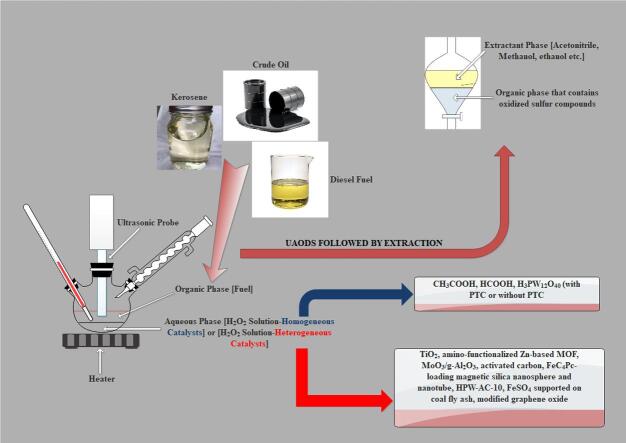
Graphical abstract
Keywords: Ultrasound, Oxidative Desulfurization (ODS), Desulfurization Process Efficiency (DPE), Phase Transfer Catalyst (PTC), Heterogeneous catalyst
Highlights
-
•
In the presence of homogeneous and heterogeneous catalysts, UAODS studies are reviewed and summarized. The use of heterogeneous catalysts is advantageous over homogeneous catalysts as they can be easily separated from the reaction mixture and reused for subsequent reactions.
-
•
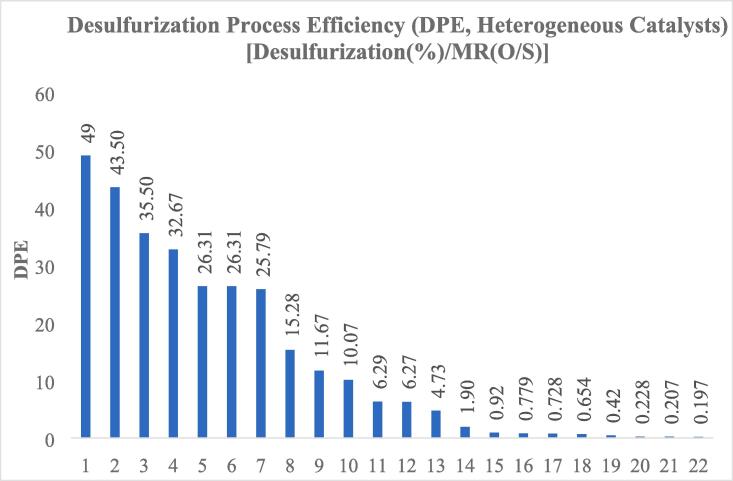
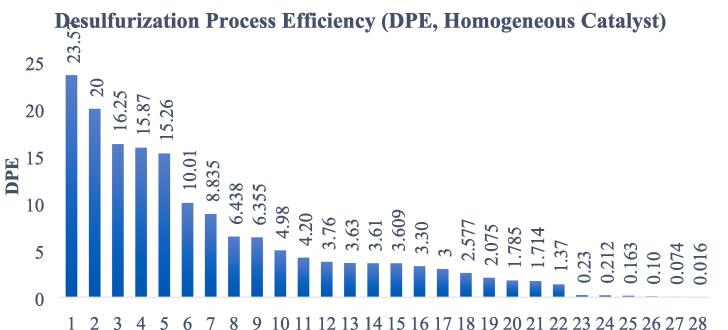
Higher desulfurization process efficiencies (DPEs) are usually reached with heterogeneous catalysts.
-
•
The effects of different sonoreactor configurations and pressure on UAODS are presented. Using a continuous-flow jacketed glass sonoreactor with glass nozzle, very high DPE was achieved in a short time in the absence of PTC for homogeneous catalyst. At low (i.e. atmospheric pressure) and relatively high operating pressures (above atmospheric pressure), the boiling point of solvent in which model sulfur compounds are dissolved or the boiling range of fuel such as diesel oil, kerosene has a very important effect on UAODS.
-
•
In the presence of oxygen as oxidant and modified heteropoly catalysts, the oxidation mechanism in UAODS is uniquely elucidated by electron transfer-oxygen transfer reaction. For nearly all the UAODS reactions, direct sonication is generally more efficient than indirect sonication in terms of DPE.
-
•
The oxidation mechanism involving persulfate agent and H2O2 was revealed in the presence of Si-Al/Al2O3 as a solid catalyst. High desulfurization efficiency was obtained in a short time like 15 min at ambient temperature with simultaneous extraction and oxidation using modified Metal-Organic Framework (MOF) prepared by ultrasonic irradiation.
-
•
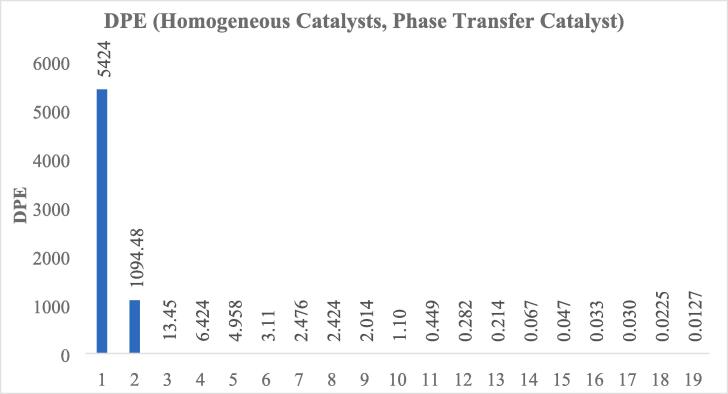
In the presence of quaternary ammonium salt containing the fluoride anion, the reason for the high UAODS yields was clarified. Since potassium ferrate is a much stronger oxidant than hydrogen peroxide, the highest DPEs were obtained in UAODS reactions in the presence of PTC.
Abstract
Recently, environmental pollution has increased significantly due to petroleum-based fuels widely used in vehicles. This environmental pollution is mainly due to the acidic SO2 gas generated by the combustion of fuels and emitted into the atmosphere. SO2 gas causes not only acid rain but also corrosion of metal parts of engines in vehicles. In addition, it functions as a catalyst poison in catalytic converters in exhaust system. Due to these damages, strict regulations have been introduced to reduce the amount of sulfur in fuels. As of 2005, the permissible amount of sulfur in diesel fuels in Europe and America has been limited to 10 and 15 ppm by weight, respectively.
Due to the decreasing oil reserves in the world, high viscosity petroleums containing high sulfur and heavier fractions (i.e., low-quality oils) are increasing, thus making desulfurization difficult and leading to high costly process. Since time and economic loss are very important today, these two terms have to be reduced to a minimum. Recently, ultrasound wave in ODS shown as an alternative to HDS is utilized to further increase desulfurization in shorter times. Ultrasound wave locally creates high temperatures and high pressures (hot-spot theory) in liquid, causing the desulfurization reaction to accelerate further.
In this review, the advantages and difficulties of oxidative desulfurization, the economics of ultrasound-assisted oxidative desulfurization are summarized and recommendations for improving the process are presented.
1. Introduction
Today, lower quality fuels (high viscosity) containing high amounts of sulfur are extracted due to decreasing oil reserves [1]. As it is known, when fuels are burned, organic sulfur compounds in them are oxidized and emit SO2 gas which is harmful to the atmosphere and the environment, and these gases cause acid rain and corrosion [2]. Therefore, it is of crucial importance to desulfurize these low quality fuels.
Hydrodesulfurization (HDS) as conventional desulfurization is widely used in the world. In HDS, organic sulfur compounds react with H2 gas and H2S is released as a result of the carbon–sulfur bond cleavage in organic compound [3]. However, HDS has some disadvantages [4], [5], [6]: the use of high temperature, high pressure, expensive H2 gas and expensive catalysts with high chemical stability and high thermal resistance that must not be affected by severe operating conditions. Also, aliphatic sulfur compounds are easy to remove in HDS, while refractory aromatic sulfur compounds are difficult to remove [7].
To eliminate these disadvantages, alternative desulfurization processes such as adsorptive desulfurization [8], extractive desulfurization [9], oxidative desulfurization [10], biodesulfurization [11] are used. Among them, the most advantageous and promising method is oxidative desulfurization (ODS). In ODS, at relatively low temperatures such as 20–60 °C, at atmospheric or near atmospheric pressures, organic sulfur compounds are oxidized by using H2O2 and a catalyst to convert first to their sulfoxides and then to their sulfones, which are more polar compounds, and finally these oxidized sulfur compounds are removed from the fuel by extraction with a polar extractant such as methanol, acetonitrile, dimethyl formamide etc. or by adsorption [12].
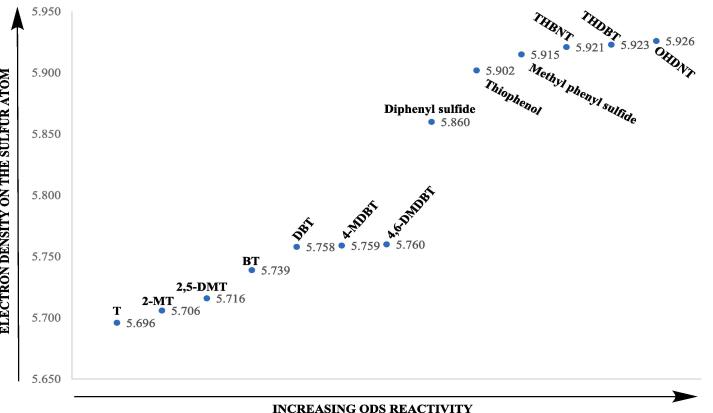
Desulfurization is also carried out with simultaneous oxidation and extraction [13]. In HDS, it is difficult to remove aromatic sulfur compounds, especially alkyl-substituted aromatic sulfur compounds which are prevented from accessing into the catalyst pores due to steric hindrance [14]. On the contrary, in ODS, using a liquid homogeneous catalytic system such as formic acid or acetic acid- H2O2 (HP) oxidant [15], alkyl-substituted aromatic sulfur derivatives are easier to remove due to an increase in electron density [1], [16], [17], [18], [19] on the sulfur atom as shown in Fig. 1. In particular, bonding the naphthenic ring to the thiophenic ring significantly increases the ODS yield of the compounds such as THBNT, THDBT and OHDNT [20]. When the phosphotungstic acid-HP system is used, the molecular size of the catalyst becomes important. Since phosphotungstic acid is a bulky molecule [21], the ODS reactivity of aromatic sulfur compounds having alkyl groups adjacent to the sulfur atom decreases due to spatial obstacle [22]. In a study [23] in which ODS of model sulfur compounds was performed by phosphotungstic acid-HP, it was reported that sulfur removal decreased in the order DBT > 4-MDBT > 4,6-DMDBT. When the solid heterogeneous catalyst is used, the sulfur atom is prevented from entering the catalyst pore and its interaction with the sulfur atom due to the steric hindrance of alkyl groups adjacent to sulfur becomes weak, consequently causing a decline in the ODS reactivity [24]. Desulfurization using t-butylhydroperoxide in the presence of Mo/Al2O3 catalyst is in the order DBT > 4-MDBT > 4,6-DMDBT≫ BT [25]. With the use of TiO2 anatase-supported V2O5 catalyst and HP, the ODS yield is in the order DBT > BT > 4-MDBT > 2-MT > 2,5-DMT > 4,6-DMDBT [26]. In the H3PW12O40/TiO2-HP system, the desulfurization at 30 °C increases in the order 4,6-DMDBT < BT < DBT [27].
Fig. 1.
ODS reactivity of various sulfur compounds for HCOOH-HP oxidant system. (THBNT: Tetrahydrobenzonaphthothiophene, THDBT: Tetrahydrodibenzothiophene, OHDNT: Octahydrodinaphthothiophene).
In ODS reactions, the mixture consists of two immiscible liquid phases as organic phase (real fuel or model fuel solution containing sulfur compounds such as DBT, 4,6-DMDBT dissolved in a non-polar solvent such as n-hexane, n-heptane or iso-octane) and aqueous phase (H2O2 solution). Therefore, quaternary ammonium salts as phase transfer catalysts (PTCs), one end of which is hydrophilic and the other end hydrophobic, are generally used, reducing the liquid–liquid interface tension [28] and enabling the transfer of oxidizing species to organic phase, so that the ODS increases significantly [29]. Sometimes using ionic liquid (IL) instead of aqueous phase, ODS is further increased such that the IL acts as extractant during oxidation [30]. For the last 20–30 years, ultrasound wave has been used to accelerate oxidation reactions and increase ODS more. Sonication has two simultaneous effects in accelerating ODS reactions. The ultrasound wave creates cavitation bubbles in liquid and the implosion of these bubbles produces very high temperatures and pressures locally in the liquid. At the extremely high temperatures, chemical bonds of organic compounds are broken and reactive radicals are generated (Sonochemical effect). Microjet, microturbulence and shock waves created by imploding cavitation bubbles significantly accelerate the mass transfer by increasing the emulsification of the organic and aqueous phase (Sonophysical effect). Thus higher desulfurization efficiencies are achieved in a shorter time [31].
ODS reactions are generally heterogeneous reactions, i.e., there are two or more phases in the mixture that are immiscible with each other. The solution of the organic phase, which is formed by dissolving model sulfur compounds in a non-polar solvent such as hexane, heptane or toluene, has been referred as to denotations such as model fuel, model diesel, model liquid fuel, model sulfur solution. The aqueous phase consists of an oxidant and a catalyst. In many studies, the reactivity of the model sulfur compounds has been determined and the optimum conditions (temperature, oxidant volume, catalyst amount, organic phase/aqueous phase volume ratio, time etc.) for maximum desulfurization have been found. These conditions have then been applied to real fuels to achieve desulfurization.
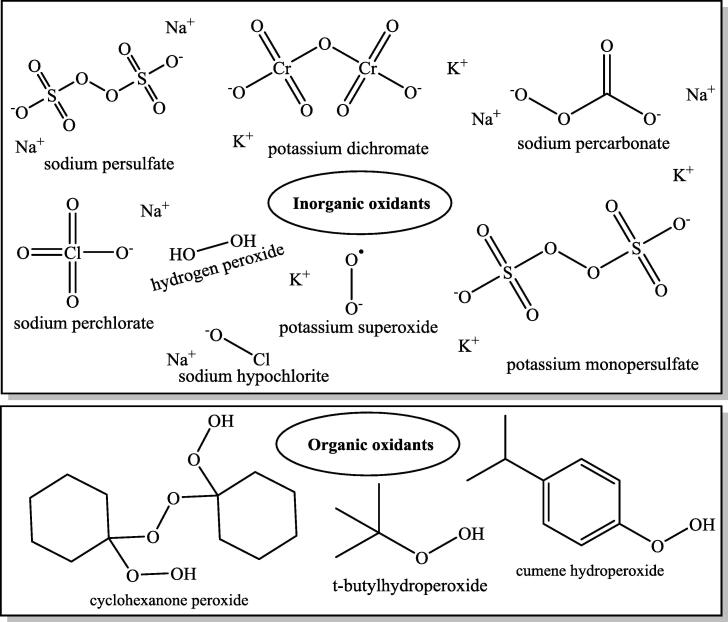
Many solid, liquid and gas oxidizers have been evaluated. Inorganic chemicals such as oxone [32], sodium persulfate [33], potassium superoxide [34], potassium dichromate [35], sodium percarbonate [36], sodium perchlorate [37], hydrogen peroxide [38], sodium hypochlorite [39], solid oxidizers such as cyclohexanone peroxide [40] and organic chemicals such as t-butylhydroperoxide [41] and cumene hydroperoxide [42] as liquid oxidizers are used. The most distinctive feature of cyclohexanone peroxide as solid organic oxidizers and cumene hydroperoxide and t-butylhydroperoxide as liquid organic oxidizers is that they can all dissolve in the organic phase or fuel, thereby directly oxidizing sulfur compounds [43], [44]. The structural formulas of oxidizing substances are shown in Fig. 2. Gaseous oxidants are generally oxygen [45], nitrogen dioxide [46] and ozone [47], and the solubility [48], [49], [50], [51] of these gases in non-polar solvents is generally higher than that in water.
Fig. 2.
Oxidizing chemicals.
Catalysts used in ODS are divided into two types; homogeneous catalysts soluble in liquid phase and heterogeneous catalysts insoluble in liquid phase.
2. Types of catalysts in UAODS
2.1. Heterogeneous catalysts
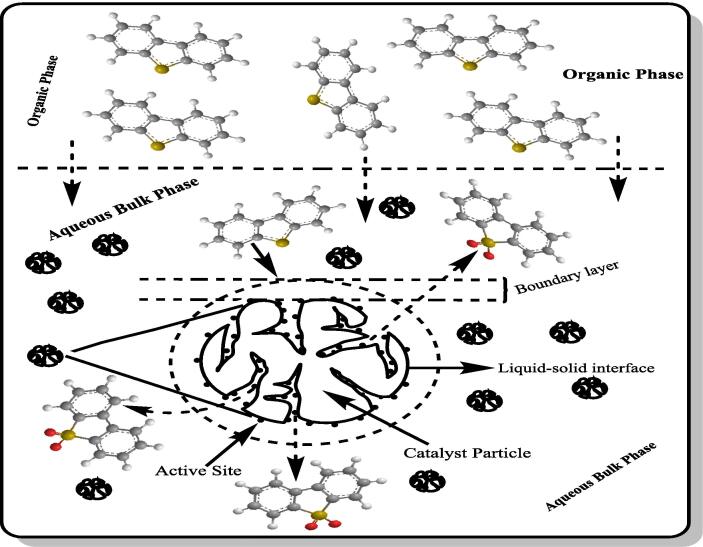
Catalysts used in heterogeneous catalysis are solid and insoluble in liquid mixture [52]. Nanoparticles improve the adsorption of sulfur compounds due to their large surface area [53]. Photocatalyst under UV [54] or visible light [55], nano-sized silica particles including mesoporous silica [56], aluminum oxide particles [57], transition metal oxides [58], activated carbons [59], modified metal–organic frameworks [60], Ni catalyst also called sponge metal [61], nanocomposite [62], graphene oxide [63], activated carbon (AC)-supported phosphotungstic acid [64] and fly ash-modified fenton catalysts [65] are used. In the case of using heterogeneous catalysts, the catalytic ODS mechanism [66], [67], [68], [69], [70] is illustrated in Scheme 1. DBT, which is transferred from the organic phase to the aqueous bulk phase by ultrasound, diffuses to the outer surface of the solid catalyst by passing across the liquid film (boundary layer) around the supported catalyst particle. DBT is adsorbed on active sites on the external surface of the catalyst or on active sites on the internal surface of the inner pores by diffusing through the pore. HP interacts with active sites on the inner and outer surface and forms oxidizing active complexes. After DBT adsorbed on these active centers is converted into its sulfones by undergoing an oxidation reaction, DBT sulfone is desorbed and transferred successively to the boundary layer, aqueous phase and organic phase. In addition to enhancement of adsorption and desorption, ultrasound significantly increases not only the external and internal diffusion but also the collision frequency of reactants with active sites, thus causing increased UAODS performance.
Scheme 1.
The ODS mechanism with heterogeneous catalysts.
2.1.1. Photocatalysts
Matsuzawa et al. [71] carried out the photocatalytic oxidation of DBT using a Hg-Xe lamp of 200 W at wavelength > 290 nm in the presence of anatase-type TiO2 (P25) as a heterogeneous photocatalyst and air (in which oxygen acts as an electron scavenger [72], thus causing oxidation only by electron vacancy (h+) [73] of TiO2 [74], [75], [76]) in a polar acetonitrile solution. They found the photooxidation rate in combination with H2O2, photocatalyst and indirect ultrasound (45 kHz and 50 W) was higher than the oxidation rate with H2O2 and photocatalyst, and this effect was due to the reactivation of the TiO2 surface and increased mass transfer. However, they stated that the direct oxidation rate of 4,6-DMDBT using only H2O2 under photoirradiation was higher than the photooxidation rates in the cases of HP-photocatalyst-US and HP-photocatalyst. In addition, it is reported that the oxidation reaction rate of the methyl group in 4,6-DMDBT increased by using aliphatic and cyclic alkanes as a non-polar solvent instead of the polar solvent acetonitrile, since oxygen was more soluble in non-polar solvents [77].
In another study [78], using photocatalytic anatase TiO2, 30 wt% HP and ultrasound with duty cycle, the catalytic oxidative desulfurization of gum turpentine, which is similar to crude sulphated turpentine and a by-product of Kraft process [79] to obtain wood pulp, spiked with dimethyl disulfide was investigated and a desulfurization efficiency of 100% was found at 28 °C, 120 W power dissipation and 20 kHz US frequency, 70% duty cycle, 15 g L–1 HP concentration, 4 g L–1 TiO2 loading for 50 ppmw DMDS initial concentration. Also, it was reported that total treatment cost (0.31 $ L–1) with (US + H2O2 + TiO2) system is less as compared to US, only 30 wt% H2O2, only Fenton, only TiO2, US + 30 wt% H2O2, US + Fenton and US + TiO2. In addition, the authors investigated the effects of individually US/Fenton and US/TiO2 processes on desulfurization, but it was found that the desulfurization efficiencies of those processes were lower than the desulfurization efficiency of the US/HP/TiO2 process. It has been explained that the reason for the very high desulfurization of the US/HP/TiO2 process is the production of more hydroxyl radicals from HP along with the support of the TiO2 catalyst and the generation of additional hydroxyl radicals as a result of the increase of active sites by deformation of the catalyst under US. It was also stated that homogeneous distribution of the catalyst particles and better mixing due to the high turbulence caused by the collapsed bubbles enhance the sulfur removal. Cavitational yields (4.65 × 10–9, 4.71 × 10–9 and 6.61 × 10–9 g J−1 for US/Fenton reagent, US/TiO2 and US/HP/TiO2, respectively) were calculated by the authors to confirm the differences in desulfurization in the three processes. In this study, it was determined that the total cost of the other treatment methods was 2.22, 43.12, 14.69, 17.50, 1.255, 0.70 and 0.595 $ L−1 for US, only HP(30%(v/v), only Fenton, only TiO2, US + HP(30%(v/v), US + Fenton and US + TiO2, respectively. Although a high sulfur removal is obtained from gum turpentine in the presence HP and TiO2 under US, oxidative desulfurization of DMDS as an aliphatic sulfur compound is quite easy, the initial sulfur quantity (50 ppm DMDS) is very low, and the reaction time is 120 min. Therefore, it is not a favorable method.
In the studies performed by Yu et al. [80] and Zhao et al. [81], sonophotocatalytic oxidative desulfurization of hydrotreated diesel oil and model diesel oil using CdO as semiconductor and H2O2 as oxidant was investigated and desulfurization efficiencies were found to be 72.7 and 99.47%, respectively. The high desulfurization in the latter under 20 kHz and 150 W US can be attributed to primarily the use of the model sulfur solution prepared by dissolving the organosulfur compound in a solvent instead of hydrogenated diesel fuel, which consists of a complex mixture of aliphatic hydrocarbons and aromatic hydrocarbons [82], and acetic acid to increase the oxidizing power of H2O2 and secondarily to the catalyst with a smaller grain size (i.e. larger surface area) and more homogenized structure, which is synthesized under ultrasound [83], hence causing a higher catalytic activity.
Behin and Farhadian [84] performed the ODS (followed by extraction with a binary solvent of methanol and water in ratio of 1:1 in volume) of nonhydrotreated kerosene with a total S content of 1553 ppmw at 0.05 cm s−1 superficial gas velocity for 15 min. by passing ozone as a homogeneous photocatalyst through an airlift reactor and using H2O2 under both US of 20 kHz frequency (60 W power) and UV in a wavelength range of 280–400 nm. Despite a 48% loss of aromaticity due to ozone, and to a lesser extent polar solvent, a desulfurization efficiency of 91.7% was reached. It is revealed that the high desulfurization yield at optimum conditions was due to HO· (oxidation potential [85] of hydroxyl radical, 2.80 V) and HO2· (oxidation potential [85] of hydroperoxyl radical, 1.7 V) radicals formed in the mixture during the reaction rather than the increased mass transfer and the physical properties of raw kerosene are almost unchanged.
In addition, sonolysis of sulfur compounds in water was carried out at high ultrasonic frequencies without using catalysts and oxidants. The dilute solution containing 21.46 ppm S BT in water was subjected to sonodegradation at 21 °C under 352 kHz and 80 W US, and it was explained that the dominant mechanism was the oxidation of BT as a result of the formation of hydroxyl radicals from water [86]. However, in the sonolysis of a dilute T solution containing 32 ppm S in water at 22 °C under 850 kHz and 40 W US power, it was revealed that the dominant mechanism was pyrolysis as a result of high temperature caused by collapsed cavitation bubbles rather than hydroxyl radical formed in the medium since T can diffuse readily into the cavitation bubble due to T's lower boiling point (i.e., more volatile) than BT [87].
AOPs were utilized in combination with sonolysis. Despite high desulfurization under both US and UV or visible light in AOPs [71], [80], [81], where photocatalysts are used, these high desulfurization yields were reached in 6,5 and 3 h, respectively, for the respective studies. In photocatalysis, a light energy such as UV or visible light is absorbed by photocatalyst (e.g., TiO2), and the electron is excited by passing from the valence band to the conduction band, and thus an electron-hole pair is formed on photocatalyst. The positive electron holes (h+) react with the water adsorbed on the catalyst to produce hydroxyl radicals. In addition, oxygen on catalyst surface reacts in series with the excited electron (e–) to produce hydroxyl radicals and also US generates hydroxyl radical from HP. Consequently, enhanced hydroxyl radical production renders sonophotocatalytic ODS yield high. The reactions are as follows [88], [89]:
| H2O + h+ → H+ + HO• |
In the Sono-Fenton process, FeSO4 is used along with HP under US irradiation. In the Fenton reaction, Fe2+ is first oxidized by HP to produce the HO· radical and then the reaction of Fe3+ with HP produces the complex intermediate Fe-OOH2+ which decomposes rapidly to form HO2· radical and Fe2+ under US [90]. Fenton reaction is substantially accelerated by US [91]. As a result, sulfur removal further increases due to enhancement of hydroxyl radicals in organic-aqueous phase interfacial area. The medium must be acidic to maximize production of free radicals [92]. The reactions in the Sonofenton process are as follows [93]:
As noted above, reaction times are very high in studies [71], [80], [81], where photocatalyst was used. Therefore, this will lead to higher electrical energy consumption for US, UV and heating, if any, increasing the operating cost in AOP.
In the study [84], in which ozone and HP were used as oxidant under US-UV, it was explained that the reason for high sulfur removal in a short time was indirect hydroxyl radical production from O3 and direct hydroxyl radical from HP by UV and US. In addition, it is stated that ultrasound greatly accelerates the gas–liquid mass transfer through micro-streaming produced by the violent collapse of bubbles and allows ozone to react with sulfur compounds by increasing the gas–liquid interfacial area. Moreover, dissolved ozone gas acts as nucleation sites to form cavitation bubbles, causing the formation of more cavitation bubbles [94]. Thus, this synergistic effect accelerates significantly the ultrasound-assisted photo oxidative desulfurization reaction rate.
