Abstract
Background
Elevated serum ferritin levels (SFLs) was previously reported to be related with hepatic histologic severity and advanced liver fibrosis among non‐alcoholic fatty liver disease (NAFLD) patients. However, whether NAFLD influences SFLs remains uncertain and needs more clinical evidences. This study explored the differences of SFLs in US adults with or without NAFLD.
Methods
We conducted a cross‐sectional study of 3689 participants aged 18–80 years using the National Health and Nutrition Examination Survey (NHANES) 2017–2018 cycle. NAFLD status was confirmed based on controlled attenuation parameter (CAP) values ≥274 dB/m through vibration controlled and transient elastography (VCTE). We performed weighted multivariable logistic regression models to evaluate the associations between NAFLD and SFLs in different age and gender.
Results
There was a positive association between NAFLD and SFLs in all three models (model 1:β = 23.07, 95% CI: 10.32, 35.81; model 2:β = 23.68, 95% CI: 10.86, 36.50; model 3:β = 13.86, 95% CI: 0.29, 27.43). After adjusting for the covariates, this positive association persisted in females (β = 16.22, 95% CI: 2.81, 29.62). Further, relationships between NAFLD and SFLs were significantly different in various age groups. In the subgroup stratified by gender, their associations further differed. In males, the positive association was more prominent in 50–64 age group (β = 70.89, 95% CI: 25.14, 116.64). In females, this positive association was more prominent in 18–34 age group (β = 20.72, 95% CI: 7.45, 33.99). However, no correlations between severe steatosis, significant fibrosis, advanced fibrosis, cirrhosis, and SFLs in adults with NAFLD were found.
Conclusion
This study indicated that US adults suffered with NAFLD had significantly higher SFLs compared with their counterparts in non‐NAFLD group. Moreover, the associations between NAFLD and SFLs further differed by age and gender.
Keywords: ferritin, NAFLD, NHANES, steatosis, vibration controlled and transient elastography
Elevated serum ferritin levels were adequately proved to be related with histologic severity and advanced fibrosis among non‐alcoholic fatty liver disease (NAFLD) patients. However, whether NAFLD status influence serum ferritin levels remain uncertainly and are still lack of evidence form large scale cohort studies. This current study explored the differences in serum ferritin levels in US adults with or without NAFLD in a large, nationwide database. The present study primarily demonstrated that US adults with NAFLD had significantly higher serum ferritin levels compared with those without NAFLD. Serum ferritin levels in NAFLD group have an average elevation with 13.86 ng/ml compared with that in non‐NAFLD group on the whole. Moreover, the association between NAFLD status and serum ferritin levels differed by age and gender.
![]()
1. INTRODUCTION
The prevalence and incidence of non‐alcoholic fatty liver disease (NAFLD) has increased rapidly in the past two decades and now affects more than 25% of the global population. 1 Especially in the United States, NAFLD has also become the most common chronic liver disease that affects approximately 30% among adults. 2 Histological changes of NAFLD range from isolated steatosis (non‐alcoholic fatty liver, NAFL), progressive non‐alcoholic steatohepatitis (NASH), significant or progressive fibrosis, to irreversible cirrhosis, even hepatocellular carcinoma (HCC) based on FLIP‐SAF scores. 3 , 4 NAFLD is a type of metabolic stress‐induced liver injury closely related to insulin resistance (IR) and genetic susceptibility. 5 It cannot only cause chronic liver injury and advanced fibrosis but also is closely associated with a high incidence of metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), atherosclerotic cardiovascular disease, and colorectal tumors. 6 What is noteworthy that NAFLD greatly raised both hepatic and extrahepatic mortality rate. Therefore, it is worthwhile to early diagnose and properly manage NAFLD patients in order to prevent NAFLD‐related end‐stage diseases and death.
Ferritin, the primary iron‐storage protein, is commonly detected in both the liver and blood circulating in the setting of systemic inflammation. 7 Higher serum ferritin levels (SFLs) have previously been reported to be related with higher prevalence of NAFLD, severe steatosis or advanced fibrosis in NAFLD patients. 8 , 9 In fact, researchers are seeking to develop serum ferritin as a biomarker to reflect physiopathologic changes of the liver in NAFLD patients. However, some controversial findings were also reported in the correlations of NAFLD status and SFLs. 10 A study by Angulo P et al. revealed serum ferritin levels had a weak diagnostic accuracy in picking liver fibrosis out from NAFLD patients. Little is known about how genders and different age stages influence their relationship. Moreover, the effect of NAFLD status on the SFLs is still lack of evidences form large scale cohort studies. Therefore, the correlation of NAFLD status and SFLs is largely unknown and need to be further clarified.
