Abstract
OBJECTIVES
The study sought to define the risk of stent thrombosis (ST) and myocardial infarction (MI) in cancer patients compared with noncancer patients after percutaneous coronary intervention (PCI).
BACKGROUND
Cancer patients are considered to be at high thrombotic risk, but data on whether this is the case after PCI remain inconclusive.
METHODS
Cancer patients undergoing PCI at Mayo Clinic Rochester from January 1, 2003, to December 31, 2013, were identified by cross-linking institutional cancer and PCI databases and by propensity score matching to noncancer patients. The combined primary endpoint was all-cause mortality, MI, and revascularization rate at 5-year follow-up. Secondary endpoints were the individual primary endpoint components, cause of mortality, ST, and Bleeding Academic Research Consortium 2+ bleeding.
RESULTS
The primary endpoint occurred in 48.6% of 416 cancer and in 33.0% of 768 noncancer patients (p < 0.001). In competing risk analyses, cancer patients had a higher rate of noncardiac death (24.0% vs. 10.5%; p < 0.001) and a lower rate of cardiac death (5.0% vs. 11.7%; p < 0.001). Cancer patients had a higher rate of MI (16.1% vs. 8.0%; p < 0.001), ST (6.0% vs. 2.3%; p < 0.001), repeat revascularization (21.2% vs. 10.0%; p < 0.001), and bleeding (6.7% vs. 3.9%; p = 0.03). The most critical period for ST in cancer patients was in the first year after PCI. The dual antiplatelet therapy score was predictive of thrombotic and ischemic events in both groups.
CONCLUSIONS
Cancer patients have a higher risk of thrombotic and ischemic events after PCI, identifiable by a high dual antiplatelet therapy score. These findings have important implications for antiplatelet therapy decisions.
Keywords: cancer, coronary artery disease, myocardial infarction, stent, thrombosis
Cancer patients have a higher risk of thromboemboic events, especially in the immediate active cancer period. This holds true not only for venous thromboembolic events, but also for arterial thromboembolic events (ATEs) (1–3). Based on analyses from the SEER-Medicare database the risk for ATEs, including myocardial infarction (MI), starts to emerge a few months prior, peaks around the time of, and persists for 1 to 2 years after the diagnosis of malignancy (4–6). An ATE risk is eminent especially in patients with advanced malignancies (stages 3 and 4), undifferentiated cancers and adenocarcinomas, possibly related to the expression of prothrombotic factors such as von Willebrand factor (4–7). Indeed, a “platelet-cancer loop” has been suggested, and most notably in colon cancer patients, aspirin has been found to yield improved survival outcomes (1,8,9).
Stent implantation generates a nidus for thrombus formation, and seminal studies with exclusive or predominant bare-metal stent use outlined a several-fold increased risk of stent thrombosis (ST) in cancer patients (10,11). This, however, has never been confirmed in a larger cohort of patients and in the era of newer drug-eluting stents (DES). Contemporary studies agree on a higher mortality in cancer patients but disagree if this is due to a higher cardiovascular (CV) or non-CV mortality (12–19). Further uncertainty remains in terms of risk of ischemic and thrombotic events in cancer patients and whether this risk can be stratified. These are important questions in view of the implications for dual antiplatelet therapy (DAPT) recommendations, especially in a population that is also considered to be at a higher bleeding risk.
This study was designed to assess the thrombotic, ischemic, and bleeding risk after percutaneous coronary intervention (PCI) in cancer and noncancer patients. All patients in this study had complete follow-up of at least 5 years, and all major adverse cardiovascular events (MACE) were individually reviewed in detail. This rigorous approach allowed for the definition of the etiology of MI, type of ST, reason for repeat revascularization, and bleeding event. Furthermore, the results were stratified by both the DAPT and the PRECISE-DAPT (Predicting Bleeding Complication in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy) score to evaluate their applicability in cancer patients (20,21).
METHODS
The data underlying this article will be made available upon reasonable request.
The study received the proper ethical oversight. For this analysis, all patients enrolled in the Mayo Clinic Cath Lab PCI Registry database from January 1, 2003, to December 31, 2013 (end date to allow for a decade of clinical practice and 5-year follow-up data) were screened according to a pre-designed search strategy to identify those with a cancer history (crossreferencing with the Mayo Clinic cancer database and clinical data repository search software using International Classification of Diseases–Ninth Revision and International Classification of Diseases–Tenth Revision coding). Of a total of 13,488 patients identified with Mayo Clinic–related first-time PCI with stent implantation between 2003 and 2013 and 585 did not grant research authorization, leaving 12,903 patients for consideration. Cross-matching of these patients with the cancer database identified 2,134 unique cancer patients, of which those with borderline malignancies (nonmelanoma skin tumors) and a cancer diagnosis after PCI were excluded (16). For both cancer and control groups, given the potential impact of chronic inflammatory disease conditions and immune modulatory drugs on the course of atherosclerosis, patients with organ transplantation (including bone marrow transplantation), autoimmune disease, or HIV/AIDS were excluded as well. The final cancer cohort constituted 549 patients, which were matched to cohort of 3,813 PCI patients with no history of cancer as outlined subsequently.
The charts of cancer patients were reviewed for details of their cancer history as well as cancer therapy. Active cancer was defined as receiving cancer therapy at the time of PCI. Cancer types that were considered as high risk of thrombosis included pancreas, lung, and gastrointestinal cancers as well as lymphomas (4,5). Advanced cancers were defined as those in stages 3 or 4. Metastatic cancer referred to those with systemic metastases. Cancer therapeutics were considered vasotoxic if they had been associated with symptomatic coronary, carotid, or peripheral arterial disease; these include cisplatin, cyclophosphamide, 5-fluorouracil, capecitabine, gemcitabine, paclitaxel, bleomycin, interferon alpha 2B, lenalidomide, carfilzomib, everolimus, tems vascular endothelial growth factor inhibitors, dasatinib, nilotinib, ponatinib, dacomitinib, erlotinib, and cabozantnib (6). Radiation therapies were divided by radiation field (i.e., chest and non-chest based).
Acute in-hospital outcomes were adjudicated as outlined before (16). Following PCI, each patient was surveyed for MACE by telephone with a standardized questionnaire at 6 months and 1 year and then annually after the procedure by trained data technicians. MACE included all-cause mortality, MI, and repeat revascularization. All long-term follow-up events were confirmed and adjudicated by review of medical records as detailed previously (16). Patients lost to follow-up were treated as censored on the last day of contact. The definition of MI and subtyping was performed in keeping with the fourth edition of the Universal Definition of MI (22). ST was defined in keeping with the criteria set forth by the Academic Research Consortium (23). Bleeding events were categorized using the Bleeding Academic Research Consortium definitions (24). DAPT and PRECISE-DAPT scores were calculated for the prediction of ischemic/thrombotic and bleeding risks (20,21). A high DAPT and PRECISE-DAPT score was defined by a value ≥2 and ≥25, respectively
STATISTICAL ANALYSIS.