In a sonophoto-fenton process [95] in which oxalic acid was used, a sulfur removal of>93% was achieved from 100 ppm DBT in toluene at 0.05 mol L−1 Fe2+ concentration, 0.15 mol L−1 oxalate concentration, pH = 2, a volume ratio (organic phase/HP) of 10:1, 25 °C and 15 min under both 37 kHz, 95 W indirect US and UV in the presence of air. It was revealed that FeII(C2O4), which is formed by the reaction of Fe2+ with oxalate anion (C2O42−) in the reaction medium, as well as complex which is formed by the reaction of FeII(C2O4) with C2O42−, is responsible for this high desulfurization. The authors reported that FeII(C2O4) and caused the formation of HO·, HO2· and O· radicals in the aqueous phase to oxidize DBT under US and UV irradiation. It was stated that Fe(II)-oxalate complex as catalyst can be reused three times (a decrease of 1.33 and 1.56% for the first and second run, respectively) without significantly losing its activity by regenerating it after each reaction.
2.1.2. Solid catalysts
The effect of solid catalysts to increase ODS has also been studied [96], and it was found that the use of US for total desulfurization of 2,3-DMBT and 2,3,7-TMBT, which are the two most abundant components in JP-8 fuel, in the presence of H2O2, formic acid and phosphoric acid-activated carbon increases the total desulfurization in the absence of US (mechanical stirring) by around 2.4-fold. It is also reported that desulfurization by chemically activated carbon (MW-99) with phosphoric acid is superior to desulfurization by thermally activated carbon (Norit SX-1) due to the larger surface area of MW-99 and the greater number of its surface acid centers. Sulfur removal of 98 and 94% (followed by adsorption with activated alumina) from JP-8 and diesel, respectively, was performed with MW-99 under optimum conditions (65 °C, 2 h, 60% amplitude, 20 kHz sonication, pH = 1.4).
Khlaif and Bded [97] carried out the ODS (followed by extraction) of crude oil containing 1.95% total S by weight in the presence of US and AC using different volumes of acetic acid and 50 wt% H2O2. As a result of the increase of the amount of AC used from 3 to 9 g, the number of active sites in AC increased, thus improving ODS and an optimum desulfurization of 81.325% was obtained by using 9 g AC, 40 mL H2O2, 30 mL acetic acid at 50 °C.
Using phosphotungstic acid (H3PW12O40@ TMU-17-NH2) incorporated in robust zinc-based MOF with enhanced efficiency as a solid catalyst, simultaneous extraction and oxidation of model oil containing BT, DBT and 4,6-DMDBT, each of which has concentration of 500 mg L–1, were performed in the presence of acetonitrile under indirect sonication of 37 kHz [98]. Although the pore volume and surface area (137 cm3 g−1 and 814 m2 g−1) of the composite MOF catalyst formed by encapsulating H3PW12O40 in TMU-17-NH2 were lower as compared to those of the neat MOF (239 cm3 g−1 and 1050 m2 g−1), a sulfur removal of 98, 87 and 71% was reached with 20 mg of the MOF composite containing 20 wt% phosphotungstic acid at model oil/MeCN 1:1 vol ratio, O/S ratio of 2:1 and room temperature for DBT, 4,6-DMDBT and BT, respectively, at the end of 15 min. The reason for the lower reactivity of 4,6-DMDBT compared to DBT is that the alkyl substituted aromatic compound is sterically prevented from entering the 3D framework. Also lower desulfurization was achieved with DMF solvent instead of MeCN depending on the fact that adsorption of solvent on the heterogeneous catalyst increases with increasing boiling point [99], [100] and polarity [101], [102], [103]. The low desulfurization with DMF can be attributed to the fact that not only the boiling point of DMF (153 °C) is significantly higher than that of MeCN (82 °C) [104] but also higher polarity [105] of the former compared to the latter causes stronger interaction with Zn2+ in the modified MOF composite [106], thus reducing adsorption of DBT. The former is bound to Zn2+ cations in the MOF composite [106]. The three possible adsorption mechanisms [107], [108] are π-π interaction between sulfur compounds and aromatic rings of modified MOF, hydrogen bonding between NH2 groups and S, and strong Zn2+-S interaction between phosphotungstic acid-TMU-17-NH2 and aromatic sulfur compounds. TMU-17-NH2 is probably structurally similar to TMU-16-NH2 with positive zeta potential [109]. H2O2 and aromatic sulfur compounds are adsorbed on the catalyst, the phosphotungstic acid anion is oxidized with hydrogen peroxide and as a consequence, the polyoxoperoxo complex anion formed oxidizes aromatic sulfur compounds [110]. In addition, water in the reaction medium can result in the radical decomposition of H2O2 by forming an aqueous complex with Zn2+ in Zn(II)-based MOF, hence generating a strong oxidant radical HO·[111] and electrophilic activation of hydrogen peroxide to convert sulfur compounds to their sulfoxides as oxidized sulfur compounds is caused by Zn-based MOF [112].
Metalloporphyrin [113] and metallophthalocyanine [114] catalysts, which are metal complexes, are also used in ODS reactions. Metal removal from the latter is not easy compared to the former [115]. The degree of ODS can be changed by adding different electron-withdrawing or electron-donating substituents to these complexes [113], [116]. In addition, the stability of these complexes can be increased by forming nanocomposite catalysts, thus ensuring that they can be reused in oxidation reactions [117].
Wang et al. fulfilled two separate studies [118], [119] concerning sonocatalytic ODS (followed by extraction with methanol) of benzothiophene in the presence of H2O2 at 60 °C using core–shell nanosphere modified with metallophthalocyanine (tetra-substituted carboxyl iron phthalocyanine, FeC4Pc) encapsulated into magnetic mesopore silica nanoparticles and silica nanotube catalyst with magnetite nanoparticles-coated interior surface and FeC4Pc-modified inner and outer surface. Higher desulfurization of the former (at the same conditions, desulfurization near 94.5%) compared to the latter (76% desulfurization yield at 30 min and molar ratio of H2O2/S = 15) can be considerably clarified by the fact that the particle size (60 nm) and the average pore size (2.6 nm) of the nanosphere composite catalyst are smaller than the outer diameter of the nanotube catalyst (200 nm), hence providing larger surface area for adsorption, though the catalyst loading is not specified in the latter. In these two studies, it was reported that high desulfurization is due to the radical decomposition of H2O2 to HO· on metallophthalocyanines. HO· radical from H2O2 by ultrasound wave can also be formed [120]. It is also stated that both catalysts can be easily isolated from the mixture by applying an external magnetic field after the reactions due to their superparamagnetic properties and reused in the next reactions.
Uniform Ni skeletal catalyst was synthesized at a size of 2.5–10 µm under 90 kHz ultrasound and crude oil containing 2.645% S by weight is subjected to oxidation with two treatment cycles using a mixture of ozone-air and 0.2% by weight catalyst based on the oil volume for 5 min in a US bath with frequency of 22 kHz [61]. Sulfur removals from gasoline and diesel fractions in crude oil were found to be 52 and 27.4%, respectively, as well as improvement of gasoline and diesel fractions.
By using 0.5 g of the modified GO/COOH solid catalyst with increased surface acidity formed by the addition of –CH2COOH group to the epoxy or hydroxyl groups of GO as a result of the reaction of graphene oxide (GO) with chloroacetic acid, a desulfurization of 95%, which is higher than desulfurization in the case of using non-acidified GO, was performed from the DBT solution containing 1000 ppm S with 30 wt% H2O2 within 300 min on sonication [121]. It was put forward that the adsorption-oxidation mechanism is the conversion of DBT to DBT sulfone by the peroxyacid group formed on the GO/COOH surface via activation of H2O2 by the carboxyl group in GO, and then π-π interaction of DBT sulfone with GO/COOH and adsorption of DBT sulfone through hydrogen bonding. In addition, it was stated that ultrasound contributes to high desulfurization due to the increase in the surface area caused by the exfoliation of GO/COOH as well as the increased collision frequency of the reactants due to the significantly increased mass transfer.
As phosphotungstic acid hydrate as oxidizing agent is dissolved in the aqueous phase, thus making it difficult to be reused by recovery [122], activated carbon-supported phosphotungstic acid (PTA) catalysts were synthesized and two separate studies [123], [124] were carried out on UAODS of 2000 ppmw DBT. In the first study [123], a DBT conversion of 93.4% was reached using 40 mL of model oil, at PTA/AC-10 catalyst/model oil 1.25: 100 mass ratio and H2O2/model oil 0.1 vol ratio under 70 W US power at 60 °C and 10 min, while in the second study [124] under the same conditions except the use of US at 100 W power, DBT conversion well below the conversion reached in the first study was obtained. The reason for the low conversion can be attributed to the weakening of the ultrasound wave (bubble shielding effect) as a consequence of absorption and scattering of US waves by these bubbles by resulting in the formation of dense cavitation bubble cloud around the transducer under high power [125]. Therefore, an optimum power intensity is needed as an important factor for high conversion in liquid phase reactions. In both studies, it was reported that desulfurization improved due to the increase in the number of surface acid sites by the increase in the amount of phosphotungstic acid in AC, and beyond a certain phosphotungstic acid amount, the sulfur removal is unchanged due to the reduction in surface area as a result of the destruction of microchannels in AC and the occupation of pores in AC by phosphotungstic acid.
In a similar study [126] where the same catalyst (HPW/AC-10) was synthesized, the optimum conditions were determined using RSM for reasonable desulfurization of the model oil containing 2800 ppm S consisting of a mixture of DBT, BT and T in the presence of individually, 30, 20 and 10 wt% H2O2 at different catalyst quantities, different AP/OP volume ratios and different times under 37 kHz US. By applying these optimum parameters to kerosene with 1370 ppmw S, a 99% desulfurization was successfully achieved, followed by four-cycle extraction.
In a study [127] where O2 in air was used as oxidant instead of thermally unstable H2O2, modified heteropolyacid catalysts (H5PV2Mo10O40/SiO2 and H5PV2W10O40/SiO2) supported on silica were synthesized. At optimum conditions (catalyst weight/model oil volume 11.09 g L–1, POM weight /SiO2 (wt. %) 39.879, sonication time 199.209 min.) found using the response surface method at 65 °C and 1.3 L min−1 air flow rate, a higher desulfurization (90 vs. 70%) of DBT was achieved in a shorter time (199 vs. 360 min.) under 20 kHz and 360 W direct US compared to the desulfurization in the case in which ultrasound is not used. It was demonstrated that the reason for low desulfurization is the polymerization of DBT due to the low concentration of oxygen dissolved in the organic phase (limited aerobic medium) under magnetic stirring, thus causing the polymer formed to accumulate on the modified heteropolyacids. While this polymerization is thought to be probably initiated by the DBT cation radical formed as a result of electron transfer from DBT to vanadium incorporated heteropolyacid [128], it was found that US increases the dissolved oxygen concentration and prevents polymer deposition on the catalyst surface. DBT conversion 10% more with H5PV2W10O40/SiO2 than the conversion percentage with H5PV2Mo10O40/SiO2 was obtained since the standard reduction potential of V5+ and W6+ (1 and −0.090 eV, respectively) is higher than that of Mo6+ (-0.913 eV), thus having stronger oxidizing power [129], [130]. The oxidation mechanism [131], [132] in the UAODS system can be elucidated by the electron transfer-oxygen transfer (ET-OT) reaction, in which oxygen is involved, between the modified heteropolyacid and DBT as follows:
Model oil with 1000 ppm total S content containing BT, DBT and 4,6-DMDBT was sonicated at 300 W, 45% amplitude and 20 kHz fixed frequency using 30 wt% H2O2 in the presence of MoO3 supported on γ-Al2O3 catalyst for 30 min [133] and at the optimum conditions (H2O2/S = 3 molar ratio, 45 °C, 30 g L–1 catalyst/model oil ratio) found by RSM with central composite design, a DBT → DBT sulphone conversion above 98% was found. Moreover, a desulfurization improvement of over 95% was achieved for DBT even after 6 cycles without losing the catalyst effect, due to US, which prevents the agglomeration of catalyst particles and H2O2 and causes desorption of adsorbed polar sulfones and water impurities from the catalyst surface. For BT, DBT and 4,6-DMDBT, the highest desulfurization was achieved when the MoO3 content on the catalyst was 10 wt% and at this loading, it was proved by XRD analysis that MoO3 is homogeneously dispersed on the support and MoO3 crystals are not seen. It was suggested that the sulfur compounds are oxidized by highly reactive molybdenum peroxide and molybdenum diperoxides formed in situ.
In a similar study [134] where the same reagents and the same ultrasonic parameters were used, complete oxidation of DBT in the model oil containing 600 ppmw total S was achieved in the presence of MoO3 loading of 10 wt.%/Al2O3 at H2O2/S = 3.8 molar ratio, 30 g L–1 catalyst/model oil ratio, 45 °C and 30 min. Besides, the addition of aromatic compounds (tetralin, naphthalene and 2-methyl naphthalene) individually to the model oil formed by dissolving DBT in hexane to mimic diesel fuel appreciably reduced the UAODS yield although the resulting DBT selectivity is high due to the competitive adsorption of the aromatic compounds on the catalyst surface. Further, in both studies [133], [134] it was shown that the active sites responsible for the adsorption of sulfur compounds are tetrahedrally coordinated Mo6+ oxides, above a Mo-saturated monolayer coverage (which is at 10 wt% Mo loading), agglomeration of amorphous MoO species results in the formation of MoO3 crystals and cause a reduction in the number of active sites, as well as the reduction of surface area, by blocking micropores of the catalyst [135], thus reducing the UAODS.
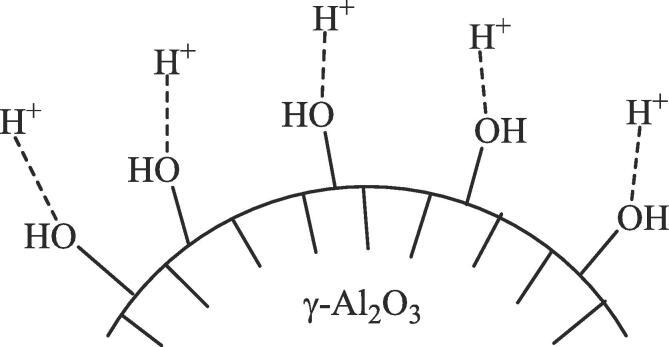
Using persulfate agent in toluene and hexane as solvent, 98 wt% H2O2 and 1% Si-Al/Al2O3 as solid catalyst, 99.72% of sulfur (followed first by extraction with acetone, then by adsorption with activated charcoal and ultimately by sonication under 30 kHz US of the diesel sample treated with acetic acid) in hydrotreated diesel fuel containing 766.73 ppmw total S was removed at around 65 °C and atmospheric pressure [136]. It can be thought that the oxidation mechanism [137] is based on sulfate ion radical caused by thermal activation of persulfate, hydroxyl radical formed as a result of the reaction of sulphate ion radical with H2O2 and activation of S2O82− by hydrogen peroxide, which causes the formation of hydroxyl radical. Moreover, US can cause homolytic cleavage of the persulfate agent [138] and hydrogen peroxide [90]. The surface hydroxyl groups [139] on Al2O3 (Fig. 3) in the solid catalyst in the reaction medium can induce the formation[137] of radical from persulfate by interacting with H+ formed by the reaction (4) and, hence accelerating the UAODS reaction.
| (1) |
| (2) |
| (3) |
| (4) |
Fig. 3.
The interaction of the hydrogen ion with two lone pairs on the oxygen atom of the hydroxyl groups on ɣ-Al2O3.
Since homogeneous Fenton catalysts (FeSO4) dissolve in the aqueous phase and consequently, making their recovery difficult [140] after ODS reactions, water-insoluble Fenton-like catalysts supported on coal fly ash (which is a very cheap waste from coal-fired power plants) were synthesized [141]. Approximately 30% desulfurization was carried out as a result of simultaneous oxidation and extraction of sulfur compounds from commercial diesel fuel containing 595 ppm S using 10 wt% H2O2 and ethanol solvent in the presence of the Fenton-like catalyst in an ultrasonic bath at 47 kHz frequency and 147 W power [65]. It has been suggested that the oxidation stems from the hydroxyl radicals formed from the reaction between Fe2+ and H2O2. Hydroxyl radicals [90] formed from the decomposition of H2O2 by US may also contribute to this desulfurization. Furthermore, since coal fly ash contains metal oxides [142], H2O2 helps desulfurization by being adsorbed on the supported catalyst as well as forming surface-bound hydroxyl radicals on the support [143].
US has also been applied to oil sands [144] as an oil deposit consisted of a mixture of clay, sand, bitumen and water. A total sulfur removal efficiency of 82% has been reported by simultaneous oxidative and extractive desulfurization of semi-solid Alberta bitumen containing 5.2 wt% S using 3 wt% H2O2, saturated NaOH and tetrahydrofuran under a 28 kHz frequency and 200 W powerful indirect ultrasound at 20 °C and 20 min [145]. Then, an 88% bitumen recovery from oil sand and a 42% sulfur reduction from bitumen was fulfilled using the same reagents, the same reaction conditions and ultrasonic parameters simultaneously. In addition, possible metalloporphyrins [146], [147], [148] in bitumen can accelerate the UAODS reaction of bitumen. Moreover, it was stated that since ionic NaOH cannot dissolve oil sand sufficiently and effectively, mid-polar THF is used owing to its high dissolving power.
The UAODS process was not limited to liquid fuels, but also applied to mesophase materials [149]. It was demonstrated a sulfur removal (followed by extraction with equal volumes of methanol and sodium hydroxide (0.5 wt%)) of 91.1% from coal tar pitch with 0.9 wt% S containing predominantly polycyclic aromatic hydrocarbons (also called polynuclear aromatic hydrocarbons) was carried out using xylene as dispersant and solvent, trichloroacetic acid as catalyst, 30 wt% H2O2 in the absence of surfactant under 20 kHz and 300 W direct US at 60 min. and 70 °C [150]. On the other hand, the use of surfactant did not increase UAODS.
Apart from hydrogen peroxides, organic peroxide has also been used as oxidant. In this type of study [151], approximately 35% desulfurization (followed by extraction three times with acetonitrile) was performed from a high-viscosity bunker-C oil MFO 380 (max kinematic viscosity 380 cSt) with 3.17 wt% S using viscosity-reducing heptane and 3 mL of t-butyl hydroperoxide as oxidant in the presence of 0.2 g MoO3 as solid catalyst under direct US at a frequency of 20 kHz and 70% amplitude at atmospheric pressure, 90 min and 80 °C. Unlike HP, TBHP has the advantage of being soluble in both aqueous and organic phases, therefore, in desulfurization reactions where the aqueous phase is not used, it is in direct contact with sulfur compounds without the need for mass transfer. It was reported that the much higher-reactivity peroxo molybdenum complex formed as a result of the reaction of t-BHP with MoO3 is responsible for the oxidation of sulfur compounds to their sulfones. When ultrasonic cavitation bubbles in sonochemistry implode, very high temperatures and pressures occur locally in the liquid (hot spot theory) [152]. Therefore, it can be deduced that reactive oxygen species, which are generated by thermal decomposition of t-BHP in this reaction, such as t-butoxyl (H3C)3 - O·, hydroxyl HO· and t-butyl peroxyl (H3C)3 - O - O· radicals [153], further contributes to the oxidation of bunker-C oil.
2.2. Homogeneous catalysts
In the presence of heterogeneous catalysts with which sulfur compounds interact electronically on the solid surface, adsorption, where mass transfer is an important factor, takes place through catalyst pores [154], whereas homogeneous catalysts dissolve in liquid (ie, aqueous phase). After UAODS reactions, isolation, recovery and reuse of homogeneous liquid catalysts, as well as the homogeneous solid catalysts dissolved in the aqueous phase, from the reaction mixture are quite problematic since they are in the same phase as reactants, which increases the process cost [155].
Reactions, in which homogeneous catalysts are involved, can be divided into two classes; 1) Reactions in the absence of PTC 2) Reactions in the presence of PTC. Among the homogeneous solid catalysts, catalysts such as phosphotungstic acid [156] as polyoxometallate class, Fe(II)SO4 [157] and CuSO4 [158] were employed, while organic acids such as acetic acid [159] and formic acid [160] were utilized as homogeneous liquid catalysts.
2.2.1. Reactions in the absence of PTC
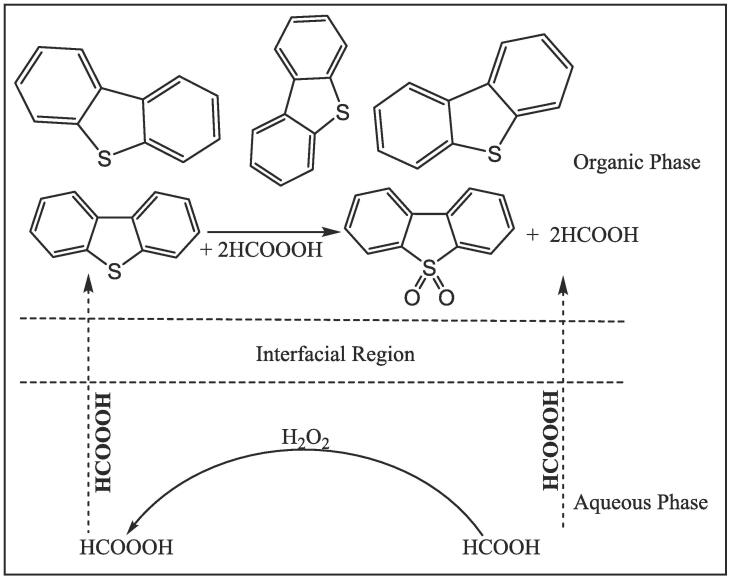
In the absence of PTC, the ODS mechanism [161] is shown in Scheme 2. Peroxyformic acid formed in situ by the reaction of HP and formic acid in aqueous phase is transferred to the organic phase where DBT is oxidized, by the effect of ultrasound.
Scheme 2.
The ODS mechanism with HP-FA system.
2.2.1.1. The use of acetic acid
In a study [162] where the sonoreactor was optimized to increase the UAODS yield, a sulfur removal of 98.25% was achieved from model fuel containing 1000 ppmw DBT in n-decane using 16 mL of 34.5 wt% H2O2 and 40 mm-diameter sonotrode with an immersion depth of 3 cm at acetic acid/H2O2 64: 300 molar ratio in 7.4 cm-diameter glass reactor under 20 kHz, 500 W and 80% amplitude direct US at 48 °C within 30 min.
UAODS of a model fuel containing 100 ppmw DBT (10.8 mM/l) in toluene was performed using FeSO4, acetic acid and 30 vol% hydrogen peroxide (HP) [163]. It was stated that the hydroperoxyl radicals formed were responsible for the oxidation of the sulfur compound rather than the hydroxyl radicals formed, hence by explaining that lower scavenging of HO2· radicals is important. An DBT removal of approximately 33.34 wt% from model oil has been reached at acetic acid/HP = 2 vol ratio, toluene/HP = 10 vol ratio, at 1.5 M Fe2+ concentration, 90 min and atmospheric pressure under 70 W and 35 kHz indirect US at 25 °C.
In a similar study [164] in which desulfurization of benzothiophene (BT), 3-methyl thiophene (3-MT) and thiophene (T) was performed using 25 mL of 30 vol% HP + CH3COOH and Fe2+, sulfur removals of 79.4, 77.9, 77% − 76.3, 76.9, 77.6% and 77.5, 76.5, 76.1% were obtained from concentrations of 100, 300 and 500 ppm for BT, 3-MT and T, respectively, under 2.5 bar, 35 W and 35 kHz indirect US at 90 min and 25 °C, such that these conversions were higher than those obtained at atmospheric pressure due to the elimination of transient cavitations at high pressure. In addition, according to the cavitation bubble dynamics model, it was revealed that the high desulfurization is caused by the sonophysical effect (microconvection) of US.
In a study [165] in which a sample of raw coal containing 2.16 wt% total S as solid fuel was treated with peroxyacetic acid, oxidative desulfurization of raw coal improved due to the increased reactivity of the coal depending on the increased specific surface area, the total pore volume and the mean pore size of the treated coal compared to those of the untreated coal since abrasion of coal particles upon sonication occurs; 17.59% of the total sulfur present in the coal was removed using 10 mL 98 wt% acetic acid and 50 mL 30 wt% HP under 20 kHz and 720 W direct US at 30 °C within 5 min. It was shown that the greatest contribution to desulfurization is that US increases the production of hydroxyl radical in the presence of HP and acetic acid in the mixture, whereas the hydroxyl radical production rate is significantly low when there is only HP.
In a similar study [166] in which the same reactants were used, the raw coal was subjected to ultrasonic treatment followed by microwave. The US applied reduced the particle size of the coal, increased its total porosity (i.e., specific surface area, total pore volume and average pore diameter of the raw coal are 0.88 m2.g−1, 0.00213 cm3.g−1 and 9.68 nm, respectively, whereas specific surface area, total pore volume and average pore diameter of the coal sample after US treatment are 1.66 m2.g−1, 0.00771 cm3.g−1 and 18.56 nm, respectively) and increased hydroxyl radicals. But at the same time, microwave increased the reaction rate dramatically as the reactants in the mixture absorbed the electromagnetic radiation generated [167]. At the end of the ultrasonic treatment at acetic acid (98 wt%)/HP (30 wt%) 1:5 vol ratio under 20 kHz and 720 W direct US for 50 min at 40 °C, followed by microwave treatment under 600 W power at a frequency of 2.45 GHz at 100 °C for 6 min, a desulfurization of nearly 22% was obtained from raw coal containing 1.93 wt% organic S, which results from the resonance nature of the thiophenic compound according to mercaptan and sulfoether, whereas the percentage of pyritic sulfur (in the form of FeS2) removed as inorganic sulfur was reported to be about 85%.
In another study [168] using the same reactants, two coal samples (XS with 0.85 wt% organic S and YN with 2.69 wt% organic S) completely free of inorganic sulfur as a result of pretreatment with dilute nitric acid were subjected simultaneously to ultrasonic and microwave treatment with a power of 560 W each for 50 min. Sulfur removals of 23.53 and 76.58% were achieved for XS and YN, respectively. Consequently, it turns out that from these three studies concerning coal, simultaneous operation (US-MW) is more efficient.
In desulfurization of model fuels prepared by dissolving model sulfur compounds in a non-polar solvent such as octane, heptane or hexane, an extraction step is not required since the sulfones as oxidized sulfur compounds are easily determined by instrumental devices such as GC-FID, HPLC, hence easily finding the conversion to sulfones. However, as there are also aliphatic and aromatic hydrocarbons in addition to sulfur compounds in real fuels, it is not possible to determine the sulfur compounds with these devices. After separating sulfones by an extractant, the total sulfur percentage in the fuel can be determined by using devices such as microcoulometric analyzer, sulfur analyzer with UV fluorescence, XRF and GC-SCD.
