Abstract
Hyperphosphatemia is present in most patients with end‐stage renal disease (ESRD) and has been associated with increased cardiovascular mortality. Phosphate binders (calcium‐based and calcium free) are the mainstay pharmacologic treatment to lower phosphorus levels in patients with ESRD. We evaluated biochemical markers of vascular calcification, inflammation, and endothelial dysfunction in patients with chronic kidney disease (CKD) treated with sevelamer carbonate (SC) versus calcium acetate (CA). Fifty patients with CKD (stages 3 and 4) were enrolled and assigned to treatment with SC and CA for 12 weeks. At the end of the study the biomarkers of vascular calcification, inflammation, and endothelial dysfunction were analyzed. A significant increase in HDL‐cholesterol was observed with SC but not with CA in patients with CKD. Treatment with SC reduced serum phosphate, calcium phosphate, and FGF‐23 levels and there was no change with CA treatment. The inflammatory markers IL‐8, IFN‐γ, and TNFα decreased with response to both treatments. The levels of IL‐6 significantly increased with CA treatment and no change was observed in the SC treatment group. SC showed favorable effects on anti‐inflammatory and vascular calcification biomarkers compared to CA treatment in patients with CKD stages 3 and 4 with normal phosphorous values.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Non‐calcium‐based phosphate binders are effective in the patients with end stage kidney disease for lowering serum phosphorus and have demonstrated anti‐inflammatory effects.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study demonstrates a favorable reduction in systemic, vascular, and bone‐related inflammatory markers from treatment with sevelamer carbonate (SC) in the patients with chronic kidney disease (CKD) not on dialysis with normal serum phosphorus levels.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study suggests that patients with CKD not on dialysis may benefit from SC phosphate binders despite having a normal serum phosphorus level.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study offers insight to the role phosphates binder may play in lowering inflammation and vascular calcification in patients with CKD not on dialysis.
INTRODUCTION
Abnormalities of mineral metabolism lead to the development of vascular calcification and aortic stiffness in patients with chronic kidney disease (CKD) that contributes to cardiovascular disease. 1 , 2 , 3 Despite normal values of calcium and phosphorus in the CKD population, 40% of patients with CKD not receiving dialysis display evidence of calcification on imaging. 4 , 5 Opportunities to reduce or slow the progression of vascular calcification in early stages of CKD should be explored to hinder the establishment of vascular calcification. Phosphate binders for the management of secondary hyperparathyroidism in patients with CKD not receiving dialysis is mainly limited to the correction of hyperphosphatemia. Several studies have illustrated the development of inflammation and abnormal mineral metabolism prior to the development of frank hyperphosphatemia. 6 , 7 , 8 The trade‐off in the nephron hypothesis suggests that a high concentration of phosphate in the cortical distal nephron reduces the concentration of ionized calcium in that segment, and thereby necessitates increased parathyroid hormone to maintain normal calcium reabsorption and normocalcemia. 9 As such, the relative normal serum phosphate provides limited value in determining the detrimental effects of phosphate exposure. 10 , 11 , 12 In this study, we sought to compare the effects of sevelamer carbonate (SC) and calcium acetate (CA)for their role on biomarkers indicative of vascular calcification, inflammation, and endothelial dysfunction in patients with CKD stages 3 and 4 without hyperphosphatemia.
METHODS
Patients
This was a randomized, prospective, open‐label, parallel group study approved by both the Institutional Review Board (IRB) at the Albany College of Pharmacy and Health Sciences and the IRB at the Albany Medical Center. Fifty patients with CKD (stages 3 and 4) were recruited from the Albany Medical Center South Campus facility between 2011 and 2015. Patients were included in the study if they were men or women greater than or equal to 18 years of age at the start of screening, CKD stage 3 or 4 (eGFR 15–60 ml/min/1.73 m2), not expected to start dialysis for 8 months, serum intact PTH less than 500 pg/ml during the screening period, and a stable dose of ACEi/ARB regimen 30 days prior to screening. Over 80% of study subjects were White and men. Patients with diabetes, taking oral steroids, chemotherapy, and radiotherapy, and children below the age of 16 years were excluded from the study.
The duration of the study was 15 weeks in total spilt into three phases: screening phase, washout phase, and treatment phase (Figure 1). Written informed consent was obtained prior to any screening procedures. Study subjects’ laboratory data was screened to ensure they fulfilled the inclusion criteria. The following examinations and assessments were conducted one time during the 30‐day screening period: demographics, medical history, iPTH, pregnancy test (if applicable), and medication history. Study subjects were randomized by a computer program in a 1:1 ratio to receive either SC or CA. If applicable, phosphate binder therapy was discontinued in study subjects receiving phosphate binder therapy 3 weeks prior to starting study medications during the washout phase. Study subjects only took phosphate binder if they were randomized to during the treatment phase. Baseline characteristics of the study subjects are presented in Table 1.