Continuous variables were presented as mean ± SD or median (interquartile range [IQR]), and discrete variables were presented as frequency and percentage. For the unmatched cohort, differences between groups were tested by Student’s t test or Kruskal-Wallis test for continuous variables and Pearson’s chi-square test for nominal variables. All p values are 2-sided with a 0.05 type I error rate. All analyses were conducted using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
A greedy nearest-neighbor matching algorithm was used to match cancer patients with a noncancer reference group using a ratio of 1:n where n ranged from 1 to 2 control subjects and a caliper of 0.1. The patients were matched on propensity scores calculated using characteristics with p values of <0.10 in the unmatched comparisons (Supplemental Table 2) as well as thrombolytics, sex, and dyslipidemia. Stent type was forced to be an exact match between groups. Any missing values among the cases prior to matching were imputed by a nonparametric process using random forest. Additionally, DAPT and PRECISE-DAPT parameters were also imputed. The matching algorithm was unable to find a suitable match for 133 cancer patients, leaving 416 in the matched cohort. Balance was assessed using standardized mean differences (SMDs), which were calculated between groups using a pooled SD. All matching variables had an SMD of <0.10, which is the generally accepted threshold for excellent balance (25). The interpretation of this SMD is that the mean difference between groups was <0.10 times the SD. All comparisons between the cancer patients and the matched reference group were tested using the conditional logistic regression with the exception of long-term outcomes.
For long-term outcomes, cumulative incidence curves were completed for both the full 5-year period and a landmark study at 1 year. These curves are the marginal event rates in the presence of the competing risks of death or the alternative cause of death when mortality is the event of interest. In the analyses involving type of bleed, type of MI, or ST type, a competing risk model was used. The 5-year event rates presented are estimated using the cumulative incidence curves and are compared using Gray’s test. Cox proportional hazards regression models using a robust variance estimator that accounts for the clustering of matched sets were fit for each outcome to provide both unadjusted and covariate adjusted hazard ratios (HRs) and tests for statistical significance. When competing risks were present, Cox proportional hazards models were ht as cause-specihc outcomes as the goals of this study are etiological in nature (26). Models were built for the cancer and noncancer subgroups that included age, sex, cardiogenic shock, acute coronary syndrome, body mass index, cerebrovascular disease, chronic lung disease, DAPT score, and PRECISE-DAPT score. In addition to these, models for the cancer subgroup included high (thrombotic) risk malignancy, advanced malignancy, active cancer, vasotoxic drugs, DAPT score, and PRECISE-DAPT score. Univariate models including these same variables were also created. Outcomes with too few events to support the full multivariable model were reduced by using variables with p < 0.1 in univariate analysis. Of the variables that were not imputed including outcomes, patients with missing data were not included in long-term analyses. For cases that are removed, their matched controls were also removed. If both controls of a match were removed, their respective case was also removed.
RESULTS
STUDY POPULATION.
A total of 549 patients with malignancies (most commonly prostate and breast cancer as well as hematological malignancies) (Supplemental Table 1) and 3,813 patients without cancer were considered for this study (Supplemental Table 2). After adjusting for baseline differences by propensity score matching, a total of 416 cancer patients (124 [30%] on active cancer therapy) and 768 noncancer patients were included. As outlined in Table 1, the 2 groups were well balanced, and only average creatinine and hemoglobin values were slightly but statistically still significantly different between the groups.
TABLE 1.
Baseline Characteristics of the Study Cohort
| Variable | Cancer Group (n = 416) | Noncancer Group (n = 768) | p Value | SMD |
|---|---|---|---|---|
| CV risk factors | ||||
|
| ||||
| Age, yrs | 72.5 ± 9.8 | 72.3 ± 9.7 | 0.82 | 0.007 |
| Female | 137 (32.9) | 249 (32.4) | 0.64 | 0.028 |
| Hypertension | 323 (77.6) | 607 (79.0) | 0.70 | 0.023 |
| Diabetes mellitus | 116 (27.9) | 187 (24.3) | 0.20 | 0.078 |
| Body mass index, kg/cm2 | 29.2 ± 5.0 | 29.3 ± 5.5 | 0.94 | 0.007 |
| Dyslipidemia | 321 (77.2) | 595 (77.5) | 0.90 | 0.017 |
| Family history of CAD | 63 (15.1) | 122 (15.9) | 0.78 | 0.020 |
| Current smoker | 57 (13.7) | 103 (13.4) | 0.84 | 0.021 |
|
| ||||
| CV disease | ||||
|
| ||||
| Prior MI | 73 (17.5) | 161 (21.0) | 0.12 | 0.088 |
| Prior CABG | 69 (16.6) | 127 (16.5) | 0.87 | 0.016 |
| Prior PCI | 24 (5.8) | 45 (5.9) | 0.96 | 0.005 |
| Prior/current HF | 76 (18.3) | 130 (16.9) | 0.57 | 0.044 |
| Pre-PCI LVEF ≤40% | 46 (11.1) | 83 (10.8) | 0.95 | 0.019 |
|
| ||||
| Presentation | ||||
|
| ||||
| Acute coronary syndrome | 0.84 | |||
| Unstable angina | 201 (48.3) | 392 (51.0) | 0.017 | |
| STEMI | 84 (20.2) | 154 (20.1) | <0.001 | |
| NSTEMI | 110 (26.4) | 201 (26.2) | 0.008 | |
| Predominant symptom | 0.86 | |||
| Chest pain | 301 (89.9) | 563 (91.5) | 0.053 | |
| CHF | 6 (1.8) | 9 (1.5) | 0.031 | |
| Arrhythmia | 4 (1.2) | 13 (2.1) | 0.073 | |
| Asymptomatic | 1 (0.3) | 1 (0.2) | 0.027 | |
