L, Klein S, Holloszy JO, Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordrecht, Netherlands) 32, 97–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harper JM, Leathers CW, Austad SN, Does caloric restriction extend life in wild mice? Aging Cell 5, 441–449 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Škop V et al. , Mouse Thermoregulation: Introducing the Concept of the Thermoneutral Point. Cell reports 31, 107501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stechmiller JK, Understanding the role of nutrition and wound healing. Nutr Clin Pract 25, 61–68 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF, Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swindell WR, Dietary restriction in rats and mice: a meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev 11, 254–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schleit J et al. , Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell 12, 1050–1061 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin K et al. , Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila. PLoS Genet 16, e1008835 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crook MA, Refeeding syndrome: Problems with definition and management. Nutrition 30, 1448–1455 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Levine ME et al. , Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Most J, Tosti V, Redman LM, Fontana L, Calorie restriction in humans: An update. Ageing Res Rev 39, 36–45 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G, Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab 29, 592–610 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Kennedy BK, Lamming DW, The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab 23, 990–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson SC, Rabinovitch PS, Kaeberlein M, mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altschuler RA et al. , Rapamycin Added to Diet in Late Mid-Life Delays Age-Related Hearing Loss in UMHET4 Mice. Front Cell Neurosci 15, 658972 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.An JY et al. , Rapamycin rejuvenates oral health in aging mice. eLife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolfson RL et al. , Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel NV, Finch CE, The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol Aging 23, 707–717 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP, Glucocorticoid-induced skeletal muscle atrophy. The international journal of biochemistry & cell biology 45, 2163–2172 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Weichhart T, mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 64, 127–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS, Modulating mTOR in aging and health. Interdisciplinary topics in gerontology 40, 107–127 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Baur JA et al. , Dietary restriction: standing up for sirtuins. Science 329, 1012–1013; author reply 1013–1014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fok WC et al. , Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci 68, 108–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy BK et al. , Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaeberlein M, How healthy is the healthspan concept? GeroScience 40, 361–364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selvarani R, Mohammed S, Richardson A, Effect of rapamycin on aging and age-related diseases—past and future. GeroScience, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee MB, Kaeberlein M, Translational geroscience: From invertebrate models to companion animal and human interventions. Translational Medicine of Aging 2, 15–29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colman RJ et al. , Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 325, 201–204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colman RJ et al. , Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5, 3557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie K et al. , Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice. Nat Commun 8, 155 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]