Alkaline solutions have also been used in UAODS. In simultaneous oxidative and extractive desulfurization [169] of ultra low-sulfur diesel spiked with 500 ppmw 4,6-DMDBT, it has been shown that desulfurization in single step can be improved without an extraction step mainly due to the hydroxyl radicals formed as well as secondarily the formation of carbonate radical by resulting in radical decomposition of HP under US in the range of pH 6 ∼ 8 with basic sodium carbonate. Approximately 94% desulfurization was reported at diesel/acetonitrile 1: 2 vol ratio, 0.8 M HP 30 wt%, 30 mM Na2CO3 under 23 kHz frequency direct ultrasonic pulse at 60 °C in 2 h.
As shown in Table 1, the other studies [170], [171], [172], [173], [174], [175] using acetic acid as organic acid in addition to HP are common in the literature. In addition, acetic acid is relatively low-cost [176]. In studies [97], [165], [177] in which desulfurization of crude oil, coal and model diesel fuel with the help of US by using acetic acid-HP oxidant system was performed, it was indicated that high desulfurization efficiency is reached in a short time at relatively low temperatures. The oxidation of sulfur compounds is caused by peroxyacetic acid and hydroxyl radicals formed in situ in the aqueous phase. It has also been shown that nitrogen compounds have an inhibitory effect on oxidative desulfurization as the oxidation reactivity of the nitrogen compounds present in the fuel (e.g. quinoline) is higher than that of the sulfur compounds [172]. Moreover, the effects of different US loop reactor types on UAODS were also examined [173]. It is stated that the aqueous phase separated after the UAODS reaction and the extractant separated after the extraction step can be reused for the fresh feedstocks containing 208 ppmw S DBT and the same feedstocks subjected to oxidation treatment, respectively, though the desulfurization efficiencies in reuses are lower than those in their first uses [171]. UAODS efficiencies of diesel fuel feeds containing different sulfur amounts in the presence of acetic acid under the relevant reaction conditions are shown in Table 1.
Table 1.
Desulfurization results of low- and high-sulfur diesel fuels under different reaction conditions.
| Feed | Reaction conditions | Desulfurization yield |
|---|---|---|
| DBT in a hydrotreated petroleum product feedstock (containing 211 ppmw S) [170] | 15 mL glacial acetic acid, 10 mL 50% (v/v) H2O2, 9 min, 20 kHz, 750 W, 40% amplitude direct US, extraction with methanol, n = 3, Volume ratio (oil/solvent) = 1:1 | 95% |
| Diesel Oil A (136 ppm S), Diesel Oil B (319 ppm S), Diesel Oil C (249 ppm S)A and C: Deep hydrotreated sample B: Hydrotreated sample [171] | Mole ratio (H2O2 (50 wt%)/Glacial Acetic Acid/S) = 64:300:1, 90 °C, 9 min., 20 kHz, 40% amp. direct US, 750 W, extraction with methanol, n = 3, volume ratio (solvent/oil) = 0.36 | 75.8, 87.7 and 76.8%, respectively. |
| Hydrotreated petroleum product feedstock (211 ppmw S DBT) [172] | 2 mL glacial acetic acid, 0.25 mL 50 wt% H2O2, 90 °C, 9 min., 20 kHz, 40% amp. direct US, 750 W, extraction with methanol, n = 3, volume ratio (solvent/diesel oil) = 0.36 | 96% |
| Hydrotreated diesel feedstock (241 ppmw S and 161 ppmw N) [173] Reactor types: Sonitube, Emitting Plate, Multi-horn, Single horn | 80 °C, 80 min, Molar ratio (S + N): H2O2 35 wt%: glacial CH3COOH = 1:56:1114, direct US, followed by silica gel adsorption n = 3 | 99.7, 99.7, 98.6 and 99.95%, respectively. |
| 4000 ppmw S real diesel enhanced with DBT [174] | 14 mL 30% H2O2, 21 mL 99 wt% CH3COOH, 30 min, 70 °C, 80% amp., 20 kHz, 80 W direct US, extraction (mixing at 25 °C for 25 min.) with DMF, solvent ratio = 1 | 99.92% |
| Diesel Fuel (849 ppmw S and 445 ppmw –SH group (thiol)) [175] | H2O2 30 wt%/oil ratio = 4 wt%, acetic acid (30%)/diesel ratio = 1.50 wt%, 120 W, 37 kHz indirect US, 25 °C, 15 min. | 76% sulfur removal 79% thiol group conversion |
The desulfurization [172] decreases as the amount of nitrogen in the sample increases. (The sulfur removal at a molar ratio of S:N = 1:2 is approximately 75%.)
One of the most important reasons why HDS is still widely used today is that fuel loss after HDS process is very low [178]. In laboratory-scale studies, after the ODS process, the properties of the fuel are almost unchanged [172], [179], [180], [181], [182], [183], [184], [185], [186], but the loss of fuel in the extraction step (i.e., the reduction of fuel recovery) after the ODS process on large scales can pose a major problem. Moreover, whether the properties such as density, viscosity, cetane number, boiling range distribution of the desulfurized fuel produced in large quantities (factory scale) have changed is a matter of investigation separately and must be checked one by one. In most research papers [171], [187], [188], [189], when H2O2/S mole ratio initially increases, desulfurization generally increases, then reaches a certain value and decreases slowly after this optimum value. It was reported that this decline is due to dilution of the aqueous phase.
In a study [172] in which nitrogen was removed by US from a synthetic fuel solution with 252 ppmw N prepared by dissolving quinoline in a hydrotreated petroleum product feed containing 3.6 ppm S, 92% nitrogen removal (followed by extraction with methanol) was achieved in the case where only acetic acid is used in the absence of HP as oxidant. It is stated that this value is higher than the value (79% nitrogen removal) obtained without oxidation treatment by only liquid–liquid extraction with methanol, hence underlining that acetic acid has the capacity to extract nitrogen compounds.
The effects of different sonoreactor types on desulfurization and denitrogenation (followed by silica gel adsorption) of hydrotreated diesel fuel containing 241 ppmw S and 161 ppmw N were also evaluated [173]. It was shown that the most effective reactor in terms of cost and performance optimization was sonitube.
In an oxidation study [190] accomplished under 20 kHz and 70 W direct US followed by extraction with DMF, it was stated that while the initial sulfur content in the model fuel containing DBT increased from 1220.80 ppmw to 3976.86 ppmw, desulfurization also increased to 98.35%. In the UAODS [175] followed by extraction, as acetic acid/oil ratio increased to 1.50 wt%, the desulfurization of diesel containing 849 ppmw S improved. This was attributed to the strong oxidant peracetic acid formed in situ.
Heterogeneous reactions with solid–liquid systems using solid oxidants were also carried out. HP-acetic acid at S/oxidant 1:10 molar ratio, KO2-Acetic acid, Na2S2O8 alone, Na2S2O8-acetic acid and oxone alone at S/oxidant 1:10 and 1:30 molar ratios at different times at 80 °C were used [186] for UAODS of model oils and diesel fuel. Sulfur and nitrogen removal were individually performed by ultrasonic horn device under 21.1 kHz and 80 W direct US and ultrasonic cup horn device under 19.9 kHz and 80 W US from mild hydrotreated diesel feedstock containing 226 ± 2.17 ppmw total S and 158 ± 2.81 ppmw total N as well as three model solutions containing 1.2 mg mL−1 DBT or DMDBT and 1.2 mg mL−1 quinoline individually. In UAODS reactions of model solutions in both reactor types, when oxone alone is used at a molar ratio of S/oxidant = 1:30 without acetic acid, very high desulfurization efficiencies compared to other oxidant systems (100% sulfur removal for DBT and DMDBT in 90 min, a nitrogen removal of 40% for quinoline in the same time) were achieved. For scale-up purposes, the US cup horn was chosen as it closely resembles the geometry of continuous flow reactors and sulfur was removed (followed by SiO2 adsorption) from hydrotreated diesel fuel at molar ratios of (S + N)/oxidant 1:10, 1:20 and 1:30 by oxone at different times. In addition to obtaining a diesel fuel containing 0.91 ± 0.48 ppmw N (a nitrogen removal of 99.4%) at a molar ratio of 1:30 in 90 min, a sulfur removal of 99% was achieved. In the case of extraction with MeOH instead of adsorption, significantly low desulfurization (65%) was obtained for the same molar ratio and the same time, but diesel fuel recovery with SiO2 adsorption was lower than that with methanol extraction by 11%. It was stated that excess oxone can be reused for the same diesel fuel without losing its activity in four treatment cycles followed by adsorption with SiO2 each (from 84% sulfur removal at the end of the 1st cycle up to 95% at the end of the 4th cycle). Although oxone is a relatively inexpensive oxidant and provides high desulfurization, a 15% diesel loss after adsorption with SiO2 makes it very difficult to use in large scales, on the contrary, low desulfurization efficiencies were obtained by extraction with methanol due to low extractive performance of the extractant selected for oxidized sulfur compounds. This major difference between extraction and adsorption performance could possibly be due to SiO2 adsorbing not only oxidized sulfur compounds but also sulfur compounds [191].
After biphasic UAODS reactions in the presence of HP and acetic acid, how to valorize the aqueous phase or eliminate the sulfur compounds and their oxidized counterparts in the aqueous phase is a crucial environmental issue.
A 96.45% sulfur removal [192] (followed by extraction with acetonitrile at 1000 rpm mechanical stirring speed for 25 min at room temperature) was achieved from model diesel fuel containing 3976.861 mg S L–1, which is prepared by dissolving DBT in homogeneous solution (n-dodecane + n-heptane + n-hexadecane), using 10 mL HP and 10 mL acetic acid under 20 kHz frequency, 70 W power and 80% amplitude direct US at 70 °C in 30 min. The aqueous phase (total organic carbon TOC content 1200 mg L–1) containing DBT, DBTO2 and acetic acid, that is separated after the heterogeneous UAODS reaction and called diesel wastewater, was diluted individually 10- and 20-fold with distilled water and subsequently subjected to homogeneous ODS reaction at C(Fe2+) = 2 mmol L–1 and C(HP) = 20 mmol L–1 Fenton's reagent concentration (with acetic acid by adjusting pH to 3.1) under 200 W and 20 kHz direct US for 120 min. At the end of the homogeneous ODS reactions of the two aqueous phase samples diluted 10- and 20-fold with pure water, a removal of 75 and 76% for DBTO2, 28 and 66% for TOC, respectively, were obtained. HPLC analysis of the treated diesel wastewater confirmed the formation of benzoic acid followed by aliphatic carboxylic acids (e.g., oxalic acid) after 30 min as a result of oxidative degradation of small amounts of remaining DBT. It was stated that this sono-Fenton process has the potential to remove organic pollutants from diesel waste water and the treated water can be reused.
In order to further remove the sulfur in the fuel (i.e., to obtain ultra-low or low-sulfur fuel), advanced oxidation processes, which are used in the removal of organic pollutants from wastewater, have also been utilized in UAODS reactions. For this purpose, FeSO4 was added to the aqueous phase containing HP-acetic acid and a 98.32% desulfurization degree [193] (followed by extraction two times at DMF/oil 1:1 vol ratio for 2 min each at room temperature) of hydrotreated Middle Eastern diesel fuel containing 568.75 ppmw total S was obtained at optimum conditions (40 °C, Fe2+/HP 0.05 mol/mol, pH = 2.10 and reaction time of 15 min) under 200 W and 28 kHz direct US. Explaining that the high desulfurization is due to the Fe2+ ion which generates more hydroxyl radicals from HP, it has been determined that the US-Fenton’s reagent system follows the second order reaction kinetics.
In a similar study [184] where Fenton’s reagent as oxidizer and acetic acid were used, 97.5% sulfur removal from original diesel fuel containing 1936.48 ppmw total S (followed by extraction at DMF/oil 1:1 vol ratio under vigorous mixing at room temperature) has been achieved at optimum operating conditions (70 °C, 10 min, 8 W cm−2 ultrasonic intensity, O/S molar ratio 6: 1, FeSO4/HP mass ratio 2:10 and acetic acid/HP volume ratio 1:2) under direct US at 28 kHz frequency. It was reported that the diesel loss after oxidation-extraction is less than 8 wt% and although the density and cetane index decreased a little, the other properties of diesel fuel did not change much.
By virtue of very severe process conditions (Hydrotreated diesel fuel with 421.45 ppmw total S obtained as the feeding material by hydrotreatment of diesel fuel containing 9997 ppmw total S for two-stage HDS, 7 MPa, 628 K, LHSV 1.8 h−1) necessary to reduce very high-sulfur diesel fuels by HDS to less than 10 ppmw S (9.5 ppmw S), diesel fuel containing 9997 ppmw total S was first processed by HDS in milder conditions (with 99.8% diesel fuel recovery) to obtain a fuel containing 421.45 ppmw S and then subjected to oxidation reaction (followed by extractions two times at DMF/oil 1: 1 vol ratio for 2 min each at room temperature) at 70 °C, HP/Diesel Oil 3/100 vol ratio, pH = 2.1 and Fe2+/HP 0.05 mol g−1 in the presence of Fenton’s reagent and acetic acid under 28 kHz and 200 W direct US in 15 min [178]. Along with the 92.2% diesel fuel recovery, diesel fuel containing 9 ppmw total S (97.86% sulfur removal) was obtained. Therefore, it was stated that integrating the ODS unit as a complement to the HDS unit is potentially advantageous in terms of overall process cost and efficiency.
It was reported that by using individually Fenton’s Reagent and Fenton-type reagent (Cu2+-HP), which is used to enrich hydroxyl radicals, in the presence of acetic acid (pH = 1.9 ∼ 2.1), a desulfurization degree (followed by extraction twice at DMF/fuel 1:1 vol ratio at room temperature for 10 min each) of 95.2 and 89.2%, respectively, was achieved for FCC diesel fuel [185] with 1936.48 ppmw total S at 60 °C, HP/S 6:1 molar ratio and M2+ (Fe2+ or Cu2+)/HP 0.05 mol mol−1 under 28 kHz and 200 W direct US in 15 min, which is an indication that metal ions catalyze the UAODS reaction creating a synergistic effect.
2.2.1.2. The use of formic acid
In a study [179] conducted to remove sulfur from a straight run diesel oil sample containing 960 ppm S (followed by extraction one time with DMF at extractant/oil volume ratio of 1:2), a desulfurization yield of 94.7% was obtained at the optimum conditions (HP/formic acid (FA) 1: 1 vol ratio, (HP + FA)/oil 1:10 vol ratio, 50 °C and 10 min) under 28 kHz–40 kHz and max 200 W direct US. It was observed that the degree of desulfurization almost does not increase due to the decomposition of HP after the optimum reaction time, the sulfur removal is slightly reduced due to side reactions after the optimum oxidant/oil volume ratio, and the desulfurization removal does not change beyond the optimum temperature. Moreover, it was stated that beyond optimum conditions, oil recovery decreases and also production costs will increase.
In a similar study [180] under the same optimum conditions as the previous study [179] (except that extractant DMF/oil volume ratio is 1: 1 and extraction time is twice), the effect of HP/FA volume ratio under direct US was investigated and a sulfur removal of 92.8% has been obtained from FCC diesel oil containing 1948 ppmw total S at the end of the UAODS process. Beyond the optimum oxidant/catalyst volume ratio (1:1), it was reported that desulfurization decreases due to nonproductive decomposition of excess HP to oxygen and water as there is not enough formic acid in the medium to form high-concentration peroxyformic acid in-situ by reaction of HP with FA.
The effect of extraction on desulfurization after the oxidation reaction of sulfur compounds in FCC diesel containing 1985 ppm total S with HP-FA oxidant system under indirect US was investigated [194]. Taking into account oil recovery and the consumption of extraction solvent, a desulfurization of 94.2% was achieved as a result of extraction two times at DMF/oil volume ratio of 1:1 at 30 °C for 20 min each.
Recently, RSM-Box-Behnken Design has been used to find the optimum desulfurization, to examine the effect of reaction parameters and interactions between the parameters on UAODS yield and also to find which parameter or parametric relationships are more important on desulfurization such that fewer experiments are performed with this program, thus resulting in less time-consuming study.
Using RSM [181], a sulfur removal of 95.46% from kerosene containing 2490 ppmw total S was achieved at the ratio of nO/nS = 15.02, nacid/nS = 107.8 and US power/fuel volume = 7.6 W mL−1 (followed by extraction with acetonitrile, extractant/kerosene volume ratio = 1, extraction stage = 1, ambient temperature, 700 rpm, 30 min.) at 20 kHz frequency and 400 W direct US at 50 °C within 10.5 min. It was observed that above the optimum nacid/nS and nO/nS ratios, the desulfurization was almost unchanged as performic acid formation and decomposition reactions occur together in an acidic medium and the equilibrium concentration of peroxyformic acid was reached due to the decomposition of HP. When the two ratios in the relation of power/volume and nO/nS to sulfur removal are above a certain value, no increase in desulfurization was observed due to dilution in the aqueous phase and the weakening of the ultrasonic wave emitted to the mixture by enlargement of the bubble cloud at the probe tip at high power. The fact that there is no significant increase in desulfurization above a certain value of the two ratios in the relation between power/volume and nacid/nS is due to the reason mentioned above. While a sulfur removal of 29.92% from kerosene is achieved by extraction alone employing acetonitrile without oxidation reaction, the desulfurization is 74.9% by oxidation and water washing without extraction process, which shows that formic acid extracts oxidized sulfur compounds sulfoxides and sulfones during the oxidation reaction.
In a similar study [195] with the same oxidant system by applying RSM, a sulfur removal higher than 98% was achieved at HP/S molar ratio of 10.82, FA/S molar ratio of 379.75 and 52 °C (which are the three independent reaction parameters selected) under 70 W and 20 kHz direct US and at 15 min for model fuel containing 500 ppm total S prepared by dissolving BT in toluene. With the same values of these 3 optimum parameters found, a sulfur reduction of approximately 95.6% (followed by extraction at acetonitrile/kerosene volume ratio of 1 for 30 min at room temperature) was achieved from kerosene containing 2720 ppmw total S under 250 W direct US in 20 min. The results revealed that the decrease in desulfurization at low acid/S and high O/S values is due to the dilution of the formic acid by increased surplus HP, thus lowering peroxyformic acid concentration and also the formation of vapor-filled bubbles rather than gas-filled bubbles with increasing HP. It was found that the importance degree of the independent reaction parameters was in descending order: Acid/S molar ratio > HP/S molar ratio> (Acid/S molar ratio)2 according to the ANOVA results of the quadratic correlation equation (where the smaller than 0.05 the P value and the larger the F value, the more important the parameter).
In a study [196] with the same oxidant system, using the RSM-Box-Behnken Design (BBD), where temperature and US power/gas fuel volume (W mL−1) were selected as constant parameters and O/S, Acid/O molar ratios and sonication time as process variables, 87% sulfur removal from gas oil containing 2210 ppmw total S (followed by one-time extraction at acetonitrile/gas oil volume ratio of 1: 1 under vigorous stirring for 30 min at room temperature) was achieved at O/S 46.36 molar ratio, acid/O 3.22 molar ratio in 19.81 min for 50 °C and 7.78 W mL−1 under a direct US of 20 kHz. However, in the case of 4-step extraction, 96.2% of the sulfur present in the gas oil was removed, but it was reported that the recovery of gas oil decreased to 81.25%. After the oxidation reaction under the same conditions, the extraction performances under mechanical mixing and under direct US were compared. It was observed that the desulfurization yields were approximately the same, thus showing that US does not have a positive effect on extraction. In addition to these, as a result of the preliminary cost analysis of this batch process, it was determined that a total operating cost of $ 0.43 was incurred for the treatment of 1 L gas fuel and also 31.7 and 56.3% of this total cost were liquid–liquid extraction and US Power/gas oil volume, respectively. It was stated that this calculated cost will be less in continuous-flow UAODS systems as there are stagnant zones in the mixture in batch UAODS systems, thus leading to a higher consumption of US power density per unit volume of fuel in the batch systems. According to ANOVA analysis, it was determined that the importance of variables is in the order: sonication time > acid/O molar ratio > O/S molar ratio> (acid/O × sonication time)> (sonication time)2> (acid/O molar ratio)2> (O/S molar ratio) × (acid/O molar ratio)> (O/S molar ratio)2. It was explained that sulfur removal decreased due to the scavenge of hydroxyl radicals at high acid/O molar ratio and enhancement of side reactions in case there is excess HP in the medium towards high O/S molar ratio. In high acid/O and high O/S molar ratios, it was explained that peroxyformic acid stabilizes at low pH of the aqueous phase as a result of very high concentration of formic acid after a certain value, thus resulting in a lower desulfurization by limiting the production of active oxidizing radicals, which are generated by the decomposition of performic acid.
The RSM-BBD was applied to a batch reactor in a continuous study [188] in which the aqueous phase consisting of HP and FA is injected by nozzles of different diameter to just below the bottom end of the probe (which is the active site where radicals are produced). O/S molar ratio, acid/S molar ratio and sonication time were selected as independent variables at 50 °C under 20 kHz and 360 W direct US and the optimum parameters (nO/nS = 38.88, nacid/nS = 116.47 and sonication time 29.2 min.) were determined under batch conditions. According to ANOVA, it is stated that the most important terms are in the order: acid/S molar ratio> (O/S molar ratio × acid/S molar ratio) > sonication time. These optimum parameters have been applied to two continuous reactors in series (where in the first reactor, the aqueous phase was injected to the lower end of the probe) at different feed rates (thus causing different retention times) and different fuel phase/aqueous phase volume ratios (herein (Vacid/VO) = 1.117). For non-hydrogenated diesel fuel containing 1550 ppmw total S, a desulfurization of 83.39% (followed by a single extraction with acetonitrile/organic phase volume ratio of 1:1 at 1000 rpm mixing speed for 30 min at room temperature) was reached at Vf (volume of the fuel phase)/Vaq (volume of the aqueous phase) 5: 1 vol ratio, 40 mL min−1 total outlet flow rate (33.33 mL min−1 diesel fuel + 6.67 mL min−1 aqueous phase), a residence time of 3 min in the first reactor and 2.5 min in the second reactor using 1.5-mm-diameter nozzle from the point of the lowest retention time and lowest aqueous phase volume to minimize the process cost. It was explained that when the nozzle diameter decreases from 1.5 mm to 0.43 mm, the desulfurization decreased to 68.74% due to a decline in the ratio of the hydrodynamic momentum flow rate generated by the US probe to the hydrodynamic momentum flow rate of the dispersed aqueous phase (in which case, aqueous phase will stay in the active zone for much less time as the increasing flow rate by use of the smaller nozzle diameter leads to the increased momentum). In addition, it was shown that the increase of the aqueous phase flow rate from 10 to 40 mL min−1 for all the nozzle diameters leads to a decrease in desulfurization due to the reason mentioned above. Batch sonoreactor and sonoreactors in series operating at different times at a constant volume ratio of Vf/Vaq = 2.96 mL mL−1 and at different Vf/Vaq ratios at constant sonication times of 5.5 min were compared and it was reported that in all cases, the sulfur removal per power density consumed in continuous sonoreactors in series is higher than that in the batch sonoreactor.
The effect of pressure on UAODS in a sonoreactor was investigated [197] and the optimum conditions (390 W US power at 20 kHz frequency, gauge pressure 0.03 barg and 22 min) were found by applying RSM-BBD in which pressure, US Power and sonication time were selected as independent variables at T = 50 °C, nO/nS = 15.02 and nacid/nS = 107.8. A sulfur removal of 96.7% (followed by one-time extraction at acetonitrile/kerosene 1:1 vol ratio under 500 rpm stirring speed for 30 min at room temperature) was obtained from kerosene with 2490 ppmw total S. Also, it was disclosed that according to computational fluid dynamics (CFD), desulfurization decreased at pressures above atmospheric pressure (1 barg and 2 barg) due to the progressively decreasing vapor volume fraction, the decreasing bubble collapse pressure, the low dispersion of the aqueous phase into the organic phase and a significant increase in the aqueous phase volume fraction. The authors suggested that the marked rise in the aqueous phase volume fraction did not result in finer emulsion droplets, thus causing the interfacial area between the aqueous and organic phase to diminish. In addition, it was stated that when the US Power increased from 100 to 400 W, the max acoustic pressure and micro-streaming speed increased according to the calorimetric analysis, thus desulfurization was improved due to the increase in mass transfer rate. It was determined that the most important terms affecting desulfurization are in the order: time > Pressure > Pressure × Power > Power according to ANOVA.
In a continuous cylindrical sonoreactor with multiple probes (3 probes) and two nozzles [182], through which the aqueous phase is injected just below the first and the second probe tips from the left side of the inside of the reactor, the optimum conditions (Vacid/VO (mL mL−1) 1.12, Vaq = (Vacid + VO) 733.33 mL, Vf = 3666.67 mL, Vf/Vaq (mL mL−1) 5 and temperature 50 °C) were determined under direct ultrasound, each of which has a power of 400 W and a frequency of 20 kHz (all ultrasonic processors ON). >97% of sulfur (followed by extraction with DMF) from diesel fuel containing 1550 ppmw total S was removed using two 1.5-mm-diameter nozzles at 15 min residence time, 277.2 W electrical power, 48.90 mL min−1 total aqueous phase volumetric flow rate (flow rate of each nozzle 24.45 mL min−1) and fuel phase volumetric flow rate of 244.44 mL min−1. According to the CFD simulation results, it was explained that this high desulfurization is due to the higher hydrodynamic momentum ratio (momentum of ultrasonic jet-like streaming/momentum of the aqueous phase injected by the nozzle) as well as secondarily, further oxidation reactions of DBT derivatives with oxidizing radicals (HO2·, O· and HO·) in the active zone just below the probe tips not only when larger-diameter (1.5 mm) nozzles are used instead of 0.4- and 0.9-mm-diameter nozzles but also when each of the aqueous phase flow rates is lower (using two nozzles with an aqueous phase flow rate of 24.45 mL min−1 each instead of using a single nozzle with the aqueous phase flow rate of 48.89 mL min−1). In this case, it was suggested that the aqueous phase is dispersed more homogeneously into fuel when compared to smaller diameter nozzles at higher flow rates.