| Positive stress test | 11 (3.3) | 13 (2.1) | 0.056 | |
| Other/unknown | 12 (3.6) | 16 (2.6) | 0.045 | |
| CCS class 3+ | 236 (56.7) | 443 (57.7) | 0.88 | 0.019 |
| Cardiac arrest | 2 (0.5) | 2 (0.3) | 0.61 | 0.035 |
| Cardiogenic shock | 11 (2.6) | 24 (3.1) | 0.72 | 0.015 |
|
| ||||
| Comorbidities | ||||
|
| ||||
| Peripheral artery disease | 47 (11.3) | 92 (12.0) | 0.73 | 0.019 |
| Cerebrovascular disease | 43 (10.3) | 79 (10.3) | 0.92 | <0.001 |
| Moderate/severe renal disease | 13 (3.3) | 38 (5.0) | 0.18 | 0.100 |
| Chronic lung disease | 60 (14.4) | 103 (13.4) | 0.88 | 0.007 |
| Peptic ulcer disease | 28 (6.7) | 46 (6.0) | 0.87 | 0.010 |
|
| ||||
| Procedural data | ||||
|
| ||||
| Femoral access | 379 (91.1) | 684 (89.1) | 0.34 | 0.055 |
| Radial | 37 (8.9) | 86 (11.2) | 0.28 | 0.063 |
| Mechanical support including IABP | 0.87 | |||
| Pre-PCI | 7 (1.7) | 12 (1.6) | 0.019 | |
| Post-PCI | 3 (0.7) | 4 (0.5) | 0.028 | |
| Target vessel | ||||
| LM | 40 (9.6) | 68 (8.9) | 0.83 | 0.024 |
| LAD | 255 (61.3) | 473 (61.6) | 1.00 | 0.010 |
| RCA | 214 (51.4) | 415 (54.0) | 0.40 | 0.041 |
| LCX | 206 (49.5) | 355 (46.2) | 0.46 | 0.050 |
| RIM | 29 (7.0) | 47 (6.1) | 0.93 | <0.001 |
| SVG | 28 (6.7) | 50 (6.5) | 0.81 | 0.014 |
| Number of diseased vessels | 1.72 ± 0.93 | 1.69 ± 0.87 | 0.62 | 0.045 |
| Complex lesion | 181 (48.8) | 310 (44.3) | 0.24 | 0.074 |
| Thrombus | 113 (27.2) | 205 (26.7) | 0.74 | 0.022 |
| Bifurcation | 72 (18.7) | 131 (17.9) | 0.78 | 0.026 |
| Pre-PCI TIMI flow grade 3 | 294 (70.7) | 558 (72.7) | 0.57 | 0.037 |
| Post-PCI TIMI flow grade 3 | 382 (96.7) | 727 (96.9) | 0.76 | 0.012 |
| Stent type | 1.00 | <0.001 | ||
| DES | 290 (69.7) | 529 (68.9) | ||
| BMS | 126 (30.3) | 239 (31.1) | ||
| Paclitaxel-coated stent | 31 (7.5) | 47 (6.1) | 0.50 | 0.041 |
| Number of stents used | 1.53 ± 0.85 | 1.52 ± 0.82 | 0.75 | 0.012 |
| Number of BMS used | 0.45 ± 0.83 | 0.47 ± 0.83 | 0.98 | 0.007 |
| Number of DES used | 1.08 ± 0.99 | 1.05 ± 0.96 | 0.67 | 0.015 |
| Maximum device diameter | 3.26 ± 0.52 | 3.25 ± 0.58 | 0.71 | 0.016 |
| Low-molecular-weight heparin | 7 (1.7) | 10 (1.3) | 0.61 | 0.019 |
| Unfractionated heparin | 387 (97.0) | 742 (96.9) | 1.00 | 0.007 |
| Glycoprotein IIb/IIIa inhibitors | 235 (56.5) | 432 (56.2) | 0.90 | 0.005 |
| Thrombolytics | 13 (3.1) | 16 (2.1) | 0.47 | 0.028 |
| Creatinine, mg/dl | 1.1 (0.9-1.2) | 1.0 (0.9-1.2) | 0.03 | 0.226 |
| Hemoglobin, g/dl | 13.0 (11.4-14.1) | 13.4 (12.3-14.3) | <0.001 | 0.254 |
| WBC count, 109/l | 7.4 (6.3-9.2) | 7.9 (6.6-9.6) | 0.26 | 0.335 |
Values are mean ± SD, n (%), or median (interquartile range). The following imputed variables had the percentage missing: 2% for age, 2% for sex, 5% for dyslipidemia, 4% for hypertension, 3% for diabetes, 4% for smoking status, 3% for infarct locations, 1% thrombolytics, 3% for cardiogenic shock, 2% for unstable angina, 3% for body mass index, 4% for cerebrovascular disease, 4% for chronic lung disease, 5% for prior PCI, 3% for maximum device diameter, 3% for paclitaxel, 8% for prior or current HF, 23% for LVEF, 8% for pre-procedure creatinine, 8% for pre-procedure hemoglobin, 13% for WBC count, and 24% for SVG.
BMS = bare-metal stent; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCS = Canadian Cardiovascular Society; CHF = •••; CV, cardiovascular; DES = drug-eluting stent; HF = heart failure; IABP = intra-aortic balloon pump; LAD = left anterior descending artery; LCX = left circumflex; LM = left main; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NSTEMI = non-ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention; RCA = right coronary artery; RIM = ramus intermedius; SMD = standardized mean difference; STEMI = ST-segment elevation myocardial infarction; SVG = saphenous vein graft; TIMI, Thrombolysis In Myocardial Infarction; WBC, white blood cell.
In terms of in-hospital post-procedural events, noncancer patients showed a higher rate of MI; no other significant differences were noted (Table 2). Discharge medications were similar for cancer and noncancer patients (Table 2). Post-discharge median follow-up time was 6.2 (IQR: 4.2 to 9.0) years in cancer patients and 5.1 (IQR: 3.6 to 8.2) years in noncancer patients (p = 0.003).
TABLE 2.
In-Hospital Outcomes and Dismissal Medications
| Variable | Cancer Group (n = 416) | Noncancer Group (n = 768) | p Value | SMD |
|---|---|---|---|---|
| In-hospital complications | ||||
|
| ||||
| Death | 1 (0.2) | 10 (1.3) | 0.05 | 0.221 |
| CABG | 3 (0.8) | 5 (0.7) | 0.80 | 0.018 |
| MI | 10 (2.5) | 36 (4.7) | 0.02 | 0.151 |
| Cardiogenic shock | 6 (1.5) | 17 (2.2) | 0.49 | 0.054 |
| HF | 13 (3.3) | 18 (2.4) | 0.34 | 0.046 |
| Cerebrovascular stroke | 1 (0.3) | 4 (0.5) | 0.51 | 0.046 |
| Tamponade | 1 (0.3) | 2 (0.3) | 0.63 | 0.001 |
| Transfusion | 19 (4.8) | 28 (3.7) | 0.43 | 0.060 |
| Length of stay, days | 2 (1-3) | 2 (1-3) | 0.459 | 0.047 |
|
| ||||
| Dismissal medications | ||||
|
| ||||
| Aspirin | 385 (96.2) | 742 (97.1) | 0.45 | 0.045 |
| P2Y12 receptor inhibitor | 393 (98.2) | 742 (96.6) | 0.12 | 0.123 |
| Anticoagulant | 45 (11.2) | 79 (10.3) | 0.74 | 0.027 |
| Statin | 224 (88.2) | 485 (90.3) | 0.17 | 0.076 |
| Beta-blocker | 346 (86.5) | 654 (85.6) | 0.62 | 0.029 |
| ACE inhibitor or ARB | 249 (62.2) | 465 (60.9) | 0.70 | 0.011 |
| Calcium-channel blocker | 68 (17.0) | 138 (18.1) | 0.50 | 0.033 |
| Nitrate | 63 (15.8) | 150 (19.6) | 0.05 | 0.101 |
| DAPT meds + anticoagulant | 38 (9.5) | 73 (9.5) | 0.86 | 0.004 |
Values are n (%) or median (interquartile range).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; DAPT = dual antiplatelet therapy; other abbreviations as in Table 1.