Here, we analyzed data from National Health and Nutritional Examination Survey (NHANES) 2017–2018 and investigated the correlation between NAFLD status and SFLs in American adult population. We also further evaluated the relationships between NAFLD and SFLs based on different age and gender groups.
2. RESEARCH DESIGN AND METHODS
2.1. Data source
Our present study is relied on data collected from the NHANES 2017–2018 cycle. It was conducted by the National Center for Health Statistics (NCHS) of the Center for Disease Control and Prevention (CDC) in the United States. 11 As a large ongoing well‐designed cross‐sectional survey program, NHANES included individuals based on a stratified, multistage, clustered probability sampling design of the American general population to guarantee nationally representative. 12 The design has a superiority in obtaining enough data on minorities in the United States through oversampling of Asian, Hispanic, and non‐Hispanic black, older individuals with low income. 12
The complete survey was composed of a structured interview implemented in home, followed by a standardized health checkup including questionnaires, physical examination, and laboratory tests at a mobile examination center (MEC). 12 Full methodology of data collection is available elsewhere. 13 The NHANES survey protocol was approved by the NCHS Research Ethics Review Board and informed consents were signed by all participants. Since information in the dataset cannot be identified, our analysis was waived by the Institutional Review Board at our institution. 12
2.2. Analysis sample
Among the 9254 individuals participated in 2017–2018 NHANES cycle, we excluded 550 participants without available data of MEC exam, 258 participants considered ineligible for different reasons (currently pregnant, unable to lie down, existing implanted electronic medical device or lesions in measurement location), 2459 participants because of participant refusal, physical, or technical limitations or limited time for vibration controlled and transient elastography (VCTE), 493 participants with partial VCTE exam (including 257 participants with fasting for less than 3 h, 129 participants with inability of obtaining 10 valid measurements, and 107 participants with an IQR/median LSM values ≥ 30%) resulting in a population of 5494 participants with attendant MEC visit and valid VCTE data. Further, we excluded 112 participants with evidence of viral hepatitis B and C, 752 participants with significant alcohol consumption, 258 participants without available ferritin data, and 682 participants aged <18 years. Finally, a sample of 3689 participants was enrolled in our analysis (Figure 1).
FIGURE 1.
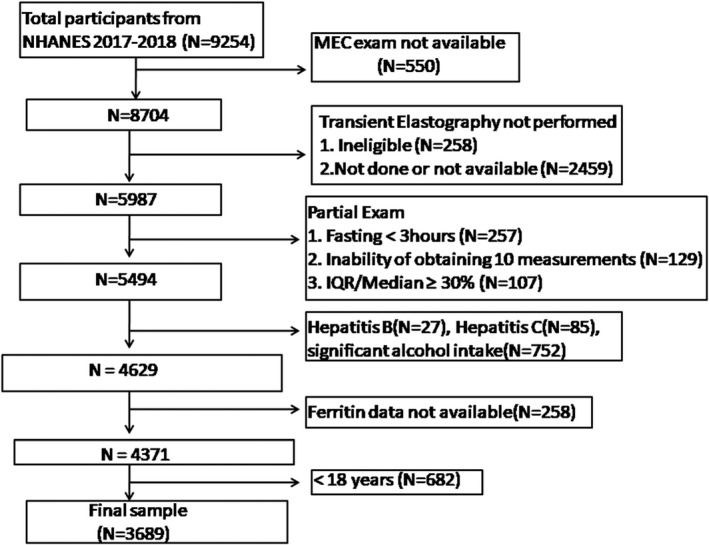
Flow‐chart of the study participants
2.3. Laboratory tests and clinical data
Body measurements of these participants including weight (kg), height (cm), and waist circumference (cm) were performed by NHANES staff during the MEC visit. Body mass index (BMI) was then calculated as dividing kilograms by weight in meters squared. According to the American Diabetes Association Criteria, a diagnosis of T2DM was made if any of the following conditions existed 14 : (1) Self‐reported diabetes; (2) Using antidiabetic medicines; (3) Fasting plasma glucose ≥126 mg/dl (7 mmol/L);(4) Random plasma glucose ≥200 mg/dl (11.1 mmol/L); (5) Hemoglobin A1c (HbA1c) level ≥6.5%(48 mmol/mol).
