Abstract
The serum Chitinase 3‐like protein 1 (CHI3L1) protein level can distinguish the stages of liver fibrosis to a great extent. However, the diagnostic and prognostic significance of serum CHI3L1 in hepatocellular carcinoma (HCC) is not clarified. To evaluate the diagnostic and prognostic value of CHI3L1 in HCC, a total of 128 HCC patients treated in the HwaMei Hospital, University of Chinese Academy of Sciences, from December 2018 to April 2020 were collected retrospectively. Matched age and gender subjects, 40 patients with liver cirrhosis, 40 patients with chronic hepatitis, and 40 healthy subjects were enrolled in the control group. The relevant clinical laboratory and examination data and the overall survival time (OS) of the HCC patients were collected. The serum CHI3L1 expression level is related to α‐fetoprotein (AFP), tumor‐node‐metastasis (TNM) stage, maximum tumor diameter, liver cirrhosis, and HCC patient's OS (p < 0.05). The area under the curve (AUC) of CHI3L1 was 0.7875 with the cutoff value of 91.36 ng/ml. Combining the serum CHI3L1 and α‐fetoprotein (AFP) by a binary logistic regression model can increase the diagnostic sensitivity to 97.5%. Multivariate Cox regression analysis indicated that CHI3L1 is an independent prognostic factor in patients with HCC.
Keywords: biomarker, CHI3L1, hepatocellular carcinoma, prognosis
This study mainly focuses on the prognostic value of CHI3L1 in HCC patients. The formula of the prognostic risk model is as follows: Risk score = 1.333 × SAFP + 2.384 × STNM + 0.879 × SCHI3L1 − 2.544 × Shepatectomy − 1.110 × STACE. Based on the median risk score, HCC patients were divided into the high‐risk group and the low‐risk group, and K‐M analysis showed that the OS of the low‐risk group was significantly longer than that of the high‐risk group.
1. INTRODUCTION
Primary liver cancer is the sixth diagnosed cancer and the fourth cause of cancer‐related death worldwide. 1 Hepatocellular carcinoma (HCC) is the major pathological category and comprises a 75%–85% incidence of primary liver cancer cases. 1 Because of the occult attacks and high recurrence rate of HCC, the overall survival (OS) rate of HCC patients is unsatisfactory. 2 Despite the progress that has been made in screening and forecasting the prognosis of HCC, there is still a lack of reliable and simple biomarkers that can detect and monitor the prognosis of patients with HCC. 3
CHI3L1 belongs to the family 18‐glycosyl hydrolase but lacks chitinolytic activity. 4 The Omic study conducted by Huang et al. has shown that CHI3L1 is specifically expressed in the liver. 5 Our previous study has shown that CHI3L1 has better performance in diagnosing liver fibrosis than other noninvasive methods in chronic hepatitis B patients. 6 In some cancers such as gastrointestinal tumors, ovarian cancer, melanoma, urinary system tumors, lung cancer, and glioblastoma, CHI3L1 can help to distinguish patients between a good prognosis and poor prognosis. 7 Studies have shown that deficiency of CHI3LI can ameliorate liver fibrosis and that upregulation of CHI3L1 can exaggerate HCC cells proliferation, invasion, and migration. 2 , 3 It is promising that CHI3L1 can help to screen and monitor the prognosis of patients with HCC.
So, we designed a retrospective study to evaluate the diagnostic value of serum CHI3L1 and determine serum CHI3L1 prognostic relevance in HCC patients.
2. MATERIALS AND METHODS
2.1. Clinical data
A total of 128 HCC patients treated in the HwaMei Hospital, University of Chinese Academy of Sciences, from December 2018 to April 2020 were enrolled in the HCC patient group. The eligibility of enrolled patients meets the criteria according to Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition) 8 ; patients had not received any specific treatment (surgery, radiotherapy, and chemotherapy, etc.). Exclusion criteria included severe organ dysfunction (such as heart, lung, and kidney), diseases associated with immunity or blood system, metastatic liver cancer, and malignancy other than HCC. During the follow‐up period, 30 patients in the HCC group underwent transcatheter arterial chemoembolization (TACE) and 17 patients underwent hepatectomy.