FIGURE 1.
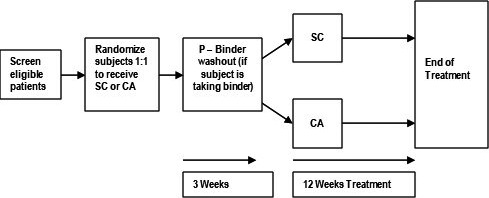
The flow chart of study design and procedure. SA, sevelamer carbonate; CA, calcium acetate
TABLE 1.
Baseline characteristics of the CKD stages 3 and 4 study subjects
| Calcium acetate (n = 15) | Sevelamer Carbonate (n = 15) | |
|---|---|---|
| Age, years (mean ± SD) | 69.26 ± 8.52 | 67.40 ± 7.77 |
| Sex, male/female | 12/3 | 13/2 |
| Race, White/Black | 12/3 | 14/1 |
| eGFR (ml/min/1.73 m2) | 38.26 ± 11.21 | 37.60 ± 6.12 |
| Height (cm) | 171.60 ± 7.82 | 172.21 ± 6.80 |
| Weight (kg) | 99.11 ± 23.31 | 103.29 ± 20.13 |
| BMI (kg/m2) | 33.60 ± 7.06 | 35.09 ± 7.92 |
All the data are mean ± SD.
Abbreviation: BMI, body mass index.
Study subjects were treated with SC 1600 mg three times daily with meals or CA 1334 mg three times daily during the treatment phase for a total of 12 weeks. All patients received cholecalciferol 400 units daily during the treatment phase of the study. The treatment phase consisted of five total study visits: baseline study (day 1 and day 2), week 4, week 8, and week 12. The following assessments and laboratory tests were obtained on day 1: fibroblast growth factor‐23 (FGF‐23), serum chemistry (calcium, phosphorous albumin, alkaline phosphatase, and cholesterol panel), iPTH, 25‐OH, 1,25 OH, markers of calcification (fetuin‐A and osteoprotegerin), inflammation (IL‐6, TNF‐α, IL‐10, IL‐17, and INF‐γ), and vascular adhesion molecules (sICAM‐1, sVCAM‐1, P‐selectin, and E‐selectin). Patients were given a urine collection container and instructed to start a 24‐h urine collection. Study subjects would return on day 2 to return their 24‐h urine collection sample, which was analyzed for urinary calcium, phosphorous, protein, and creatinine. The following laboratory assessments were collected during weeks 4 and 8: FGF‐23, phosphorous, calcium, and iPTH. The levels of FGF‐23, serum chemistry (calcium, phosphorous albumin, alkaline phosphatase, and cholesterol panel), iPTH, 25‐OH, 1,25 OH, markers of calcification (fetuin‐A and osteoprotegerin), inflammation (IL‐6, TNF‐α, IL‐10, IL‐17, and INF‐γ), and vascular adhesion molecules (sICAM‐1, sVCAM‐1, P‐selectin, and E‐selectin) were assessed at the end of the treatment phase (12 weeks).
Statistics
Continuous variables were expressed as mean ± SD. Comparison of the biomarkers’ concentrations within groups and between the two groups were performed using either Student’s t‐test or Wilcoxon rank sum test for paired differences and nonparametric data with GraphPad Prism 7 software (GraphPad) and statistical significance was defined as p < 0.05.
RESULTS
Markers of mineral metabolism
Changes in markers of mineral metabolism are presented in Table 2. The average serum phosphate level was within normal range for both treatment groups, 3.7 ± 0.73 and 3.3 ± 0.70 for CA and SC, respectively. In both treatments, within groups the levels of phosphate and calcium remained clinically unchanged. Sevelamer treatment resulted in a significant decrease in levels of FGF‐23, calcidiol, and calcitriol, whereas FGF‐23 and calcitriol remained unchanged in the CA group. A significant increase in calcidiol, was observed in the CA group. PTH decreased significantly in the CA group but not in the SC group.
TABLE 2.