Information on age, gender, race, smoked over 100 cigarettes in lifetime, and drink at least 12 alcohol drinks in lifetime were obtained through self‐report. Laboratory methods for measuring serum glucose, HbA1c, triglyceride, total cholesterol, high density lipoprotein (HDL)‐cholesterol, platelet count (PLT), alanine aminotransferase (ALT),aspartate aminotransferase (AST), γ‐glutamyltranspeptidase (GGT), serum albumin, serum creatinine, uric acid, and ferritin were clarified in detail. 13 Hepatitis C virus (HCV) infection was confirmed by positive for antibody test and/or presence of viral RNA and hepatitis B virus (HBV) infection was defined as positive for surface antigen test, as previously described. 15 Alcohol intake was assessed according to investigation on the frequency and amounts of alcohol drinking during the past year. It was regarded significant alcohol intake if >3 drinks per day for male and >2 drinks per day for female. 16
2.4. Vibration controlled and transient elastography
As a noninvasive technique to assess the prevalence and severity of NAFLD, VCTE is widely adopted by physicians in clinical practice. It was well validated to have a high accuracy in detecting presence of both liver steatosis through controlled attenuation parameter (CAP) and fibrosis through liver stiffness measurement (LSM). 17 In 2017–2018 cycle, NHANES technicians used FibroScan® model 502 V2 Touch (Echosens) equipped with a medium (M) and extra‐large (XL) probes to perform VCTE exam for the participants. 12 Initially, the M probe was selected unless the machine indicated to use the XL probe. NHANES staff placed the participants in a supine position with the right arm completely abducted and then scanned the right liver lobe via an intercostal pathway to measure liver steatosis and stiffness. CAP values were described in decibels per meter (dB/m) and LSM values were expressed in kilopascals (kPa).
Vibration controlled and transient elastography exams were considered reliable on the condition that at least 10 LSM values were acquired after a fasting time of at least 3 h, with an interquartile (IQRe) range/median <30%. 18 The participants were regarded indicative of NAFLD status if CAP values ≥274 dB/m, as the cut‐off manifested 90% sensitivity in identifying any degree of liver steatosis according to a recent landmark research. 19 A cut‐off of median LSM ≥8 kPa was adopted to identify the participants with significant (≥F2) fibrosis since it demonstrated a high accuracy when compared with liver biopsy as previously reported. 20 In line with a remarkable research, 18 severe steatosis (S3) defined as CAP ≥ 302, advanced fibrosis (≥F3) defined as LSM ≥ 9.7 kPa, and cirrhosis (F4) defined as LSM ≥ 13.6 kPa were identified. We confirmed the cut‐off selection before analyzing the data to avoid possible bias.
2.5. Exposure and outcome factors
Exposure factor in this study is NAFLD status. NAFLD was defined as the following criteria 19 : CAP values ≥274 dB/m after exclusion of Hepatitis B or C virus infection and significant alcohol intake. Outcome factor in this study is SFLs. Ferritin was measured on the Roche Cobas® e601 starting from the first incubation period that combined a specific antibody and a labeled specific antibody into sandwich complex. The main task of the second incubation period is to add particles, causing the complex bind to the solid phase. The reaction mixture is sucked into a measuring cell where the microparticles are magnetically captured to the surface of the electrode. Unbound substances are then removed. Application of a voltage to the electrode then induces chemiluminescent emission which is measured by a photomultiplier. Results are determined via a calibration curve. The more detailed information can be found in https://wwwn.cdc.gov/Nchs/Nhanes/2017‐2018/FERTIN_J.htm
2.6. Statistical methods
The NHANES sample weights were taken into account as recommended by NCHS. Data are expressed as numbers and weighted proportions for categorical variables and weighted Mean ± SD for continuous variables. Statistical analyses were undertaken using package R version 3.4.3 (http://www.R‐project.org) and EmpowerStats software (http://www.empowerstat.com). A two‐tailed value of p value <0.05 was considered statistically significant. The association of NAFLD status with SFLs in US adults was assessed by multivariable logistic regression models. According to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines, 21 three models were created in our study: model 1: no covariates were adjusted; model 2: gender, age, and race were adjusted. Model 3: the covariates presented in Table 1 were adjusted. Subgroup analysis stratified by age and gender were also performed.
TABLE 1.
