Abstract
Background and Objective
Stress hyperglycemia may occur in diabetic patients with acute severe cerebrovascular disease, but the results regarding its association with stroke outcomes are conflicting. This study aimed to examine the association between stress‐induced hyperglycemia and the occurrence of in‐hospital death in patients with diabetes and acute ischemic stroke.
Research Design and Methods
All data were from the Chinese Stroke Center Alliance (CSCA) database and were collected between 2016 and 2018 from >300 centers across China. Patients’ demographics, clinical presentation, and laboratory data were extracted from the database. The primary endpoint was in‐hospital death. The ratio of fasting blood glucose (FBG) to HbA1c was calculated, that is, the stress‐induced hyperglycemia ratio (SHR), to determine stress hyperglycemia following acute ischemic stroke.
Results
A total of 168,381 patients were included. The mean age was 66.2 ± 10.7, and 77,688 (43.0%) patients were female. The patients were divided into two groups: survivors (n = 167,499) and non‐survivors (n = 882), as well as into four groups according to their SHR quartiles (n = 42,090–42,099/quartile). There were 109 (0.26%), 142 (0.34%), 196 (0.47%), and 435 (1.03%) patients who died in the Q1, Q2, Q3, and Q4 quartiles, respectively. Compared with Q1 patients, the death risk was higher in Q4 patients (odds ratio (OR) = 4.02) (adjusted OR = 1.80, 95% confidence interval [CI] = 1.10–2.92, p = 0.018 after adjustment for traditional cardiovascular risk factors). The ROC analyses showed that SHR (AUC = 0.667, 95% CI: 0.647–0.686) had a better predictive value for mortality than that of fasting blood glucose (AUC = 0.633, 95% CI: 0.613–0.652) and HbA1c (AUC = 0.523, 95% CI: 0.504–0.543).
Conclusions
The SHR may serve as an accessory parameter for the prognosis of patients with diabetes after acute ischemic stroke. Hyperglycemia in stroke patients with diabetes mellitus is associated with a higher risk of in‐hospital death.
Keywords: diabetes, hyperglycemia, ischemia, mortality, physiological, stress, stroke
Stress‐induced hyperglycemia ratio may serve as an accessory parameter for the prognosis of patients with diabetes after acute ischemic stroke. Hyperglycemia in stroke patients with diabetes mellitus is associated with a higher risk of in‐hospital death.

1. BACKGROUND
Acute stress hyperglycemia is a common manifestation found in patients presenting to the emergency room with acute cerebrovascular disease. Acute stress hyperglycemia is not only associated with the severity of stroke 1 , 2 but also with poor outcomes of stroke, especially in patients without diabetes mellitus. 3 On the other hand, the association between acute hyperglycemia and the outcomes of patients with diabetes mellitus is controversial, not only for stroke but also for other critical illnesses. 1 , 4 , 5 Indeed, a meta‐analysis of stroke and hyperglycemia demonstrated that stress hyperglycemia in non‐diabetic patients was associated with an increased risk of mortality after stroke (pooled relative risk (RR) = 3.07, 95% confidence index (CI): 2.50–3.79), but this was not observed in patients with a history of diabetes. 6 This phenomenon is supported by other cohort studies 4 , 7 and was also observed in other critical illnesses, that is, that acute hyperglycemia with pre‐existing diabetes mellitus led to lower mortality and shorter length of ICU stay than in patients without diabetes. 6 , 8 , 9
There is no unified definition of stress hyperglycemia, 10 and the patients are generally classified as known diabetes, newly diagnosed diabetes, and hospital‐related hyperglycemia. 3 , 11 , 12 Most of the previous studies simply used fasting glucose or initial blood glucose at admission to determine the presence of stress hyperglycemia, without considering the usual glucose levels before stroke onset. This might explain why stress hyperglycemia cannot predict the outcome of stroke in patients with a history of diabetes because of high background glucose levels. Therefore, background blood glucose levels should be considered when assessing the relationship between stress hyperglycemia and the outcomes of critical illness, especially for patients with pre‐existing diabetes. The stress hyperglycemia ratio (SHR) is a new method for determining blood glucose stress. It also considers glycated hemoglobin (HbA1c) (which represents the blood glucose levels over the last 2–3 months) and random blood glucose after the stress events. 13
A study on acute ischemic stroke in patients with diabetes showed that the use of the glycemic gap and SHR as indicators of stress hyperglycemia could be better predictors for the severity and poor outcome of stroke, 14 but this study was a single‐center, small sample study, limiting the generalizability of its results. In addition, most previous studies used admission glucose, which can be influenced by the diabetic status and the food consumed over the previous hours. 10 , 13 , 15 , 16 , 17 Therefore, fasting blood glucose (FBG) instead of random or admission glucose could be a more reliable marker, as previously suggested. 18 China is a large country with a high burden of cerebrovascular diseases. 19 , 20 With the support of the Chinese government, an important database was built at Tiantan Hospital. The aim of the present study was to examine the association between stress‐induced hyperglycemia and the occurrence of in‐hospital death in patients with diabetes and acute ischemic stroke.
2. METHODS
2.1. Study participants
All data for the present study were from the Chinese Stroke Center Alliance (CSCA) database and were collected between August 01, 2015, and July 31, 2019, from 1476 centers across China. The National Clinical Research Center for Nervous System Diseases of Beijing Tiantan Hospital is mandated to manage the CSCA. The CSCA is funded by the Chinese government, and the data are not accessible to the general public. The CSCA aims to establish a continual stroke registry of patients with stroke in China. It contains the data of millions of patients with stroke from all over China, and it aims to help reduce the burden of stroke in China. 21 The present study was approved by the ethics committee of Beijing Tiantan Hospital. Individual consent was waived by the committee because the data were anonymized.
The patient population of the CSCA includes (a) patients ≥18 years of age; (b) primary diagnosis of stroke or transient ischemic attack confirmed by brain computed tomography (CT) or magnetic resonance imaging (MRI); (c) within 7 days of onset; and (d) admitted directly to wards or from the emergency department.