The operating cost of the UAOD system was investigated [183] in a continuous flow jacketed glass reactor where the glass nozzle through which the aqueous phase (85 wt% FA + 35 wt% HP) flows is placed 3 cm below the US probe tip. Residence time (min), FA/S molar ratio and oxidant/S molar ratio were selected as independent variables at a reaction temperature of 50 °C as constant value and RSM based on BBD was applied. A sulfur removal of 86.90% (followed by one-time extraction at DMF:oil 1:1 vol ratio at room temperature and 875 rpm stirring speed for 30 min) was obtained from the partially hydrotreated diesel fuel containing 2760 ppmw total S at optimum conditions (retention time of 16 min, molar ratio of na/nS 54.47 and molar ratio of nO/nS 8.24) under 360 W and 20 kHz direct US. Under these optimum conditions, it was reported that the organic phase/aqueous phase volume ratio is 4.34 and the operating cost (chemical consumption + electricity due to ultrasound irradiation) is 7.73 cents per liter of oxidized diesel fuel. As the largest part of the operating cost was HP consumption, the organic phase/aqueous phase volume ratio was increased to 10 in order to significantly reduce the aqueous phase consumption at residence time 16 min and FA/HP volume ratio 3.16. Eventually, a sulfur removal of 84.38% was achieved with an operating cost of 4.66 cents per liter of oxidized diesel fuel at na/nS 23.64 molar ratio, nO/nS 3.58 molar ratio, 7.07 mL min−1 diesel flow rate and 0.71 mL min−1 aqueous phase flow rate (0.54 mL min−1 85 wt% FA + 0.17 mL min−1 35 wt% HP). According to ANOVA results, it was determined that the most important terms affecting desulfurization in this process are in the order: residence time ≈ na/nS > (residence time)2 > (na/nS)2 > (na/nS × nO/nS) > (nO/nS)2.
Sono-desulfurization of gasoline and crude oil was performed at optimum conditions found by applying RSM-BBD in which ultrasonic power, irradiation time and oxidant amount are selected as independent variables [198]. A desulfurization of 80.87% (followed by extraction three times at DMSO/gasoline 1:1 vol ratio and water washing four times) was obtained for gasoline containing 1207 ppmw S at optimum conditions (464.7 W direct ultrasonic power (pulsed ultrasound 2 s on, 2 s off), 5.5 min irradiation time and 8.1 mL HP (HP: FA volume ratio 1:1)), whereas a sulfur removal of 73.37% (followed by first magnetic stirring of oil sample for one h and then extraction with 60 mL of a mixture at acetonitrile:methanol:water 1:1:1 vol ratio) was achieved from the crude oil containing 28,620 ppmw S at optimum conditions (785.1 W direct ultrasonic power, 6.2 min irradiation time, 11.4 mL HP (HP: FA, the same volume ratio) with the same pulsed ultrasound. It was stated that after the oxidation of the gasoline sample, adding distilled water up to 1% of the DMSO volume to DMSO for the extraction of oxidized sulfur compounds decreases desulfurization by 20% compared to extraction alone with DMSO. It was explained that this low desulfurization is due to the fact that water reduces the extraction ability of DMSO as the DMSO and water dipole moments [199] are 3.96 and 1.85 D, respectively, (hence DMSO has greater polarity). The differences between mechanical stirring-heating and desulfurization under US were compared and these differences were reported to be approximately 10 and 30% for gasoline and heavy crude oil, respectively, which demonstrates that UAODS is more effective for high-sulfur fuels. This threefold higher difference can be attributed to the emergence of the higher cavitation intensity [200] as heavy crude oil has higher density, higher viscosity and higher surface tension than gasoline. In addition, the high vapor pressure of extremely volatile gasoline compared to heavy crude oil can limit violent implosion of cavitation bubbles in the liquid mixture [125].
RSM-Box-Behnken Design (BBD) was used to evaluate the effects of nformic acid/nS, nO/nS, ultrasound power (UP)/simulated oil volume and temperature on UAODS and to optimize these reaction parameters on the purpose of max attainable desulfurization efficiency [187]. A sulfur removal of approximately 97% from DBT containing 500 ppmw S in toluene is reported at nO/nS = 26.7, nformic acid/nS = 74.6, UP/model oil volume = 7 W cm−3 and at 50 °C under 20 kHz and 400 W direct US in 630 s. Besides, it was stated that the FA (formic acid)/HP molar ratio should be at a certain value (1.4–2.8) in order to maximize the concentration of peroxyformic acid (HCOOOH), which is formed in the equilibrium reaction between HP and HCOOH in the aqueous phase in desulfurization reactions and oxidizes the sulfur compounds.
In a study [161] where a computational fluid dynamic (CFD) model was used to examine the hydrodynamic and mass transfer characteristics of model fuel in the ultrasonic horn reactor, it was explained that high desulfurization is caused by physical effects such as jet stream, high turbulence intensity rather than the chemical effect of ultrasound, and the reaction is controlled by chemical kinetic due to the very high mass transfer rate. In the mentioned study, a sulfur removal of 96.35% from the model fuel containing 500 ppmw DBT in toluene was achieved at nO/nS = 26.7, nformic acid/nS = 74.6, UP/Model Oil Volume = 26.7 W mL−1 under 20 kHz direct US at 50 °C in 210 s.
It was observed in the studies [178], [181], [182], [183], [184], [185] that the properties of diesel fuels (density at 15 °C, kinematic viscosity at 40 °C, flash point, water content, cetane index) almost did not change after UAODS process followed by extraction.
Three organic acid catalysts (FA, acetic acid and trifluoroacetic acid) were compared and a 76.5% sulfur reduction [201] (followed by extraction at a DMF/oil volume ratio of 1:1) was achieved for the catalytic cracking diesel containing 1452 ppmw total S by using trifluoroacetic acid at oxidant/oil 1:10 vol ratio, 70 °C and 60 min as the optimum operating conditions under indirect 20 kHz US, which is higher than the sulfur removals obtained in the case of using acetic acid and FA catalysts as the acidity [202] of trifluoroacetic acid (pKa = 0.18) is higher than that of formic acid (pKa = 3.75) and acetic acid (pKa = 4.75), thus causing the oxidizing power of the peroxycarboxylic acid formed to increase further.
In a study [189] where 1-butyl-3-methyl imidazolium hydrogen sulfate [Bmim][HSO4] and 1-octyl-3-methyl imidazolium hydrogen sulphate [Omim][HSO4] with two different alkyl lengths were synthesized and used instead of aqueous phase, approximately 100% desulfurization yield of the model fuel containing 500 ppmw DBT in n-decane was obtained using [Omim][HSO4] at O/S = 5 molar ratio and mass ratio IL/model fuel = 2 under 30 W power and 25 kHz direct US at 25 °C in 3 min. In the experiments in the absence of ultrasound, it was explained that the desulfurization with [Omim][HSO4] is higher than the desulfurization with [Bmim][HSO4] by applying the same optimum operating conditions as the case of using ultrasound under stirring at 900 rpm. It was noted this high desulfurization is due to the longer alkyl chain of the cation of [Omim][HSO4]. In addition, the reactivity of different sulfur compounds under the same operating conditions was compared and it was reported that the UAODS was in descending order DBT > BT > T > 4,6-DMDBT. It was stated that the lowest desulfurization for 4,6-DMDBT is due to the steric hindrance of two alkyl groups adjacent to the sulfur atom, hence weakening the π-π interaction between the aromatic sulfur compound and the ionic liquid. Under the same optimum conditions, a UAODS efficiency of 76.3% was obtained for the real diesel fuel containing 746 ppmw total S. Moreover, it was reported that [Omim][HSO4] can be used six times without losing its activity in UAODS reactions of the model fuel by regenerating it after each reaction and the solubility of the model fuel in this ionic liquid is very low (1.45 wt%), thus suggesting that the synthesized ionic liquid has the potential to be used both as an extractant and as a catalyst.
However, the high viscosity of ionic liquids, their costly synthesis [203], and the change in the solubility [204] of the fuel in the ionic liquid according to the anions and cations formed depending on the starting raw materials, and more importantly, the presence of aromatic groups [177], [189] such as imidazolium in IL significantly that reduces the desulfurization reactivity of thiophenes, especially abundant in petroleum products, due to steric hindrance make the UAODS process very difficult to be feasible using ionic liquid.
One of the two identical hydrotreated diesel feeds containing 231 ppmw S and 115.5 ppmw N to use expensive oxidants in lower quantities was subjected to pre-extractive desulfurization and the other to pre-adsorptive desulfurization (diesel/methanol volume ratio 1:1 for EDS/N and diesel/fuller's earth (V/W) = 1:0.2 for ADS/N) and then, the UAODS/UAODN reaction (followed by EDS/N and ADS/N individually at the same ratios as those in the pre-treatments) of the two partially desulfurized and denitrogenized fuel samples (S = 196 ppmw and N = 85 ppmw after pre-EDS/N and S = 184 ppmw and N = 52 ppmw after pre-ADS/N) was performed using oxone or HP in US Cup Horn at 80 °C under 80 W and 19.9 kHz direct US for 90 min [205]. As a result of all these processes, diesel fuel with 11 ppmw S and 6 ppmw N is obtained by the pre- and post-ADS/N process, while diesel fuel with 78 ppmw S and 25 ppmw N is obtained by the pre- and post-EDS/N process, thus suggesting that it would be economically feasible to use cheap and efficient adsorbent fuller’s earth instead of expensive extractant methanol. It was stated that this process can be proposed to be complementary to HDS.
2.2.2. UAODS reactions in the presence of PTC
According to the ODS mechanism [28], [29], [206] (Scheme 3) using phosphotungstic acid in the presence of PTC, the phosphotungstate anion in aqueous phase is oxidized to the peroxophosphotungstate anion (1) by HP, then this active oxidizing complex anion is transferred (3) to organic phase by forming an ion pair (2) with the lipophilic cation of PTC. This complex anion is reduced to phosphotungstate anion by oxidizing the sulfur compounds in organic phase (4). The phosphotungstate anion is transferred to the aqueous phase by the lipophilic cation (5) and the cycle is completed.
Scheme 3.
The ODS mechanism with HP-Phosphotungstic acid oxidant system.
A DBT removal of 100% from model fuel [206] containing 4000 ppmw S DBT in toluene was performed using HP 30 vol% (phosphotungstic acid concentration of 0.6 mM in aqueous phase and tetraoctylammonium bromide (TOAB) concentration of 7.32 mM in organic phase) under 600 W and 20 kHz direct US at 75 °C in 7 min. The same conditions were applied to diesel fuels with different sulfur content at certain times (18 min for diesel A with 7744 ppmw S, 10 min for diesel B with 3011 ppmw S and 10 min for diesel C with 1867 ppmw S) at 75 °C and a desulfurization yield (followed by extraction with acetonitrile three times at solvent/oil mass ratio of 1:2 at room temperature for 2 min each) of 98.2, 98.7 and 99.4%, respectively, was achieved along with a fuel recovery of 82.8, 87.2 and 85.5 wt%. It was reported n-paraffins, n-alkyl cyclohexanes, n-alkyl benzenes and alkyl naphthalenes as component classes in the diesel C sample selected as representative were not adversely affected during oxidation, but alkyl naphthalenes among the four main components have relatively high polarity and thus they were extracted by acetonitrile.
In a similar study [207] (where the temperature, tetraoctyl ammonium fluoride (TOAF) concentration, sonication time, phosphotungstic acid concentration and HP purity were 70 °C, 7.5 mM, 10 min, 0.7 mM and 30 vol%, respectively) with the research [206], under the same direct US power and frequency in a continuous flow sonoreactor, marine fuel with less than 23 ppmw S and jet fuel with 1 ppmw S (each followed by adsorption with activated, acidic Al2O3), respectively, were obtained from marine gas oil containing 1710 ppmw S and Jet Fuel (JP-8) containing 863 ppmw S. 33-fold lower consumption of Al2O3 compared to acetonitrile, loss of alkyl naphthalene less than 1 wt%, regeneration with 94% alumina recovery by washing with DMF solvent and maintaining 99% of its adsorption capacity by calcination at 550 °C have revealed that alumina has the potential of being used in large-scale continuous systems.
In another study [208] where 30 wt% HP and phosphotungstic acid were used, the UAODS performances of DBT in the presence of different phase transfer catalyst types at 70 °C under 20 kHz and 600 W direct US were evaluated. It was stated that desulfurization reactions of DBT took place in the presence of TOAB (49.57% conversion), tetrabutylammonium bromide (TBAB) (38.34% conversion), methyltributylammonium chloride (MBAC) (11.4%), methyltributylammonium hydroxide (MBAH) (11.10%) and tetramethylammonium fluoride (8.20%) as cationic-type PTCs, whereas desulfurization reactions did not occur in the presence of 1-octanesulfonic acid as anionic-type PTC, Tween 80 as non-ionic PTC and in the absence of PTC. In addition, in the presence of TOAF and tetraoctadecylammonium bromide (TODAB), 90.30% (97.53% in 20 min for TOAF) and 56.89% conversions were performed in 10 min, respectively. From these results, it was emphasized that the biggest positive effect on UAODS is the long alkyl chain (hence more lipophilic cation) bound to the quaternary cation, and the less positive effect is the hydrophilic anion of quaternary salt. It was stated that the smaller (i.e., the more hydrophilic) the size of the monoatomic anion of quaternary salt for the same alkyl chain length, the more effective the PTC. It was determined by GC-PFPD analysis that 3-bromobenzothiophene and 2-bromobenzothiophene sulfone were formed as intermediates when TOAB was used in UAODS reactions of BT, while in the case of TOAF, intermediate products were not formed. The formation of the byproducts can be shown representatively in Scheme 4: either by the radical mechanism [209], [210] where aromatic sulfur compounds react with bromine radical which is formed by homolytic cleavage [211] of molecular bromine on sonication or by direct reaction [212] with Br2 formed. Bromine radical can also be formed by the reaction of hydrogen peroxide with bromide anion [213].
Scheme 4.
The formation mechanism of brominated compounds.
The reason for the absence of intermediates can be explained as follows: the standard reduction potential [214] of fluorine and HP is E° (V) = +2.87 and E °(V) = +1.77, respectively. In case of quaternary ammonium salt containing fluoride anion, H2O2 cannot oxidize the fluoride anion to fluorine as the standard reduction potential of F2/F− is +2.87 V. Therefore, fluoride-containing organosulfur compounds are not found in the reaction products. But as a result of the dissociation of the quaternary ammonium salts containing the other halide anions except fluoride in aqueous acidic media, the halide ions reduce hydrogen peroxide to water, causing the decomposition of hydrogen peroxide [215]. For example, when TOAB is used, HP in the aqueous acidic phase is reduced by oxidizing the bromide anion released by dissociation [216] of the quaternary ammonium salt in water according to the following reaction as E° (V) of Br2 is + 1.07 [214].
Catalytic decomposition [217], [218] of HP in acidic medium in the presence of bromide ion is as follows:
| (1) |
| (2) |
Br2 formed in reaction 1 reacts with H2O2 in reaction 2 forming bromide ion again. The sum of reaction 1 and 2 is written as
Br2, which is formed according to reaction (1), participates in bromination reaction with sulfur compounds in organic phase and forms bromo intermediates. Besides, as mentioned before, bromination reaction can be carried out by bromine radical Br· formed by homolytic decomposition of Br2 molecule under US. The reason of the decreased desulfurization in this case can be explained as follows: as HP is decomposed in an acidic environment, the amount of peroxo-phosphotungstate formed in situ may decrease significantly. Additionally, a small amount of peroxo-phosphotungstate, which has a higher ability to oxidize organic compounds than hydrogen peroxide [219], reacts very quickly with Br2 in the medium, causing the amount of peroxo-phosphotungstate to decrease much more. Therefore, the desulfurization under US can be significantly lower. In the case of the quaternary ammonium salt containing fluoride for the same alkyl chain length, HP is not reduced by fluoride, thus high desulfurization efficiencies can be achieved by the high amount of peroxotungstate formed and no intermediates are formed. A similar phenomenon can occur when carboxylic acids such as formic acid are used instead of phosphotungstic acid. The reaction mechanism under ultrasound irradiation in the presence of a quaternary ammonium salt with bromide anion can be explained as follows:
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
Accordingly, Br2 formed through the reaction 1, the PFA formed in-situ and HP in the reaction solution bring about a series of reaction 3–12 generating hydroxyl [158], [161], [220] and bromine radicals [211] by the decomposition of PFA and HP and the homolytic bond cleavage of Br2. Consequently, the hydroxyl and hydroperoxyl radicals play a dominant role in oxidation of the organosulfur compounds. In a study [221] in which HP reacts with FA at 30 °C in the presence of TBAB, it was confirmed by titrimetric analysis that the HP concentration decreased significantly by the decomposition of HP and the peroxyformic acid concentration was too low. The change of the transparent color of the aqueous solution containing HP, FA and PFA in the absence of TBAB to the yellow color of the bromine water formed by the dissolution of Br2 in water in the presence of TBAB is an additional indicative of the decomposition. The resulting performic acid (or peracetic acid formed in the case of using acetic acid) can also react as follows:
| (13) |
where R is H or CH3 and its concentration may decrease depending on the concentration of Br ion in the medium.
Moreover, formic acid can react with the resulting Br2 according to the following reaction [222], [223] (14), thus causing formic acid concentration to decrease.
| (14) |
In the reaction mechanism in the case of using HP, FA and TOAF, peroxyformic acid generates formyloxyl radical and hydroxyl radical by homolytic cleavage under US [220]. As a result of the reaction of peroxyformic acid with hydroxyl radicals, formyl radical and peroxyformyl radical are formed, which is similar to the reactions [224] of peracetic acid with the hydroxyl radicals. Therefore, in addition to the hydroxyl radicals formed and the high concentration of performic acid, highly reactive formyloxyl and peroxyformyl radicals may also be responsible for the high desulfurization.
Diesel fuel [208] containing 0.1 g TOAF was undergone ODS reaction (followed by extraction four times at acetonitrile/oil 1:1 mass ratio at room temperature for 1.5 min each) with an equal volume of 30 vol% HP solution containing 0.2 g of phosphotungstic acid under the same US frequency and power at the same temperature as the previous study [207]. After UAODS reactions of 10 min followed by extraction four times, a sulfur removal of 95, 98.8, 87.5, 99.9 and 96.1% was achieved from F-76 containing 4222 ppmw S, MGO containing 1710 ppmw S, JP-5 containing 113.7 ppmw S, JP-8 containing 863 ppmw S and transportation fuel containing 259 ppmw S, respectively. In addition, after a 98.8% UAODS yield from MGO containing 1710 ppm S in the presence of TOAF, the aqueous phase was reused for two fresh MGO samples with 1710 ppmw S each in the presence and absence of TOAF and a UAODS of 98.15 and 96.01%, respectively, was obtained. Again, under the same conditions, this time using dilute HP (3 vol%), a UAODS of 97.90 and 94.8% was obtained for MGO and F-76, respectively. It was stated that after the UAODS reaction of organic sulfur compounds, 99.49% of the tungsten remained in the aqueous phase according to ICP analysis, hence it could be completely recovered.
In a study [225] investigating the effect of quaternary ammonium salts with four different alkyl lengths as PTC on UAODS, using PTC (optimum concentration 0.0116 mol L–1) in the range of 0.03–0.25 g, 12 mL 30% HP and 12 mL formic acid, 28.37, 42.37, 70.02, 86.57 and 94.67% sulfur removal, respectively, were obtained without PTC and in the presence of TMAB, TEAB, TPAB and TBAB at 50 °C in 1.5 h under direct US for 0.028 mL of thiophene dissolved in 24 mL of n-heptane. The highest desulfurization with TBAB was attributed to the bigger radius (thus more stable complex formation [HCOOO–--Q---Br] by higher electron delocalization) of the phase transfer cation TBA+ compared to the radii of the other phase transfer cations for the transfer of [HCOOO–] to the organic phase in the presence of the same anion (Br–) and the higher extraction constant of TBAB. It was revealed that the reaction follows pseudo first order kinetics.
In a study [226] in which the effect of two different types of continuous flow reactors on UAODS (followed by extraction at acetonitrile/oil 1: 1 mass ratio at room temperature for 1.5 min with vigorous shaking) of MGO was investigated, a 92.74% sulfur removal was performed using 25 g 30 vol% HP, 0.1 g TOAF, 0.2 g phosphotungstic acid under 600 W US in power at 70 °C in 20 min for treating 20 g MGO containing 1710 ppmw total S in a probe-type reactor operating at 20 kHz, while using 625 g 30 vol% HP, 2.5 g TOAF, 5 g of phosphotungstic acid to treat 500 g of MGO per h in a portable tubular sonoreactor operating at 40 kHz, a desulfurization degree of 92.36 and 89.78% was achieved at 25 °C for 100 W US power-60 min and 200 W US power-30 min, respectively. Then, this tubular sonoreactor was scaled up to a treatment rate of 12.5 lb MGO h−1 and a 92.42% desulfurization performance was accomplished using 7.09 kg 30 vol% HP, 56.75 g TOAF and 28.13 g phosphotungstic acid under 100 W US power at 25 °C in 60 min. It has also been stated that sonoreactors can be connected in parallel to treat more fuel (25 lb h−1) with the same removal percentage. In addition, by using diluted HP (3 vol%), a sulfur removal of 91% was reached in this sonoreactor in 120 min. Moreover, it is predicted that chemical costs can be reduced by recycling the processed phosphotungstic acid, TOAF and HP by connecting sonoreactors in parallel to treat larger quantities of fuel (four times the recycle rate) and electricity consumption can be reduced by using low power US. Thus, in terms of total cost, it was reported that this parallel sonoreactor type has the potential to be applied in large-scale processes and has a greater advantage over batch-operated probe-type reactors for industrial and commercial applications.
In a study [227] where ionic liquid was used instead of the aqueous phase, 97.6, 99.4 and 98.9% sulfur removal (followed by stirring for 170 min), respectively, was obtained from 511 ppmw thiophene, 524 ppmw benzothiophene and 530 ppmw dibenzothiophene using 5 g 30 vol% HP, 1.5 g 20% trifluoroacetic acid and 0.3 g TOAF at 50 °C in 10 min in the presence of 5 g of 1-n-butyl-3-methyl imidazolium methylsulfate ionic liquid under 600 W and 20 kHz direct US. A 100% desulfurization was achieved by applying the same conditions for Navy diesel (F-76) containing 4220 ppmw total S instead of model compounds. It is reported that the limitation of this method is that the ionic liquid used can extract sulfur-free aromatic compounds present in the fuel.
Nowadays, due to the increase in oil consumption, urban and industrial wastes have been used as an energy source. In the presence of 0.1 g TOAB, 30 vol% HP and 0.2 g phosphotungstic acid, a sulfur removal [228] of 27.5 and 61.8% (followed by extraction three times at acetonitrile/oil 1: 1 mass ratio with vigorous agitation at room temperature for two min each) under 20 kHz direct US at 88 °C in 20 min, respectively, was achieved from pyrolysis oil containing 8800 ppmw total S obtained by pyrolysis of the waste tire at 650 °C for use as clean fuel and also diesel fuel containing 960 ppmw total S. As high carbon black and different hydrocarbon compounds in pyrolysis oil led to low desulfurization efficiency, after UAODS reaction, oxidized compounds were adsorbed in a 6-cm-length column filled with 30 g Al2O3 and a sulfur removal of 68.2 and 99.7% was performed for pyrolysis oil and diesel, respectively. Nevertheless, this sulfur removal value was not considered sufficient as pyrolysis oil contains more benzothiophene and thiophene groups with the lowest ODS reactivity compared to diesel fuel according to GC-SCD analysis. Therefore, two continuous UAODS reactors were connected in series and a desulfurization efficiency of 89% was obtained for the pyrolysis oil containing 0.88 wt% total S under the same UAODS reaction conditions, followed by adsorption in a 6-cm-length column filled with 30 g Al2O3.
A series of UAODS experiments [229] were conducted by selecting sonication time, thiophene solution/phosphotungstic acid mass ratio, thiophene solution/HP mass ratio and thiophene solution/TOAB mass ratio as independent variables. At the optimum conditions found by principal component analysis (T:HP:TOAB:Phosphotungstic acid = 1:1.5:0.005:0.01 mass ratio), an approximately 73.5% conversion of thiophene at 500 ppmw concentration to its sulfones has been carried out in the range from 75 to 85 °C in 20 min under 20 kHz direct US. The same conditions were applied to solutions of other model sulfur compounds and the ODS reactivity following the pseudo first-order reaction kinetics was in the order: 4,6-DMDBT > 4-MDBT > DBT > 2-MBT > BT > T. It was explained that the low reactivity of thiophene is due to the low electron density on S atom and the relatively high reaction temperature near the boiling point (84 °C) of thiophene.
The conversion of 99% (55.5% at 0.02 M HP) and 99.9% (99.1% at 0.02 M HP), respectively, was achieved from model fuel 1 (500 µg BT mL−1) and model fuel 2 (500 µg DBT mL−1) using 0.2 g phosphotungstic acid, 0.1 g TOAB, 0.65 M HP at 80 °C in 15 min under 20 kHz direct US [230]. The activation energies for the oxidation reactions of DBT and BT following the pseudo first-order reaction kinetics were found to be 45.01 and 60.52 kJ mol−1, respectively.
An economic analysis of the study [228] was also evaluated. A sulfur removal [231] (each followed by adsorption in a 6-cm-length column filled with Al2O3) of 68 and 90.91%, respectively, was obtained from pyrolysis oil with high-sulfur content (8800 ppmw total S) obtained by pyrolysis of waste tires in one continuous sonoreactor and two continuous sonoreactors connected in series at pyrolysis fuel/phosphotungstic acid 100:1 mass ratio, 30 vol% HP sol./TOAB 250:1 mass ratio and the convenient feed rates of aqueous and organic phase in such a way that fuel/water volume ratio is 1:1 in the reactors at room temperature and atmospheric pressure in 20 min under 20 kHz direct US. As a result of the benefit-cost analysis, it is explained that a single UAODS unit can be feasible at industrial scales as the benefit/cost ratio is 1.16 and 0.86 for a single reactor and reactors in series, respectively. A recycle rate of 95, 92, 99 (which is obtained by regeneration at 500–600 °C) and 95% was reached for phosphotungstic acid, HP, Al2O3 and PTC, respectively, in a single sonoreactor.
A 47% yield (which is higher than the desulfurization efficiency at atmospheric pressure under the same conditions) of UAODS was obtained for the model fuel [232] with 100 ppmw DBT concentration prepared by dissolving DBT in toluene using 0.05 g of TOAB, 2 mL of 30 vol% HP and 4 mL of formic acid at 25 °C in 90 min under high pressure of 1.8 bar and 35 kHz and 70 W indirect US. It was explained that this relatively high desulfurization is caused by the stable complex formation of TOAB with HP and the elimination of transient cavitation by high pressure, thus preventing the production of reducing species such as H2 and CO, which consume oxidizing species formed by the collapse of transient cavitation bubbles in the organic phase. It was stated that as US emulsifies the aqueous and organic phase highly (hence creating a higher interface area) and the mass transfer resistance is relatively large in the absence of PTC under mechanical mixing, the effect of PTC under US on sulfur removal is lower than that under stirring.