Weight characteristics of study sample with and without NAFLD status
|
Non‐NAFLD (n = 2085) |
NAFLD (n = 1604) |
p Value | |
|---|---|---|---|
| Age (years) | 45.16 ± 18.03 | 52.73 ± 16.26 | <0.0001 |
| Gender (%) | |||
| Male | 45.56 | 53.35 | 0.0010 |
| Female | 54.44 | 46.65 | |
| Race/Ethnicity (%) | |||
| Mexican American | 6.46 | 10.72 | 0.0007 |
| Other Hispanic | 7.60 | 5.62 | |
| Non‐Hispanic White | 61.56 | 63.88 | |
| Non‐Hispanic Black | 12.85 | 9.61 | |
| Non‐Hispanic Asian | 6.11 | 6.81 | |
| Other Race | 5.43 | 3.36 | |
| Diabetes (%) | |||
| No | 84.60 | 67.00 | <0.0001 |
| Yes | 15.40 | 33.00 | |
| BMI (Kg/m2) | 26.62 ± 5.53 | 32.98 ± 6.80 | <0.0001 |
| Waist circumference (cm) | 92.58 ± 13.84 | 109.53 ± 14.93 | <0.0001 |
| Laboratory features | |||
| Total cholesterol (mg/dl) | 185.65 ± 40.34 | 189.52 ± 42.71 | 0.0553 |
| HDL‐cholesterol (mg/dl) | 56.58 ± 14.44 | 49.79 ± 14.35 | <0.0001 |
| Triglyceride (mg/dl) | 90.78 ± 57.54 | 143.85 ± 138.32 | <0.0001 |
| HbA1c (%) | 5.47 ± 0.66 | 5.99 ± 1.14 | <0.0001 |
| AST (IU/L) | 20.57 ± 10.81 | 21.93 ± 9.31 | 0.0064 |
| ALT (IU/L) | 19.27 ± 14.89 | 25.53 ± 16.24 | <0.0001 |
| GGT (IU/L) | 23.11 ± 25.09 | 32.66 ± 33.61 | <0.0001 |
| Serum albumin (g/L) | 40.92 ± 3.17 | 39.97 ± 3.07 | <0.0001 |
| Platelet count (109/L) | 235.69 ± 60.44 | 241.35 ± 62.18 | 0.0569 |
| Serum creatinine (mg/dl) | 0.87 ± 0.29 | 0.89 ± 0.30 | 0.2627 |
| Uric acid (mg/dl) | 5.14 ± 1.36 | 5.82 ± 1.42 | <0.0001 |
| Ferritin (ng/ml) | 131.90 ± 142.36 | 166.41 ± 161.36 | <0.0001 |
| CAP (dB/m) | 221.78 ± 36.85 | 322.20 ± 36.09 | <0.0001 |
| LSM (kPa) | 4.94 ± 3.57 | 6.37 ± 4.84 | <0.0001 |
Mean ± SD was for continuous variables. p‐Value was calculated by weight linear regression model. % was for categorical variables. p‐Value was calculated by weighted chi‐square test.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; LSM, liver stiffness measurement; NAFLD, non‐alcoholic fatty liver disease.
3. RESULTS
A total of 3689 participants aged 18–80 years were included, with the weighted characteristics according to NAFLD or non‐NAFLD was presented in Table 1. There were significant differences in the distribution of baseline characteristics between NAFLD group and non‐NAFLD group. Compared with non‐NAFLD group, the participants with NAFLD were elder, more likely to be male, more Mexican American or non‐Hispanic White and less non‐Hispanic Black, had much higher BMI and waist circumference, higher LSM values, triglyceride, glycohemoglobin, AST, ALT, uric acid, and had lower serum albumin and HDL‐cholesterol levels (p < 0.05 for each). It is noteworthy that SFLs in US adults in NAFLD group were significantly higher compared with their counterpart in non‐NAFLD group. However, no significant differences were found in levels of PLT and serum creatinine.
According to three different multivariate linear regression models, the results for the participants aged 18–80 years, and 18–34 years, 35–49 years, 50–64 years, 65–80 years were shown in Tables 2, 3, 4, 5, 6, respectively. There was a significantly positive association between NAFLD status and SLFs in all three models (model 1:β = 23.07, 95% CI:10.32, 35.81; model 2:β = 23.68, 95% CI:10.86, 36.50; model 3:β = 13.86, 95% CI: 0.29, 27.43). After adjusting for all the covariates, this positive association only persisted in females (β = 16.22, 95% CI: 2.81, 29.62), but not existed in males (β = 10.96, 95% CI: −12.56, 34.48). These results are presented in Table 2.
TABLE 2.
Associations between NAFLD status and serum ferritin (ng/ml) in adults aged 18–80 years
| Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p | |
|---|---|---|---|
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 23.07 (10.32, 35.81), 0.0004 | 23.68 (10.86,36.50), 0.0003 | 13.86 (0.29, 27.43), 0.0454 |
| Females | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 10.59 (−3.00, 24.18), 0.1268 | 11.43 (−2.22, 25.07), 0.1008 | 16.22 (2.81, 29.62), 0.0178 |
| Males | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 32.79 (11.01, 54.57), 0.0032 | 32.12 (9.99, 54.26), 0.0045 | 10.96 (−12.56, 34.48), 0.3611 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender (not adjusted for in the subgroup analyses), race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
TABLE 3.