MACE AND MORTALITY RATES.
Cancer patients had a significantly higher 5-year MACE rate (48.6% vs. 33.0%; HR: 1.61; 95% confidence interval [CI]: 1.34 to 1.94; p < 0.001) (Figure 1A). All-cause mortality was higher in cancer patients over the 5-year follow-up period (29.1% vs. 22.3%; HR: 1.31; 95% CI: 1.05 to 1.63; p = 0.02) (Figure 1B), emerging after the first 1 year following PCI (Supplemental Figure 1B). In competing risk analyses, cancer patients had a higher 5-year rate of non-CV mortality (24.0% vs. 10.5%; HR: 2.28; 95% CI: 1.70 to 3.05; p < 0.001) but a lower 5-year rate of CV mortality (5.0% vs. 11.7%; HR: 0.43; 95% CI: 0.27 to 0.70; p < 0.001) (Figures 1C and 1D). The predictors of non-CV and CV mortality in cancer and noncancer patients are listed in Supplemental Table 3. Among cancer patients, the strongest independent predictors for all-cause and noncardiac mortality were active and metastatic malignancy and for cardiac mortality the DAPT score.
Figure 1.
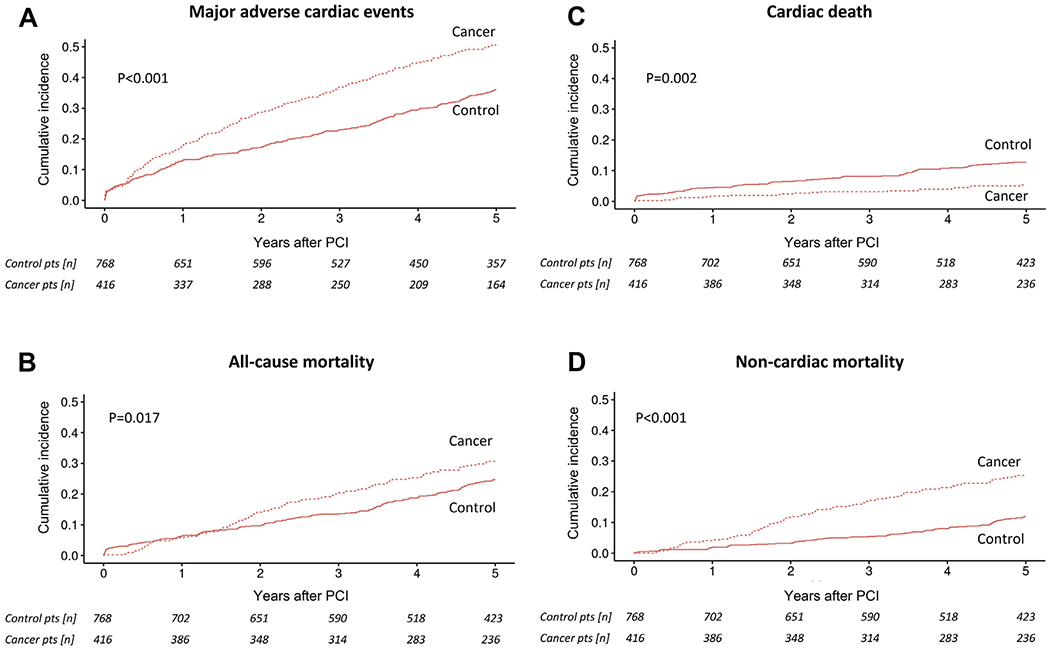
Competing risk cumulative incidence curves for (A) major adverse cardiac events, (B) all-cause mortality, (C) cardiac mortality, and (D) noncardiac mortality in cancer and noncancer (control) patients after percutaneous coronary intervention (PCI) and group comparison by Gray’s test.
MI, ST, AND REVASCULARIZATION RATES.
Cancer patients had a higher rate of MI after PCI over the 5-year follow-up period (16.1% vs. 8.0%; HR: 2.10; 95% CI: 1.49 to 2.96; p < 0.001) (Central Illustration). The cumulative increase in risk of MI in cancer patients was evident in the first year after PCI as well as in subsequent years (Supplemental Figure 2A). Sudden cardiac death and ST were the 2 types of MI that were significantly more common in cancer patients (Table 3). The 5-year rate of ST in cancer patients was nearly 3-fold higher (6.0% vs. 2.3%; HR: 2.74; 95% CI: 1.49 to 5.05; p < 0.001) (Central Illustration) and was noted for Bristol Myers Squibb and DES (Table 4). As indicated in landmark analyses (Supplemental Figure 2B), the period of increased risk of ST in the cancer cohort overall was confined to the first year after PCI. In line with the previous results, the 5-year rate of repeat revascularization was higher in cancer patients than in noncancer patients (21.2% vs. 10.9%; HR: 2.04; 95% CI: 1.52 to 2.74; p < 0.001) (Central Illustration) and was noted both within and after the first year following PCI (Supplemental Figure 2C). The independent predictors of MI, ST, and repeat revascularization are outlined in Supplemental Table 3. At the time of ST, 39.3% and 56.5% of the patients in the cancer and noncancer groups were off DAPT (p = 0.22). Among the different subgroups, the difference in ST outcomes between cancer and noncancer patients was mainly seen in non-ST-segment elevation MI patients. Among patients with malignancies, metastatic cancer (HR: 4.05; 95% CI: 1.43 to 11.44; p = 0.008) and a high DAPT score (HR: 1.81; 95% CI: 1.38 to 2.38; p < 0.001) were predictive of future non-type 2 MIs among cancer patients. Furthermore, in landmark analyses high thrombotic risk cancers had a higher risk of ST in the first year after PCI (Supplemental Figure 3C).
CENTRAL ILLUSTRATION.