The age‐ and gender‐matched control group consists of 120 subjects: 40 patients with liver cirrhosis and 40 patients with chronic hepatitis (CH) from the HwaMei Hospital, University of Chinese Academy of Sciences, and 40 healthy subjects from International Health Care Center of Ningbo Mingzhou Hospital, excluding subjects with any liver disease and severe heart, lung, kidney, and other organ dysfunction. This study was approved by the Human Research Ethics Committee from HwaMei Hospital, University of Chinese Academy of Sciences, and Zhejiang University MingZhou Hospital. Each patient signed an informed consent form.
2.2. Sample collection and detection
The serum CHI3L1 level was determined by enzyme‐linked immunosorbent assay (ELISA) and in a blinded manner. The CHI3L1 ELISA Kit was produced by Proprium Biotech Company Limited, Hangzhou, China. The expression level of CHI3L1 was detected once the subjects first visit the clinic or admit to hospital.
2.3. Follow‐up of patients
128 patients with HCC were followed up. Follow‐up data were obtained by hospital electronic medical records, telephone calls, and outpatient clinics. The follow‐up time was 8–22 months. During the follow‐up period, laboratory monitoring was performed every 3–6 months, including routine blood chemistry tests and imaging studies using computed tomography (CT) or magnetic resonance imaging (MRI) to monitor disease status. The OS is from the day of the patient's first serum sample test to the day of death; for patients who are still alive on the follow‐up deadline, the period between the day of the first serum sample test and the last follow‐up date will be obtained.
2.4. Establishment of an HCC prognostic model
According to multivariate Cox regression analysis, a prognostic model was demonstrated using a small number of clinicopathological features. The risk score was calculated as ∑ Ni = 1 (Ci × Si), where “N” was the number of prognostic factors, “C” represented the regression coefficient, and “S” represented the risk score of clinical characteristics.
2.5. TCGA data analysis
The clinicopathological data of 50 paired HCC and adjacent non‐tumor tissues as well as 278 unpaired HCC tissues, and the relative expression levels of CHI3L1 were downloaded from The Cancer Genome Atlas (TCGA)‐Liver Cancer RNA sequencing database (https://genome‐cancer.ucsc.edu).
2.6. Statistical analysis
In this study, the SPSS18.0 software package and the GraphPad8.0 software package were used for statistical analysis and graphing. An independent sample t test was used for comparing the continuous variables of normal distribution. Mann‐Whitney U test was used for comparing the continuous variables of non‐normal distribution. The ROC curve was drawn to evaluate diagnostic power, and the optimal cutoff was deemed as the value that maximized the sum of sensitivity and specificity. Kaplan‐Meier (K‐M) curve and log‐rank test were used to describe the survival of patients. Univariate and multivariate analyses were evaluated by the Cox regression method, and p < 0.05 indicates a significant difference.
3. RESULTS
3.1. Serum CHI3L1 expression in the HCC group and the control group
The median serum level of CHI3L1 in the HCC group was 168.84 (95%CI = 96.24, 322.61) ng/ml, which was significantly higher than the median serum level of CHI3L1 in the control group (median = 71.36 ng/ml, 95% CI = 48.63, 114.77 ng/ml) (p < 0.0001, Figure 1).
FIGURE 1.