Associations between NAFLD status and serum ferritin (ng/ml) in adults aged 18–34 years
| Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p | |
|---|---|---|---|
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 52.74 (37.76, 67.73), <0.0001 | 48.52 (33.46,63.58), <0.0001 | 25.37 (9.17, 41.56), 0.0022 |
| Females | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 22.84 (11.50, 34.18) <0.0001 | 23.33 (11.84,34.81), <0.0001 | 20.72 (7.45, 33.99) 0.0024 |
| Males | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 74.41 (48.33,100.48), <0.0001 | 63.47 (37.20,89.74), <0.0001 | 18.85 (−9.41, 47.12), 0.1919 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender (not adjusted for in the subgroup analyses), race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
TABLE 4.
Associations between NAFLD status and serum ferritin (ng/ml) in adults aged 35–49 years
| Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p | |
|---|---|---|---|
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | −0.92 (−22.51, 20.67), 0.9334 | −3.59 (−25.54, 18.36), 0.7487 | −27.26 (−53.47,−1.05), 0.0419 |
| Females | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | −1.52 (−15.31, 12.27) 0.8287 | −2.27 (−16.29, 11.76) 0.7517 | −8.13 (−24.65, 8.39) 0.3356 |
| Males | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | −0.18 (−45.98, 45.62) 0.9938 | −8.27 (−56.19, 39.65) 0.7354 | −49.11 (−105.46,7.24) 0.0887 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender (not adjusted for in the subgroup analyses), race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
TABLE 5.
Associations between NAFLD status and serum ferritin (ng/ml) in adults aged 50–64 years
| Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p | |
|---|---|---|---|
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 36.76 (8.83, 64.69) 0.0100 | 36.35 (8.40, 64.31) 0.0110 | 52.41 (25.67, 79.14) 0.0001 |
| Females | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 17.39 (−7.37, 42.15) 0.1692 | 17.23 (−7.56, 42.01) 0.1736 | 16.61 (−6.42, 39.63) 0.1581 |
| Males | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 59.47 (6.35, 112.60) 0.0287 | 53.15 (−0.57,106.87) 0.0530 | 70.89 (25.14,116.64) 0.0025 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender (not adjusted for in the subgroup analyses), race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
TABLE 6.
Associations between NAFLD status and serum ferritin (ng/ml) in adults aged 65–80 years
| Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p | |
|---|---|---|---|
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 0.63 (−27.08, 28.33) 0.9647 | 6.53 (−21.63, 34.69) 0.6495 | −1.54 (−29.40, 26.32) 0.9140 |
| Females | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | 5.32 (−33.76, 44.40) 0.7897 | 12.11 (−27.85,52.06) 0.5529 | 24.25 (−13.93,62.43) 0.2138 |
| Males | |||
| Non‐NAFLD | Reference | Reference | Reference |
| NAFLD | −3.91 (−43.20, 35.38) 0.8455 | 5.85 (−34.21, 45.91) 0.7749 | −25.71 (−67.35,15.92), 0.2268 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender (not adjusted for in the subgroup analyses), race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
After controlling for potential confounding factors, the associations between NAFLD status and SFLs were different in various age groups. The association remained positive in 18–34 age group (β = 25.37, 95% CI: 9.17, 41.56) and 50–64 age group (β = 52.41, 95% CI: 25.67, 79.14), became negative in 35–49 age group (β = −27.26, 95% CI: −53.47, −1.05), but did not remain significant in 65–80 age group (β = −1.54, 95% CI: −29.40, 26.32). In the subgroup analysis stratified by gender, this association further differed after adjusting for all the covariates. In males, this significantly positive association was more prominent (β = 70.89, 95% CI: 25.14, 116.64) in the 50–64 age group, but not existed in 18–34 age group (β = 18.85, 95% CI: −9.41, 47.12), 35–49 age group (β = −49.11, 95% CI: −105.46, 7.24) and 6580 age group (β = −25.71, 95% CI: −67.35, 15.92). In females, this significantly positive association was more prominent (β = 20.72, 95% CI: 7.45, 33.99) in the 18–34 age group, but not existed in 50–64 age group (β = 16.61, 95% CI: −6.42, 39.63), 65–80 age group (β = 24.25, 95% CI: −13.93, 62.43), and the 35–49 age group (β = −8.13, 95% CI: −24.65, 8.39). These results are presented in Tables 3, 4, 5, and 6.