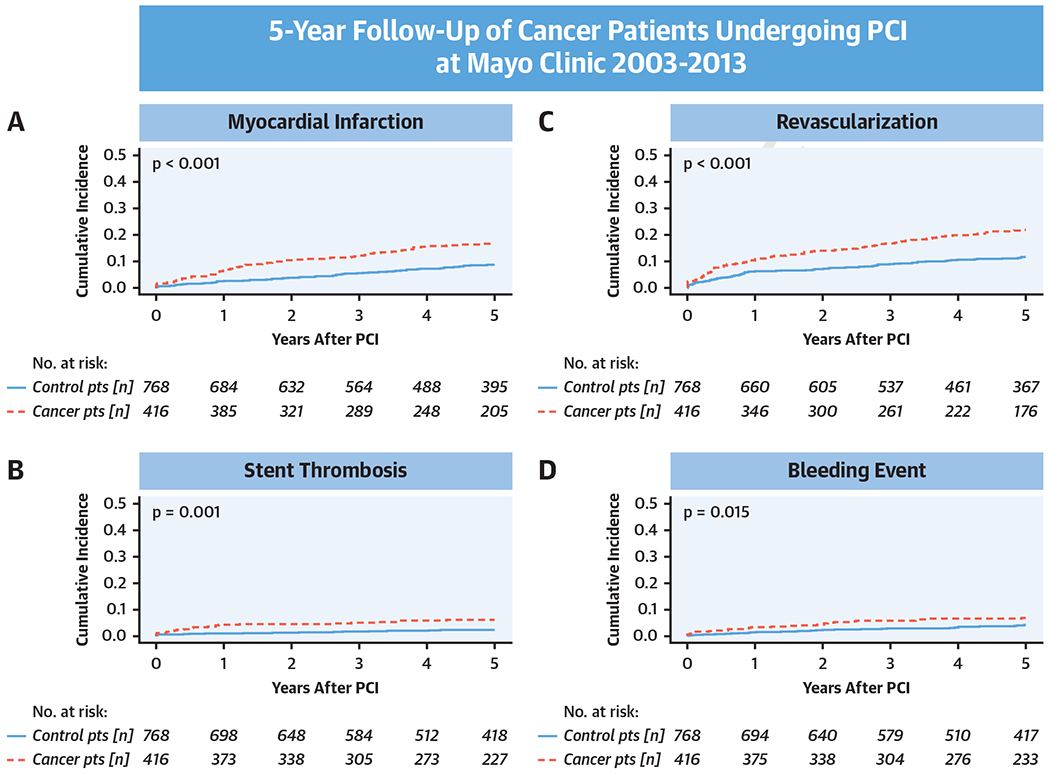
Competing risk cumulative incidence curves for (A) myocardial infarction, (B) stent thrombosis, (C) revascularization, and (D) bleeding in cancer and noncancer (control) patients after percutaneous coronary intervention (PCI) and group comparison by Gray’s test.
TABLE 3.
5-Year MI Event Rate, Stratified by Type of MI
| Variable | Cancer Group (n = 416) | Noncancer Group (n = 768) | p Value |
|---|---|---|---|
| Type of MI | |||
|
| |||
| 1—spontaneous | 8 (1.9) | 9 (1.2) | 0.78 |
| 2—demand supply | 34 (8.2) | 41 (5.4) | 0.07 |
| 3—sudden cardiac death | 9 (2.2) | 2 (0.3) | 0.001 |
| 4a—periprocedural MI | 1 (0.2) | 0 (0) | 0.20 |
| 4b—stent thrombosis | 15 (3.6) | 9 (1.2) | 0.02 |
Values are n (%).
MI = myocardial infarction.
TABLE 4.
5-Year ST Event Rate, Stratified by Type of ST and Stent Type
| Variable | Cancer Group | Noncancer Group | p Value |
|---|---|---|---|
| Timing | (n = 25) | (n = 18) | |
| Time to ST, days | 181.0 (60.0-448.0) | 547.5 (44.0-1,112.3) | 0.29 |
| Early ST | 5 (20.0) | 5 (27.8) | 0.06 |
| Late ST | 13 (52.0) | 3 (16.7) | |
| Very late ST | 7 (28.0) | 10 (55.6) | |
| DES | (n = 290) | (n = 529) | |
| ST | 16 (5.5) | 13 (2.5) | 0.03 |
| Definite | 12 (4.1) | 7 (1.3) | 0.01 |
| Probable | 1 (0.3) | 2 (0.4) | 0.91 |
| Possible | 3 (1.0) | 4 (0.8) | 0.71 |
|
| |||
| First-generation DES | (n = 192) | (n = 310) | |
| ST | 10 (5.2) | 10 (3.2) | 0.27 |
| Definite | 8 (4.2) | 5 (1.6) | 0.08 |
| Probable | 1 (0.5) | 1 (0.3) | 0.73 |
| Possible | 1 (0.5) | 4 (1.3) | 0.39 |
|
| |||
| Second-generation DES | (n = 98) | (n = 219) | |
| ST | 6 (6.1) | 3 (1.4) | 0.02 |
| Definite | 4 (4.1) | 2 (0.9) | 0.07 |
| Probable | 0 (0.5) | 1 (0.5) | 0.48 |
| Possible | 2 (2.0) | 0 (0.0) | 0.04 |
|
| |||
| BMS | (n = 126) | (n = 239) | |
| ST | 9 (7.1) | 5 (2.1) | 0.02 |
| Definite | 5 (4.0) | 5 (2.1) | 0.30 |
| Probable | 2 (1.6) | 0 (0) | 0.06 |
| Possible | 2 (1.6) | 0 (0) | 0.05 |
Values are median (interquartile range) or n (%).
Abbreviations as in Table 1.
BLEEDING RATES.
Compared with noncancer patients, cancer patients had a higher overall 5-year bleeding rate after PCI (6.7% vs. 3.9%; HR: 1.73; 95% CI: 1.06- 2.83; p = 0.03) (Central Illustration). Even before PCI, cancer patients more often had a history of bleeding events (5.8% vs. 2.7%; p = 0.025). The predictors of post-PCI bleeding are listed in Supplemental Table 3. By Bleeding Academic Research Consortium bleeding definitions, cancer patients had more type 2 bleeding events than noncancer patients did (2.4% vs. 0.9%; p = 0.04). No significant differences were found between the other types of bleeding. Active cancer was the strongest predictor of bleeding in cancer patients. In landmark analyses, patients with high thrombotic risk cancers had a higher risk of bleeding after the first year following PCI (Supplemental Figure 3D).
DAPT AND PRECISE-DAPT SCORE.
The DAPT score had a similarly predictive value in cancer and noncancer patients in univariate analysis for cardiac mortality (HR: 1.35; 95% CI: 0.94 to 1.93; p = 0.10; and HR: 1.20; 95% CI: 1.02 to 1.41; p = 0.03), MACE (HR: 1.05; 95% CI: 0.94 to 1.17; p = 0.41; and HR: 1.23; 95% CI: 1.11 to 1.36; p < 0.001), MI (HR: 1.64; 95% CI: 1.38 to 1.95; p < 0.001; and HR: 1.65; 95% CI: 1.36 to 2.01; p < 0.001), ST (HR: 2.00; 95% CI: 1.51 to 2.66; p < 0.001; and HR: 1.51; 95% CI: 0.99 to 2.31; p = 0.06), and repeat revascularization (HR: 1.18; 95% CI: 0.99 to 1.41; p = 0.07; and HR: 1.23; 95% CI: 1.03 to 1.48; p = 0.02), respectively. A high DAPT score was predictive of MI and ST within and after the first year following PCI (Figures 2, 3, and 4). Furthermore, a high versus a low DAPT score remained highly predictive of ST in cancer patients after second-generation DES (13.3% vs. 1.4%; p < 0.001).