In a similar study [233] where the effects of PTC on UAODS were elucidated by cavitation bubble dynamics and thermodynamic analysis, at HP/HCOOH 0.6 molar ratio, HP/TBAB 16.11 molar ratio (0.5 g of TBAB) and solvent/oxidant 3.33 vol ratio, a sulfur reduction of approximately 96.65 and 77.63% was achieved from 20 mL of model fuel containing 100 ppmw DBT in toluene with 35 kHz and 70 W indirect US at 40 °C in 90 min under atmospheric pressure and nitrogen atmosphere of 1.8 bar, respectively. On the contrary to the study [232], it was reported that this low desulfurization at high pressure occurs due to lower emulsification and lower interfacial area compared to the situation at atmospheric pressure although transient cavitation is eliminated. It was declared that DBT undergoes almost complete oxidation due to the intensive microconvection with the help of US and the enhanced UAODS by transferring fast the oxidant anion of PTC to the organic phase by a large amount of PTC and oxidant in the medium compared to DBT although UAODS in the presence of PTC is based on an ionic mechanism (with higher activation energy than the activation energy of the UAODS reaction in the absence of PTC) rather than radical mechanism. In addition, it was reported that the effect of PTC under mechanical mixing is less pronounced than the effect under US due to the higher activation energy, the higher ΔG and the lower ΔS value of the stirring system compared to the ultrasonic system.
UAODS reactions [234] of two model fuels containing 500 ppmw model sulfur compound each prepared by dissolving BT and DBT in toluene were carried out using 50 wt% HP and TOAB with different polyoxomethalate catalysts at 30, 50 and 70 °C in the range of 2 to 30 min and it was found that the highest reactivity was obtained with a DBT conversion of 94.8% after 30 min of reaction by using NaPW under 500 W power 20 kHz and 40% amplitude (200 W power output) direct US at 70 °C. According to the BT and DBT conversion results, it was found that the UAODS catalytic activity was in the order Na3PW12O40 > H3PW12O40 > H3PMo12O40 > H4SiW12O40 as well as an increase in sulfur removal with increasing temperature for each catalyst. It was stated that the reason for the activity order H3PW12O40 > H3PMo12O40 is that the peroxotungsten complex formed is more catalytically active than the peroxomolybdenum complex even though the standard reduction potential of Mo(VI) is higher than W(VI). However, it was noted that the acidity of the aqueous phase in the case of phosphotungstic acid does not affect UAODS yield much when compared to the desulfurization results obtained in the case of the most active catalyst, sodium phosphotungstate.
At optimum conditions (21.96 mL oxidant volume, 1 g catalyst, 0.1 g PTC and 100% amplitude) found by RSM, in which volume of oxidant (40 vol% HP), catalyst (phosphotungstic acid) mass, TOAB mass and ultrasonic wave amplitude are selected as independent variables, using Minitab 15 software, a desulfurization (followed by extraction at acetonitrile/oil 1:1 mass ratio) of 94.5% for gas oil [235] containing 250 ppmw total S was performed at 65 °C in 20 min under 20 kHz and 750 W direct US. In this study, it was reported that the importance of process independent variables and their interactions according to UAODS results was in the order oxidant volume > ultrasonic wave amplitude > oxidant volume × ultrasonic wave amplitude > catalyst mass > PTC mass > oxidant volume × PTC mass > catalyst mass × PTC mass > PTC mass × ultrasonic wave amplitude and after a certain HP volume, excess HP causes a reduction in sulfur removal by creating a radical scavenging effect.
In a study [236] aimed at reducing the kinematic viscosity and sulfur of diesel oil, using the Box-Behnken design as RSM by Design Expert v.7.0.0 software, HP volume (X1), acetic acid volume (X2), PTC (TOAB) mass (X3), the amount of transition metal catalyst (phosphotungstic acid) (X4) and time (X5) were chosen as independent variables. As a result of the screening of the variables, time was found to be insignificant with respect to the desulfurization performances. After applying RSM by screening out the time variable, the importance of the relevant four variables and their interactions with each other for UAODS according to the results of ANOVA was in the order X12 > X42 > X32 > X22 > X1X2 > X2 > X1. Under the optimum conditions found (13.17 mL HP, 17.26 mL acetic acid, 0.15 g TOAB and 1.5 g phosphotungstic acid), an S removal (followed by extraction one time at 166.7 g L–1 NaOH (caustic soda solution)/oil 1:1 vol ratio for 2 min) of 68.85% was achieved from diesel oil containing 5044 ppmw total S at 50 °C in 5 min under 20 kHz frequency, 700 W power and 40% amplitude direct US. After a certain amount of PTC, the mass transfer was slowed down due to the formation of a thick turbid layer in the mixture, thus leading to a reduction in UAODS. A similar trend of sulfur removal to the trend with PTC has been also observed for the transition metal catalyst, but due to the large volume of phosphotungstic acid and the small surface area of the particles. As a result of the screening analysis, it was stated that as the viscosity of diesel fuel, which has a kinematic viscosity of 3.96 cSt at 40 °C, decreases by max 20% after UAODS process, the relevant independent variables have no effect on the viscosity, and therefore the kinematic viscosity as a dependent variable was not taken into account.
In a study [237] investigating the mechanism of the UAODS system in the presence of different catalysts (phosphotungstic acid, acetic acid and formic acid), it has been underlined that the desulfurization reaction is based on the ionic mechanism (caused by the transport of the peroxo-metallate anion and the anion of peracids from the aqueous phase into the interface by the lipophilic cation of PTC) in the presence of phase transfer catalyst, whereas in the absence of PTC, the desulfurization reaction is based on the radical-based mechanism (caused by the formation of active oxygen radicals such as acetyl radical CH3CO· and hydroperoxy radical HO2· by resulting in decomposition of peracids and HP by the collapse of cavitation bubbles formed). It was found that the sulfur removal efficiencies achieved at 1.8 bar for all three catalysts were lower than the desulfurization performances at atmospheric pressure, mainly due to the reduction in microconvection intensity within the mixture under high pressure, resulting in lower mass transport. In this study, in contrast to the other two studies [164], [233] in which n-hexane and toluene were used as solvents, it was reported that as n-decane has a high boiling point and therefore has a very low vapor pressure, no reducing species such as H2 and CO, which reduces oxidizing species, were formed as a result of ultrasonic cavitation at atmospheric pressure. At n-decane (organic phase)/HP (aqueous phase) volume ratio of 10, a maximum desulfurization of about 74% with a rate constant of 0.0155 min−1 was performed using 60 mg L–1 TBAB, 4 mL FA and 2 mL HP at 50 °C in 90 min under 35 kHz and 70 W indirect US for the model fuel containing 100 ppmw DBT in n-decane. Excessive use of PTC prevented mass transfer, decreasing UAODS relatively. The excess of the transition metal catalyst acts as an emulsion in the mixture by covering the emulsion droplets with a thin film and creating a barrier in the mass transport of the oxidant into the interface, thus causing the UAODS yield to be levelled off.
The optimum conditions, which led to a sulfur removal of 60.75% without extraction, found for the batch reactor in the study [236], were applied to the continuous tube-type flow-through sonoreactor [238] by scaling up 2.5 times and under direct US with two transducers operating at a frequency of 20 kHz and a sonication power of 48 W each, a sulfur removal efficiency of 80.79% was achieved from final gas oil containing 5044 ppmw total S using 30 mL HP, 45 mL acetic acid, 0.375 g TOAB and 3.75 g phosphotungstic acid at equal feed and outlet flow rates in 5 min. It was explained that this higher conversion compared to that in the batch reactor is due to the lack of temperature control (hence leading to an increase in the temperature of the mixture as a result of cavitation under US) in the continuously operating sonoreactor and the fact that every fluid element does not reside for exactly 5 min as in the batch reactor (i.e., resided for 5 min on average). The kinematic viscosity of the relevant gas oil decreased by 9.40% within 5 min under the UAODS conditions, while a 13.5% reduction in kinematic viscosity was achieved by using US alone in the same minute. It has been noted that US gives off some of its energy to split HP and peracetic acid into their radicals under oxidation conditions, while under US alone, it converts the gas oil into lighter fractions by giving off its energy to cleave the C - C and C - S bonds. However, for the cases of US alone and UAODS, no significant change was observed in kinematic viscosity at treatment times of 15 min, compared to the kinematic viscosity before the treating of gas oil. In the absence of acetic acid, besides final gas oil containing 5044 ppmw total S, other feedstocks (atmospheric gas oil with 10,700 ppmw total S, atmospheric kerosene with 4980 ppmw S, Isomax gas oil with 181 ppmw total S) were subjected to oxidation reaction under direct US with 48 W max power and it was stated that the UAODS efficiency is in the order atmospheric kerosene > atmospheric gas oil > final gas oil > Isomax gas oil and the sulfur removal from high-sulfur gas oils is higher. As for kerosene, since lighter fractions as well as the small number of condensed aromatic sulfur compounds (thus lower specific gravity, lower kinematic viscosity, and lower boiling range of kerosene, compared to gas oils) were present, the best desulfurization improvement has been achieved.
In a study [239] where crude oil containing 2133 ppmw total S was desulfurized and upgraded (simultaneous extraction and oxidation process) under 40 kHz indirect US, 65.28% S removal was achieved with 200 ppm oxidant, 60 ppm demulsifier dosage and distilled water at 65 °C in 10 min and it was determined that the physical properties of the treated crude oil have improved (ie, decrease in density, decrease in kinematic viscosity at 20 °C, increase in cetane number, decrease in 10% carbon residue on residuum/%).
At optimum conditions (17 min, 180.3 mmol HP and 25 ppm FeSO4) found by applying RSM based on central composite design (CCD) in which HP amount, catalyst (FeSO4) amount and time are selected as independent variables, a 90% desulfurization of gas oil [240] containing 9500 ppmw total S was performed by three-stage UAODS process (followed by extraction three times at a volume ratio of methanol/oil 4:5 at room temperature for 2 min each after every UAODS reaction) using isobutanol as PTC in the presence of acetic acid (ie, in acidic medium where the catalyst is active at pH less than 3) at 62 °C under 24 kHz and 400 W direct US. In the presence of TOAB as PTC instead of isobutanol, 21.99% sulfur removal from gas oil was performed by a one-step UAODS process under the same conditions, while in the presence of isobutanol, a 67.70% reduction in total sulfur was achieved by one-step UAODS (followed by extraction). Moreover, it was stated that isobutanol is very cheap, can be mixed into the fuel and burned, and it has economic viability as it does not require separation after UAODS reactions. After the oxidation reactions, the extractions with methanol were carried out under US and the sulfur removal was the same as that obtained by the extraction under stirring, thus demonstrating that ultrasound has no effect on extraction in this study. According to the F-test of the regression model, it was revealed that the effect of time variable and time × HP interaction on UAODS is not of importance.
At the optimum conditions (16.4 min sonication time, 122.1 mg TOAB, organic phase/aqueous phase 29.7 mL/10.3 mL volume ratio and 204.8 ppm Fe(VI) for BT, 29.5 min sonication time , 111.6 mg TOAB, organic phase/aqueous phase 16.2 mL/23.8 mL volume ratio and 245.3 ppm Fe(VI) for DBT) found by applying RSM based on BBD for which the ultrasonication time, TOAB amount, organic phase/aqueous phase volume ratio and Ferrate concentration in ppm unit are selected as independent variables, a sulfur removal of 88.3 and 91.8%, respectively, was obtained using 0.1 N acetic acid (pH = 4) from two model fuels (500 ppmw BT in toluene and 500 ppmw DBT in toluene) at 70 °C [241]. The optimum conditions found for BT and DBT were individually applied to diesel fuel containing 1428.6 ppmw total S, resulting in 85.7% BT and 91% DBT reduction in diesel oil. It was explained that these lower desulfurization yields compared to model fuels is due to the presence of many different sulfur compounds in diesel fuel that make oxidation difficult. The effect of different amounts of Ferrate and TOAB on UAODS was also investigated under 20 kHz frequency, 500 W and 40% amplitude direct US. When the ferrate concentration increased to a certain value, sulfur removal gave a maximum and after a certain value, sulfur removal decreased. This was attributed to the fact that as the ferrate concentration increased, the pH of the aqueous phase slightly increased (i.e., more basic medium), thus leading to a decrease in the oxidation capacity of the ferrate in basic medium (lower reduction potential (+0.72 V) in basic medium [242]). However, the standard reduction potential [243] of ferrate in acidic medium is + 2.20 V. With the excessive use of TOAB, the sulfur removal decreased, which has been attributed to the slowing of mass transfer due to turbidity of the mixture and to sterically prevention of electrophilic oxidation of sulfur compounds by the high concentration of alkyl groups. According to ANOVA results, it was reported that OP/AP volume ratio, PTC × (OP:AP) volume ratio interaction and PTC2 have the greatest effect on UAODS for BT, whereas OP/AP, US time × PTC interaction, US time × Ferrate concentration interaction, PTC × ferrate concentration, PTC2, (OP:AP)2 and (Ferrate conc.)2 have the greatest effect on the sulfur removal for DBT. It was determined that the amount of PTC for both model sulfur compounds is not important to UAODS. It has been pronounced that potassium ferrate has higher oxidation capacity and higher stability than HP and HP decomposes thermally at high temperature despite its lower cost, which is another important advantage of this process. Moreover, thermal decomposition [244] of potassium ferrate occurs above 198 °C. The oxidation mechanism is based on the formation of protonated Fe(VI) as a reactive complex [245], [246], [247] (which is much stronger oxidant than FeO42 −) by reaction of ferrate with acetic acid and, subsequently the transfer of the complex into the organic phase (where organic sulfur compounds are oxidized) by binding to the lipophilic cation of the phase transfer agent.
By applying the Pareto-optimal analysis-based fuzzy logic model [248] in which US time, TOAB amount, organic phase/aqueous phase volume ratio and ferrate concentration are selected as four independent variables to maximize the sulfur reduction and, also US energy consumption, TOAB amount and the Ferrate amount are selected as three independent variables to minimize the operating cost, in the presence of acetic acid (pH = 4) at 70 °C under 20 kHz direct US with 200 W power output (500 W, 40% amplitude), it was reported that a conversion of 93.79% was achieved per operating cost of $ 0.830 at the optimum conditions (15.86 min US time, 107.7 mg TOAB, 30 mL:10 mL organic phase/aqueous phase volume ratio and 100 ppm ferrate concentration) for 500 ppmw BT, while a conversion of 88.36% was achieved per $ 0.769 operating cost at the optimum operating conditions (10 min US time, 100.1 mg TOAB, organic phase/aqueous phase volume ratio 16.96 mL/23.04 mL and 300 ppm ferrate concentration) for 500 ppmw DBT. It was shown that the desulfurization efficiencies obtained in this study are comparable with two sonoreactors in series in the previous studies [228], [231], whereas the operating cost in this study is lower than that in the continuous sonoreactors connected in series, hence having the potential to be applicable for scaling up purposes.
3. Outlooks
UAODS is performed at relatively much lower temperatures (i.e., in the range of room temperature to 90 °C), atmospheric or near atmospheric pressures, and generally shorter times than HDS. Process efficiency in UAODS is very important in terms of commercial applicability. In addition, US power intensity [125], defined as the power transferred to the liquid per surface area of the ultrasonic probe, and amplitude are important. It is beneficial to use low-amplitude ultrasound from the point of lower power and lower electricity consumption.
As mentioned before, reaction and ultrasonic parameters have a very important effect on desulfurization. Increasing the amount of PTC up to a certain value improves UAODS by allowing more PTC-oxidant complexes to transfer into the organic phase and then ODS decreases slightly as a result of the slowing down of mass transfer between the aqueous-organic phase in the liquid mixture due to the formation of a thick turbid layer above an optimum amount of PTC [236], [237], [241]. As known, the reaction rate constant increases exponentially with increasing temperature according to Arrhenius equation, consequently increasing the reaction rate as well [249]. Nevertheless, above an optimum temperature, the collapse intensity of cavitation decreases as more solvent vapors will accumulate in cavitation bubble [120], [250], [251] in addition to decomposition of HP into water and oxygen, thus decreasing UAODS yield. Temperature can be increased unless the collapse intensity of the cavitation bubble reduces the total reaction rate [200]. Above an optimum reaction volume, sulfur removal decreases due to the lower ultrasonic power density [78], [196]. With increasing HP concentration (i.e., a more concentrated HP solution) up to a certain value in aqueous phase, UAODS usually increases due the formation of more HO· radicals than HP [65], [126]. Above an optimum concentration, HP can have a scavenging effect on hydroxyl radicals [157]. The sulfur removal increases up to a certain ultrasonic intensity, whereas dense bubble clouds, which show the cavitation shielding effect, will accumulate near the probe above a certain intensity [184]. Therefore, UAODS yield can decrease at high intensities and consequently, an optimum US intensity is required. Although generally, dissolved gases such as helium and oxygen in liquid mixture act as nucleation sites, facilitating the formation of the cavitation bubble, reaction rates change depending on the solubility, the thermal conductivity and the specific heat of the gases used [91], [200]. However, dissolved gas above a certain concentration in cavitation bubble can cushion the collapse of the cavitation bubble, consequently causing a lower collapse intensity [252], [253]. Therefore, it is necessary to find the optimum dissolved gas concentration in liquid mixture to increase the UAODS reaction rates unless the dissolved gas quantity decreases the cavitation effect. Pressure can have two opposite effects. As pressure increases, the intensity of the cavitation bubble implosion increases [254]. However, above an optimum pressure, much less bubbles, which can have almost no impact on overall reaction rate, can be produced due to increasing cavitation threshold of the liquid mixture [200]. The effect of pressure on sulfur removal varies as shown in Table S1 in the Supplementary Information and the boiling point of the solvent in the organic phase or the boiling range of fuel becomes crucial. For low boiling point solvents such as hexane, toluene, it is observed that sulfur removal increases with increasing pressure at relatively low operating temperature [164], [232], while sulfur removal decreases with increasing pressure at high operating temperature [233]. For high boiling point solvents, it was reported that sulfur removal decreases with increasing pressure at relatively high temperature [197], [237]. These differences observed in sulfur removal at high pressures can be attributed to a decrease or increase in the collapse intensity of cavitation bubbles. Nonetheless, much more effort is needed to establish a clear relationship between pressure and temperature in terms of cavitation intensity. In summary, in order to maximize total UAODS reaction rate, it is necessary to consider in combination the effects of reaction and ultrasonic parameters on UAODS yield.
Desulfurization process efficiency (DPE = UAODS yield/MR(H2O2/S)) can be defined as the UAODS yield per molar ratio of reactants used (i.e., the molar ratio of hydrogen peroxide to sulfur). The less the amount of HP, the larger the quantity of fuel used to remove sulfur and the higher the UAODS yield, the higher the process efficiency. Fig. 4, Fig. 5, Fig. 6 show DPEs calculated using heterogeneous catalysts, homogeneous catalysts in the absence of PTC and homogeneous catalysts in the presence of PTC, respectively. The operating conditions of UAODS reactions with heterogeneous catalyst, homogeneous catalyst in the absence of PTC and homogeneous catalyst in the presence of PTC are given in Tables S2, S3 and S4, respectively. From the three figures, it can be seen that the DPEs under indirect US (ultrasonic bath) are mostly lower than the DPEs under direct US. This low process efficiency can be attributed to the fact that the intensity of the indirect US (in this case, the ultrasonic wave generated by the transducer passes first through the walls of the sample container and then through the liquid) is much lower compared to the intensity of ultrasound in direct contact with liquid using the ultrasonic probe [255]. Also, in an ultrasonic bath, ultrasonic wave cannot propagate equally in all directions into each fluid element in a liquid, thus resulting in heterogeneous dissipation [256], [257], [258].
Fig. 4.
DPEs in the presence of heterogeneous catalysts (where 1–3, 4, 5–7, 8, 9, [10,14], 11, 12, 13, [15,19], 16, 17, 18, 20, 21 and 22 represent the references [98], [133], [134], [84], [151], [145], [97], [119], [118], [126], [124], [123], [150], [65], [78], [121], respectively).*49, 43.50 and 35.50 are DPE values calculated for DBT, 4,6-DMDBT and BT, respectively, pertaining to reference [98]. **DPE values calculated for 4,6-DMDBT, DBT and BT pertaining to reference [134] are 26.31, 26.31 and 25.79, respectively. ***10.07 and 1.90 are DPE values calculated for Alberta Bitumen and Oil Sand, respectively, pertaining to reference [145]. ****0.92 and 0.42 are DPE values calculated for simulated fuel (2800 ppm S) and Kerosene (1370 ppm S), respectively, pertaining to reference [126].
Fig. 5.
DPEs with homogeneous catalysts in the absence of PTC (where 1, [2,5], 3, 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and 28 represent the references [183], [189], [184], [185], [180], [195], [197], [181], [179], [182], [178], [187], [188], [161], [186], [172], [174], [196], [173], [175], [171], [169], [162], [166], [165], [170], [163], respectively).*20 and 15.26 are DPE values calculated for DBT and real diesel oil, respectively, pertaining to reference [189].
Fig. 6.
DPEs with homogeneous catalysts in the presence of PTC (where 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and 19 represent the references [241], [248], [230], [238], [236], [240], [231], [228], [206], [208], [226], [225], [235], [227], [233], [229], [237], [232], [234], respectively).
It can be seen that in the case of using heterogeneous catalysts, the DPEs are generally higher than DPEs with and without PTC using homogeneous catalysts. These high DPEs can be due to both the adsorption of sulfur compounds on the catalyst surface and the oxidation of sulfur compounds by forming an active oxidizing complex caused by HP on the surface, as well as the adsorption of oxidized sulfur compounds. There are many advantages of using solid catalyst in liquid under US irradiation: solid particles function as nucleation sites to form cavitation bubbles, thus causing free radicals to increase further. Sonication results in an increase in surface area by reducing the particle size of solid catalysts and inactive catalyst becomes reactive as a result of desorption of adsorbed sulfones (passivating surface coating) due to the surface cleaning caused by liquid jet streams which are formed by implosion of cavitation bubbles [259]. In addition, more collision occurs between reactants and catalysts due to microstreaming [250] and agglomeration of catalysts is prevented [260]. Moreover, the high heat generated by the collapse of cavitation bubbles near solid catalysts can propagate inside catalyst, consequently leading the reaction rate to be higher and it is emphasized that the largest sonochemical effect occurs in macropores >50 nm in diameter [261]. On the other hand, too many catalyst particles can attenuate US waves propagating through liquid [125]. Therefore, an optimum catalyst loading is necessary in UAODS reactions.
There is an exception in the case of using potassium ferrate in Fig. 6. As potassium ferrate is a stronger oxidant in acidic environment than HP and the active complex consisting of ferrate and acetic acid has higher oxidation power than ferrate alone, DPEs are very high.
DPEs for acetic acid-HP and formic acid-HP in Fig. 5 are generally higher than those for the phosphotungstic acid-HP system in Fig. 6, which is due to the small molecular size of acetic acid [262] (ca. 0.4 nm) and formic acid [263] (ca. 0.3 nm), thus alkyl substituted aromatic sulfur compounds do not cause steric hindrance. The reason that DPEs are lower in the case of using phosphotungstic acid-HP system in the presence of PTC in Fig. 6 compared to DPEs in the case of using homogeneous catalysts without PTC is that the alkyl groups adjacent to the sulfur atom of compounds such as 2,5-DMT, 4-MDBT and 4,6-DMDBT in fuel lead to the steric hindrance due to bulky size of the oxidizing polyoxoperoxo complex composed of phosphotungstic acid and HP. However, when organic acids such as formic acid and acetic acid are used in combination with phosphotungstic acid, DPE increases considerably by creating a synergistic effect due to the polyoxoperoxo complexes and peracids formed [236], [238]. The reason for using PTCs in the case of phosphotungstic acid is the transfer of the formed polyoxoperoxo complex anion to the organic phase, otherwise DPE without PTC may be low. Also, phosphotungstic acid decomposes as pH increases from 1 to 8.3 [264] and thus an acidic medium is favorable to the UAODS reactions. Since phosphotungstic acid is thermally stable [265] up to 400 °C, it can form stable polyoxoperoxo complexes with HP and hence ODS can be performed at relatively higher temperatures, which are below 100 °C, compared to the temperatures in the case of acetic acid and formic acid. Performic acid [266] and peracetic acid [267] undergo dramatically thermal decomposition, especially at temperatures of 45 °C and above.
Formic acid and acetic acid have the capacity to extract sulfur compounds and peracids formed as a result of emulsification by US effect can easily be transferred into the organic phase or the organic-aqueous phase interface. Therefore, it can be deduced that PTC has no significant effect on DPE. In the studies in Fig. 6, it is seen that PTC is used in addition to phosphotungstic acid. The reason for using PTC may be due to the low desulfurization obtained by using phosphotungstic acid in the absence of PTC.
In Fig. 4, modified Metal-organic Framework (MOF) was used in the study where DPE of 49, 43.5 and 35.5% was obtained. The reason for the high DPE can be both the entrapment of phosphotungstic acid into amino-functionalized MOF with large surface area and pore volume (hence aromatic sulfur compounds are effectively adsorbed and oxidized on phosphotungstic acid@TMU-17-NH2), and the simultaneous extraction of oxidized sulfur compounds using acetonitrile. In addition, ultrasonic synthesis, which is more environmentally friendly and performed at lower reaction time at room temperature than solvothermal process carried out at high temperature, may have contributed to high desulfurization as MOFs synthesized under ultrasound have generally higher surface area, lower particle size, higher crystallinity, more uniform morphology and size distribution compared to those obtained by conventional preparation methods.
Reactor configurations also affect DPE. In Fig. 5, the high DPE of 23.57 is due to the nozzle, through which the aqueous phase consisting of FA and HP flows in a very low amount (0.71 mL min−1), placed just below the tip of ultrasonic probe, thus causing an increase in sulfur removal by generating active radicals in this efficient region and dispersing the aqueous phase more homogeneously into the organic phase.
In ODS, ionic liquids have also been tried instead of the aqueous phase. However, their synthesis is generally high cost and it is difficult to transport them due to their high viscosity. In addition, as more US power is needed to fully emulsify the high viscosity ionic liquid phase and organic phase, the operating cost will increase due to electrical energy consumption. Moreover, since the ionic liquid loses its activity after a certain recycle, its regeneration will also lead to an additional cost. Therefore, the use of ionic liquids in continuous processes is not practical.
In the studies, one of the biggest problems of UAODS is fuel loss during extraction and/or adsorption process to remove oxidized sulfur compounds after oxidative treatment. During the separation processes, other polar hydrocarbons in fuel pass into the extractant phase or are adsorbed on the adsorbent. Although it has been shown in laboratory and pilot studies that the physicochemical properties of the fuel after the UAODS process change in acceptable ranges according to the fuel specifications for petroleum fractions, how these properties will change in large-scale industrial production is a separate research topic. In addition, the ultrasonic probe must be replaced with the new one as the tip surface erodes by pitting in long service life [125], [268], otherwise it becomes inoperable.
One of the biggest reasons for the widespread use of HDS is that it has a high fuel recovery as well as a very little negative effect on fuel properties. In addition, hydrotreatment of diesel consisting of paraffinic, aromatic and naphthenic components saturates the aromatic compounds in the diesel, resulting in an increase in the cetane number [269].