We further investigate the correlation between degree of steatosis and SFLs in adults with NAFLD; however, no significant differences were found in severe steatosis with SFLs (β = −7.3, 95% CI: −29.0, 14.4) after controlling for potential confounding factors. Similarly, there were no significant differences found in significant fibrosis (β = 5.9, 95% CI: −29.5, 41.2), advanced fibrosis (β = −0.7, 95% CI: −29.8, 28.4), and cirrhosis (β = 38.9, 95% CI: −15.2, 93.0) with SFLs (Table 7).
TABLE 7.
Associations between degree of liver steatosis or fibrosis and serum ferritin (ng/ml) in adults with NAFLD
| Exposure | Model 1:β (95% CI), p | Model 2:β (95% CI), p | Model 3:β (95% CI), p |
|---|---|---|---|
| Severe steatosis (S3) | |||
| CAP < 302 | Reference | Reference | Reference |
| CAP ≥ 302 | 9.1 (−14.1, 32.2), 0.443 | −1.1 (−23.6, 21.5), 0.925 | −7.3 (−29.0, 14.4), 0.508 |
| Significant fibrosis (≥F2) | |||
| LSM < 8.0 | Reference | Reference | Reference |
| LSM ≥ 8.0 | 95.4 (59.4, 131.4), <0.001 | 84.3 (49.3,119.4), <0.001 | 5.9 (−29.5, 41.2), 0.745 |
| Advanced fibrosis (≥F3) | |||
| LSM < 9.7 | Reference | Reference | Reference |
| LSM ≥ 9.7 | 74.2 (44.8, 103.7), <0.001 | 65.5 (36.8, 94.1), <0.001 | −0.7 (−29.8, 28.4), 0.960 |
| Cirrhosis (F4) | |||
| LSM < 13.6 | Reference | Reference | Reference |
| LSM ≥ 13.6 | 147.9 (93.2,202.6), <0.001 | 141.6 (88.5,194.6),<0.001 | 38.9 (−15.2, 93.0), 0.159 |
Model 1: no covariates were adjusted. Model 2: age, gender, race were adjusted. Model 3: age, gender, race, BMI, diabetes, waist circumference, HDL‐cholesterol, glycohemoglobin, AST, ALT, GGT, serum albumin, serum creatinine, and uric acid were adjusted.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transpeptidase; HDL, high density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
4. DISCUSSION
This study investigated the associations of NAFLD status with SFLs. In conclusion, our results primarily demonstrated that the participants in NAFLD group had significantly higher SFLs compared with their counterparts in non‐NAFLD group based on the large, nationwide cross‐sectional study. On the whole, SFLs in NAFLD group have an average elevation with 13.86 ng/ml compared with that in non‐NAFLD group. Moreover, we further found that the relationship between NAFLD status and SFLs differed in various age groups, and differed by gender. However, no correlations between severe steatosis, significant fibrosis, advanced fibrosis, cirrhosis, and SFLs in adults with NAFLD were found. To the best of our perspective, this study is so far the largest sample size study on the correlation between NAFLD and serum ferritin in a general American population.
Previously, researches from different countries draw similar conclusions on the associations of SFLs and NAFLD. In a multicenter study of 628 participants, SFLs >1.5 × ULN was associated with increased prevalence of NASH, more severe steatosis, lobular inflammation, hepatocellular ballooning, and advanced fibrosis. 22 Importantly, a meta‐analysis analyzed 14 studies focusing on the SFLs in NAFL and/or NASH patients, and found SFLs was actually increased in NAFLD patients compared with individuals without NAFLD, and SFLs was increased in NASH adults compared with NAFL. 10 However, the connection between SFL and NAFLD patients in pediatric or adolescent populations was observed inconsistently, still disputable. 10 In our present study, no correlations between severe steatosis, advanced fibrosis, NASH cirrhosis, and SFLs were found. The divergent conclusions may be resulted from the differences in inclusion criterion, methods to define NAFLD/NASH, examination technologies for serum ferritin, and study design type.
Previous studies mainly investigated the effect of different SFLs on the prevalence and severity of NAFLD or hepatic fibrosis, but clinical data about how NAFLD status influences SFLs were relatively inadequate. A study from Japan in 86 patients with histopathologically verified NAFLD reconfirmed elevated SFLs in both NAFLD patients and NASH patients. 23 In NAFLD patients, ferritin levels were reported to primarily differ according to fibrosis stage, increasing from early to moderate fibrosis, and declining in cirrhosis. How is the real relationship between NAFLD status and SFLs in a large cohort database? Our results reveal that those individuals with NAFLD had an increased level of SFLs when compared with those without NAFLD. But in individuals with NAFLD, no correlations between severe steatosis, significant fibrosis, advanced fibrosis, cirrhosis, and SFLs were detected. Further studies were needed based on biopsy‐proved fibrosis or cirrhosis.