The study population included patients with acute cerebral infarction within 72 h of onset and with a previous history of diabetes or previous use of hypoglycemic drugs for > 6 months. Diabetes diagnosis was based on a self‐reported history of diabetes confirmed by the medical records. Potential patients were excluded from the study if they had incomplete information on in‐hospital mortality or had missing HbA1c and FBG data.
2.2. Data collection and outcome assessment
In the CSCA, data are directly entered by each center using a web‐based patient data collection interface by trained registrars. 21 Baseline information was extracted from the database, including demography, vascular risk factors, infarction location, laboratory data, and clinical data. Vascular risk factors included a medical history of hypertension, atrial fibrillation, coronary artery disease, and current smoking. Clinical data included systolic and diastolic blood pressure, baseline National Institutes of Health Stroke Score (NIHSS), leukocytes, alcohol intake, body mass index (BMI), and brain natriuretic peptide (BNP) at admission. Current smoking was defined as smoked at least one cigarette per day for the previous year or more. Laboratory data included baseline HbA1c, fasting blood sugar, and routine blood biochemical variables that were obtained following an 8–12 h fast within the first 24 h after admission. Laboratory data were from the certified central hospital laboratories.
2.3. Assessment of initial fasting glucose levels, HbA1c, and SHR
To determine the presence of stress hyperglycemia following an acute ischemic stroke, the ratio of AG (random glucose at admission) to HbA1c was used, which is named the SHR. 13 In order to eliminate the confounding effect of food, AG in the formula was replaced by FBG (fasting blood glucose) in the present study, as supported by a previous study. 18 HbA1c was measured within 24 h after admission using high‐performance liquid chromatography (HPLC) (G8 HPLC Analyzer; Tosoh Bioscience). The analyses were conforming with the Diabetes Control and Complications Trial and National Glycohemoglobin Standardization Program (NGSP) standards.
2.4. Grouping
The included patients were separated into four groups based on the quartiles of SHR. The ranges of the Q1, Q2, Q3, and Q4 of SHR were 0–0.90, 0.90–1.08, 1.08–1.32, and > 1.32.
2.5. Statistical analysis
The data were tested for normal distribution using the Kolmogorov‐Smirnov test. The continuous variables were expressed as means ± SD or as medians with interquartile ranges according to their distribution (normal or skewed). Statistical comparisons of continuous variables were performed using Student's t test or Wilcoxon rank‐sum test (comparisons of two groups), or ANOVA or the Kruskal‐Wallis U test (comparison of more than two groups). Categorical variables were expressed as numbers and percentages and analyzed using the chi‐square test or Fisher's exact test. We evaluated the association between SHR and in‐hospital death using multivariable logistic regression analysis adjusted for potential confounders, including age, sex, BMI, NIHSS on admission, hypertension, atrial fibrillation, previous ischemic stroke, previous myocardial infarction, SAH, antiplatelets, anticoagulation, lipid‐lowering drug, smoking, alcohol, LDL‐C, FBG, HbA1c, eGFR, HCY, systolic blood pressure, and diastolic blood pressure. Receiver operating characteristics (ROC) curves were generated to examine the predictive value of SHR, FBG, and HbA1c, based on the area under the ROC curve (AUC). All statistical analyses were performed using SAS 9.4 (SAS Institute). A SAS macro named %ggBaseline was used to analyze and report the baseline characteristics automatically. 22 P‐values < 0.05 (two‐sided) were deemed statistically significant.
3. RESULTS
3.1. Characteristics of the patients
A total of 181,111 diabetic patients with acute ischemic stroke were available in the CSCA database; 341 patients were excluded for incomplete data regarding in‐hospital mortality, and 12,389 patients were excluded for missing glucose data. Therefore, 168,381 patients were included in this study (Figure 1). The mean age was 66.2 ± 10.7, and 77,688 (43.0%) patients were female.
FIGURE 1.
Patient flow diagram. CSCA, China Stroke Center Alliance
3.2. Characteristics of the patients according to the vital status
The patients were divided into two groups according to the outcome during hospitalization: survivors (n = 167,499) and non‐survivors (n = 882). The characteristics of the two groups are shown in Table 1. The patients in the non‐survivor group were more likely to display traditional cardiovascular risk factors such as being older (p < 0.0001), hypertension (p = 0.0004), history of myocardial infarction (p < 0.0001), higher initial fasting blood glucose (p < 0.0001), higher HbA1c (p = 0.018), lower eGFR (p < 0.0001), higher homocysteine (p < 0.0001), and higher systolic blood pressure (p < 0.0001), as well as factors associated with poor stroke prognosis, such as higher NIHSS (p < 0.0001), history of transient ischemic attack (p = 0.024), history of stroke (p < 0.0001), cerebral hemorrhage (p = 0.033), subarachnoid hemorrhage (p = 0.004). Regarding treatments, the non‐survivors were more likely to be on antiplatelet (p = 0.001), anticoagulants (p < 0.0001), and lipid‐lowering drugs (p = 0.003) and to have received reperfusion therapy (p < 0.001), suggesting that they had the indications for such treatments. Otherwise, the non‐survivors showed characteristics usually associated with a better prognosis, such as not smoking (p < 0.0001) and not drinking (p = 0.007). Regarding the specific objective of the present study, the non‐survivors had a higher SHR compared with the survivor group (p < 0.0001).
TABLE 1.