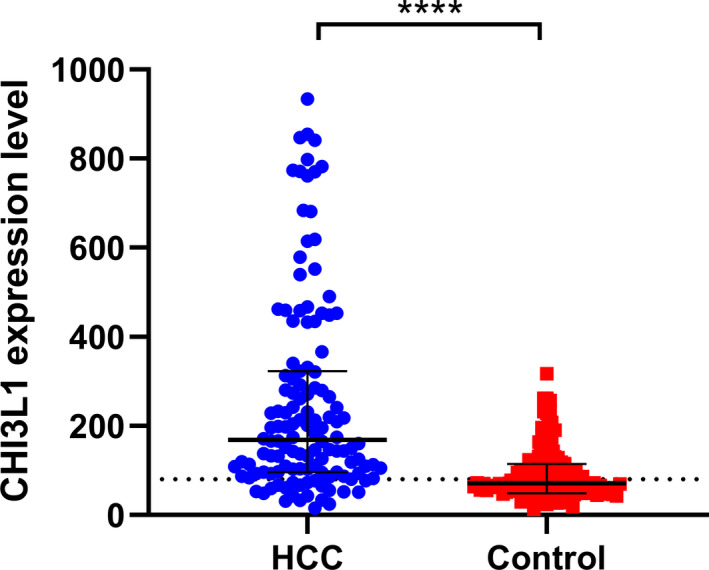
Expression of serum CHI3L1 in the HCC group (n = 128) and the control group (n = 120); ****p < 0.0001
3.2. Diagnostic value of serum CHI3L1 for HCC group
In our study, to evaluate the diagnostic significance of CHI3L1 and AFP in HCC, we screened out 120 cases in the HCC group and the control group, which can meet the requirements of collecting AFP and CHI3L1 expression levels at the same time, and there is no significant difference in gender and age. As is shown in Figure 2, an AUC of CHI3L1 was 0.7875 (p < 0.0001); according to the Youden index, the sensitivity and specificity were 78.33% and 66.67% when the optimal cutoff was 91.36 ng/ml. However, the diagnostic value of AFP (AUC = 0.8346, sensitivity = 85.83%, specificity = 76.67%, the optimal cutoff value = 6.05) was better than that of CHI3L1. Combining serum CHI3L1 and AFP by a binary logistic regression model, the regression model formula is logit(P) = −1.86 + 0.008 × CHI3L1 + 0.018 × AFP. We transformed the formula and got the combining predictors' formula as CHI3L1 + 9/4AFP. The sensitivity of the combined predictors reached 97.50%, and the AUC was 0.8636 (p < 0.001) when the cutoff is 280.8 (Figure 2).
FIGURE 2.
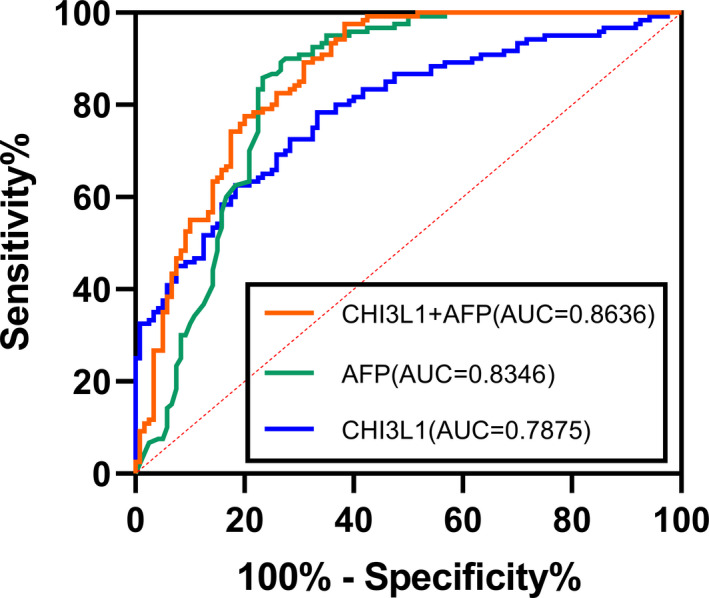
Diagnostic value of serum CHI3L1 (orange) or serum AFP (green) alone, and the combination of these two markers for the diagnosis of HCC (blue)
3.3. Relevance between serum CHI3L1 expression and clinicopathological characteristics
The clinical data of 128 HCC patients were analyzed. Serum CHI3L1 level was closely correlated with serum AFP level, tumor‐node‐metastasis (TNM) stage, Child‐Pugh stage, the maximum diameter of tumor, and presence of liver cirrhosis (p < 0.05), but there was no significant correlation between gender and age (p > 0.05). The patients with high AFP level (>400 ng/L), TNM III + IV stage, large tumor diameter, liver cirrhosis, and Child‐Pugh B + C had higher serum CHI3L1 levels (Table 1).
TABLE 1.