Figure 2.
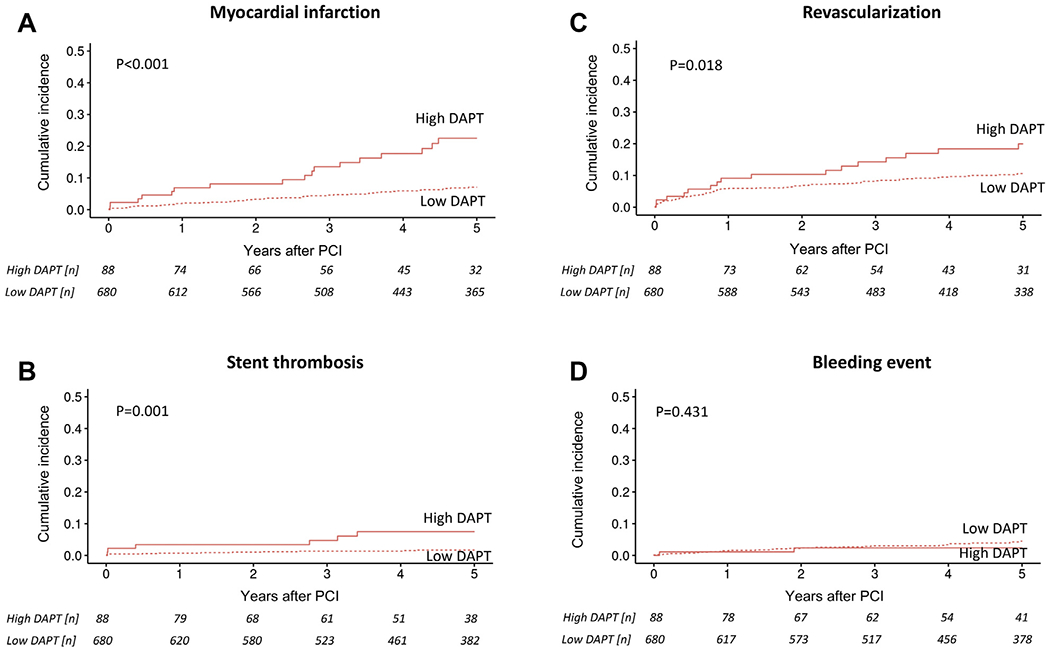
Competing risk cumulative incidence curves for (A) myocardial infarction, (B) stent thrombosis, (C) revascularization, and (D) bleeding stratified by high and low dual antiplatelet therapy (DAPT) score in noncancer patients after percutaneous coronary intervention (PCI) and group comparison by Gray’s test.
Figure 3.
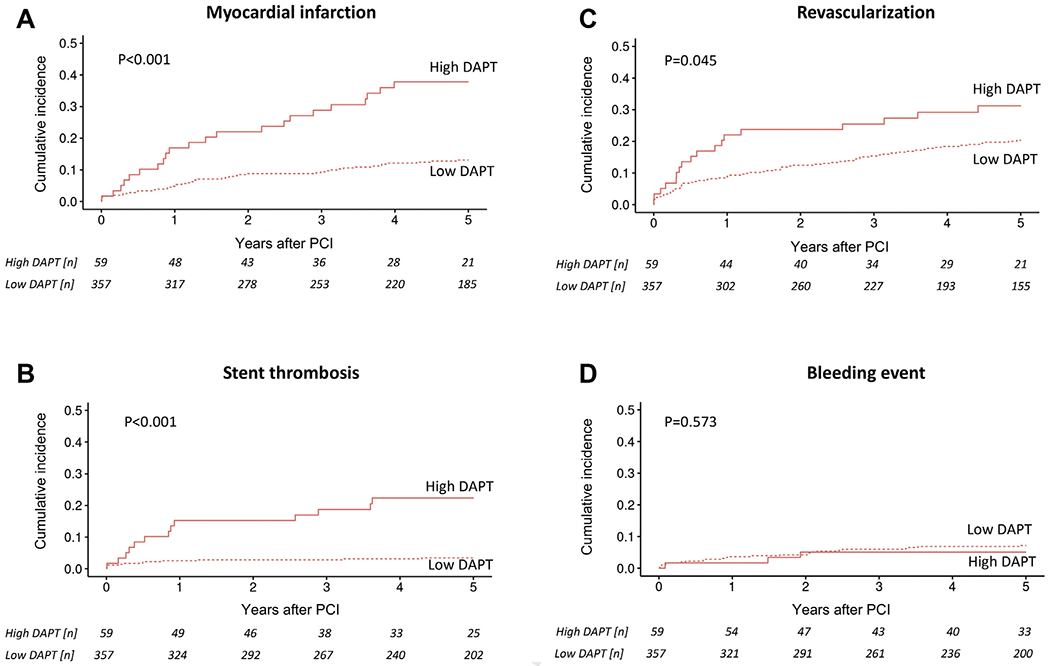
Competing risk cumulative incidence curves for (A) myocardial infarction, (B) stent thrombosis, (C) revascularization, and (D) bleeding stratified by high and low DAPT score in cancer patients after PCI and group comparison by Gray’s test. Abbreviations as in Figure 3.
Figure 4.