Clinical features of the survivors and non‐survivors
| Variables | All (N = 168,381 [100%]) | Survivors (N = 167,499 [99.5%]) | Non‐survivors (N = 882 [0.5%]) | p |
|---|---|---|---|---|
| Demography | ||||
| Age (years) | 66.2 ± 10.7 | 66.1 ± 10.7 | 73.2 ± 11.1 | <0.0001 |
| Female | 72,422 (43.0) | 72,017 (43.0) | 405 (45.9) | 0.0803 |
| Body mass index (kg/2) | 24.5 ± 4.7 | 24.5 ± 4.7 | 24.4 ± 6.6 | 0.6065 |
| Previous history | ||||
| Previous transient ischemic attack | 2871 (1.7) | 2854 (1.7) | 17 (1.9) | 0.0238 |
| Previous ischemic stroke | 65,665 (39.0) | 65,230 (38.9) | 435 (49.3) | <0.0001 |
| Previous myocardial infarction | 4436 (2.6) | 4345 (2.6) | 91 (10.3) | <0.0001 |
| Cerebral hemorrhage | 4066 (2.4) | 4035 (2.4) | 31 (3.5) | 0.0329 |
| Subarachnoid hemorrhage | 467 (0.3) | 460 (0.3) | 7 (0.8) | 0.0035 |
| Risk factors | ||||
| Glasgow Coma Scale | 15.0 (1.0–15.0) | 15.0 (1.0–15.0) | 5.0 (5.0–5.0) | 0.5067 |
| NIH Stroke Score | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | 15.0 (9.0–21.0) | <0.0001 |
| Hypertension | 130,265 (77.4) | 129,539 (77.3) | 726 (82.3) | 0.0004 |
| Atrial fibrillation | 7988 (4.7) | 7757 (4.6) | 231 (26.2) | <0.0001 |
| Smoking | 32,647 (19.4) | 32,544 (19.4) | 103 (11.7) | <0.0001 |
| Alcohol | 35,369 (21.0) | 35,213 (21.0) | 156 (17.7) | 0.0073 |
| Previous drugs | ||||
| Antiplatelet | 49,346 (29.3) | 49,047 (29.3) | 299 (33.9) | 0.0011 |
| Anticoagulation | 8424 (5.0) | 8358 (5.0) | 66 (7.5) | <0.0001 |
| Lipid‐lowering drug | 26,620 (15.8) | 26,458 (15.8) | 162 (18.4) | 0.0028 |
| Reperfusion therapy | 9191 (5.5) | 9003 (5.4) | 188 (21.3) | <0.0001 |
| Biochemical indexes | ||||
| Low‐density lipoprotein (mmol/L) | 2.8 ± 1.3 | 2.8 ± 1.3 | 2.9 ± 1.6 | 0.9406 |
| Fasting blood glucose (mmol/L) | 8.1 (6.4–10.9) | 8.1 (6.4–10.9) | 10.2 (7.3–14.0) | <0.0001 |
| HbA1c (%) | 7.6 (6.5–9.2) | 7.6 (6.5–9.2) | 7.4 (6.3–9.0) | 0.0178 |
| eGFR (ml/min/1.73 m2) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.3 (1.0–1.8) | <0.0001 |
| Homocysteine (µmol/L) | 98.7 ± 97.8 | 98.8 ± 98.0 | 75.8 ± 41.7 | <0.0001 |
| Diastolic blood pressure (mmHg) | 13.8 ± 6.8 | 13.8 ± 6.8 | 15.2 ± 8.3 | <0.0001 |
| HbA1c (%) | 151.1 ± 22.5 | 151.1 ± 22.4 | 155.3 ± 26.8 | <0.0001 |
| Homocysteine (µmol/L) | 86.0 ± 13.1 | 86.0 ± 13.1 | 86.6 ± 15.5 | 0.2755 |
| SHR | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.3 (1.0–1.7) | <0.0001 |
3.3. SHR and in‐hospital death
The patients were divided into four groups according to their SHR quartiles. The characteristics are presented in Table 2. We identified a potential association between the occurrence of in‐hospital death and SHR in diabetic patients with acute ischemic stroke in the unadjusted (P for trend <0.0001) and adjusted (P for trend = 0.0141) models (Table 3). Multicollinearity was investigated, and no multicollinearity was found. There were 109 (0.26%), 142 (0.34%), 196 (0.47%), and 435 (1.03%) patients who died in the Q1, Q2, Q3, and Q4 quartiles, respectively. Q1 was used as the reference. Although the P for trend was significant, there were significant differences in Q2 in the unadjusted analysis (odds ratio [OR] = 1.30, p < 0.0001) but not in the adjusted analysis (OR = 1.20, 95% confidence interval [CI] = 0.79–1.81, p = 0.382), and there were significant differences for Q3 (adjusted OR = 1.51, 95% confidence interval [CI] = 1.00–2.26, p = 0.048). Compared with Q1, the death risk was increased in Q4 (OR = 4.02) (adjusted OR = 1.80, 95% confidence interval [CI] = 1.10–2.92, p = 0.018) after adjusting for age, sex, BMI, NIHSS on admission, hypertension, atrial fibrillation, previous ischemic stroke, previous myocardial infarction, SAH, antiplatelet, anticoagulation, lipid‐lowering drug, smoking, alcohol, LDL‐C, FBG, HbA1c, eGFR, HCY, systolic blood pressure, diastolic blood pressure, and reperfusion therapeutic in a Cox regression model (P for trend = 0.014).
TABLE 2.
