Abstract
High vaccine reactogenicities may reflect stronger immune responses, but the epidemiological evidence for coronavirus disease 2019 (COVID-19) vaccines is sparse and inconsistent. We observed that a fever of ≥38℃ after two doses of the BNT162b2 vaccine was associated with higher severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike IgG titers.
Keywords: Reactogenicity, Spike IgG titer, SARS-CoV-2, Fever, BNT162b2 vaccine
1. Introduction
A SARS-CoV-2 vaccine based on mRNA technology, developed by Pfizer and Moderna, has shown 94–95 % efficacy in preventing COVID-19 [1]; the second dose of this mRNA vaccine substantially increases IgG antibody titers against SARS-CoV-2 spike protein [2], while accompanying much greater side effects than that of the first dose [1], [3]. The hypothesized mechanism underlying this phenomenon is that the type I interferons and multiple pro-inflammatory cytokines and chemokines produced by the booster vaccine, which induce injection-site and systemic inflammation (side effects), promote an antibody-producing immune response [4]. The vaccine reactogenicity may thus correlate with the immune response.
As with vaccines against other infectious diseases [5], epidemiological evidence on the relationship between reactogenicity and immunogenicity of COVID-19 vaccines is limited and inconsistent [6], [7], [8]. A Japanese study among healthcare workers (HCWs) observed higher levels of anti-spike IgG antibodies after the second dose of the BNT162b2 vaccine among those who experienced systemic symptoms [8]. In contrast, a Korean study of HCWs showed no difference in IgG spike titers according to local and systemic reactogenicity grades after the first and second dose of the AZD1222 or BNT162b2 vaccines [6]. However, no studies have reported the immunogenicity in relation to post-vaccination fever, which is an objective measure of systemic reactogenicity as well as an indicator of immunity activation [9], [10]. Here, we report anti-spike IgG antibodies in relation to reactogenicity following COVID-19 vaccination, with particular attention to the onset of post-vaccination fever.
2. Methods
We performed a repeated survey after the start of the vaccination program in March 2021 in a cohort of staff members of the National Center for Global Health and Medicine (NCGM), a national medical institution in Japan. Details of the study design are explained in the Supplementary appendix 1.
We recruited 100 staff members who were not taking immunosuppressive medication. Out of the 100 participants, 12 members were excluded who lacked data on symptoms and fever after vaccination, and 88 members were included for the analyses. The participants were vaccinated with the mRNA-based SARS-CoV-2 vaccine BNT162b2 (Pfizer-BioNTech) according to the standard protocol (two doses of 30 µg administered 3 weeks apart). As part of the vaccination program, the vaccinated recipients were asked to report their worst local and systemic symptoms and highest body temperature that they might experience within 4 days of vaccination via a web questionnaire. To assess humoral response by the vaccine, we quantitatively measured the IgG against SARS-CoV-2 spike protein (the AdviseDx SARS-CoV-2 IgG II assay, Abbott ARCHITECT®) in accordance with the manufacturer's package insert (positive threshold: ≥50.0 [AU/mL]). We also qualitatively measured the IgG against the SARS-CoV-2 nucleocapsid protein (Abbott ARCHITECT®; positive threshold: ≥1.40 [S/C]) to exclude those with previous undiagnosed SARS-CoV-2 infection. We measured the antibodies using blood samples donated 7, 39, and 60–74 days after the second vaccination. The study protocol was approved by the Ethics Committee of NCGM, Japan (the approved number: NCGM-A-004175). Written informed consent was obtained from all participants.
We used linear regression models to estimate the means of log10-transformed spike IgG titers at 7, 39, and 60–74 days after the second dose of the vaccine according to the body temperature (<37.5 ℃, 37.5–37.9 ℃, 38.0–38.4 ℃, or ≥ 38.5 ℃) with adjustment for age (continuous) and sex, and then back-transformed these values to present the geometric means. We also calculated the mean spike IgG titers according to the grade of local or systemic reactogenicity (Supplementary Appendix 2), with reference to the U.S. Food and Drug Administration guidance [11]. We tested the statistical significance of the differences by comparing the coefficients (i.e., ratios of means) between groups. As a sensitivity analysis, we ran the above model after excluding participants with the prophylaxis use of antipyretics on vaccination (n = 5).