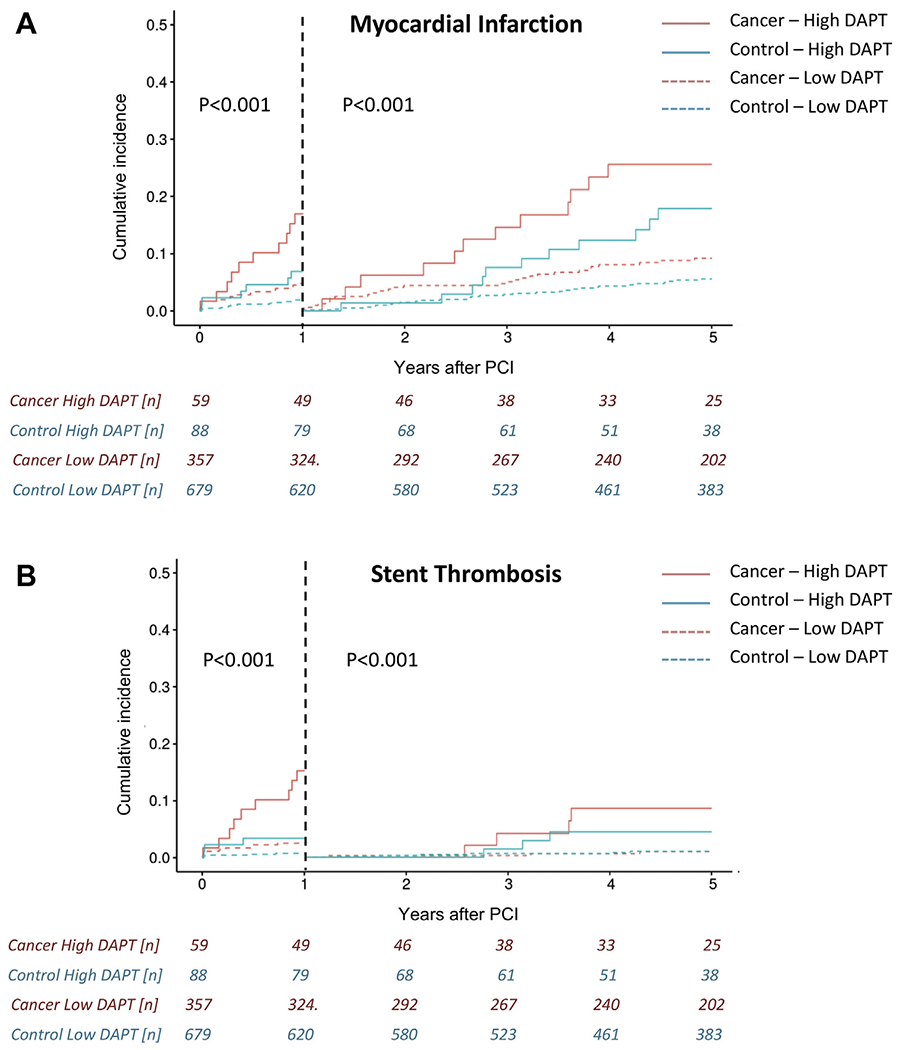
Landmark analyses for myocardial infarction (top) and stent thrombosis (bottom) by patient group (cancer vs. noncancer) and DAPT score (high vs. low) after PCI. Graphs reflect competing risk cumulative incidences and group comparisons were made by Gray’s test. Abbreviations as in Figure 3.
The PRECISE-DAPT score predicted all-cause mortality (HR: 1.04; 95% CI: 1.03 to 1.05; p < 0.001), cardiac mortality (HR: 1.05; 95% CI: 1.03 to 1.06; p < 0.001), noncardiac mortality (HR: 1.03; 95% CI: 1.02 to 1.04; p < 0.001), MACE (HR: 1.02; 95% CI: 1.01 to 1.03; p < 0.001), and bleeding (HR: 1.04; 95% CI: 1.01 to 1.06; p < 0.001) in all patients and in cancer patients: all-cause mortality (HR: 1.03; 95% CI: 1.02 to 1.04; p < 0.001), noncardiac mortality (HR: 1.03; 95% CI: 1.00 to 1.06; p < 0.001), and bleeding (HR: 1.03; 95% CI: 1.00 to 1.06; p = 0.04).
DISCUSSION
The current study in a well-characterized cohort of PCI patients over a 10-year treatment window in the DES era finds that cancer patients compared with noncancer patients have a higher rate of MI, ST, and repeat revascularization as well as bleeding. Although cancer patients remained at an increased risk of MI and repeat revascularization over the entire followup period, the increased ST risk in the cancer cohort was evident over the first year after PCI only. A high DAPT score could identify cancer patients at high ischemic and thrombotic risk, both early and late after PCI.
Prior studies have outlined worse outcomes after PCI in cancer patients, mainly an increased mortality (18,19). This has been variably attributed to an increased non-CV or CV mortality, irrespective of type of presentation at the time of PCI (13–16,27–30). Of note, non-CV death has emerged as the leading cause of death in patients after PCI in general. This seems to be even more so the case in the cancer population with important implications for competing risk analyses as conducted herein: an increase in the risk of one type of mortality must result in a proportional decrease in risk of the other. Thus, the current analysis is not to convey that cancer patients do not have a CV risk, but this CV mortality risk is outweighed by the non-CV mortality risk in these patients.
Data from the National Heart, Lung, and Blood Institute Dynamic Registry indicated that among patients presenting with an acute MI for PCI, those with a history of cancer not only have a higher 1-year mortality, but also have a higher 1-year recurrent MI rate (12). This has not been demonstrated in the PCI population at large, and the current analysis adds in this regard. A detailed analysis of MI subtypes in the current work furthermore indicates that cancer patients have a higher risk of a sudden cardiac death and ST but not of the most commonly reported type 1 or 2 MIs, which may harmonize some of the divergent results across studies. Of note, metastatic cancer was independently predictive of non-type 2 MIs, possibly relating to a higher thrombotic risk in these patients, though this remains speculative (6).
Several case reports and 1 focused analysis of patients undergoing Bristol Myers Squibb framed a risk of ST in cancer patients over a decade ago. The Dutch Stent Thrombosis Registry, with two-thirds of patients undergoing Bristol Myers Squibb, pointed out malignancy as the strongest patient-related risk factor for early and late ST. However, only 87 of 1,303 patients included in the analysis had cancer, and only patient with present (active) malignancy were included. Conducted in the same early DES era (2005 to 2007), the CREDO-Kyoto (Coronary REvascularization Demonstrating Outcome Study in Kyoto) PCI/coronary artery bypass grafting cohort-2 registry noted a trend of a higher risk of ST in cancer patients (27). The current analysis therefore is an important addition to the existing literature, confirming an increased ST risk in cancer patients with Bristol Myers Squibb as well as with newer-generation DES. Importantly, the ST rates among Bristol Myers Squibb patients are rather similar to the Dutch registry and are also in line with some of the most recent reports, such as those from the ADAPT-DES study (11,31).
There was no difference in the percentage of patients off DAPT at the time of ST between the groups, indicating that DAPT discontinuation rates at the time of ST were not the deciding factor. Although various prespecified cancer-related factors were not predictive of ST and MI over the entire 5-year follow-up period, when stratified in landmark analyses, patients with a high thrombotic risk malignancies, as defined by Navi et al. (6), did have a higher risk of ST and MI over the first year after PCI. Intriguingly, the bleeding risk showed an opposite trend in these patients, indicating that 1 year would present the optimal duration of DAPT after PCI in these patients. The bleeding dynamics for the entire cancer cohort, though, pointed toward a higher risk in the first year of PCI. The relatively high utilization of femoral access may contribute to this and is an aspect of consideration to lower the bleeding risk in cancer patients undergoing PCI as recommended in a Society for Cardiovascular Angiography and Interventions consensus document (32).