Characteristics according to the stress hyperglycemia states measured by the glucose‐to‐HbA1c ratio
| Variables | Q1 (N = 42,099 [0–0.90]) | Q2 (N = 42,094 [0.90–1.08]) | Q3 (N = 42,098 [1.08–1.32]) | Q4 (N = 42,090 [>1.32]) | p |
|---|---|---|---|---|---|
| Demography | |||||
| Age (years) | 67.1 ± 10.6 | 66.4 ± 10.6 | 65.7 ± 10.8 | 65.5 ± 10.8 | <0.0001 |
| Female | 17,708 (42.1) | 17,753 (42.2) | 18,152 (43.1) | 18,809 (44.7) | <0.0001 |
| Body mass index (kg/m2) | 24.29 ± 4.57 | 24.58 ± 4.77 | 24.58 ± 4.13 | 24.55 ± 5.23 | <0.0001 |
| Previous history | |||||
| Previous transient ischemic attack | 768 (1.8) | 682 (1.6) | 692 (1.6) | 729 (1.7) | 0.1894 |
| Previous ischemic stroke | 16,689 (39.6) | 16,320 (38.8) | 16,060 (38.1) | 16,596 (39.4) | 0.0003 |
| Previous myocardial infarction | 1177 (2.8) | 1161 (2.8) | 1004 (2.4) | 1094 (2.6) | <0.0001 |
| Cerebral hemorrhage | 1064 (2.5) | 1030 (2.4) | 983 (2.3) | 989 (2.3) | 0.2269 |
| Subarachnoid hemorrhage | 146 (0.3) | 120 (0.3) | 100 (0.2) | 101 (0.2) | 0.0075 |
| Risk factors | |||||
| Glasgow coma scale | 11.00 (1.00–15.00) | 15.00 (1.00–15.00) | 15.00 (12.00–15.00) | 15.00 (13.50–15.00) | 0.1752 |
| NIH Stroke Scale | 3.00 (2.00–6.00) | 3.00 (2.00–6.00) | 3.00 (2.00–6.00) | 4.00 (2.00–7.00) | <0.0001 |
| Hypertension | 32,739 (77.8) | 3,3046 (78.5) | 32,510 (77.2) | 31,970 (76.0) | <0.0001 |
| Atrial fibrillation | 1983 (4.7) | 1993 (4.7) | 1877 (4.5) | 2135 (5.1) | 0.0001 |
| Smoking | 8039 (19.1) | 8221 (19.5) | 8439 (20.0) | 7948 (18.9) | <0.0001 |
| Alcohol | 8096 (19.2) | 8861 (21.1) | 9304 (22.1) | 9108 (21.6) | <0.0001 |
| Previous drugs | |||||
| Antiplatelet | 12,941 (30.7) | 12,729 (30.2) | 12,011 (28.5) | 11,665 (27.7) | <0.0001 |
| Anticoagulation | 2223 (5.3) | 2131 (5.1) | 1898 (4.5) | 2172 (5.2) | <0.0001 |
| Lipid lowering | 6915 (16.4) | 6895 (16.4) | 6506 (15.5) | 6304 (15.0) | <0.0001 |
| Reperfusion therapy | 1954 (4.6) | 2193 (5.2) | 2258 (5.4) | 2786 (6.6) | <0.0001 |
| Biochemical indexes | |||||
| Low‐density lipoprotein (mmol/L) | 2.73 ± 1.28 | 2.82 ± 1.26 | 2.87 ± 1.23 | 2.96 ± 1.39 | <0.0001 |
| Fasting blood glucose (mmol/L) | 5.86 (4.94–7.08) | 7.26 (6.25–8.70) | 8.90 (7.60–10.80) | 12.55 (10.04–15.50) | <0.0001 |
| HbA1c (%) | 7.90 (6.79–9.69) | 7.30 (6.30–8.70) | 7.50 (6.50–9.10) | 7.60 (6.20–9.20) | <0.0001 |
| eGFR (ml/min/1.73 m2) | 97.84 ± 126.93 | 99.16 ± 92.42 | 100.16 ± 83.99 | 97.74 ± 80.83 | <0.0001 |
| Homocysteine (µmol/L) | 13.97 ± 6.71 | 13.97 ± 6.72 | 13.82 ± 6.72 | 13.54 ± 6.92 | <0.0001 |
| Systolic blood pressure (mmHg) | 149.50 ± 21.97 | 150.42 ± 21.91 | 151.79 ± 22.27 | 152.77 ± 23.48 | <0.0001 |
| Diastolic blood pressure (mmHg) | 84.62 ± 12.84 | 85.83 ± 12.83 | 86.60 ± 13.03 | 87.14 ± 13.69 | <0.0001 |
TABLE 3.
Adjusted hazard ratios of outcomes at 12 months according to glucose‐to‐HbA1c ratio quartiles
| Covariate | Level | Death N (%) | Crude Results | Adjusted Results a | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p | P for trend b | Odds Ratio (95% CI) | p | P for trend b | |||
| Glucose‐to‐HbA1c ratio groups | Q1 | 109 (0.26) | – | – | <0.0001 | – | – | 0.0141 |
| Q2 | 142 (0.34) | 1.304 (1.016–1.674) | <0.0001 | 1.201 (0.796–1.813) | 0.3822 | |||
| Q3 | 196 (0.47) | 1.802 (1.425–2.278) | 0.6677 | 1.505 (1.004–2.256) | 0.0477 | |||
| Q4 | 435 (1.03) | 4.023 (3.260–4.965) | <0.0001 | 1.797 (1.104–2.924) | 0.0184 | |||
Adjusted for age, sex, BMI, NIHSS on admission, hypertension, atrial fibrillation, previous ischemic stroke, previous myocardial infarction, SAH, antiplatelet, anticoagulation, lipid‐lowering drug, smoking, alcohol, LDL‐C, FBG, HbA1c, eGFR, HCY, systolic blood pressure, diastolic blood pressure, and reperfusion therapeutic.
Test for trend based on the variable containing the median value for each quintile.
Table 4 shows the subgroup analyses according to the type of stroke. After adjusting for age, sex, BMI, NIHSS on admission, hypertension, atrial fibrillation, previous ischemic stroke, previous myocardial infarction, SAH, antiplatelet, anticoagulation, lipid‐lowering drug, smoking, alcohol, LDL‐C, FBG, HbA1c, eGFR, HCY, systolic blood pressure, and diastolic blood pressure in a Cox regression model, compared with Q1, Q4 had a higher risk of death in patients with ischemic stroke (OR = 2.27, 95% CI: 1.18–4.40, p = 0.015), and Q3 had a higher risk of death in patients with reperfusion therapy (OR = 2.91, 95% CI: 1.12–7.57, p = 0.029).
TABLE 4.