3. Results
The mean age (standard deviation) was 43 (12), and 64% were women. The major occupations of the members were administrative staff (20%), nurses (15%), doctors (8%), allied health professionals (5%), and others (52%). None of the participants had a history of COVID-19 and showed positive nucleocapsid/spike IgG antibody test results, removing the possibility of a previously undiagnosed SARS-CoV-2 infection.
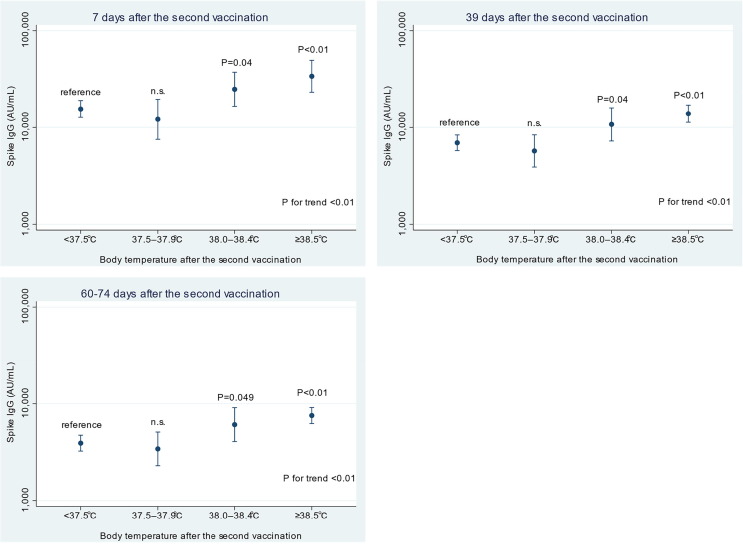
All participants were seropositive for spike IgG from 7 through 60–74 days after the second dose of the vaccine. Participants who had a fever of 38.0–38.4℃ (ratio of means, 1.60; 95% CI, 1.02 to 2.51) and ≥ 38.5℃ (ratio of means, 2.18; 95 %CI, 1.41 to 3.36) had significantly higher spike IgG titers at 7 days after the second dose (Fig. 1 and Supplemental table 1) compared with that in the participants who reported no or slight fever (<37.5℃) after the second dose of vaccination. The spike IgG titers tended to decrease with time among all the participants, but participants who experienced a high fever had higher titers than those who had no or slight fever even at both 39 and 60–74 days. The grades of local or systemic reactions were not significantly associated with spike IgG titers (Table 1 and Supplementary Table 2). These associations were virtually unchanged after excluding those with the prophylaxis use of antipyretics on vaccination.
Fig. 1.
Estimated geometric means of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike IgG titers with 95 % confidence intervals (CIs) by body temperature after the second dose of vaccination. Data are shown as geometric means with 95% CIs estimated by the linear regression model adjusting for age (continuous) and sex. The number of participants in the categories of < 37.5 ℃, 37.5–37.9 ℃, 38.0–38.4 ℃, and ≥ 38.5 ℃ were 58, 12, 12, and 6, respectively. The P value for trend was calculated by treating the categorical variable as a continuous term in the model. n.s.: non-significance (P > 0.05).
Table 1.
Estimated geometric means of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike IgG titers with 95% confidence intervals (CIs) by the grade of local or systemic reactogenicity after the second vaccination.