With this being said, the DAPT score emerged as the main differentiating factor for thrombotic versus bleeding risk in cancer patients in this study (20). This score has never been evaluated in a larger cancer cohort, and the current results indicate it performs just as well in this patient population as it does in noncancer patients. Although the DAPT score was developed to guide therapy after 12 months of DAPT, it risk-stratified for both early and late ischemic and thrombotic events after PCI and even for STunrelated MIs, and more so in cancer patients. The risk of bleeding was not higher for cancer patients with a high DAPT score than for those with a low DAPT score. The DAPT score may therefore serve as an ideal tool to gauge the balance between ischemic and thrombotic risk on the one hand and bleeding risk on the other hand, so important for decisions on the duration of DAPT therapy. However, as not all factors possibly influencing this balance could be captured and accounted for in this study, the current findings are most supportive of a randomized clinical trial on optimal DAPT management in cancer patients. This is a very timely need, as recent trials including the LEADERS FREE and TWILIGHT trials, point out options for short duration of DAPT even in cancer patients (33,34).
STUDY LIMITATIONS.
The retrospective character and the single-center nature of the current study links to its limitations. This being said, the goal of the current study was to clearly define events related and unrelated to a defined stenting procedure. The ability to define events such as ST in meticulous detail by chart and angiographic review in a large cohort of patients is the advantage of analyses such as this one compared, for example, with studies based on claims or large databases. Confounding (e.g., based on selection bias) remains a concern, but we took particular care herein to matching with a variable number of control subjects (one-to-many matching) to increase precision. Three different matching approaches were taken: non-propensity score matching, 1 propensity score match based on a priori defined variables that are associated with increased risk of adverse outcomes after PCI, and 1 propensity score match adjusting for all baseline differences (as presented herein), always with the same study results.
CONCLUSIONS
Cancer patients have a higher risk of thrombotic and ischemic events as well as of bleeding after PCI. A high DAPT score identifies patients who are at a high ischemic and thrombotic risk and yet at a bleeding risk that is similar to those with a low DAPT score. These findings have important implications for antiplatelet therapy decisions.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Cancer patients are at high thrombotic risk, especially early after diagnosis. Whether this is the case after PCI and for patients with active cancer and survivors alike remains undefined.
WHAT IS NEW?
Patients with active cancer and a history of malignancy have a higher risk of thrombotic and ischemic events after PCI. The increased ST risk in cancer patients was confined to the first year after PCI while the risk of MI persisted. Cancer patients at risk of early and late thrombotic and ischemic events after PCI can be identified by a high DAPT score.
WHAT IS NEXT?
These findings emphasize the importance of adequate DAPT in cancer patients, especially those with a high DAPT score, and support the design of a prospective randomized controlled trial to define optimal type and duration of DAPT in cancer patients after PCI.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This work is supported by the National Cancer Institute (CA233610 [to Dr. Herrmann]), Miami Heart Foundation, and Mayo Clinic Department of Cardiovascular Medicine. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ATE
arterial thromboembolic event
- CI
confidence interval
- DAPT
dual antiplatelet therapy
- DES
drug-eluting stent
- HR
hazard ratio
- IQR
interquartile range
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- SMD
standardized mean difference
- ST
stent thrombosis
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Oren O, Herrmann J. Arterial events in cancer patients-the case of acute coronary thrombosis. J Thorac Dis 2018;10:S4367–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458–64. [DOI] [PubMed] [Google Scholar]
- 3.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- 4.Navi BB, Reiner AS, Kamel H, et al. Reply: Arterial thromboembolism in non-Hodgkin lymphoma, as the presentation of occult cancer, and with cancer therapies. J Am Coll Cardiol 2018;71:260–2. [DOI] [PubMed] [Google Scholar]
- 5.Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019;133:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrmann J Vascular toxic effects of cancer therapies. Nat Rev Cardiol 2020;17:503–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang AJ, Wang M, Wang Y, et al. Cancer cellderived von Willebrand factor enhanced metastasis of gastric adenocarcinoma. Oncogenesis 2018;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018;131:1777–89. [DOI] [PubMed] [Google Scholar]
- 9.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross CM, Posch MG, Geier C, et al. Subacute coronary stent thrombosis in cancer patients. J Am Coll Cardiol 2008;51:1232–3. [DOI] [PubMed] [Google Scholar]
- 11.van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009;53:1399–409. [DOI] [PubMed] [Google Scholar]
- 12.Abbott JD, Ahmed HN, Vlachos HA, Selzer F, Williams DO. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2007;100:190–5. [DOI] [PubMed] [Google Scholar]
- 13.Kurisu S, Iwasaki T, Ishibashi K, Mitsuba N, Dohi Y, Kihara Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int J Cardiol 2013;167:2335–7. [DOI] [PubMed] [Google Scholar]
- 14.Velders MA, Boden H, Hofma SH, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol 2013;112:1867–72. [DOI] [PubMed] [Google Scholar]
- 15.Hess CN, Roe MT, Clare RM, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc 2015;4:e001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Gulati R, Lennon RJ, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc 2016;91:1680–92. [DOI] [PubMed] [Google Scholar]
- 17.Ueki Y, Vögeli B, Karagiannis A, et al. Ischemia and bleeding in cancer patients undergoing percutaneous coronary intervention. J Am Coll Cardiol CardioOnc 2019;1:145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roule V, Verdier L, Blanchart K, et al. Systematic review and meta-analysis of the prognostic impact of cancer among patients with acute coronary syndrome and/or percutaneous coronary intervention. BMC Cardiovasc Disord 2020;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintana RA, Monlezun DJ, Davogustto G, et al. Outcomes following percutaneous coronary intervention in patients with cancer. Int J Cardiol 2020;300:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018;72:2231–64. [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115: 2344–51. [DOI] [PubMed] [Google Scholar]
- 24.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 25.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387–98. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatsuma K, Shiomi H, Morimoto T, et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur Heart J Qual Care Clin Outcomes 2018;4:200–7. [DOI] [PubMed] [Google Scholar]
- 28.Landes U, Kornowski R, Bental T, et al. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron Artery Dis 2017;28:5–10. [DOI] [PubMed] [Google Scholar]
- 29.lannaccone M, D’Ascenzo F, Vadala P, et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care 2018;7:631–8. [DOI] [PubMed] [Google Scholar]
- 30.Gong IY, Yan AT, Ko DT, et al. Temporal changes in treatments and outcomes after acute myocardial infarction among cancer survivors and patients without cancer, 1995 to 2013. Cancer 2018;124:1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chau KH, Kirtane AJ, Easterwood RM, et al. Stent thrombosis risk over time on the basis of clinical presentation and platelet reactivity: analysis from ADAPT-DES. J Am Coll Cardiol Intv 2021;14:417–27. [DOI] [PubMed] [Google Scholar]
- 32.Iliescu C, Grines CL, Herrmann J, et al. SCAI expert consensus statement: Evaluation, management, and special considerations of cardiooncology patients in the cardiac catheterization laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv 2016;87:895–9. [DOI] [PubMed] [Google Scholar]
- 33.Urban P, Meredith IT, Abizaid A, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–47. [DOI] [PubMed] [Google Scholar]
- 34.Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019;381:2032–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