Subgroup analyses for adjusted hazard ratios of outcomes at 12 months according to glucose‐to‐HbA1c ratio quartiles
| Covariate | Level | Death N (%) | Crude results | Adjusted results a | ||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p‐value | P for trend b | Odds ratio (95% CI) | p‐value | P for trend b | |||
| History of ischemic stroke | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 57 (0.34) | – | – | <0.0001 | – | – | 0.0076 |
| Q2 | 65 (0.40) | 1.167 (0.817–1.666) | 0.3961 | 1.207 (0.668–2.183) | 0.5331 | |||
| Q3 | 92 (0.57) | 1.681 (1.207–2.341) | 0.0021 | 1.593 (0.899–2.824) | 0.1109 | |||
| Q4 | 221 (1.33) | 3.938 (2.941–5.273) | <0.0001 | 2.274 (1.176–4.397) | 0.0146 | |||
| MI | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 14 (1.19) | – | – | <0.0001 | – | – | 0.3871 |
| Q2 | 11 (0.95) | 0.795 (0.359–1.758) | 0.5703 | 0.510 (0.136–1.919) | 0.3196 | |||
| Q3 | 24 (2.39) | 2.034 (1.047–3.954) | 0.0362 | 1.493 (0.437–5.102) | 0.5223 | |||
| Q4 | 42 (3.84) | 3.317 (1.801–6.107) | 0.0001 | 1.499 (0.280–8.013) | 0.6360 | |||
| Hemorrhage | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 5 (0.47) | – | – | 0.0070 | – | – | 0.0506 |
| Q2 | 4 (0.39) | 0.826 (0.221–3.084) | 0.7759 | 3.253 (0.192–55.201) | 0.4142 | |||
| Q3 | 8 (0.81) | 1.738 (0.567–5.330) | 0.3338 | 1.014 (0.038–26.991) | 0.9935 | |||
| Q4 | 14 (1.42) | 3.042 (1.092–8.477) | 0.0333 | 16.826 (0.771–367.381) | 0.0728 | |||
| Subarachnoid | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 1 (0.68) | – | – | 0.0513 | – | – | – |
| Q2 | 1 (0.83) | 1.218 (0.075–19.688) | 0.8893 | – | – | |||
| Q3 | 1 (1.00) | 1.465 (0.091–23.693) | 0.7882 | – | – | |||
| Q4 | 4 (3.96) | 5.980 (0.658–54.310) | 0.1121 | – | – | |||
| Reperfusion therapeutic | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 17 (0.87) | – | – | <0.0001 | – | – | 0.2319 |
| Q2 | 25 (1.14) | 1.313 (0.707–2.439) | 0.3884 | 1.406 (0.513–3.851) | 0.5074 | |||
| Q3 | 48 (2.13) | 2.473 (1.418–4.314) | 0.0014 | 2.911 (1.119–7.572) | 0.0285 | |||
| Q4 | 98 (3.52) | 4.152 (2.473–6.971) | <0.0001 | 2.235 (0.683–7.314) | 0.1838 | |||
| Without a history of previous infarction | ||||||||
| Glucose‐to‐HbA1c ratio groups | Q1 | 52 (0.20) | – | – | <0.0001 | – | – | 0.3870 |
| Q2 | 77 (0.30) | 1.461 (1.027–2.078) | 0.0348 | 1.111 (0.626–1.973) | 0.7188 | |||
| Q3 | 104 (0.40) | 1.956 (1.401–2.729) | <0.0001 | 1.295 (0.727–2.307) | 0.3800 | |||
| Q4 | 214 (0.84) | 4.128 (3.047–5.592) | <0.0001 | 1.369 (0.662–2.831) | 0.3968 | |||
Adjusted for age, sex, BMI, NIHSS on admission, hypertension, atrial fibrillation, antiplatelet, anticoagulation, lipid‐lowering drug, smoking, alcohol, LDL‐C, FBG, HbA1c, eGFR, HCY, systolic blood pressure, and diastolic blood pressure.
Test for trend based on the variable containing the median value for each quintile.
3.4. ROC analysis of SHR for predicting in‐hospital death
The ROC analyses showed that SHR (AUC = 0.667, 95% CI: 0.647–0.686; 65.1% sensitivity, and 60.4% specificity) had a better predictive value for mortality than fasting blood glucose (AUC = 0.633, 95% CI: 0.613–0.652; 54.3% sensitivity, and 66.7% specificity) and HbA1c (AUC = 0.523, 95% CI: 0.504–0.543; 55.8% sensitivity, and 48.8% specificity) (Figure 2).
FIGURE 2.
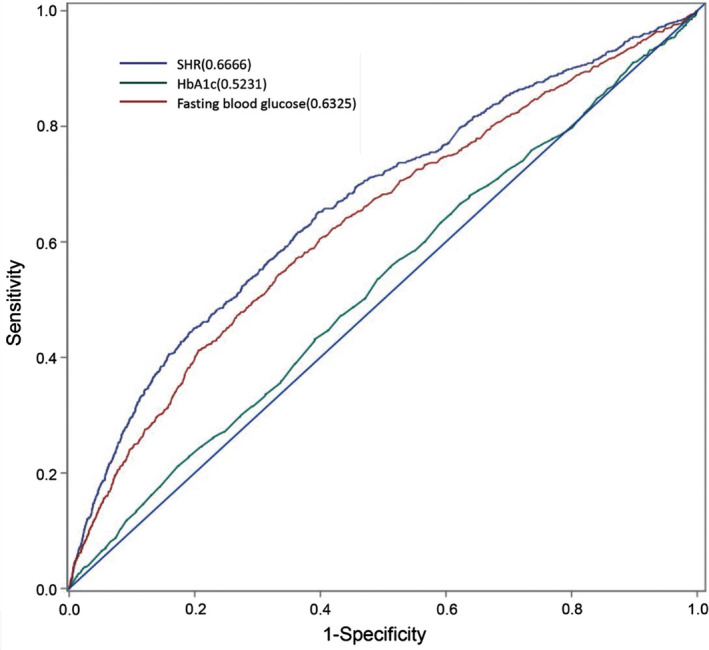
Receiver operating characteristic (ROC) analysis of stress‐induced hyperglycemia ratio (SHR), fasting blood glucose, and HbA1c for predicting in‐hospital death
4. DISCUSSION
Stress hyperglycemia can occur in diabetic patients with acute severe cerebrovascular disease, 1 , 3 , 11 , 14 but the results regarding its association with stroke outcomes are conflicting. Therefore, this study aimed to examine the association between stress‐induced hyperglycemia and the occurrence of in‐hospital death in patients with diabetes and acute ischemic stroke. The study used the data from the CSCA, which is a national initiative to improve the burden of stroke in China. 21 The results suggest that the SHR may serve as an accessory parameter for the prognosis of patients with diabetes after acute ischemic stroke.