After UAODS, how to eliminate the waste sulfones generated and accumulated is an environmental issue. Elemental sulfur, which is mainly used for sulfuric acid production [270], can be produced by the reaction of SO2 with H2S generated in HDS units after the waste sulfones are converted to SO2 as a result of thermal decomposition [271] by burning them in high temperature furnace operating at 1093–1427 °C in the Claus process [272], [273] or by pyrolysis [274].
In a study [275] evaluating the desulfurization process economics by using Aspen Plus simulation, it has been shown that the UAODS process is not cost-effective for fuels containing high sulfur (i.e., in the range of several thousand ppmw) due to high chemical consumption to drastically reduce the sulfur content of fuel and very high amounts of extraction solvent required to separate the huge amounts of sulfones formed, therefore it is not competitive with HDS. Therefore, detailed research taking fuel loss into account is still needed to achieve cost savings and high sulfur removal in the UAODS process by using low amounts of reagents, performing reactions at the lowest possible temperature in the shortest possible time and using the most efficient extraction solvent in the lowest possible amounts.
Concluding remarks and future directions can be presented as follows:
-
-
In order to increase the sulfur removal per power density consumed as well as to reduce the process cost, one continuous-flow sonoreactor or two continuous-flow sonoreactors in series can be used at low flow rate of the aqueous phase feed and, short retention times. At high conversions, continuous sonoreactors can be connected in parallel to treat more fuel.
-
-
Desulfurization can be increased by the addition of heterogeneous catalysts to continuous sonoreactors connected in series.
-
-
Potassium ferrate with a much higher reduction potential than HP under acidic conditions can be activated by HCl, HNO3, H2SO4, HClO4 and HCOOH instead of CH3COOH. To reduce the process cost, UAODS reactions can be carried out using potassium ferrate in acidic medium in the absence of relatively expensive PTCs.
-
-
Low temperatures in the range of 20–40 °C favor UAODS reactions since the decomposition of performic and peracetic acid increases drastically above 40 °C in the case of homogeneous catalysts. To observe the change of concentration of peroxycarboxylic acid over time, the reactions of HP and carboxylic acids (i.e., HCOOH or CH3COOH) can be carried out at different temperatures, different times and various molar ratios in the absence of both PTC and organic phase under US irradiation and consequently, peroxyformic acid or peroxyacetic acid (HCOOOH or CH3COOOH) concentration at any time t during the reaction can be readily determined by titrimetric analysis. Eventually, the time, at which peroxyformic acid or peroxyacetic acid concentration is maximum, is found for each temperature. Therefore, UAODS reactions can be performed at those times, thus reducing the process cost due to short reaction times and increasing the sulfur removal efficiency. Alternatively, UAODS reactions can be performed at different temperatures and by taking an aliquot of the aqueous phase at certain times during the UAODS reaction for each temperature, the change of the concentration of the peroxycarboxylic formed can be followed by titrimetric analysis. Consequently, a relationship between the sulfur removal and peroxycarboxylic acid concentration can be established and sono-oxidative desulfurization reaction conditions can be optimized.
-
-
Indirect ultrasonic application in UAODS reactions is not as effective as direct US application from the point of view of DPE.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105845.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Houda S., Lancelot C., Blanchard P., Poinel L., Lamonier C. Oxidative desulfurization of heavy oils with high sulfur content: a review. Catalysts. 2018;8:344. doi: 10.3390/catal8090344. [DOI] [Google Scholar]
- 2.Jain R.K., Cui Z., Domen J.K. Environmental impact of mining and mineral processing. Elsevier. 2016 doi: 10.1016/C2014-0-05174-X. [DOI] [Google Scholar]
- 3.J.R. Anderson, M. Boudart, Catalysis, 1., Springer Berlin Heidelberg, Berlin, Heidelberg, Heidelberg, 1996. https://doi.org/10.1007/978-3-642-61040-0.
- 4.Campos-Martin J.M., Capel-Sanchez M.C., Perez-Presas P., Fierro J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010;85(7):879–890. doi: 10.1002/jctb.2371. [DOI] [Google Scholar]
- 5.Y.T. Shah, Chemical Energy from Natural and Synthetic Gas, 1., CRC Press, 2017. https://doi.org/10.1201/9781315302355.
- 6.N.S. El-Gendy, H.N. Nassar, Biodesulfurization in Petroleum Refining, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2018. https://doi.org/10.1002/9781119224075.
- 7.H. Rase, Petroleum Refining, in: Handb. Commer. Catal. Heterog. Catal., CRC Press, 2000: p. 520. https://doi.org/10.1201/b21367-2.
- 8.Yaseen M., Ullah S., Ahmad W., Subhan S., Subhan F. Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel. 2021;284:119102. doi: 10.1016/j.fuel.2020.119102. [DOI] [Google Scholar]
- 9.Wang Q., Zhang T., Zhang S., Fan Y., Chen B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep. Purif. Technol. 2020;231:115923. doi: 10.1016/j.seppur.2019.115923. [DOI] [Google Scholar]
- 10.Al-Shahrani F., Xiao T., Llewellyn S.A., Barri S., Jiang Z., Shi H., Martinie G., Green M.L.H. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl. Catal. B Environ. 2007;73(3-4):311–316. doi: 10.1016/j.apcatb.2006.12.016. [DOI] [Google Scholar]
- 11.Nassar H.N., Abu Amr S.S., El-Gendy N.S. Biodesulfurization of refractory sulfur compounds in petro-diesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN4. Environ. Sci. Pollut. Res. 2021;28(7):8102–8116. doi: 10.1007/s11356-020-11090-7. [DOI] [PubMed] [Google Scholar]
- 12.Ismagilov Z., Yashnik S., Kerzhentsev M., Parmon V., Bourane A., Al-Shahrani F.M., Hajji A.A., Koseoglu O.R. Oxidative desulfurization of hydrocarbon fuels. Catal. Rev. 2011;53(3):199–255. doi: 10.1080/01614940.2011.596426. [DOI] [Google Scholar]
- 13.Gomez Bernal H., Cedeño Caero L. Solvent effects during oxidation-extraction desulfurization process of aromatic sulfur compounds from fuels. Int. J. Chem. React. Eng. 2005;3 doi: 10.2202/1542-6580.1246. [DOI] [Google Scholar]
- 14.Sikarwar P., Gosu V., Subbaramaiah V. An overview of conventional and alternative technologies for the production of ultra-low-sulfur fuels. Rev. Chem. Eng. 2019;35:669–705. doi: 10.1515/revce-2017-0082. [DOI] [Google Scholar]
- 15.Hossain M., Park H., Choi H. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts. 2019;9:229. doi: 10.3390/catal9030229. [DOI] [Google Scholar]
- 16.Ma X., Sakanishi K., Mochida I. KINETICS, CATALYSIS, AND REACTION ENGINEERING hydrodesulfurization reactivities of various sulfur compounds in vacuum gas oil. Ind. Eng. Chem. Res. 1996;35(8):2487–2494. [Google Scholar]
- 17.Otsuki S., Nonaka T., Takashima N., Qian W., Ishihara A., Imai T., Kabe T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels. 2000;14(6):1232–1239. doi: 10.1021/ef000096i. [DOI] [Google Scholar]
- 18.De Filippis P., Scarsella M. Oxidative desulfurization: oxidation reactivity of sulfur compounds in different organic matrixes. Energy Fuels. 2003;17:1452–1455. doi: 10.1021/ef0202539. [DOI] [Google Scholar]
- 19.K. Yazu, A. Matsumura, S. Sato, Oxidative desulfurization of naphtha with hydrogen peroxide in presence of acid catalyst in naphtha/acetic acid biphasic system, J. Japan Pet. Inst. 53 (2010) 251–255. https://doi.org/10.1627/jpi.53.251.
- 20.Shiraishi Y., Hirai T. Desulfurization of vacuum gas oil based on chemical oxidation followed by liquid-liquid extraction. Energy Fuels. 2004;18(1):37–40. doi: 10.1021/ef0301396. [DOI] [Google Scholar]
- 21.Estephane G., Lancelot C., Blanchard P., Toufaily J., Hamiye T., Lamonier C. Sulfur compounds reactivity in the ODS of model and real feeds on W-SBA based catalysts. RSC Adv. 2018;8(25):13714–13721. doi: 10.1039/C8RA01542B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., He L., Lu J., Zhu W., Jiang X., Wang Y., Yan Y. Deep oxidative desulfurization of fuels catalyzed by phosphotungstic acid in ionic liquids at room temperature. Energy Fuels. 2009;23(3):1354–1357. doi: 10.1021/ef800797n. [DOI] [Google Scholar]
- 23.Te M., Fairbridge C., Ring Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2 and formic acid/H2O2 systems. Appl. Catal. A Gen. 2001;219(1-2):267–280. doi: 10.1016/S0926-860X(01)00699-8. [DOI] [Google Scholar]
- 24.Crucianelli M., Bizzarri B.M., Saladino R. SBA-15 anchored metal containing catalysts in the oxidative desulfurization process. Catalysts. 2019;9:984. doi: 10.3390/catal9120984. [DOI] [Google Scholar]
- 25.Wang D., Qian E.W., Amano H., Okata K., Ishihara A., Kabe T. Oxidative desulfurization of fuel oil: part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide. Appl. Catal. A Gen. 2003;253(1):91–99. doi: 10.1016/S0926-860X(03)00528-3. [DOI] [Google Scholar]
- 26.Caero L.C., Hernández E., Pedraza F., Murrieta F. Oxidative desulfurization of synthetic diesel using supported catalysts. Catal. Today. 2005;107–108:564–569. doi: 10.1016/j.cattod.2005.07.017. [DOI] [Google Scholar]
- 27.Yue D.u., Jiaheng L., Lina Z., ran G.Z., Xiaodi D.u., Junsheng L.i. Highly efficient deep desulfurization of fuels by meso/macroporous H3PW12O40/TiO2 at room temperature. Mater. Res. Bull. 2018;105:210–219. doi: 10.1016/j.materresbull.2018.04.048. [DOI] [Google Scholar]
- 28.C.M. Starks, C.L. Liotta, M.E. Halpern, Phase-Transfer Catalysis, Springer Netherlands, Dordrecht, 1994. https://doi.org/10.1007/978-94-011-0687-0.
- 29.Sachdeva T.O., Pant K.K. Deep desulfurization of diesel via peroxide oxidation using phosphotungstic acid as phase transfer catalyst. Fuel Process. Technol. 2010;91(9):1133–1138. doi: 10.1016/j.fuproc.2010.03.027. [DOI] [Google Scholar]
- 30.Mohumed H., Rahman S., Imtiaz S.A., Zhang Y. Oxidative-extractive desulfurization of model fuels using a pyridinium ionic liquid. ACS Omega. 2020;5(14):8023–8031. doi: 10.1021/acsomega.0c00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Z. Fang, R.L. Smith, Jr., X. Qi, Production of Biofuels and Chemicals with Ultrasound, Springer Netherlands, Dordrecht, 2015. https://doi.org/10.1007/978-94-017-9624-8.
- 32.Xu H., Zhang D., Wu F., Cao R. Deep oxidative desulfurization of fuels based on[C4mimCl]CoCl2 ionic liquid oxone solutions at room temperature. Fuel. 2017;208:508–513. doi: 10.1016/j.fuel.2017.07.060. [DOI] [Google Scholar]
- 33.Hao R., Yang S., Yuan B., Zhao Y. Simultaneous desulfurization and denitrification through an integrative process utilizing NaClO2/Na2S2O8. Fuel Process. Technol. 2017;159:145–152. doi: 10.1016/j.fuproc.2017.01.018. [DOI] [Google Scholar]
- 34.Hayyan M., Alakrach A.M., Hayyan A., Hashim M.A., Hizaddin H.F. Superoxide ion as oxidative desulfurizing agent for aromatic sulfur compounds in ionic liquid media. ACS Sustain. Chem. Eng. 2017;5(2):1854–1863. doi: 10.1021/acssuschemeng.6b02573. [DOI] [Google Scholar]
- 35.Pandey S., Srivastava V.C. Oxidative-extractive desulfurization of liquid fuel using stannous chloride-acetic acid mixture as catalyst. Pet. Sci. Technol. 2018;36(1):40–47. doi: 10.1080/10916466.2017.1403451. [DOI] [Google Scholar]
- 36.Gómez M.V., Caballero R., Vázquez E., Moreno A., de la Hoz A., Díaz-Ortiz Á. Green and chemoselective oxidation of sulfides with sodium perborate and sodium percarbonate: nucleophilic and electrophilic character of the oxidation system. Green Chem. 2007;9(4):331–336. doi: 10.1039/B614847F. [DOI] [Google Scholar]
- 37.Shakirullah M., Ahmad W., Ahmad I., Ishaq M. Oxidative desulphurization study of gasoline and kerosene role of some organic and inorganic oxidants. Fuel Process. Technol. 2010;91(11):1736–1741. doi: 10.1016/j.fuproc.2010.07.014. [DOI] [Google Scholar]
- 38.Yu G., Lu S., Chen H., Zhu Z. Oxidative desulfurization of diesel fuels with hydrogen peroxide in the presence of activated carbon and formic acid. Energy Fuels. 2005;19(2):447–452. doi: 10.1021/ef049760b. [DOI] [Google Scholar]
- 39.Khurana J.M., Panda A.K., Ray A., Gogia A. RAPID OXIDATION OF SULFIDES AND SULFOXIDES WITH SODIUM HYPOCHLORITE. Org. Prep. Proced. Int. 1996;28(2):234–237. doi: 10.1080/00304949609356529. [DOI] [Google Scholar]
- 40.Wang T., Zhao D.S., Sun Z.M., Li F.T., Song Y.Q., Kou C.G. One-step oxidative desulfurization of dibenzothiophene using cyclohexanone peroxide in N-alkyl-imidazolium–based ionic liquid extraction systems. Pet. Sci. Technol. 2012;30(4):385–392. doi: 10.1080/10916466.2011.601510. [DOI] [Google Scholar]
- 41.Yu G.X., Zhong Q., Jin M., Wang J.H., Lu P. Deep desulfurization of diesel fuel oxidized with TBHP coupled with solvent extraction intensified by ultrasound. Adv. Mater. Res. 2014;910:57–60. doi: 10.4028/www.scientific.net/AMR.910.57. [DOI] [Google Scholar]
- 42.Safa M.A., Al-Shamary T., Al-Majren R., Bouresli R., Ma X. Reactivities of various alkyl dibenzothiophenes in oxidative desulfurization of middle distillate with cumene hydroperoxide. Energy Fuels. 2017;31(7):7464–7470. doi: 10.1021/acs.energyfuels.7b01272. [DOI] [Google Scholar]
- 43.Anisimov A.V., Tarakanova A.V. Oxidative desulfurization of hydrocarbon raw materials. Russ. J. Gen. Chem. 2009;79(6):1264–1273. doi: 10.1134/S1070363209060437. [DOI] [Google Scholar]
- 44.Safa M.A., Bouresli R., Al-Majren R., Al-Shamary T., Ma X. Oxidative desulfurization kinetics of refractory sulfur compounds in hydrotreated middle distillates. Fuel. 2019;239:24–31. doi: 10.1016/j.fuel.2018.10.141. [DOI] [Google Scholar]
- 45.Lü H., Gao J., Jiang Z., Yang Y., Song B.o., Li C. Oxidative desulfurization of dibenzothiophene with molecular oxygen using emulsion catalysis. Chem. Commun. 2007;(2):150–152. doi: 10.1039/B610504A. [DOI] [PubMed] [Google Scholar]
- 46.P.S. Tam, J.R. Kittrell, J.W. Eldridge, Desulfurization of Fuel Oil by Oxidation and Extraction. 1. Enhancement of Extraction Oil Yield, Ind. Eng. Chem. Res. 29 (1990) 321–324. https://doi.org/doi.org/10.1021/ie00099a002.
- 47.Ma C., Dai B., Liu P., Zhou N.a., Shi A., Ban L., Chen H. Deep oxidative desulfurization of model fuel using ozone generated by dielectric barrier discharge plasma combined with ionic liquid extraction. J. Ind. Eng. Chem. 2014;20(5):2769–2774. doi: 10.1016/j.jiec.2013.11.005. [DOI] [Google Scholar]
- 48.Stoker H.S. Cengage Learning; 2013. General, Organic, and Biological Chemistry, 6th Editio. [Google Scholar]
- 49.Squadrito G.L., Postlethwait E.M. On the hydrophobicity of nitrogen dioxide: could there be a “lens” effect for NO2 reaction kinetics? Nitric Oxide. 2009;21(2):104–109. doi: 10.1016/j.niox.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.J. Wei, J.L. Anderson, K.B. Bischoff, M.M. Denn, J.H. Seinfeld, G. Stephanopoulos, eds., Advances in Chemical Engineering, Volume 19, Academic Press, 1994.
- 51.Biń A.K. Ozone solubility in liquids. Ozone Sci. Eng. 2006;28(2):67–75. doi: 10.1080/01919510600558635. [DOI] [Google Scholar]
- 52.Mizuno N. Modern heterogeneous oxidation catalysis. Wiley. 2009 doi: 10.1002/9783527627547. [DOI] [Google Scholar]
- 53.Palomino J.M., Tran D.T., Hauser J.L., Dong H., Oliver S.R.J. Mesoporous silica nanoparticles for high capacity adsorptive desulfurization. J. Mater. Chem. A. 2014;2(36):14890–14895. doi: 10.1039/C4TA02570A. [DOI] [Google Scholar]
- 54.Zhu W., Xu Y., Li H., Dai B., Xu H., Wang C., Chao Y., Liu H. Photocatalytic oxidative desulfurization of dibenzothiophene catalyzed by amorphous TiO2 in ionic liquid. Korean J. Chem. Eng. 2014;31(2):211–217. doi: 10.1007/s11814-013-0224-3. [DOI] [Google Scholar]
- 55.Zarrabi M., Entezari M.H., Goharshadi E.K. Photocatalytic oxidative desulfurization of dibenzothiophene by C/TiO 2 @MCM-41 nanoparticles under visible light and mild conditions. RSC Adv. 2015;5(44):34652–34662. doi: 10.1039/C5RA02513C. [DOI] [Google Scholar]
- 56.Wang J., Wu W., Ye H., Zhao Y., Wang W.-H., Bao M. MoO3 subnanoclusters on ultrasmall mesoporous silica nanoparticles: an efficient catalyst for oxidative desulfurization. RSC Adv. 2017;7(71):44827–44833. doi: 10.1039/C7RA08566D. [DOI] [Google Scholar]
- 57.Abdullah W.N.W., Bakar W.A.W.A., Ali R. Catalytic oxidative desulfurization of diesel fuel utilizing a polymolybdate alumina supported catalyst: characterization, catalytic activity and mechanistic study. React. Kinet. Mech. Catal. 2015;114(2):547–560. doi: 10.1007/s11144-014-0801-4. [DOI] [Google Scholar]
- 58.Bakar W.A.W.A., Ali R., Kadir A.A.A., Mokhtar W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012;101:78–84. doi: 10.1016/j.fuproc.2012.04.004. [DOI] [Google Scholar]
- 59.Timko M.T., Wang J.A., Burgess J., Kracke P., Gonzalez L., Jaye C., Fischer D.A. Roles of surface chemistry and structural defects of activated carbons in the oxidative desulfurization of benzothiophenes. Fuel. 2016;163:223–231. doi: 10.1016/j.fuel.2015.09.075. [DOI] [Google Scholar]
- 60.Liao X., Wang X., Wang F., Yao Y., Lu S. Ligand modified metal organic framework UiO-66: a highly efficient and stable catalyst for oxidative desulfurization. J. Inorg. Organomet. Polym. Mater. 2021;31(2):756–762. doi: 10.1007/s10904-020-01808-y. [DOI] [Google Scholar]
- 61.Kairbekov Z.K., Anisimov A.V., Myltykbaeva Z.K., Kanseitova D.K., Rakhmanov E.V., Seisembekova A.B. Sonocatalytic oxidative desulfurization of oil from the Zhanazhol oilfield. Moscow Univ. Chem. Bull. 2017;72(1):29–33. doi: 10.3103/S0027131417010072. [DOI] [Google Scholar]
- 62.Rezvani M.A., Khandan S. Synthesis and characterization of a new nanocomposite (FeW 11 V@CTAB-MMT) as an efficient heterogeneous catalyst for oxidative desulfurization of gasoline. Appl. Organomet. Chem. 2018;32(11):e4524. doi: 10.1002/aoc.4524. [DOI] [Google Scholar]
- 63.Gu Q., Wen G., Ding Y., Wu K.-H., Chen C., Su D. Reduced graphene oxide: a metal-free catalyst for aerobic oxidative desulfurization. Green Chem. 2017;19(4):1175–1181. doi: 10.1039/C6GC02894B. [DOI] [Google Scholar]
- 64.J. Xiao, L. Wu, Y. Wu, B. Liu, L. Dai, Z. Li, Q. Xia, H. Xi, Effect of gasoline composition on oxidative desulfurization using a phosphotungstic acid/activated carbon catalyst with hydrogen peroxide, Appl. Energy. 113 (2014) 78–85. https://doi.org/https://doi.org/10.1007/s12182-019-0349-z.
- 65.Flores R., Rodas A., Gasperin R. Oxidative desulfurization of diesel fuel oil using supported Fenton catalysts and assisted with ultrasonic energy. Pet. Sci. 2019;16(5):1176–1184. doi: 10.1007/s12182-019-0349-z. [DOI] [Google Scholar]
- 66.H. Nakajima, Mass Transfer - Advanced Aspects, InTech, 2011. https://doi.org/10.5772/1432.
- 67.Chen X., Zhang M., Wei Y., Li H., Liu J., Zhang Q.i., Zhu W., Li H. Ionic liquid-supported 3DOM silica for efficient heterogeneous oxidative desulfurization. Inorg. Chem. Front. 2018;5(10):2478–2485. doi: 10.1039/C8QI00519B. [DOI] [Google Scholar]
- 68.Yu Z., Wang D., Xun S., He M., Ma R., Jiang W., Li H., Zhu W., Li H. Amorphous TiO2-supported Keggin-type ionic liquid catalyst catalytic oxidation of dibenzothiophene in diesel. Pet. Sci. 2018;15(4):870–881. doi: 10.1007/s12182-018-0268-4. [DOI] [Google Scholar]
- 69.Mokhtari B., Akbari A., Omidkhah M. Superior deep desulfurization of real diesel over MoO3/silica gel as an efficient catalyst for oxidation of refractory compounds. Energy Fuels. 2019;33(8):7276–7286. doi: 10.1021/acs.energyfuels.9b01646. [DOI] [Google Scholar]
- 70.Dizaji A.K., Mokhtarani B., Mortaheb H.R. Deep and fast oxidative desulfurization of fuels using graphene oxide-based phosphotungstic acid catalysts. Fuel. 2019;236:717–729. doi: 10.1016/j.fuel.2018.09.076. [DOI] [Google Scholar]
- 71.Matsuzawa S., Tanaka J., Sato S., Ibusuki T. Photocatalytic oxidation of dibenzothiophenes in acetonitrile using TiO2: effect of hydrogen peroxide and ultrasound irradiation. J. Photochem. Photobiol. A Chem. 2002;149(1-3):183–189. doi: 10.1016/S1010-6030(02)00004-7. [DOI] [Google Scholar]
- 72.Ai S., Li J., Yang Y.a., Gao M., Pan Z., Jin L. Study on photocatalytic oxidation for determination of chemical oxygen demand using a nano-TiO2–K2Cr2O7 system. Anal. Chim. Acta. 2004;509(2):237–241. doi: 10.1016/j.aca.2003.09.056. [DOI] [Google Scholar]
- 73.Mousavi-Kamazani M., Ashrafi S. Single-step sonochemical synthesis of Cu2O-CeO2 nanocomposites with enhanced photocatalytic oxidative desulfurization. Ultrason. Sonochem. 2020;63:104948. doi: 10.1016/j.ultsonch.2019.104948. [DOI] [PubMed] [Google Scholar]
- 74.Kormann C., Bahnemann D.W., Hoffmann M.R. Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand. Environ. Sci. Technol. 1988;22(7):798–806. doi: 10.1021/es00172a009. [DOI] [PubMed] [Google Scholar]
- 75.Venkatadri R., Peters R.W. Chemical oxidation technologies: ultraviolet light/hydrogen peroxide, fenton’s reagent, and titanium dioxide-assisted photocatalysis. Hazard. Waste Hazard. Mater. 1993;10(2):107–149. doi: 10.1089/hwm.1993.10.107. [DOI] [Google Scholar]
- 76.Tang W.Z., Zhang Z., An H., Quintana M.O., Torres D.F. TiO2/UV photodegradation of azo dyes in aqueous solutions. Environ. Technol. 1997;18(1):1–12. doi: 10.1080/09593330.1997.9618466. [DOI] [Google Scholar]
- 77.P.S. Cohen, S.L. Geffner, Contemporary Chemistry - The Physical Setting, Amsco School Publications, Inc., 2004.
- 78.Sinhmar P.S., Gogate P.R. Ultrasound assisted oxidative deep-desulfurization of dimethyl disulphide from turpentine. Ultrason. Sonochem. 2020;63:104925. doi: 10.1016/j.ultsonch.2019.104925. [DOI] [PubMed] [Google Scholar]
- 79.Speight J.G. McGRAW-HILL; 2002. Chemical and Process Design Handbook. [Google Scholar]
- 80.Yu X., Zhao M., Han P., Lu X. Ultrasound assisted catalytic oxidative desulfurization of diesel oil using CdO nanoparticles. Pet. Sci. Technol. 2018;36(15):1158–1163. doi: 10.1080/10916466.2018.1465961. [DOI] [Google Scholar]
- 81.Zhao M., Han P., Lu X. Ultrasound assisted photocatalytic oxidative desulfurization of model diesel fuel. Pet. Sci. Technol. 2018;36(1):29–33. doi: 10.1080/10916466.2017.1403447. [DOI] [Google Scholar]
- 82.Jansohn P. Woodhead Publishing; 2013. Modern Gas Turbine Systems. http://www.sciencedirect.com/science/article/pii/B9781845697280500082. [Google Scholar]
- 83.S. Manickam, M. Ashokkumar, Cavitation: A novel energy-efficient technique for the generation of nanomaterials, Taylor & Francis, CRC Press, 2014. https://doi.org/10.4032/9789814411554.