Relationship between serum CHI3L1 level and clinicopathological characteristics in the HCC group
| All patients (n = 128) | CHI3L1 (ng/ml) | ||
|---|---|---|---|
| Variable | n | Median (P25, P75) | p‐value |
| Gender | |||
| Male | 104 | 168.76 (94.23, 329.01) | 0.7725 |
| Female | 24 | 174.65 (104.91, 290.19) | |
| Age (years) | |||
| ≤60 | 64 | 137.24 (83.97, 408.92) | 0.1635 |
| >60 | 64 | 201.08 (116.9, 310.88) | |
| AFP (ng/ml) | |||
| ≤400 | 68 | 129.16 (81.03, 232.42) | <0.0001 |
| >400 | 50 | 241.95 (143.61, 459.37) | |
| Cirrhosis | |||
| No | 36 | 109.97 (62.02, 164.29) | <0.0001 |
| Yes | 86 | 224.17 (108.37, 449.64) | |
| Child‐pugh grade | |||
| A | 53 | 146.46 (94.50, 270.66) | <0.0001 |
| B + C | 33 | 432.63 (239.79, 682.53) | |
| Size of tumor (cm) | |||
| ≤5 | 67 | 118.42 (80.80, 231.61) | 0.0379 |
| >5 | 40 | 156.50 (106.11, 438.35) | |
| TNM stage | |||
| Ⅰ + Ⅱ | 48 | 104.02 (78.07, 161.44) | <0.0001 |
| Ⅲ + Ⅳ | 75 | 242.46 (143.23, 459.25) | |
3.4. Relationship between serum CHI3L1 expression and HCC patients’ survival
According to the survival data of patients with HCC, the median expression level of CHI3L1 (168.84 ng/ml) was taken as the boundary, and the patients were divided into the low expression group (n = 64) and the high expression group (n = 64). To understand the relationship between the expression level of CHI3L1 and the prognosis of patients, the prognosis of HCC patients with high and low expression of CHI3L1 was analyzed by Kaplan‐Meier curves. The OS rate of patients with high expression of CHI3L1 was lower than that of patients with low expression of CHI3L1, and the difference was statistically significant (Log‐rank test, p < 0.0001, Figure 3).
FIGURE 3.
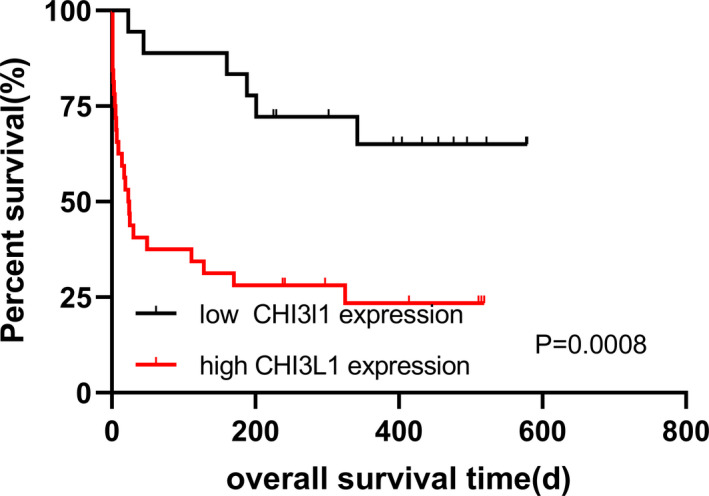
K‐M curves for OS of HCC patients with high and low CHI3L1 expression
3.5. Independent factors for the prognosis of the HCC group
Univariate Cox regression analysis demonstrated that TNM stage(p < 0.0001), the maximum diameter of tumor (p = 0.01), AFP (p < 0.0001) and CHI3L1 serum levels (p < 0.0001), hepatectomy (p = 0.001), and TACE treatment (p < 0.0001) were the prognostic factors, while multivariate Cox regression analysis showed that TNM stage (p = 0.023), the serum AFP level (p = 0.003) and CHI3L1 expression (p = 0.037), hepatectomy (p = 0.013), and TACE (p = 0.004) treatment were the independent prognostic factors (Table S1).
3.6. Subgroup analysis of HCC patients
Hepatocellular carcinoma patients were divided into subgroups according to several clinical characteristics such as TNM stage, serum AFP level, exposure to hepatectomy, and exposure to TACE treatment. Then, subgroup analysis was conducted to further explore the correlation between serum CHI3L1 expression level and the prognosis of patients with different characteristics of HCC. According to the median CHI3L1 expression level (168.84 ng/ml), the serum CHI3L1 level was divided into the low expression group and the high expression group. There was no significant difference in OS in the group with the low level of serum AFP (Figure 4A), but there was a significant difference in the group with the high level of serum AFP (Figure 4B). In the subgroup of patients without liver cirrhosis, the patients with high CHI3L1 expression have a shorter OS than that of patients with low CHI3L1 expression (Figure 4C). In the subgroup of patients with liver cirrhosis, the OS rate of patients with low expression of CHI3L1 was higher than that of patients with high expression of CHI3L1 (p < 0.0001, Figure 4D). There was a significant difference in the group with TNM III + IV stage (Figure 4E). However, in the TNM I+II stage group, there were no deaths, making it difficult to draw a survival curve.