Previous studies showed that stress hyperglycemia is associated with poor outcomes in patients with severe illnesses such as acute myocardiac infarction, trauma, and acute ischemic stroke. 6 , 8 , 9 Zarean et al. 23 showed that the glycemic gap was associated with mortality in patients with diabetes and hemorrhagic stroke. Nevertheless, the association between stress hyperglycemia and acute ischemic stroke in patients with diabetes was rarely reported. Several studies showed that admission hyperglycemia was significantly associated with poor short‐term outcomes of ischemic stroke after mechanic thrombectomy. 15 , 16 , 17 Nevertheless, these studies did not further analyze the association of admission hyperglycemia with the outcome of acute ischemic stroke in patients with diabetes. It is believed that acute hyperglycemia has a distinct association with the increased risk of poor outcome or mortality in non‐diabetic patients. 3 , 24 , 25 For patients with pre‐diabetes history, this relationship is controversial. Because hyperglycemia is a common trait among patients with DM, absolute hyperglycemia cannot reflect the changes in glucose under critical conditions without consideration of the basic glucose level, especially when blood glucose is poorly controlled. This might be a reasonable explanation for the paradoxical relationship between absolute hyperglycemia and mortality in patients with diabetes. 3 , 7 Therefore, the relative stress hyperglycemia indicators of glycemic gap and SHR, which take background blood glucose into consideration, should have a better prediction of the poor outcome of critical illness. By using the glycemic gap, Zarean et al. 23 could exclude the impact of poor glycemic control in the outcome of diabetic patients with hemorrhagic stroke. Lee et al. 26 showed that SHR could predict the in‐hospital mortality in critically ill patients across the glycemic spectrum, while absolute glycemia could not. A recent study showed that the SHR was a predictor of poor outcomes in non‐diabetic patients, but that study included only 18.1% (29/160) of diabetic patients. 27 Merlino et al. 28 showed in 414 patients (irrespective of the diabetes status) that stress hyperglycemia was associated with poor outcomes in patients with acute ischemic stroke after intravenous thrombolysis and that the quartiles of SHR had significant trends for poor outcomes and mortality. In patients with minor stroke or TIA, patients with newly diagnosed diabetes had a risk of recurrence similar to patients with known diabetes, while patients with stress hyperglycemia had a markedly higher risk of recurrence. 29 Yuan et al. 10 reported that stress hyperglycemia was associated with the risk of hemorrhagic transformation, which could explain, at least in part, the higher mortality observed with high SHR. In the present study, the glucose‐to‐HbA1c ratio was used, which also accounts for glycemic control. Indeed, blood glucose levels fluctuate widely during the course of the day and with disease conditions, while HbA1c levels represent the general glycemic control over the past 3 months. 13 In addition, the AUC of SHR for mortality was higher than for FBG and HbA1c.
Although the underlying mechanisms are too complex to be fully understood, it was proposed that the hyperactivated oxidative stress response, insulin resistance, inflammation, cytokine production, and hormonal derangements may account for the association between stress hyperglycemia and poor outcomes. 11 , 30 The excess mortality has been hypothesized to be due to the combined effects of glucagon, growth factors, catecholamines, and glucocorticoids, leading to gluconeogenesis, inflammation, and insulin resistance, 31 contributing to neuroinflammation and oxidative stress that exacerbates the brain injury. 32 In addition, high blood glucose is associated with brain‐blood barrier breakdown, brain edema, and increased apoptosis. 33 , 34 , 35 Furthermore, elevated blood glucose could aggravate inflammation and oxidative stress response, potentially creating a vicious cycle that leads to further hyperglycemia. 36 , 37 Meanwhile, hyperglycemia promotes the release of excessive circulating free fatty acids, which also aggravates hyperglycemia. The overlapping interaction of glucotoxicity, lipotoxicity, and inflammation might contribute to a detrimental physiopathological vicious cycle. In addition, hyperglycemia accelerates neuronal damage in hypoxic brain tissue. 38 Hyperglycemia also increases the production of thrombin‐antithrombin complexes and the tissue factor pathway to stimulate coagulation. 39 The activation of protein kinase C and NADPH oxidase increases reactive oxygen species (ROS) levels and reduces nitric oxide synthase, thereby leading to decreased reperfusion and possibly neuron damage. 39 Evidence from MRI studies showed that admission hyperglycemia was associated with expanded infarction core and reduced penumbra salvage. 40 , 41 Therefore, the multiple intricate molecular mechanisms and pathological changes might lead to poor outcomes after stroke with stress hyperglycemia. These mechanisms probably affect nerve repair after stroke, impair collateral circulation, increase vascular permeability, increase platelet cohesion, and increase the occurrence of complications in patients. The exact mechanisms will have to be examined.
Still, poor glycemic control has been shown to be associated with poor functional outcomes after stroke, 42 indicating that long‐term glycemic stress and damage are involved in the functional prognosis of stroke, while acute hyperglycemia after stroke might be a predictor of death. Unfortunately, the present study had no data about the functional outcomes after stroke. This will have to be examined in future studies.
Even though stress hyperglycemia is associated with poor outcomes after stroke, intensive glycemic control is not actually recommended during the acute phase. So far, no evidence showed that tight blood glucose control was associated with better outcomes after acute ischemic stroke. A meta‐analysis including 1296 patients with acute ischemic stroke from seven trials demonstrated that tight blood glucose control (i.e., maintaining glucose levels between 4.0 and 7.5 mmol/L) increased the risk of hypoglycemia events compared with the control group (OR = 25.9, 9.2–72.7). 43 The current stroke guidelines recommend that blood glucose be controlled between 140 and 180 mg/dl, 44 but the guidelines do not make any distinction between patients with or without diabetes history. The recently completed phase 3 trial Stroke Hyperglycemia Insulin Network Effort (SHINE) demonstrated that intensive blood glucose control (80–130 mg/dl) did not improve the functional outcomes and even increased the risk of severe hypoglycemia. 45 To be worth mentioning, about 80% of the included patients had a history of diabetes. In view of the risks for diabetic patients, SHR is more meaningful than absolute hyperglycemia. Such a view is supported by a study in critically ill patients. 26 As suggested by a recent review, acute management of stroke could include aggressive glucose management in patients with ischemic stroke and hyperglycemia. 46
The present study has several limitations. First, we did not assess the blood glucose control during hospitalization because such data are not included in the database. Second, the design of this study was observational, and a cause‐effect relationship cannot be determined. Although it is a national multi‐center registration study with a huge amount of data, most of the enrolled patients have mild stroke severity, which makes the study inevitable choice bias. Third, no distinction is made between types 1 and 2 diabetes in the database. Thus, a prospective randomized large‐scale study is expected to clarify this relationship.