- 84.Behin J., Farhadian N. Multi-objective optimization of oxidative desulfurization in a sono-photochemical airlift reactor. Ultrason. Sonochem. 2017;38:50–61. doi: 10.1016/j.ultsonch.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 85.Sun B., Sato M., Sid Clements J. Optical study of active species produced by a pulsed streamer corona discharge in water. J. Electrostat. 1997;39(3):189–202. doi: 10.1016/S0304-3886(97)00002-8. [DOI] [Google Scholar]
- 86.Al-Zaydi K., Petrier C., Mousally S., Arab S., Refat M. Sonochemical degradation of benzothiophene (BT) in deionized water. Nat. Water Sea Water Mol. 2019;24:257. doi: 10.3390/molecules24020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Z., Ondruschka B. Aquasonolysis of thiophene and its derivatives. Ultrason. Sonochem. 2006;13(1):86–91. doi: 10.1016/j.ultsonch.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Lazar M., Varghese S., Nair S. Photocatalytic water treatment by titanium dioxide: recent updates. Catalysts. 2012;2:572–601. doi: 10.3390/catal2040572. [DOI] [Google Scholar]
- 89.P. Pichat, ed., Photocatalysis and Water Purification, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013. https://doi.org/10.1002/9783527645404.
- 90.Pirsaheb M., Moradi N. Sonochemical degradation of pesticides in aqueous solution: investigation on the influence of operating parameters and degradation pathway – a systematic review. RSC Adv. 2020;10(13):7396–7423. doi: 10.1039/C9RA11025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Siddique M., Farooq R., Price G.J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. Sonochem. 2014;21(3):1206–1212. doi: 10.1016/j.ultsonch.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 92.Maslahati Roudi A., Chelliapan S., Wan Mohtar W., Kamyab H. Prediction and optimization of the fenton process for the treatment of landfill leachate using an artificial neural network. Water. 2018;10:595. doi: 10.3390/w10050595. [DOI] [Google Scholar]
- 93.Song Y.-L., Li J.-T., Chen H. Degradation of C.I. Acid Red 88 aqueous solution by combination of Fenton’s reagent and ultrasound irradiation. J. Chem. Technol. Biotechnol. 2009;84(4):578–583. doi: 10.1002/jctb.2083. [DOI] [Google Scholar]
- 94.N. Kerabchi, S. Merouani, O. Hamdaoui, Numerical insight into the sonolytic ozonation applied for water treatment, in: Water Eng. Model. Math. Tools, Elsevier, 2021: pp. 1–23. https://doi.org/10.1016/B978-0-12-820644-7.00021-9.
- 95.Chakrabarty S., Upadhyay P., Chakma S. Experimental and theoretical study of deep oxidative desulfurization of Dibenzothiophene using Oxalate-Based catalyst. Ultrason. Sonochem. 2021;75:105580. doi: 10.1016/j.ultsonch.2021.105580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gonzalez L.A., Kracke P., Green W.H., Tester J.W., Shafer L.M., Timko M.T. Oxidative desulfurization of middle-distillate fuels using activated carbon and power ultrasound. Energy Fuels. 2012;26(8):5164–5176. doi: 10.1021/ef201289r. [DOI] [Google Scholar]
- 97.Hameed Khlaif T., Salim Bded A. Decreasing the sulfur content of crude oil by ultra-sound and activated carbon assisted oxidative method. IOP Conf. Ser. Mater. Sci. Eng. 2019;579(1):012012. doi: 10.1088/1757-899X/579/1/012012. [DOI] [Google Scholar]
- 98.Afzalinia A., Mirzaie A., Nikseresht A., Musabeygi T. Ultrasound-assisted oxidative desulfurization process of liquid fuel by phosphotungstic acid encapsulated in a interpenetrating amine-functionalized Zn(II)-based MOF as catalyst. Ultrason. Sonochem. 2017;34:713–720. doi: 10.1016/j.ultsonch.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Li L., Sun Z., Li H., Keener T.C. Effects of activated carbon surface properties on the adsorption of volatile organic compounds. J. Air Waste Manage. Assoc. 2012;62(10):1196–1202. doi: 10.1080/10962247.2012.700633. [DOI] [PubMed] [Google Scholar]
- 100.K.B. Schnelle Jr., R.F. Dunn, M.E. Ternes, Air Pollution Control Technology Handbook, CRC Press, 2016. https://doi.org/10.1201/b19286.
- 101.Espeel P.H.J., Vercruysse K.A., Debaerdemaker M., Jacobs P.A. Solvent effects in liquid phase Friedel-crafts alkylation over zeolites: control of reaction rate and selectivity by adsorption. Stud. Surf. Sci. Catal. 1994:1457–1464. doi: 10.1016/S0167-2991(08)63688-5. [DOI] [Google Scholar]
- 102.Krishnakumar S., Somasundaran P. Role of surfactant-adsorbent acidity and solvent polarity in adsorption-desorption of surfactants from nonaqueous media. Langmuir. 1994;10(8):2786–2789. doi: 10.1021/la00020a046. [DOI] [Google Scholar]
- 103.A.T. Hubbard, Encyclopedia of Surface and Colloid Science: Volume 1, 1st ed., Marcel Dekker Inc, 2002.
- 104.C. Cooper, R. Purchase, Organic Chemist’s Desk Reference, Third Edition, CRC Press, 2018. https://doi.org/10.4324/9781315120768.
- 105.M. Loudon, J. Parise, Organic Chemistry, Sixth, W.H. Freeman, 2016. https://www.cambridge.org/core/product/identifier/CBO9781107415324A009/type/book_part.
- 106.Brozek C.K., Michaelis V.K., Ong T.-C., Bellarosa L., López N., Griffin R.G., Dincă M. Dynamic DMF binding in MOF-5 enables the formation of metastable cobalt-substituted MOF-5 analogues. ACS Cent. Sci. 2015;1(5):252–260. doi: 10.1021/acscentsci.5b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dias E.M., Petit C. Towards the use of metal–organic frameworks for water reuse: a review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A. 2015;3(45):22484–22506. doi: 10.1039/C5TA05440K. [DOI] [Google Scholar]
- 108.Hao L., Hurlock M.J., Ding G., Zhang Q. Metal-organic frameworks towards desulfurization of fuels. Top. Curr. Chem. 2020;378:17. doi: 10.1007/s41061-020-0280-1. [DOI] [PubMed] [Google Scholar]
- 109.Roushani M., Saedi Z., Musa beygi T. Anionic dyes removal from aqueous solution using TMU-16 and TMU-16-NH 2 as isoreticular nanoporous metal organic frameworks. J. Taiwan Inst. Chem. Eng. 2016;66:164–171. doi: 10.1016/j.jtice.2016.06.012. [DOI] [Google Scholar]
- 110.Lin Z.-J., Zheng H.-Q., Chen J., Zhuang W.-E., Lin Y.-X., Su J.-W., Huang Y.-B., Cao R. Encapsulation of phosphotungstic acid into metal-organic frameworks with tunable window sizes: screening of PTA@MOF catalysts for efficient oxidative desulfurization. Inorg. Chem. 2018;57(20):13009–13019. doi: 10.1021/acs.inorgchem.8b02272. [DOI] [PubMed] [Google Scholar]
- 111.Kuznetsov M.L., Teixeira F.A., Bokach N.A., Pombeiro A.J.L., G.B. Shul’pin, Radical decomposition of hydrogen peroxide catalyzed by aqua complexes [M(H2O)n]2+ (M=Be, Zn, Cd) J. Catal. 2014;313:135–148. doi: 10.1016/j.jcat.2014.03.010. [DOI] [Google Scholar]
- 112.Kholdeeva O., Maksimchuk N. Metal-organic frameworks in oxidation catalysis with hydrogen peroxide. Catalysts. 2021;11:283. doi: 10.3390/catal11020283. [DOI] [Google Scholar]
- 113.Tripathi D., Yadav I., Negi H., Singh R.K., Srivastava V.C., Sankar M. Highly efficient Co(II) porphyrin catalysts for the extractive oxidative desulfurization of dibenzothiophene in fuel oils under mild conditions. J. Porphyr. Phthalocyanines. 2021;25(01):24–30. doi: 10.1142/S1088424620500443. [DOI] [Google Scholar]
- 114.Wang F., Xiao K., Shi L., Bing L., Han D., Wang G. Catalytic oxidative desulfurization of model fuel utilizing functionalized HMS catalysts: characterization, catalytic activity and mechanistic studies. React. Chem. Eng. 2021;6(2):289–296. doi: 10.1039/D0RE00373E. [DOI] [Google Scholar]
- 115.McCleverty J.A., Meyer T.J. Comprehensive coordination chemistry II: applications of coordination chemistry. Elsevier Science. 2003 doi: 10.1108/S1479-363620170000009011. [DOI] [Google Scholar]
- 116.Zhang J., Li J., Ren T., Hu Y., Ge J., Zhao D. Oxidative desulfurization of dibenzothiophene based on air and cobalt phthalocyanine in an ionic liquid. RSC Adv. 2014;4(7):3206–3210. doi: 10.1039/C3RA43765E. [DOI] [Google Scholar]
- 117.Zhang G., Liu B., Zhou H., Yang Y., Chen W., Zhao J. Graphene wrapped phthalocyanine: enhanced oxidative desulfurization for dibenzothiophene in fuel. Appl. Organomet. Chem. 2018;32(9):e4477. doi: 10.1002/aoc.v32.910.1002/aoc.4477. [DOI] [Google Scholar]
- 118.Wang F., Wang G., Sun W., Wang T., Chen X. Metallophthalocyanine functionalized magnetic mesoporous silica nanoparticles and its application in ultrasound-assisted oxidation of benzothiophene. Microporous Mesoporous Mater. 2015;217:203–209. doi: 10.1016/j.micromeso.2015.06.038. [DOI] [Google Scholar]
- 119.Wang F., Wang G., Cui H., Sun W., Wang T. Preparation of metallophthalocyanine functionalized magnetic silica nanotubes and its application in ultrasound-assisted oxidative desulfurization of benzothiophene. Mater. Res. Bull. 2015;63:181–186. doi: 10.1016/j.materresbull.2014.12.019. [DOI] [Google Scholar]
- 120.J.C. Colmenares, G. Chatel, Sonochemistry: From Basic Principles to Innovative Applications, Springer International Publishing, Cham, 2017. https://doi.org/10.1007/978-3-319-54271-3. [DOI] [PubMed]
- 121.Abdi G., Ashokkumar M., Alizadeh A. Ultrasound-assisted oxidative-adsorptive desulfurization using highly acidic graphene oxide as a catalyst-adsorbent. Fuel. 2017;210:639–645. doi: 10.1016/j.fuel.2017.09.024. [DOI] [Google Scholar]
- 122.Xiong J., Zhu W., Ding W., Yang L., Chao Y., Li H., Zhu F., Li H. Phosphotungstic acid immobilized on ionic liquid-modified SBA-15: efficient hydrophobic heterogeneous catalyst for oxidative desulfurization in fuel. Ind. Eng. Chem. Res. 2014;53(51):19895–19904. doi: 10.1021/ie503322a. [DOI] [Google Scholar]
- 123.Liu L., Zhang Y., Tan W. Synthesis and characterization of phosphotungstic acid/activated carbon as a novel ultrasound oxidative desulfurization catalyst. Front. Chem. Sci. Eng. 2013;7(4):422–427. doi: 10.1007/s11705-013-1353-2. [DOI] [Google Scholar]
- 124.Liu L., Zhang Y., Tan W. Ultrasound-assisted oxidation of dibenzothiophene with phosphotungstic acid supported on activated carbon. Ultrason. Sonochem. 2014;21(3):970–974. doi: 10.1016/j.ultsonch.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 125.H.A. D. Chen, S.K. Sharma, A. Mudhoo (Eds.), Handbook on Applications of Ultrasound: Sonochemistry for Sustainability, CRC Press, 2012. https://doi.org/10.1201/b11012.
- 126.Gildo P.J., Dugos N., Roces S., Wan M.-W., Kumoro A.C., Hadiyanto, Roces S.A., Yung L., Rong X., Lothongkum A.W., Phong M.T., Hussain M.A., Daud W.R.W., Nam P.T.S. Optimized ultrasound-assisted oxidative desulfurization process of simulated fuels over activated carbon-supported phosphotungstic acid. MATEC Web Conf. 2018;156:03045. doi: 10.1051/matecconf/201815603045. [DOI] [Google Scholar]
- 127.Mansourian S.H., Shahhosseini S., Maleki A. Optimization of oxidative polymerization-desulfurization of a model fuel using polyoxometalate: Effect of ultrasound irradiation. J. Ind. Eng. Chem. 2019;80:576–589. doi: 10.1016/j.jiec.2019.08.040. [DOI] [Google Scholar]
- 128.R. Van Eldik, L. Cronin, Advances in Inorganic Chemistry, Volume 69: Polyoxometalate Chemistry, 1st ed., Academic Press, 2017.
- 129.D.W. Ball J.A. Key Introductory Chemistry- 1st Canadian Edition, BCcampus 2014 http://uilis.unsyiah.ac.id/oer/files/original/dcded6f2f4b25f5b280bd6494723b8b3.pdf.
- 130.Ryan L., Norris R. 3rd ed. Cambridge University Press; 2020. Cambridge International AS & A Level Chemistry Coursebook with Digital Access. [Google Scholar]
- 131.Gao X., Xu J. The oxygen activated by the active vanadium species for the selective oxidation of benzene to phenol. Catal. Lett. 2006;111(3-4):203–205. doi: 10.1007/s10562-006-0148-1. [DOI] [Google Scholar]
- 132.Neumann R. Activation of molecular oxygen, polyoxometalates, and liquid-phase catalytic oxidation. Inorg. Chem. 2010;49(8):3594–3601. doi: 10.1021/ic9015383. [DOI] [PubMed] [Google Scholar]
- 133.Akbari A., Omidkhah M., Darian J.T. Investigation of process variables and intensification effects of ultrasound applied in oxidative desulfurization of model diesel over MoO 3/Al2O3 catalyst. Ultrason. Sonochem. 2014;21(2):692–705. doi: 10.1016/j.ultsonch.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 134.Akbari A., Omidkhah M., Towfighi Darian J. Facilitated and selective oxidation of thiophenic sulfur compounds using MoOx/Al2O3-H2O2 system under ultrasonic irradiation. Ultrason. Sonochem. 2015;23:231–237. doi: 10.1016/j.ultsonch.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 135.Wang B., Ding G., Shang Y., Lv J., Wang H., Wang E., Li Z., Ma X., Qin S., Sun Q. Effects of MoO3 loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3. Appl. Catal. A Gen. 2012;431–432:144–150. doi: 10.1016/j.apcata.2012.04.029. [DOI] [Google Scholar]
- 136.Abdullah M.A., Sekar T. Enhanced removal of sulfur from diesel fuel using non-hydrodesulfurization technique coupled with ultrasound technique. J. Sci. Ind. Res. (India) 2016;75:258–261. [Google Scholar]
- 137.Wilson S., Farone W., Leonard G., Birnstingl J., Leombruni A. Catalyzed persulfate: advancing in situ chemical oxidation (ISCO) technology. Pollut. Eng. 2013;45:1–16. [Google Scholar]
- 138.Wang H., Cai W.-W., Liu W.-Z., Li J.-Q., Wang B., Yang S.-C., Wang A.-J. Application of sulfate radicals from ultrasonic activation: disintegration of extracellular polymeric substances for enhanced anaerobic fermentation of sulfate-containing waste-activated sludge. Chem. Eng. J. 2018;352:380–388. doi: 10.1016/j.cej.2018.07.029. [DOI] [Google Scholar]
- 139.Peri J.B., Hannan R.B. SURFACE HYDROXYL GROUPS ON γ-ALUMINA 1. J. Phys. Chem. 1960;64(10):1526–1530. doi: 10.1021/j100839a044. [DOI] [Google Scholar]
- 140.Xavier S., Gandhimathi R., Nidheesh P.V., Ramesh S.T. Comparison of homogeneous and heterogeneous Fenton processes for the removal of reactive dye Magenta MB from aqueous solution. Desalin. Water Treat. 2015;53(1):109–118. doi: 10.1080/19443994.2013.844083. [DOI] [Google Scholar]
- 141.Wang N., Zhao Q., Zhang A. Catalytic oxidation of organic pollutants in wastewater via a Fenton-like process under the catalysis of HNO 3 -modified coal fly ash. RSC Adv. 2017;7(44):27619–27628. doi: 10.1039/C7RA04451H. [DOI] [Google Scholar]
- 142.Ibrahim, Lubna A.A. Chemical characterization and mobility of metal species in fly ash–water system. Water Sci. 2015;29:109–122. doi: 10.1016/j.wsj.2015.10.001. [DOI] [Google Scholar]
- 143.Maier A.C., Iglebaek E.H., Jonsson M. Confirming the formation of hydroxyl radicals in the catalytic decomposition of H 2 O 2 on metal oxides using coumarin as a probe. ChemCatChem. 2019;11(22):5435–5438. doi: 10.1002/cctc.201901316. [DOI] [Google Scholar]
- 144.Green D.W., Southard M.Z. 9th ed., McGraw-Hill Education; 2019. Perry’s Chemical Engineers’ Handbook. [Google Scholar]
- 145.Okawa H., bin Wan Kamal W.M.I., Akazawa N., Kato T., Sugawara K. Simultaneous recovery and desulfurization of bitumen from oil sand using ultrasound irradiation. Jpn. J. Appl. Phys. 2018;57(7S1):07LE09. doi: 10.7567/JJAP.57.07LE09. [DOI] [Google Scholar]
- 146.Hunter R.N., Self A., Read J. 6th ed., ICE Publishing; 2015. The Shell bitumen handbook. [Google Scholar]
- 147.Zhou X., Lv S., Wang H., Wang X., Liu J. Catalytic oxygenation of dibenzothiophenes to sulfones based on FeIII porphyrin complex. Appl. Catal. A Gen. 2011;396(1-2):101–106. doi: 10.1016/j.apcata.2011.01.041. [DOI] [Google Scholar]
- 148.Pires S.M.G., Simões M.M.Q., Santos I.C.M.S., Rebelo S.L.H., Pereira M.M., Neves M.G.P.M.S., Cavaleiro J.A.S. Biomimetic oxidation of organosulfur compounds with hydrogen peroxide catalyzed by manganese porphyrins. Appl. Catal. A Gen. 2012;439–440:51–56. doi: 10.1016/j.apcata.2012.06.044. [DOI] [Google Scholar]
- 149.J.Y. Chen, Activated Carbon Fiber and Textiles, Woodhead Publishing, 2017. https://doi.org/10.1016/c2014-0-03521-6.
- 150.Jin L., Cao Q., Li J., Dong J. Sulfur removal in coal tar pitch by oxidation with hydrogen peroxide catalyzed by trichloroacetic acid and ultrasonic waves. Fuel. 2011;90(11):3456–3460. doi: 10.1016/j.fuel.2011.06.047. [DOI] [Google Scholar]
- 151.Tang Q., Lin S., Cheng Y., Liu S., Xiong J.-R. Ultrasound-assisted oxidative desulfurization of bunker-C oil using tert-butyl hydroperoxide. Ultrason. Sonochem. 2013;20(5):1168–1175. doi: 10.1016/j.ultsonch.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 152.J. Goddard, M. Malacria, C. Ollivier, eds., Activation Methods: Sonochemistry and High Pressure, Volume 2, Wiley, 2019. https://doi.org/10.1002/9781119687443.
- 153.Hiatt R., Clipsham J., Visser T. THE INDUCED DECOMPOSITION OF tert -BUTYL HYDROPEROXIDE. Can. J. Chem. 1964;42(12):2754–2757. doi: 10.1139/v64-408. [DOI] [Google Scholar]
- 154.G. Ertl, H. Knözinger, F. Schüth, J. Weitkamp, eds., Handbook of Heterogeneous Catalysis, 2nd ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2008. https://doi.org/10.1002/9783527610044.
- 155.Wei S., He H., Cheng Y., Yang C., Zeng G., Qiu L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016;6(105):103253–103269. doi: 10.1039/C6RA22358C. [DOI] [Google Scholar]
- 156.de la Fuente N., Chen L., Wang J.A., González J., Navarrete J. Roles of oxygen defects and surface acidity of Keggin-type phosphotungstic acid dispersed on SBA-15 catalysts in the oxidation of 4,6-dimethyldibenzothiophene. React. Kinet. Mech. Catal. 2021;132(2):1119–1135. doi: 10.1007/s11144-021-01966-1. [DOI] [Google Scholar]
- 157.More N.S., Gogate P.R. Intensified approach for desulfurization of simulated fuel containing thiophene based on ultrasonic flow cell and oxidizing agents. Ultrason. Sonochem. 2019;51:58–68. doi: 10.1016/j.ultsonch.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 158.Flores R., Rodas A., Chavarria W. Desulfurization of fuel oils using an advanced oxidation method. ACS Div. Fuel Chem. Prepr. 2004;49:341–342. [Google Scholar]
- 159.Sinhmar P.S., Gogate P.R. Ultrasound assisted oxidative desulfurization of simulated diesel using flow cell and longitudinal bath in combination with different oxidants. Chem. Eng. Process. - Process Intensif. 2020;153:107968. doi: 10.1016/j.cep.2020.107968. [DOI] [Google Scholar]
- 160.Zhang Q., Zhu M., Jones I., Zhang Z., Zhang D. Desulfurization of spent tire pyrolysis oil and its distillate via combined catalytic oxidation using H 2 O 2 with formic acid and selective adsorption over Al 2 O 3. Energy Fuels. 2020;34(5):6209–6219. doi: 10.1021/acs.energyfuels.9b03968. [DOI] [Google Scholar]
- 161.Rahimi M., Shahhosseini S., Movahedirad S. Hydrodynamic and mass transfer investigation of oxidative desulfurization of a model fuel using an ultrasound horn reactor. Ultrason. Sonochem. 2019;52:77–87. doi: 10.1016/j.ultsonch.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 162.Ebrahimi S.L., Khosravi-Nikou M.R., Hashemabadi S.H. Sonoreactor optimization for ultrasound assisted oxidative desulfurization of liquid hydrocarbon. Pet. Sci. Technol. 2018;36(13):959–965. doi: 10.1080/10916466.2018.1458112. [DOI] [Google Scholar]
- 163.Bhasarkar J.B., Chakma S., Moholkar V.S. Mechanistic features of oxidative desulfurization using sono-fenton-peracetic acid (ultrasound/Fe2+-CH3COOH-H 2O2) system. Ind. Eng. Chem. Res. 2013;52(26):9038–9047. doi: 10.1021/ie400879j. [DOI] [Google Scholar]
- 164.Bolla M.K., Choudhury H.A., Moholkar V.S. Mechanistic features of ultrasound-assisted oxidative desulfurization of liquid fuels. Ind. Eng. Chem. Res. 2012;51(29):9705–9712. doi: 10.1021/ie300807a. [DOI] [Google Scholar]
- 165.Tang L., Fan H., Guo J., Zeng W., Tao X. Investigation on the mechanism of coal desulfurization by ultrasonic with peroxyacetic acid. Energy Sources Part A Recover. Util. Environ. Eff. 2018;40(8):999–1009. doi: 10.1080/15567036.2018.1468512. [DOI] [Google Scholar]
- 166.Tang L., Fan H., Chen S., Tao X., He H., Zhu X. Investigation on the synergistic mechanism of coal desulfurization by ultrasonic with microwave. Energy Sources Part A Recover. Util. Environ. Eff. 2020;42(20):2516–2525. doi: 10.1080/15567036.2019.1607950. [DOI] [Google Scholar]
- 167.B. Bimal Krishna, B. Debasish, Advances in Microwave Chemistry, CRC Press, 2019. https://doi.org/10.1201/9781351240499.
- 168.Pu Z.T., Mi J., Kang J. Removal of organic sulfur in two coals in microwave and ultrasonic co-enhanced oxidative process. Adv. Mater. Res. 2013;781–784:923–926. doi: 10.4028/www.scientific.net/AMR.781-784.923. [DOI] [Google Scholar]
- 169.Deshpande A., Bassi A., Prakash A. Ultrasound-assisted, base-catalyzed oxidation of 4,6-dimethyldibenzothiophene in a biphasic diesel-acetonitrile system. Energy Fuels. 2005;19(1):28–34. doi: 10.1021/ef0340965. [DOI] [Google Scholar]
- 170.Mello P.de.A., Duarte F.A., Nunes M.A.G., Alencar M.S., Moreira E.M., Korn M., Dressler V.L., Flores É.M.M. Ultrasound-assisted oxidative process for sulfur removal from petroleum product feedstock. Ultrason. Sonochem. 2009;16(6):732–736. doi: 10.1016/j.ultsonch.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 171.Duarte F.A., Mello P.de.A., Bizzi C.A., Nunes M.A.G., Moreira E.M., Alencar M.S., Motta H.N., Dressler V.L., Flores É.M.M. Sulfur removal from hydrotreated petroleum fractions using ultrasound-assisted oxidative desulfurization process. Fuel. 2011;90(6):2158–2164. doi: 10.1016/j.fuel.2011.01.030. [DOI] [Google Scholar]
- 172.Nunes M.A.G., Mello P.A., Bizzi C.A., Diehl L.O., Moreira E.M., Souza W.F., Gaudino E.C., Cravotto G., Flores E.M.M. Evaluation of nitrogen effect on ultrasound-assisted oxidative desulfurization process. Fuel Process. Technol. 2014;126:521–527. doi: 10.1016/j.fuproc.2014.05.031. [DOI] [Google Scholar]
- 173.Calcio Gaudino E., Carnaroglio D., Boffa L., Cravotto G., Moreira E.M., Nunes M.A.G., Dressler V.L., Flores E.M.M. Efficient H2O2/CH3COOH oxidative desulfurization/denitrification of liquid fuels in sonochemical flow-reactors. Ultrason. Sonochem. 2014;21(1):283–288. doi: 10.1016/j.ultsonch.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 174.N. Palaic K. Sertic-Bionda D. Margeta S. Podolski Oxidative desulphurization of diesel fuels Chem. Biochem. Eng. Q. 29 2015 323 327 https://doi.org/10.15255/CABEQ.2015.2184.