Association of NAFLD status with SFLs might differ according to gender or age. Previously, the correlation of serum ferritin and NAFLD has been incompletely studied in children. Ferritin levels in children with NAFLD were obviously higher than their counterparts in non‐NAFLD group. 24 A recent study by H.B. Kim et al. 25 found serum ferritin level were positively associated with NAFLD in postmenopausal women. However, the association between NAFLD and SFLs in women participants aged 50–80 years was no statistically significant in our study. Nindy Sabrina et al. 26 suggested that serum iron to ferritin ratio accurately predicted decreased risk of severe liver steatosis in young adult women compared to middle‐aged women. Thus, we performed subgroup analyses stratified by age and gender. In this study, we found that this positive association only existed in females, but not existed in males. In females, serum ferritin levels in NAFLD group had an elevation by 16.22 ng/ml compared with that in non‐NAFLD group. Moreover, the association between NAFLD status and serum ferritin were different in various age groups. When the participants were stratified by gender, this association differed. In males, this significantly positive association was more prominent in the 50–64 age group. In female participants aged 50–64 years, serum ferritin levels in NAFLD group had an elevation by 70.89 ng/ml compared with that in non‐NAFLD group. In females, this significantly positive association was more prominent in the 18–34 age group. In male participants aged 18–34 years, serum ferritin levels in NAFLD group had an elevation by 20.72 ng/ml compared with non‐NAFLD group.
Researchers have explored pathophysiologic mechanisms of elevated serum ferritin in NAFLD. Hyperferritinemia in NAFLD patients was often attributed to hepatic inflammation and adiponectin as a marker of insulin resistance. Serum iron changes are commonly seen in adult NAFLD characterized by increased SFLs and normal transferrin saturation, known as metabolic abnormality iron overload syndrome. In addition, serum ferritin was closely associated with the iron‐regulating hormone Hepcidin and liver iron levels in NAFLD. 27 Serum ferritin in male adolescents with obesity was mainly determined by liver fat content and inflammation but not by body iron status. 28 The elevated SFLs was associated with abnormal markers of iron or inflammation in NAFLD patients, partly resulting from β‐cell dysfunction and insulin resistance. 29 The role of disordered iron homeostasis in the pathogenesis of NAFLD was rapidly emerging, indicating that treatments focused on how to rectify iron metabolism may be beneficial.
National Health and Nutrition Examination Survey is designed to provide nationally representative estimates, therefore the strength of these findings in our study lies in the size of the cohort. We enrolled 3689 adult participants, the largest cohort study focusing on the correlation of NAFLD status and serum ferritin in our perspective. Subgroup analyses could be performed due to the large sample size. However, there were still several limitations or shortcomings in our study. First, NAFLD status was defined according to CAP values through VCTE, but not biopsy‐proven NAFLD, which may cause bias in inclusion of NAFLD patients. Moreover, NAFLD status was defined as values ≥274 dB/m accordance with a landmark study, 19 with which as a cut‐off value to diagnose NAFLD the sensibility and specificity needs further confirmed. Second, lots of the participants did not perform VCTE or partially finished the exam, leading to the smaller number of the participants were included in this study. There is a possibility that the participants with NAFLD based on CAP values were misclassified. Third, self‐reported confounders might be susceptible to self‐report bias. Fourth, the nature of the cross‐sectional study limited the conclusions to an association and not causality in this study.
5. CONCLUSIONS
This study indicated that US adults with NAFLD had significantly higher SFLs compared with those without NAFLD. Meanwhile, the association between NAFLD status and serum ferritin differed by age and gender. To better understand the mechanisms involved the correlations between NAFLD and SFLs, more in‐depth studies are needed.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
Mingqin Lu made a contribution to the conception and study design. Yi Lu and Liwen Cao were in charge of execution, acquisition of data, analysis, and interpretation. Naibin Yang took charge in drafting, revising, and critically reviewing the article. All authors gave final approved of the version to be published, had agreed on the journal of which the article has been submitted and agreed to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Yang N, Lu Y, Cao L, Lu M. The association between non‐alcoholic fatty liver disease and serum ferritin levels in American adults. J Clin Lab Anal. 2022;36:e24225. doi: 10.1002/jcla.24225
Naibin Yang, Yi Lu and Liwen Cao contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data are publicly available on the Internet and researchers throughout the world http://www.cdc.gov/nchs/nhanes/.