5. CONCLUSION
This study showed that the SHR is significantly associated with an increased risk of in‐hospital mortality in diabetic patients after acute ischemic stroke. This finding suggests that careful glycemic management is important for diabetic patients after stroke onset.
CONFLICT OF INTEREST
All authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
DHM and ZXL wrote the manuscript and interpreted the data. HQG and YYJ contributed to review the manuscript and statistical problems. XQZ and YLW reviewed the manuscript. YJW interpreted data, reviewed and edited the manuscript, and contributed to discussions. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent was obtained from all participants prior to data collection. The study and consent form details were approved by the Ethics Committee of Beijing Tiantan Hospital. This study adhered to the World Medical Association's Declaration of Helsinki (1964–2008) for Ethical Human Research, including confidentiality, privacy, and data management.
ACKNOWLEDGMENTS
We thank all the participating centers in the CSCA program for their hard work in data collection.
Mi D, Li Z, Gu H, et al. Stress hyperglycemia is associated with in‐hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. 2022;28:372–381. doi: 10.1111/cns.13764
Funding information
This study was funded by the Ministry of Science and Technology of the People's Republic of China (National Key R&D Program of China, 2017YFC1310901, 2016YFC0901002, 2017YFC1307905), the National Science and Technology Major Project (2017ZX09304018), the National Natural Science Foundation of China (no. 81971092), the Beijing Municipal Science & Technology Commission (D171100003017002), and the Beijing Talents Project (2018A13, 2018000021223ZK03).
DATA AVAILABILITY STATEMENT
The analysis data are owned by China National Clinical Research Center for Neurological Diseases (http://paper.ncrcnd.ttctrc.com/). Data are not available at present but upon reasonable request and with permission.
REFERENCES
- 1. Tziomalos K, Dimitriou P, Bouziana SD, et al. Stress hyperglycemia and acute ischemic stroke in‐hospital outcome. Metabolism. 2017;67:99‐105. 10.1016/j.metabol.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 2. Szczudlik A, Slowik A, Turaj W, et al. Transient hyperglycemia in ischemic stroke patients. J Neurol Sci. 2001;189(1–2):105‐111. [DOI] [PubMed] [Google Scholar]
- 3. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426‐2432. [DOI] [PubMed] [Google Scholar]
- 4. Hu GC, Hsieh SF, Chen YM, Hsu HH, Hu YN, Chien KL. Relationship of initial glucose level and all‐cause death in patients with ischaemic stroke: the roles of diabetes mellitus and glycated hemoglobin level. Eur J Neurol. 2012;19(6):884‐891. 10.1111/j.1468-1331.2011.03647.x [DOI] [PubMed] [Google Scholar]
- 5. Roquer J, Giralt‐Steinhauer E, Cerda G, et al. Glycated hemoglobin value combined with initial glucose levels for evaluating mortality risk in patients with ischemic stroke. Cerebrovasc Dis. 2015;40(5–6):244‐250. 10.1159/000440735 [DOI] [PubMed] [Google Scholar]
- 6. Egi M, Bellomo R, Stachowski E, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249‐2255. 10.1097/CCM.0b013e318181039a [DOI] [PubMed] [Google Scholar]
- 7. Stollberger C, Exner I, Finsterer J, Slany J, Steger C. Stroke in diabetic and non‐diabetic patients: course and prognostic value of admission serum glucose. Ann Med. 2005;37(5):357‐364. 10.1080/07853890510037356 [DOI] [PubMed] [Google Scholar]
- 8. Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80(12):1558‐1567. 10.4065/80.12.1558 [DOI] [PubMed] [Google Scholar]
- 9. Kerby JD, Griffin RL, MacLennan P, Rue LW 3rd. Stress‐induced hyperglycemia, not diabetic hyperglycemia, is associated with higher mortality in trauma. Ann Surg. 2012;256(3):446‐452. 10.1097/SLA.0b013e3182654549 [DOI] [PubMed] [Google Scholar]
- 10. Yuan C, Chen S, Ruan Y, et al. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin Interv Aging. 2021;16:431‐442. 10.2147/CIA.S280808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798‐1807. 10.1016/S0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Association Diabetes in Hospitals Writing: management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553‐591. 10.2337/diacare.27.2.553 [DOI] [PubMed] [Google Scholar]
- 13. Roberts GW, Quinn SJ, Valentine N, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490‐4497. 10.1210/jc.2015-2660 [DOI] [PubMed] [Google Scholar]
- 14. Yang CJ, Liao WI, Wang JC, et al. Usefulness of glycated hemoglobin A1c‐based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am J Emerg Med. 2017;35(9):1240‐1246. 10.1016/j.ajem.2017.03.049 [DOI] [PubMed] [Google Scholar]
- 15. Kim JT, Jahan R, Saver JL, Investigators S. Impact of glucose on outcomes in patients treated with mechanical thrombectomy: a post hoc analysis of the solitaire flow restoration with the intention for thrombectomy study. Stroke. 2016;47(1):120‐127. 10.1161/STROKEAHA.115.010753 [DOI] [PubMed] [Google Scholar]
- 16. Osei E, den Hertog HM, Berkhemer OA, et al. Increased admission and fasting glucose are associated with unfavorable short‐term outcome after intra‐arterial treatment of ischemic stroke in the MR CLEAN pretrial cohort. J Neurol Sci. 2016;371:1‐5. 10.1016/j.jns.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 17. Goyal N, Tsivgoulis G, Pandhi A, et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J Neurointerv Surg. 2018;10(2):112‐117. 10.1136/neurintsurg-2017-012993 [DOI] [PubMed] [Google Scholar]
- 18. Ye XH, Cai XL, Nie DL, et al. Stress‐induced hyperglycemia and remote diffusion‐weighted imaging lesions in primary intracerebral hemorrhage. Neurocrit Care. 2019;32(2):427‐436. 10.1007/s12028-019-00747-y [DOI] [PubMed] [Google Scholar]
- 19. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16(4):203‐212. 10.1038/s41569-018-0119-4 [DOI] [PubMed] [Google Scholar]