FIGURE 4.
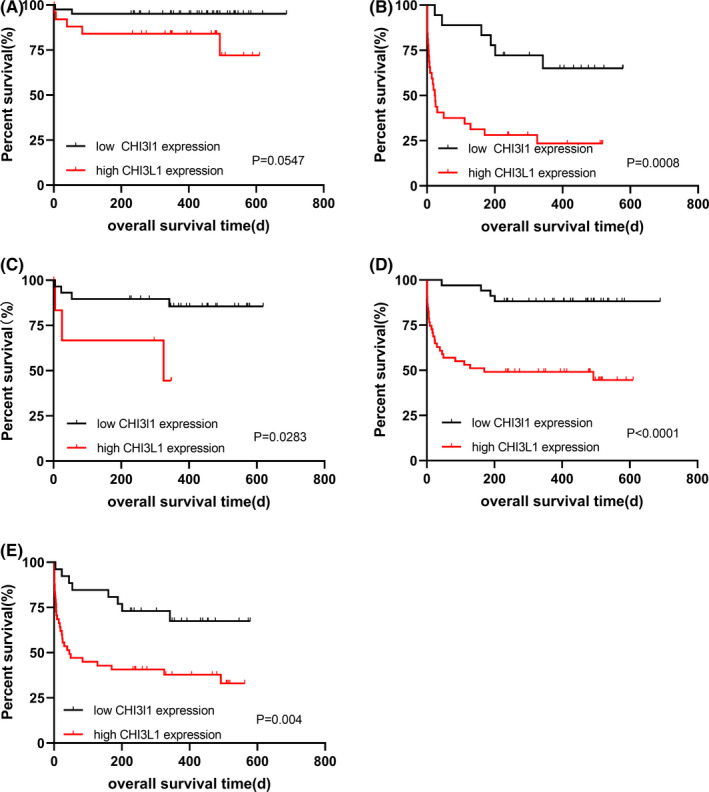
Relationship between the serum CHI3L1 expression level and the prognosis of HCC patients with different characteristics. (A) K‐M curves for OS of HCC patients with low levels of serum AFP; (B) K‐M curves for OS of HCC patients with high levels of serum AFP; (C) K‐M curves for OS of HCC patients without cirrhosis; (D) K‐M curves for OS of HCC patients with cirrhosis; (E) K‐M curves for OS of HCC patients with TNM III + IV stage
3.7. Establishment of survival prognostic model for HCC
To further evaluate the prognostic value of CHI3L1, we combine the expression level of CHI3L1 with other clinicopathological features and establish a survival and prognostic model of HCC. Based on multivariate Cox regression analysis, AFP ≤ 400ng/ml, low CHI3L1 expression, TNM I+II stage, no hepatectomy, no TACE was 0 in the score, and AFP > 400ng/ml, high CHI3L1 expression was 1 in the score. The score of TNM III + IV stage score was 2. The score was 2 for hepatectomy and 1 for the TACE as is shown in Table S2. We can get the formula of the prognostic risk model:
Risk score = 1.333 × SAFP + 2.384 × STNM + 0.879 × SCHI3L1 − 2.544 × Shepatectomy − 1.110 × STACE.
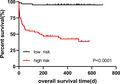
Based on the median risk score, HCC patients were divided into the high‐risk group and the low‐risk group, and K‐M analysis was used to compare the OS of the two groups. The results demonstrated that the OS of the low‐risk group was significantly longer than that of the high‐risk group (p < 0.0001, Figure 5). These results show that the performance of the prognostic model is good.
FIGURE 5.