REFERENCES
- 1. Lazarus J, Mark H, Anstee Q, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2021;19(1):60‐78. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee Q, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 3. Liou I, Kowdley K. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40:S11‐S16. [DOI] [PubMed] [Google Scholar]
- 4. EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388‐1402. [DOI] [PubMed] [Google Scholar]
- 5. Diehl A, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063‐2072. [DOI] [PubMed] [Google Scholar]
- 6. Younossi Z, Ong J, Takahashi H, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.06.048. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7. Buzzetti E, Petta S, Manuguerra R, et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non‐alcoholic fatty liver disease. Liver Int. 2019;39(7):1325‐1334. [DOI] [PubMed] [Google Scholar]
- 8. Manousou P, Kalambokis G, Grillo F, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non‐alcoholic fatty liver disease patients. Liver Int. 2011;31(5):730‐739. [DOI] [PubMed] [Google Scholar]
- 9. Jung J, Shim J, Park S, et al. Serum ferritin level is associated with liver steatosis and fibrosis in Korean general population. Hepatol Int. 2019;13(2):222‐233. [DOI] [PubMed] [Google Scholar]
- 10. Du S, Lu L, Geng N, et al. Association of serum ferritin with non‐alcoholic fatty liver disease: a meta‐analysis. Lipids Health Dis. 2017;16(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao X, Xu X, Jin F, Zhu Z. The correlation of type 2 diabetes status with bone mineral density in middle‐aged adults. Diabetes Metab Syndr Obes Targets Ther. 2020;13:3269‐3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciardullo S, Monti T, Perseghin G. Prevalence of liver steatosis and fibrosis detected by transient elastography in adolescents in the 2017–2018 national health and nutrition examination survey. Clin Gastroenterol Hepatol. 2021;19(2):384‐390.e381. [DOI] [PubMed] [Google Scholar]
- 13. Cfdcap . National Health and Nutrition Examination Survey (NHANES). U.S. Department of health and human services. 2017. Available from https://wwwncdcgov/nchs/data/nhanes/2017‐2018/manuals/2017_MEC_Laboratory_Procedures_Manualpdf. Accessed 31 March 2020.
- 14. ADA . Classification and diagnosis of diabetes: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Supplement 1):S14‐S31. [DOI] [PubMed] [Google Scholar]
- 15. NCfH . National health and nutrition examination survey 2017‐2018 laboratory data. Statistics. Internet: https://wwwcdcgov/nchs/nhanes/Search/DataPageaspx?Component=Laboratory&CycleBegin. (Accessed June 2020) 2017.
- 16. Chalasani N, Younossi Z, Lavine J, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328‐357. [DOI] [PubMed] [Google Scholar]
- 17. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264‐1281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metab. 2021;121:154752. [DOI] [PubMed] [Google Scholar]
- 19. Eddowes P, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717‐1730. [DOI] [PubMed] [Google Scholar]
- 20. Roulot D, Czernichow S, Le Clésiau H, Costes J, Vergnaud A, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48(4):606‐613. [DOI] [PubMed] [Google Scholar]
- 21. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. [DOI] [PubMed] [Google Scholar]
- 22. Kowdley K, Belt P, Wilson L, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(1):77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoneda M, Nozaki Y, Endo H, et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. 2010;55(3):808‐814. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Cao J, Xu H, et al. Ferritin as a key risk factor for nonalcoholic fatty liver disease in children with obesity. J Clin Lab Anal. 2021;35(2):e23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Lee H, Lee Y. Association of serum ferritin levels with non‐alcoholic fatty liver disease in postmenopausal women. Climacteric. 2018;21(5):509‐514. [DOI] [PubMed] [Google Scholar]
- 26. Sabrina N, Bai C, Chang C, Chien Y, Chen J, Chang J. Serum iron: ferritin ratio predicts healthy body composition and reduced risk of severe fatty liver in young adult women. Nutrients. 2017;9(8):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan J, Armitage A, Cobbold J, et al. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. 2018;38(1):164‐173. [DOI] [PubMed] [Google Scholar]
- 28. Mörwald K, Aigner E, Bergsten P, et al. Serum ferritin correlates with liver fat in male adolescents with obesity. Front Endocrinol. 2020;11:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Utzschneider K, Largajolli A, Bertoldo A, et al. Serum ferritin is associated with non‐alcoholic fatty liver disease and decreased Β‐cell function in non‐diabetic men and women. J Diabetes Complications. 2014;28(2):177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are publicly available on the Internet and researchers throughout the world http://www.cdc.gov/nchs/nhanes/.