| Estimated geometric means of spike IgG titers with 95% CIs |
P for trend a | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | ||
| Local reactogenicity | n = 9 | n = 46 | n = 33 | n = 0 | |
| 7 days after the second vaccination | 13,160 (7,824–22,136) | 17,013 (13,525–21,398) | 18,234 (13,837–24,029) | – | 0.33 |
| 39 days after the second vaccination | 5,895 (3,678–9,449) | 7,823 (6,353–9,633) | 7,666 (5,968–9,847) | – | 0.51 |
| 60–74 days after the second vaccination | 3,414 (2,124–5,486) | 4,494 (3,639–5,550) | 4,293 (3,340–5,518) | – | 0.62 |
| Systemic reactogenicity | n = 9 | n = 24 | n = 27 | n = 28 | |
| 7 days after the second vaccination | 15,209 (9,126–25,349) | 12,087 (8,886–16,441) | 21,593 (16,163–28,848) | 18,707 (14,116–24,791) | 0.07 |
| 39 days after the second vaccination | 6,427 (4,016–10,285) | 6,097 (4,593–8,092) | 9,496 (7,274–12,397) | 7,638 (5,894–9,898) | 0.20 |
| 60–74 days after the second vaccination | 3,722 (2,315–5,983) | 3,463 (2,588–4,636) | 5,273 (4,029–6,902) | 4,399 (3,389–5,711) | 0.21 |
Data are shown as geometric means with 95% confidence intervals estimated by the linear regression model adjusting for age (continuous) and sex.
Compared with the group of grade 0, those with higher grades had no significant differences in the ratios of means in both models of local and systemic reactions.
aP value for trend was calculated by treating the categorical variable as a continuous term in the model.
4. Discussion
To the best of our knowledge, this is the first study that reported higher antibody titers among recipients who experienced high fever following the two-dose BNT162b2 vaccine. This result is consistent with biological mechanisms; type-I interferon is produced as a response of innate immunity after vaccination, triggering adaptive immune response including the differentiation of T follicular helper cells, which promote B cell differentiation into antibody-secreting plasma cells [4]. Additionally, type-I interferons and other cytokines cause various side reactions, including fever. The onset of fever itself is hypothesized to augment the adaptive immune response [9]. A fever, which is more frequently observed after the second dose of vaccines [1], [3], may enhance the activation of B cells generated by the first dose of vaccine.
To date, the epidemiological data on the association between overall systemic reactions regarding the COVID-19 vaccines and antibody production are inconsistent [6], [7], [8]. In the present study, we found no association between systemic reaction gradings and the spike IgG titers. According to the clinical trial of the BNT162b2 vaccine [1], one-fourth of the placebo recipients reported systemic symptoms (fatigue 23 % and headache 24 %) after the second dose, while only 0.8 % of the recipients reported a high fever of ≥ 38.0℃. Thus, we speculate that symptom-based grading of vaccine side reactions may be a less sensitive variable to capture the association, if any, in the study of immunogenicity.
In conclusion, while the BNT162b2 vaccine induced sufficient anti-SARS-CoV-2 immune response in all participants regardless of side reactions, those who experienced a fever of 38℃ or higher after the second dose had higher concentrations of circulating spike IgG titers than those who did not. Large-scale studies are required to confirm the present findings and examine whether a post-vaccination fever can be used in the prediction of long-term immunogenicity.
Funding
This work was funded by Abbott Japan (grant number 20C050), the NCGM COVID-19 Gift Fund (grant number 19K059), the Japan Health Research Promotion Bureau Research Fund (grant number 2020-B-09), and a grant from the National Center for Global Health and Medicine (grant number 21A006).
Role of the Funder/Sponsor
The funders did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the members of the working group of this study (Yusuke Oshiro, Natsumi Inamura, Takashi Nemoto, Haruka Osawa, Maki Konishi, and Nobumi Katayama) for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.052.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019;4(1) doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang Y.H., Song K.-H., Choi Y., Go S., Choi S.-J., Jung J., et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? The. Korean J Intern Med. 2021;36(6):1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi M, Higa Y, Esaki A, Nabeshima Y, Nakazono A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? medRxiv (preprint) 2021. [DOI] [PMC free article] [PubMed]
- 8.Kawasuji H, Morinaga Y, Tani H, et al. Functional and quantitative evaluation of BNT162b2 SARS-CoV-2 vaccine-induced immunity. medRxiv (preprint) 2021. [DOI] [PMC free article] [PubMed]
- 9.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luheshi G.N. Cytokines and Fever: Mechanisms and Sites of Action. Annals NY Acad Sci. 1998;856(1 MOLECULAR MEC):83–89. doi: 10.1111/j.1749-6632.1998.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 11.Food U., Administration D. Food and Drug Administration; US Department of Health and Human Services: 2007. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.