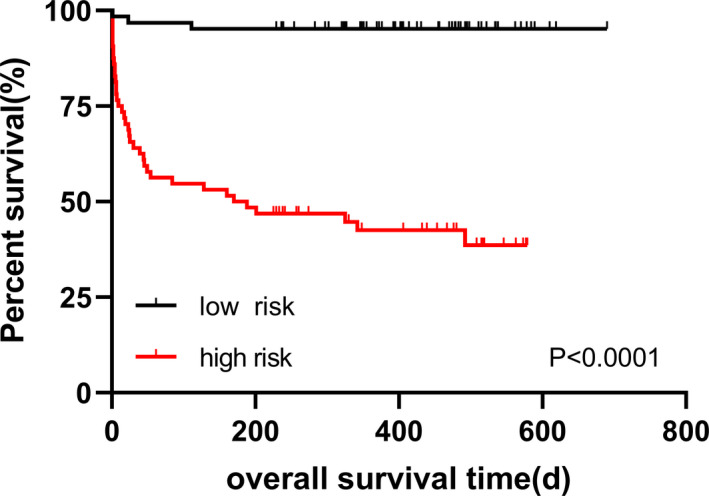
K‐M curves for OS of patients in the high‐risk and the low‐risk group
3.8. Upregulation of CHI3L1 in paired non‐tumorous tissues was associated with poor survival in patients with HCC
We examined the expression levels of CHI3L1, survival time, and survival status in patients with HCC using the TCGA database and found that CHI3L1 had no significant difference in paired HCC tissues (n = 50, p = 0.2483; Figure 6A). To assess the prognostic significance of CHI3L1 in HCC, we analyzed the prognostic value in patients with HCC. As shown in Figure 6B, the cutoff value of CHI3L1 (12.59) was acquired according to its expression levels in HCC tissue, and the patients were divided into high CHI3L1 expression and low CHI3L1 expression groups. K‐M analysis showed that HCC patients with high CHI3L1 expression in HCC tissues had no difference in OS (p = 0.7304, Figure 6C). While K‐M analysis showed that HCC patients with high CHI3L1 expression in paired non‐tumorous tissues harbored poorer survival (p = 0.0015, Figure 6D) as compared with those with low CHI3L1 expression.
FIGURE 6.
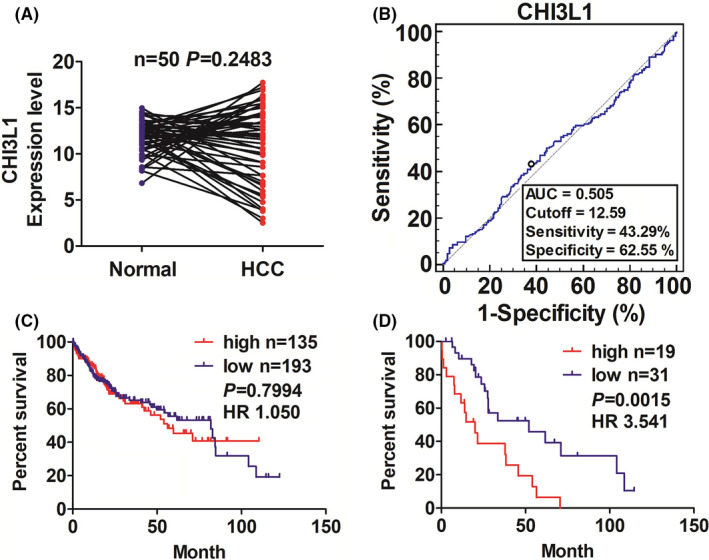
The association between CHI3L1 expression level in tissues and the prognosis in patients with HCC. (A) TCGA cohort analysis of the expression level of CHI3L1 in paired HCC tissues (n = 50). (B) ROC curve analysis of the cutoff value, sensitivity, specificity, and AUC of CHI3L1 in HCC tissues and non‐tumors tissues. (C) The patients with HCC were divided into high or low CHI3L1 expression according to its cutoff value. K‐M analysis of the association of high or low CHI3L1 expression with the OS in patients with HCC (p = 0.7994). (D) Kaplan Meier analysis of the association of high or low CHI3L1 expression in paired nontumors tissues with the survival in patients with HCC (p = 0.0015)
4. DISCUSSION
CHI3L1 plays an active role in the occurrence and progress of tumors. Studies have shown that CHI3L1 induces the expression of angiogenic molecules including C‐C motif chemokine ligand 2 (CCL2), C‐X‐C motif chemokine ligand 2 (CXCL2), and matrix metalloproteinase‐9 (MMP‐9) in macrophages, thus promoting the occurrence of lung metastasis. 9 Jeet et al. 10 found that knockout of CHI3L1 can significantly reduce the invasion and migration of prostate cancer cells, while overexpression of CHI3L1 makes prostate cancer cells have higher invasiveness and migration ability. At present, it has been found that overexpression of CHI3L1 is associated with a poor prognosis of cancer in some tumors. 11 , 12
Hepatocellular carcinoma is one of the high incidences of tumors in China and is one of the main contributors to cancer‐related deaths, 1 which indicates the importance of discovering applicable prognostic markers or simple prognostic models that can be used for HCC patients. Qiu et al. 13 found that the overexpression of CHI3L1 can significantly increase the number of S‐phase cells in vitro and can promote the proliferation of cancer cells, putting forward the hypothesis that CHI3L1 may participate in the regulation of HCC by acting on TGF‐β pathway. Pan's group reported that when compared with adjacent tissues, the mRNA and protein levels of CHI3L1 in HCC tissues were up‐regulated and further increased in tumors with metastases and that the higher expression level of CHI3L1 in HCC tissues has the worse prognosis. 