- 175.Keynejad K., Nikazar M., Dabir B. Diesel desulfurization using a ultrasound-assisted oxidative process. Pet. Sci. Technol. 2018;36(11):718–725. doi: 10.1080/10916466.2018.1445099. [DOI] [Google Scholar]
- 176.Heravi M.M., Behbahani F.K., Oskooie H.A., Shoar R.H. Catalytic aromatization of Hantzsch 1,4-dihydropyridines by ferric perchlorate in acetic acid. Tetrahedron Lett. 2005;46(16):2775–2777. doi: 10.1016/j.tetlet.2005.02.147. [DOI] [Google Scholar]
- 177.Desai K., Dharaskar S., Khalid M., Gupta T.C.S.M. Triphenyl methyl phosphonium tosylate as an efficient phase transfer catalyst for ultrasound-assisted oxidative desulfurization of liquid fuel. Environ. Sci. Pollut. Res. 2021;28(21):26747–26761. doi: 10.1007/s11356-021-12391-1. [DOI] [PubMed] [Google Scholar]
- 178.Dai Y., Qi Y., Zhao D. A novel technology for desulfurization of FCC diesel fuels: combination of hydrogenation and oxidation-assisted ultrasound. Pet. Sci. Technol. 2010;28(2):146–154. doi: 10.1080/10916460903058145. [DOI] [Google Scholar]
- 179.Zhao D.-z., Sun M.-z. Oxidative desulfurization of diesel fuel using ultrasound. Pet. Sci. Technol. 2009;27(17):1943–1950. doi: 10.1080/10916460802096402. [DOI] [Google Scholar]
- 180.Sun M.-Z., Zhang B., Wu Y.-H., Zhu J., Zhao D.-Z. Deep oxidative desulfurization of FCC diesel fuel with ultrasound. Pet. Sci. Technol. 2012;30(23):2471–2477. doi: 10.1080/10916466.2010.525978. [DOI] [Google Scholar]
- 181.Khodaei B., Sobati M.A., Shahhosseini S. Optimization of ultrasound-assisted oxidative desulfurization of high sulfur kerosene using response surface methodology (RSM) Clean Technol. Environ. Policy. 2016;18(8):2677–2689. doi: 10.1007/s10098-016-1186-z. [DOI] [Google Scholar]
- 182.Rahimi M., Shahhosseini S., Sobati M.A., Movahedirad S., Khodaei B., Hassanzadeh H. A novel multi-probe continuous flow ultrasound assisted oxidative desulfurization reactor; experimental investigation and simulation. Ultrason. Sonochem. 2019;56:264–273. doi: 10.1016/j.ultsonch.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 183.Dana M., Sobati M.A., Shahhosseini S., Ansari A. Optimization of a continuous ultrasound assisted oxidative desulfurization (UAOD) process of diesel using response surface methodology (RSM) considering operating cost. Chinese J Chem. Eng. 2020;28(5):1384–1396. doi: 10.1016/j.cjche.2019.12.007. [DOI] [Google Scholar]
- 184.Fan Q., Zhao D., Dai Y. The research of ultra-deep desulfurization in diesel via ultrasonic irradiation under the catalytic system of H2O2-CH3COOH-FeSO4. Pet. Sci. Technol. 2009;27(3):302–314. doi: 10.1080/10916460701707679. [DOI] [Google Scholar]
- 185.Dai Y., Zhao D., Qi Y. Sono-desulfurization oxidation reactivities of FCC diesel fuel in metal ion/H2O2 systems. Ultrason. Sonochem. 2011;18(1):264–268. doi: 10.1016/j.ultsonch.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 186.Carnaroglio D., Gaudino E.C., Mantegna S., Moreira E.M., Vicente de Castro A., Flores E.M.M., Cravotto G. Ultrasound-assisted oxidative desulfurization/denitrification of liquid fuels with solid oxidants. Energy Fuels. 2014;28(3):1854–1859. doi: 10.1021/ef402431e. [DOI] [Google Scholar]
- 187.Khodaei B., Sobati M.A., Shahhosseini S. Rapid oxidation of dibenzothiophene in model fuel under ultrasound irradiation. Monatshefte Fur Chemie. 2017;148(2):387–396. doi: 10.1007/s00706-016-1801-z. [DOI] [Google Scholar]
- 188.Rahimi M., Shahhosseini S., Movahedirad S. Continuous-flow ultrasound assisted oxidative desulfurization (UAOD) process: an efficient diesel treatment by injection of the aqueous phase. Ultrason. Sonochem. 2017;39:611–622. doi: 10.1016/j.ultsonch.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 189.Safa M., Mokhtarani B., Mortaheb H.R., Tabar Heidar K. Oxidative desulfurization of model diesel using ionic liquid 1-Octyl-3-methylimidazolium hydrogen sulfate: an investigation of the ultrasonic irradiation effect on performance. Energy Fuels. 2016;30(12):10909–10916. doi: 10.1021/acs.energyfuels.6b01708. [DOI] [Google Scholar]
- 190.Margeta D., Sertić-Bionda K., Foglar L. Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl. Acoust. 2016;103:202–206. doi: 10.1016/j.apacoust.2015.07.004. [DOI] [Google Scholar]
- 191.Qin B., Shen Y., Xu B., Zhu S., Li P., Liu Y. Mesoporous TiO 2 –SiO 2 adsorbent for ultra-deep desulfurization of organic-S at room temperature and atmospheric pressure. RSC Adv. 2018;8(14):7579–7587. doi: 10.1039/C8RA00112J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Margeta D., Grčić I., Papić S., Sertić-Bionda K., Foglar L. Impact of ultrasound application on oxidative desulphurization of diesel fuel and on treatment of resulting wastewater. Environ. Technol. (United Kingdom) 2016;37(3):293–299. doi: 10.1080/09593330.2015.1068870. [DOI] [PubMed] [Google Scholar]
- 193.Dai Y., Qi Y., Zhao D., Zhang H. An oxidative desulfurization method using ultrasound/Fenton’s reagent for obtaining low and/or ultra-low sulfur diesel fuel. Fuel Process. Technol. 2008;89(10):927–932. doi: 10.1016/j.fuproc.2008.03.009. [DOI] [Google Scholar]
- 194.Sun M.Z., Zhang B., Wu Y.H., Zhu J., Zhao D.Z. Effects of extraction on the desulfurization of FCC diesel by ultrasound oxidation technique. Adv. Mater. Res. 2013;634–638:751–754. doi: 10.4028/www.scientific.net/AMR.634-638.751. [DOI] [Google Scholar]
- 195.Karami E., Sobati M.A., Khodaei B., Abdi K. An experimental investigation on the ultrasound-assisted oxidation of benzothiophene in model fuel: application of response surface methodology. Appl. Therm. Eng. 2017;118:691–702. doi: 10.1016/j.applthermaleng.2017.03.028. [DOI] [Google Scholar]
- 196.Jalali M.R., Sobati M.A. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: optimization using Box-Behnken design (BBD) Appl. Therm. Eng. 2017;111:1158–1170. doi: 10.1016/j.applthermaleng.2016.10.015. [DOI] [Google Scholar]
- 197.Khodaei B., Rahimi M., Sobati M.A., Shahhosseini S., Jalali M.R. Effect of operating pressure on the performance of ultrasound-assisted oxidative desulfurization (UAOD) using a horn type sonicator: Experimental investigation and CFD simulation. Chem. Eng. Process. - Process Intensif. 2018;132:75–88. doi: 10.1016/j.cep.2018.08.006. [DOI] [Google Scholar]
- 198.Zhou C., Wang Y., Huang X., Wu Y., Chen J. Optimization of ultrasonic-assisted oxidative desulfurization of gasoline and crude oil. Chem. Eng. Process. - Process Intensif. 2020;147:107789. doi: 10.1016/j.cep.2019.107789. [DOI] [Google Scholar]
- 199.S. Ašperger, Chemical Kinetics and Inorganic Reaction Mechanisms, 2nd ed., Springer US, Boston, MA, MA, 2003. https://doi.org/10.1007/978-1-4419-9276-5.
- 200.Thompson L.H., Doraiswamy L.K. Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 1999;38:1215–1249. doi: 10.1021/ie9804172. [DOI] [Google Scholar]
- 201.Li Y., Zhao D., Lin J., Yuan Q. Preliminary study on oxidative desulfurization of diesel via power ultrasound. Energy Sources Part A Recover. Util. Environ. Eff. 2009;31(3):191–198. doi: 10.1080/009083190957612. [DOI] [Google Scholar]
- 202.Solomons T.W.G., Fryhle C.B., Snyder S.A. 12th ed. John Wiley & Sons Inc; 2016. Organic Chemistry. [Google Scholar]
- 203.C. Hardacre, V. Parvulescu, Catalysis in Ionic Liquids: From Catalyst Synthesis to Application, Royal Society of Chemistry, Cambridge, 2014. https://doi.org/10.1039/9781849737210.
- 204.Song Z., Zeng Q., Zhang J., Cheng H., Chen L., Qi Z. Solubility of imidazolium-based ionic liquids in model fuel hydrocarbons: a COSMO-RS and experimental study. J. Mol. Liq. 2016;224:544–550. doi: 10.1016/j.molliq.2016.10.026. [DOI] [Google Scholar]
- 205.Gaudino E.C., Carnaroglio D., Mantegna S., Tabasso S., Moreira E.M., Cravotto G. Fuel desulfurization/denitrification via adsorption or extraction: a complementary approach to oxidative treatments. Energy Sources Part A Recover. Util. Environ. Eff. 2016;38(15):2290–2298. doi: 10.1080/15567036.2015.1048388. [DOI] [Google Scholar]
- 206.Mei H., Mei B.W., Yen T.F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization☆. Fuel. 2003;82(4):405–414. doi: 10.1016/S0016-2361(02)00318-6. [DOI] [Google Scholar]
- 207.Etemadi O., Yen T.F. Selective adsorption in ultrasound-assisted oxidative desulfurization process for fuel cell reformer applications. Energy Fuels. 2007;21(4):2250–2257. doi: 10.1021/ef0700174. [DOI] [Google Scholar]
- 208.Wan M.W., Yen T.F. Enhance efficiency of tetraoctylammonium fluoride applied to ultrasound-assisted oxidative desulfurization (UAOD) process. Appl. Catal. A Gen. 2007;319:237–245. doi: 10.1016/j.apcata.2006.12.008. [DOI] [Google Scholar]
- 209.F.A. Carey, R.J. Sundberg, Advanced Organic Chemistry: Part A: Structure and Mechanisms, 5th ed., Springer US, Boston, MA, MA, 2007. https://doi.org/10.1007/978-0-387-44899-2.
- 210.R. Bruckner, Organic Mechanisms: Reactions, Stereochemistry and Synthesis, Springer Berlin Heidelberg, Berlin, Heidelberg, Heidelberg, 2010. https://doi.org/10.1007/978-3-642-03651-4.
- 211.G. Chatel, Sonochemistry: New Opportunities for Green Chemistry, WORLD SCIENTIFIC (EUROPE), 2017. https://doi.org/10.1142/q0037.
- 212.F.A. Carey, R.J. Sundberg, Advanced Organic Chemistry Part B: Reaction and Synthesis, 5th ed., Springer US, Boston, MA, MA, 2007. https://doi.org/10.1007/978-0-387-71481-3.
- 213.Solyanikov V.M., Denisov E.T. Formation of radicals in the reaction of hydrogen peroxide with the bromide anion. Bull. Acad. Sci. USSR Div. Chem. Sci. 1968;17(7):1415–1418. doi: 10.1007/BF00907836. [DOI] [Google Scholar]
- 214.Chang R., Overby J. 13th ed., McGraw-Hill Education; 2019. Chemistry. [Google Scholar]
- 215.Mohammad A., Liebhafsky H.A. The kinetics of the reduction of hydrogen peroxide by the halides. J. Am. Chem. Soc. 1934;56(8):1680–1685. doi: 10.1021/ja01323a009. [DOI] [Google Scholar]
- 216.Miranda O.R., Dollahon N.R., Ahmadi T.S. Critical concentrations and role of ascorbic acid (Vitamin C) in the crystallization of gold nanorods within hexadecyltrimethyl ammonium bromide (CTAB)/tetraoctyl ammonium bromide (TOAB) micelles. Cryst. Growth Des. 2006;6(12):2747–2753. doi: 10.1021/cg060455l. [DOI] [Google Scholar]
- 217.Bray W.C., Livingston R.S. THE CATALYTIC DECOMPOSITION OF HYDROGEN PEROXIDE IN A BROMINE-BROMIDE SOLUTION, AND A STUDY OF THE STEADY STATE. J. Am. Chem. Soc. 1923;45(5):1251–1271. doi: 10.1021/ja01658a021. [DOI] [Google Scholar]
- 218.Reger D.L., Goode S.R., Ball D.W. 3rd ed., Brooks/Cole Cengage Learning; 2010. Chemistry: Principles and Practice. [Google Scholar]
- 219.Idrissou Y., Mouanni S., Amitouche D., Mazari T., Marchal-Roch C., Rabia C. Cyclohexanone oxidation over H3PMo12O40 heteropolyacid via two activation modes microwave irradiation and conventional method. Bull. Chem. React. Eng. Catal. 2019;14:427–435. doi: 10.9767/bcrec.14.2.3054.427-435. [DOI] [Google Scholar]
- 220.Jolhe P.D., Bhanvase B.A., Patil V.S., Sonawane S.H., Potoroko I. Ultrasound assisted synthesis of performic acid in a continuous flow microstructured reactor. Ultrason. Sonochem. 2017;39:153–159. doi: 10.1016/j.ultsonch.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 221.S. Toksoy, Investigation of Kinetics of Formic Acid Reaction with Hydrogen Peroxide in the presence of TBAB Phase Transfer Catalyst, MSc Thesis, İstanbul University-Cerrahpaşa, 2013.
- 222.Hammick D.L., Hutchison W.K., Snell F.R. CCCLXXI.—The rate of reaction of bromine with aqueous formic acid. J. Chem. Soc., Trans. 1925;127(0):2715–2720. doi: 10.1039/CT9252702715. [DOI] [Google Scholar]
- 223.Brusa M.A., Colussi A.J. The kinetics and mechanism of the oxidation of formic acid by bromine in acid aqueous media. Int. J. Chem. Kinet. 1980;12(12):1013–1020. doi: 10.1002/kin.550121210. [DOI] [Google Scholar]
- 224.Rokhina E.V., Makarova K., Golovina E.A., Van As H., Virkutyte J. Free radical reaction pathway, thermochemistry of peracetic acid homolysis, and its application for phenol degradation: spectroscopic study and quantum chemistry calculations. Environ. Sci. Technol. 2010;44(17):6815–6821. doi: 10.1021/es1009136. [DOI] [PubMed] [Google Scholar]
- 225.Zhao D., Ren H., Wang J., Yang Y., Zhao Y. Kinetics and mechanism of quaternary ammonium salts as phase-transfer catalysts in the liquid-liquid phase for oxidation of thiopene. Energy Fuels. 2007;21:2543–2547. doi: 10.1021/ef070152g. [DOI] [Google Scholar]
- 226.Wan M.-W., Yen T.-F. Portable continuous ultrasound-assisted oxidative desulfurization unit for marine gas oil. Energy Fuels. 2008;22(2):1130–1135. doi: 10.1021/ef7006358. [DOI] [Google Scholar]
- 227.Cheng S.-S., Yen T.F. Use of ionic liquids as phase-transfer catalysis for deep oxygenative desulfurization. Energy Fuels. 2008;22(2):1400–1401. doi: 10.1021/ef700734x. [DOI] [Google Scholar]
- 228.Chen T.-C., Shen Y.-H., Lee W.-J., Lin C.-C., Wan M.-W. The study of ultrasound-assisted oxidative desulfurization process applied to the utilization of pyrolysis oil from waste tires. J. Clean. Prod. 2010;18(18):1850–1858. doi: 10.1016/j.jclepro.2010.07.019. [DOI] [Google Scholar]
- 229.Chen T.-C., Shen Y.-H., Lee W.-J., Lin C.-C., Wan M.-W. Optimization of thiophene removal by an ultrasound-assisted oxidative desulfurization process. Environ. Eng. Sci. 2012;29(7):623–629. doi: 10.1089/ees.2011.0123. [DOI] [Google Scholar]
- 230.Wan M.-W., Biel L.C.C., Lu M.-C., de Leon R., Arco S. Ultrasound-assisted oxidative desulfurization (UAOD) using phosphotungstic acid: effect of process parameters on sulfur removal. Desalin. Water Treat. 2012;47(1-3):96–104. doi: 10.1080/19443994.2012.696802. [DOI] [Google Scholar]
- 231.Chen T.C., Shen Y.H., Lee W.J., Lin C.C., Wan M.W. An economic analysis of the continuous ultrasound-assisted oxidative desulfurization process applied to oil recovered from waste tires. J. Clean. Prod. 2013;39:129–136. doi: 10.1016/j.jclepro.2012.09.001. [DOI] [Google Scholar]
- 232.Bhasarkar J.B., Chakma S., Moholkar V.S. Investigations in physical mechanism of the oxidative desulfurization process assisted simultaneously by phase transfer agent and ultrasound. Ultrason. Sonochem. 2015;24:98–106. doi: 10.1016/j.ultsonch.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 233.Bhasarkar J.B., Singh M., Moholkar V.S. Mechanistic insight into phase transfer agent assisted ultrasonic desulfurization. RSC Adv. 2015;5(125):102953–102964. doi: 10.1039/C5RA12178G. [DOI] [Google Scholar]
- 234.Choi A.E.S., Roces S., Dugos N., Wan M.-W. Oxidation by H2O2 of benzothiophene and dibenzothiophene over different polyoxometalate catalysts in the frame of ultrasound and mixing assisted oxidative desulfurization. Fuel. 2016;180:127–136. doi: 10.1016/j.fuel.2016.04.014. [DOI] [Google Scholar]
- 235.Kadijani J.A., Narimani E., Kadijani H.A. Using response surface methodology to optimize ultrasound-assisted oxidative desulfurization. Korean J. Chem. Eng. 2016;33(4):1286–1295. doi: 10.1007/s11814-015-0276-7. [DOI] [Google Scholar]
- 236.A. Shamseddini, F. Esmaeilzadeh, D. Mowla, Diesel oil upgradation by ultrasound irradiation: A study on the effects of main operational parameters, Phys. Chem. Res. 5 (2017) 691–707. https://doi.org/10.22036/pcr.2017.86142.1380.
- 237.Bal D.K., Bhasarkar J.B. Mechanistic investigation of Sono-Phosphotungstic acid/phase transfer agent assisted oxidative desulfurization of liquid fuel. Asia-Pacific J. Chem. Eng. 2019;14(1):e2271. doi: 10.1002/apj.v14.110.1002/apj.2271. [DOI] [Google Scholar]
- 238.Shamseddini A., Mowla D., Esmaeilzadeh F. Continuous treatment of petroleum products in a tailor-made flow-through sonoreactor. J. Pet. Sci. Eng. 2019;173:1149–1162. doi: 10.1016/j.petrol.2018.10.068. [DOI] [Google Scholar]
- 239.Lin Y., Feng L., Li X., Chen Y., Yin G., Zhou W. Study on ultrasound-assisted oxidative desulfurization for crude oil. Ultrason. Sonochem. 2020;63:104946. doi: 10.1016/j.ultsonch.2019.104946. [DOI] [PubMed] [Google Scholar]
- 240.Shayegan Z., Razzaghi M., Niaei A., Salari D., Tabar M.T.S., Akbari A.N. Sulfur removal of gas oil using ultrasound-assisted catalytic oxidative process and study of its optimum conditions. Korean J. Chem. Eng. 2013;30(9):1751–1759. doi: 10.1007/s11814-013-0097-5. [DOI] [Google Scholar]
- 241.Choi A.E.S., Roces S., Dugos N., Futalan C.M., Lin S.-S., Wan M.-W. Optimization of ultrasound-assisted oxidative desulfurization of model sulfur compounds using commercial ferrate (VI) J. Taiwan Inst. Chem. Eng. 2014;45(6):2935–2942. doi: 10.1016/j.jtice:2014.08.003. [DOI] [Google Scholar]
- 242.Sharma V.K. Potassium ferrate(VI): an environmentally friendly oxidant. Adv. Environ. Res. 2002;6(2):143–156. doi: 10.1016/S1093-0191(01)00119-8. [DOI] [Google Scholar]
- 243.Jiang J.-Q., Stanford Cécile, Petri M. Practical application of ferrate(VI) for water and wastewater treatment – Site study’s approach. Water-Energy Nexus. 2018;1(1):42–46. doi: 10.1016/j.wen.2018.05.001. [DOI] [Google Scholar]
- 244.Schofield K. Combustion emissions: formation, reaction, and removal of trace metals in combustion products. Elsevier. 2020 doi: 10.1016/C2018-0-04690-3. [DOI] [Google Scholar]
- 245.LIU S., WANG B., CUI B., SUN L. Deep desulfurization of diesel oil oxidized by Fe (VI) systems. Fuel. 2008;87(3):422–428. doi: 10.1016/j.fuel.2007.05.029. [DOI] [Google Scholar]
- 246.F.S. García, Waste Water - Treatment and Reutilization, InTech, 2011. https://doi.org/10.5772/656.
- 247.Xie J., Li B., Liu H., Li Y., He J.-B., Zheng Y., Lau K.-C., Lau T.-C. Hydrogen atom transfer in the oxidation of alkylbenzenesulfonates by ferrate(VI) in aqueous solutions. Dalt. Trans. 2021;50:715–721. doi: 10.1039/D0DT03245J. [DOI] [PubMed] [Google Scholar]
- 248.Choi A.E.S., Roces S., Dugos N., Wan M.-W. Operating cost study through a Pareto-optimal fuzzy analysis using commercial ferrate (VI) in an ultrasound-assisted oxidative desulfurization of model sulfur compounds. Clean Technol. Environ. Policy. 2016;18(5):1433–1441. doi: 10.1007/s10098-015-1079-6. [DOI] [Google Scholar]
- 249.Fogler H.S. 2nd ed., Pearson Education Inc; 2018. Essentials of Chemical Reaction Engineering. [Google Scholar]
- 250.T. Mason, D. Peters, Practical sonochemistry: Power Ultrasound Uses and Applications, 2nd ed., Woodhead Publishing Limited, 2002. https://doi.org/10.1533/9781782420620.
- 251.T.S.H. Leong, S. Manickam, G.J.O. Martin, W. Li, M. Ashokkumar, Ultrasonic Production of Nano-emulsions for Bioactive Delivery in Drug and Food Applications, Springer International Publishing, Cham, 2018. https://doi.org/10.1007/978-3-319-73491-0.
- 252.Liu L., Yang Y., Liu P., Tan W. The influence of air content in water on ultrasonic cavitation field. Ultrason. Sonochem. 2014;21(2):566–571. doi: 10.1016/j.ultsonch.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 253.Yamashita T., Ando K. Low-intensity ultrasound induced cavitation and streaming in oxygen-supersaturated water: Role of cavitation bubbles as physical cleaning agents. Ultrason. Sonochem. 2019;52:268–279. doi: 10.1016/j.ultsonch.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 254.R.V. Rai, J.A. Bai, Trends in Food Safety and Protection, CRC Press, 2017. https://doi.org/10.1201/9781315114859.
- 255.Capelo-Martinez J.L. Ultrasound in chemistry: analytical applications. Wiley. 2009 doi: 10.1002/9783527623501. [DOI] [Google Scholar]
- 256.A.R. Fernández-Alba, Comprehensive Analytical Chemistry, Vol. XLIII: Chromatographic-Mass Spectrometric Food Analysis for Trace Determination of Pesticide Residues, Elsevier Science, 2005.
- 257.Luque de Castro M.D., Priego-Capote F. Analytical applications of ultrasound-(Techniques and instrumentation in analytical chemistry) Elsevier B.V. 2007 [Google Scholar]
- 258.Bermudez-Aguirre D. Academic Press; 2017. Ultrasound: Advances in Food Processing and Preservation. http://marefateadyan.nashriyat.ir/node/150. [Google Scholar]
- 259.F.J. Keil, ed., Modeling of Process Intensification, Wiley, 2007. https://doi.org/10.1002/9783527610600.
- 260.Y.T. Shah, A.B. Pandit, V.S. Moholkar, Cavitation Reaction Engineering, Springer US, Boston, MA, 1999. https://doi.org/10.1007/978-1-4615-4787-7.
- 261.J.-L. Luche, Synthetic Organic Sonochemistry, Springer US, Boston, MA, MA, 1998. https://doi.org/10.1007/978-1-4899-1910-6.
- 262.Sano T., Ejiri S., Yamada K., Kawakami Y., Yanagishita H. Separation of acetic acid-water mixtures by pervaporation through silicalite membrane. J. Memb. Sci. 1997;123:225–233. doi: 10.1016/S0376-7388(96)00224-4. [DOI] [Google Scholar]
- 263.Barone J.R., Schmidt W.F. Effect of formic acid exposure on keratin fiber derived from poultry feather biomass. Bioresour. Technol. 2006;97(2):233–242. doi: 10.1016/j.biortech.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 264.Zhu Z., Tain R., Rhodes C. A study of the decomposition behaviour of 12-tungstophosphate heteropolyacid in solution. Can. J. Chem. 2003;81(10):1044–1050. doi: 10.1139/v03-129. [DOI] [Google Scholar]
- 265.Zhai L., Li H. Polyoxometalate-polymer hybrid materials as proton exchange membranes for fuel cell applications. Molecules. 2019;24:3425. doi: 10.3390/molecules24193425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.SUN X., ZHAO X., DU W., LIU D. Kinetics of formic acid-autocatalyzed preparation of performic acid in aqueous phase. Chinese J. Chem. Eng. 2011;19(6):964–971. doi: 10.1016/S1004-9541(11)60078-5. [DOI] [Google Scholar]
- 267.Kunigk L., Gomes D.R., Forte F., Vidal K.P., Gomes L.F., Sousa P.F. The influence of temperature on the decomposition kinetics of peracetic acid in solutions. Braz. J Chem. Eng. 2001;18(2):217–220. doi: 10.1590/S0104-66322001000200009. [DOI] [Google Scholar]
- 268.J.-M. Lévêque, G. Cravotto, F. Delattre, P. Cintas, Organic Sonochemistry: Challenges and Perspectives for the 21st Century, Springer International Publishing, Cham, 2018. https://doi.org/10.1007/978-3-319-98554-1.
- 269.J. Ancheyta, Experimental Methods for Evaluation of Hydrotreating Catalysts, Wiley, 2020. https://doi.org/10.1002/9781119518037.
- 270.M.J. King, W.G. Daveport, M.S. Moats, Sulfuric Acid Manufacture: Analysis, Control and Optimization, 2nd ed., Elsevier Ltd, 2013. https://doi.org/10.1016/B978-0-08-044428-4.X5000-3.
- 271.Weh R., de Klerk A. Thermochemistry of sulfones relevant to oxidative desulfurization. Energy Fuels. 2017;31(6):6607–6614. doi: 10.1021/acs.energyfuels.7b00585. [DOI] [Google Scholar]
- 272.Eow J.S. Recovery of sulfur from sour acid gas: a review of the technology. Environ. Prog. 2002;21(3):143–162. doi: 10.1002/ep.670210312. [DOI] [Google Scholar]
- 273.Wang T., Stiegel G. Integrated gasification combined cycle (IGCC) technologies. Elsevier. 2017 doi: 10.1016/C2014-0-00849-0. [DOI] [Google Scholar]
- 274.Vögtle F., Rossa L. Pyrolysis of sulfones as a synthetic method [new synthetic methods (28)] Angew. Chemie Int. Ed. English. 1979;18(7):515–529. doi: 10.1002/anie.197905151. [DOI] [Google Scholar]
- 275.Anderson K., Atkins M.P., Borges P., Chan Z.P., Rafeen M.S., Sebran N.H., van der Pool E., Vleeming J.H. Economic analysis of ultrasound-assisted oxidative desulfurization. Energy Sources Part B Econ. Plann. Policy. 2017;12(4):305–311. doi: 10.1080/15567249.2014.917131. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.