- 20. Wu S, Wu BO, Liu M, et al. China Stroke Study: Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394‐405. 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Li Z, Wang Y, et al. Chinese Stroke Center Alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3(4):256‐262. 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gu HQ, Li DJ, Liu C, Rao ZZ. %ggBaseline: a SAS macro for analyzing and reporting baseline characteristics automatically in medical research. Ann Transl Med. 2018;6(16):326. 10.21037/atm.2018.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zarean E, Lattanzi S, Looha MA, et al. Glycemic gap predicts in‐hospital mortality in diabetic patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(5):105669. 10.1016/j.jstrokecerebrovasdis.2021.105669 [DOI] [PubMed] [Google Scholar]
- 24. Ahmed N, Davalos A, Eriksson N, et al. Association of admission blood glucose and outcome in patients treated with intravenous thrombolysis: results from the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS‐ISTR). Arch Neurol. 2010;67(9):1123‐1130. 10.1001/archneurol.2010.210 [DOI] [PubMed] [Google Scholar]
- 25. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS‐II trial. Stroke. 2008;39(10):2749‐2755. 10.1161/STROKEAHA.108.514307 [DOI] [PubMed] [Google Scholar]
- 26. Lee TF, Drake SM, Roberts GW, et al. Relative hyperglycemia is an independent determinant of in‐hospital mortality in patients with critical illness. Crit Care Med. 2020;48(2):e115‐e122. 10.1097/CCM.0000000000004133 [DOI] [PubMed] [Google Scholar]
- 27. Chen X, Liu Z, Miao J, et al. High stress hyperglycemia ratio predicts poor outcome after mechanical thrombectomy for ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(6):1668‐1673. 10.1016/j.jstrokecerebrovasdis.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 28. Merlino G, Smeralda C, Gigli GL, et al. Stress hyperglycemia is predictive of worse outcome in patients with acute ischemic stroke undergoing intravenous thrombolysis. J Thromb Thrombolysis. 2021;51(3):789‐797. 10.1007/s11239-020-02252-y [DOI] [PubMed] [Google Scholar]
- 29. Guo Y, Wang G, Jing J, et al. Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging (Albany NY). 2021;13(6):9108‐9118. 10.18632/aging.202797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angeli F, Reboldi G, Poltronieri C, et al. Hyperglycemia in acute coronary syndromes: from mechanisms to prognostic implications. Ther Adv Cardiovasc Dis. 2015;9(6):412‐424. 10.1177/1753944715594528 [DOI] [PubMed] [Google Scholar]
- 31. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. 2013;17(2):305. 10.1186/cc12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Kooten F, Hoogerbrugge N, Naarding P, Koudstaal PJ. Hyperglycemia in the acute phase of stroke is not caused by stress. Stroke. 1993;24(8):1129‐1132. 10.1161/01.str.24.8.1129 [DOI] [PubMed] [Google Scholar]
- 33. Song EC, Chu K, Jeong SW, et al. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke. 2003;34(9):2215‐2220. 10.1161/01.STR.0000088060.83709.2C [DOI] [PubMed] [Google Scholar]
- 34. Olivot JM, Mlynash M, Kleinman JT, et al. Wijman and D. investigators: MRI profile of the perihematomal region in acute intracerebral hemorrhage. Stroke. 2010;41(11):2681‐2683. 10.1161/STROKEAHA.110.590638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiu CD, Chen CC, Shen CC, et al. Hyperglycemia exacerbates intracerebral hemorrhage via the downregulation of aquaporin‐4: temporal assessment with magnetic resonance imaging. Stroke. 2013;44(6):1682‐1689. 10.1161/STROKEAHA.113.675983 [DOI] [PubMed] [Google Scholar]
- 36. Ling PR, Smith RJ, Bistrian BR. Hyperglycemia enhances the cytokine production and oxidative responses to a low but not high dose of endotoxin in rats. Crit Care Med. 2005;33(5):1084‐1089. [DOI] [PubMed] [Google Scholar]
- 37. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079‐2086. [DOI] [PubMed] [Google Scholar]
- 38. Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11(3):261‐271. 10.1016/S1474-4422(12)70005-4 [DOI] [PubMed] [Google Scholar]
- 39. Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145‐155. 10.1038/nrneurol.2009.231 [DOI] [PubMed] [Google Scholar]
- 40. Els T, Klisch J, Orszagh M, et al. Hyperglycemia in patients with focal cerebral ischemia after intravenous thrombolysis: influence on clinical outcome and infarct size. Cerebrovasc Dis. 2002;13(2):89‐94. 10.1159/000047756 [DOI] [PubMed] [Google Scholar]
- 41. Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52(1):20‐28. 10.1002/ana.10241 [DOI] [PubMed] [Google Scholar]
- 42. Lattanzi S, Bartolini M, Provinciali L, Silvestrini M. Glycosylated hemoglobin and functional outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(7):1786‐1791. 10.1016/j.jstrokecerebrovasdis.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 43. Bellolio MF, Gilmore RM, Stead LG. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev. 2011;9:CD005346. 10.1002/14651858.CD005346.pub3 [DOI] [PubMed] [Google Scholar]
- 44. Powers WJ, Rabinstein AA, Ackerson T, et al. American Heart Association Stroke: 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke, 2018;49(3), e46‐e110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 45. Johnston KC, Bruno A, Pauls Q, et al. Neurological Emergencies Treatment Trials and S. T. I. the: intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE Randomized Clinical Trial. JAMA. 2019;322(4):326‐335. 10.1001/jama.2019.9346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferrari F, Moretti A, Villa RF. The treatment of hyperglycemia in acute ischemic stroke with incretin‐based drugs. Pharmacol Res. 2020;160:105018. 10.1016/j.phrs.2020.105018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysis data are owned by China National Clinical Research Center for Neurological Diseases (http://paper.ncrcnd.ttctrc.com/). Data are not available at present but upon reasonable request and with permission.