14 But, our results from the TCGA database are not consistent with the results from Pan's group, showing that there is no significant difference between HCC tissues and para‐non‐tumorous tissues and that the expression of CHI3L1 in HCC tissues has no prognostic value but that in paired non‐tumorous tissues has prognostic value. Our previous study has indicated that the expression level of serum CHI3L1 was steadily increased from CH to liver cirrhosis to HCC in monoinfected hepatitis B virus patients. Serum CHI3L1 expression levels were positively associated with non‐invasiveness liver fibrosis methods such as liver stiffness measurement (LSM), fibrosis‐4 (FIB‐4) index, aspartate aminotransferase‐to‐platelet ratio index (APRI), and serum CHI3L1 has better performance than these noninvasive methods with an area under the ROC curve (AUC) of 0.97 in diagnosing significant fibrosis. With the results from the TCGA database, the CHI3L1 expression level in paired non‐tumor tissues has the prognostic value in OS of HCC patients, which hinted that CHI3L1 may play a role in the tumor microenvironment of HCC.
In our study, the abnormal expression feature was confirmed by comparing clinical serum samples between patients with HCC and the control group including liver cirrhosis, CH, and healthy subjects during the same period. According to the ROC curve, serum CHI3L1 level has a good diagnostic performance for HCC (AUC = 0.7875, the optimal cutoff value = 91.36 ng/ml), while serum AFP has better performance with an AUC of 0.8346 when the optimal cutoff value is 6.05ng/ml. when combining CHI3L1 with AFP, the AUC reaches 0.8636 and sensitivity reaches 0.975, indicating that a combination of the serum CHI3L1 and AFP can be used as a powerful biomarker for the diagnosis of HCC.
At present, the commonly used treatment methods for HCC include hepatectomy, liver transplantation, local ablation, TACE, 15 and targeted therapy. 16 Some researchers found serum CHI3L1 is an independent prognostic biomarker for HCC patients treated with TACE. The OS of the groups whose CHI3L1 expression was higher than the 95th percentile serum level of healthy controls was shorter than those of patients with normal CHI3L1 expression. 17 Similar results were obtained in the study of HCC patients undergoing hepatectomy. 18
In our study, high CHI3L1 expression is closely related to the high level of serum AFP and TNM III + IV stage. The expression level of CHI3L1 has been determined to be negatively correlated with the survival of patients. Cox analysis of OS showed that the expression of CHI3L1 was an independent prognostic factor in HCC. Subgroup analysis showed that the low AFP expression group cannot distinguish differences in the survival of patients with high and low CHI3L1 expression. But in patients with high AFP expression or TNM III + IV stage, regardless of whether there is cirrhosis, the OS of patients with high CHI3L1 expression is shorter than that with low CHI3L1 expression. In the survival prognostic model, CHI3L1 was positively correlated with OS, indicating that it is a risk prognostic factor for HCC.
This study has some limitations. First, the number of patients receiving TACE and radical surgery is small. Second, our study is retrospective. Third, we did not distinguish HCC patients with different liver disease backgrounds. Finally, this study is only aimed at Chinese patients, and it is not clear whether the expression of serum CHI3L1 is increased in HCC patients with different races.
5. CONCLUSIONS
In summary, we compared the difference of serum CHI3L1 between the HCC group and the control group, analyzed the diagnostic performance of CHI3L1 for HCC, and evaluated the prognostic power of serum CHI3L1 in HCC patients. The results showed that serum CHI3L1 can help to increase diagnostic power and evaluate prognosis for HCC patients.
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
We would like to express our gratitude to our colleagues for collecting data.
Wang S, Chen S, Jin M, et al. Diagnostic and prognostic value of serum Chitinase 3‐like protein 1 in hepatocellular carcinoma. J Clin Lab Anal. 2022;36:e24234. doi: 10.1002/jcla.24234
Funding information
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation of China [Grant No. LQ19H160007], Applied Research Projects on Nonprofit Technology of Zhejiang Province [Grant No. LGF19H160012], Natural Science Foundation of Ningbo [Grant No. 202003N4020], Key Laboratory of Diagnosis and Treatment of Digestive System tumors of Zhejiang Province [Grant No. 2019E10020], Ningbo Clinical Research Center for Digestive System tumors [Grant No. 2019A21003], Ningbo HwaMei Key Research Fund [Grant No. 2020HMZD10, 2019HMZD13], and the K. C. Wong Magna Fund in Ningbo University.
Contributor Information
Weixin He, Email: hwxdoctor@163.com.
Liyun Fu, Email: fuliyun@ucas.ac.cn, Email: nbfly2009@126.com.
DATA AVAILABILITY STATEMENT
The original anonymized data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Janevska D, Chaloska‐Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahin T, Serin A, Emek E, Bozkurt B, Arikan BT, Tokat Y. Effectiveness of noninvasive fibrosis markers for the prediction of hepatocellular carcinoma in chronic hepatitis B and chronic hepatitis B+D induced cirrhosis. Transplant Proc. 2019;51(7):2397‐2402. [DOI] [PubMed] [Google Scholar]
- 4. Hakala BE, White C, Recklies AD. Human cartilage gP‐39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268(34):25803‐25810. [PubMed] [Google Scholar]
- 5. Huang H, Wu T, Mao J, et al. CHI3L1 is a liver‐enriched, noninvasive biomarker that can be used to stage and diagnose substantial hepatic fibrosis. OMICS. 2015;19(6):339‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang Z, Wang S, Jin J, et al. The clinical significance of serum chitinase 3‐like 1 in hepatitis B‐related chronic liver diseases. J Clin Lab Anal. 2020;34(5):e23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bian B, Li L, Yang J, et al. Prognostic value of YKL‐40 in solid tumors: a meta‐analysis of 41 cohort studies. Cancer Cell Int. 2019;19:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libreros S, Garcia‐Areas R, Keating P, Carrio R, Iragavarapu‐Charyulu VL. Exploring the role of CHI3L1 in “pre‐metastatic” lungs of mammary tumor‐bearing mice. Front Physiol. 2013;4:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeet V, Tevz G, Lehman M, Hollier B, Nelson C. Elevated YKL40 is associated with advanced prostate cancer (PCa) and positively regulates invasion and migration of PCa cells. Endocr Relat Cancer. 2014;21(5):723‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libreros S, Garcia‐Areas R, Shibata Y, Carrio R, Torroella‐Kouri M, Iragavarapu‐Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer. 2012;131(2):377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo D, Chen H, Lu P, et al. CHI3L1 overexpression is associated with metastasis and is an indicator of poor prognosis in papillary thyroid carcinoma. Cancer Biomark. 2017;18(3):273‐284. [DOI] [PubMed] [Google Scholar]
- 13. Qiu QC, Wang L, Jin SS, et al. CHI3L1 promotes tumor progression by activating TGF‐β signaling pathway in hepatocellular carcinoma. Sci Rep. 2018;8(1):15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan JJ, Ge YS, Xu G‐L, et al. The expression of chitinase 3‐like 1: a novel prognostic predictor for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2013;139(6):1043‐1054. [DOI] [PubMed] [Google Scholar]
- 15. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734‐1739. [DOI] [PubMed] [Google Scholar]
- 16. Ding XX, Zhu QG, Zhang SM, et al. Precision medicine for hepatocellular carcinoma: driver mutations and targeted therapy. Oncotarget. 2017;8(33):55715‐55730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu CB, Wang C, Chen LL, et al. Serum YKL‐40 independently predicts outcome after transcatheter arterial chemoembolization of hepatocellular carcinoma. PLoS One. 2012;7(9):e44648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu CB, Chen LL, Tian JJ, et al. Elevated serum YKL‐40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Ann Surg Oncol. 2012;19(3):817‐825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The original anonymized data used to support the findings of this study are available from the corresponding author upon request.