Changes in markers of mineral metabolism at baseline and after 12 weeks of treatment with calcium acetate or sevelamer carbonate
| Calcium acetate | Sevelamer carbonate | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Calcium (mg/dl) | 9.5 ± 0.11 | 9.7 ± 0.13* | 9.6 ± 0.12 | 9.5 ± 0.10 |
| Phosphate (mg/dl) | 3.7 ± 0.73 | 3.7 ± 0.17 | 3.3 ± 0.70 | 3.4 ± 0.16 |
| FGF−23 (pg/L) | 106.4 ± 23.9 | 101.1 ± 19.8 | 112.8 ± 25.9 | 96.3 ± 13.99* |
| Calcidiol (ng/ml) | 23.9 ± 3.0 | 27.5 ± 2.7 | 31.4 ± 3.1 | 28.9 ± 3.5 |
| Calcitriol (pg/ml) | 35.6 ± 3.9 | 36.8 ± 3.8 | 44.4 ± 4.1 | 38.4 ± 3.9* |
| Ca × P (mg2/dl2) | 35.8 ± 1.94 | 36.71 ± 1.94 | 32.03 ± 1.7 | 32.97 ± 1.56 |
| PTH (pg/ml) | 70.8 ± 14.1 | 51.2 ± 11.09* | 58.0 ± 5.34 | 66.2 ± 5.36 |
All data are mean ± SD. Paired comparison from baseline to week 12 change. *p < 0.05.
Abbreviations: Ca × P, calcium × phosphate; FGF‐23, fibroblast growth factor 23; PTH, parathyroid.
Serum lipids
Significant decreases in total cholesterol and LDL‐cholesterol (p < 0.001) were observed with SC but not with CA. Significant increases in HDL‐cholesterol were observed with SC but not with CA (p < 0.05), triglyceride did not change from baseline in either group. No statistically significant difference was observed in triglycerides in both groups compared to baseline Table 3.
TABLE 3.
Changes in levels in vascular calcification markers at baseline and after 12 weeks of treatment with calcium acetate and sevelamer carbonate
| Calcium acetate | Sevelamer carbonate | |||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| OPG (pg/ml) | 2409 ± 188 | 2437 ± 201.4 | 2813 ± 612.6 | 2817 ± 489.4 |
| SOST (pg/ml) | 234.4 ± 26.8 | 213.1 ± 27.39* | 213.1 ± 27.18 | 208.3 ± 26.08* |
| TRAcP5b (U/L) | 3.00 ± 0.21 | 2.69 ± 0.17* | 3.07 ± 0.21 | 3.05 ± 0.22 |
| AlkPO4 (IU/L) | 60.53 ± 5.23 | 56.73 ± 4.24 | 68.6 ± 5.65 | 79.6 ± 6.52 |
All data are mean ± SD, Paired comparison from baseline for each subject to week 12 change. *p < 0.05.
Abbreviations: AlkPO4, alkaline phosphate; OPG, osteoprotegerin; SOST, serum sclerostin; TRAcP5b, serum tartrate‐resistant acid phosphatase.
Treatment with SC significantly decreased total cholesterol, LDL, and triglycerides compared to CA (Figure 2). These changes were significantly different between groups (***p < 0.001 and *p < 0.05).
FIGURE 2.
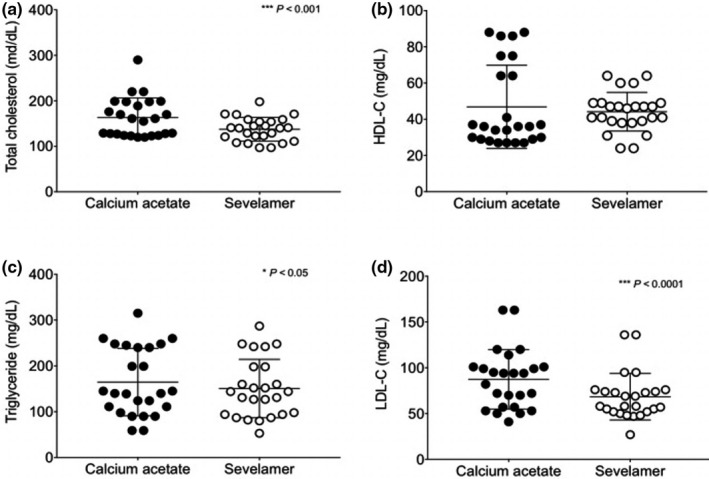
Changes in serum lipids after 12 weeks of treatment with sevelamer carbonate and calcium acetate. (a) Total cholesterol (TC), (b) high‐density‐lipoprotein cholesterol (HDL‐C), (c) triglycerides (TG), (d) low‐density lipoprotein cholesterol (LDL‐C). Mean ± SD *p < 0.05, ***p < 0.001
Inflammatory markers
Effects of phosphate binder treatment on inflammatory markers are presented in Table S1. A significant decrease in C‐reactive protein (CRP) was observed at the end of the treatment with SC, whereas no change was observed in CRP in the CA group. A significant decrease was observed in IL‐8, IFN‐γ, and TNF‐α with response to both treatments. The levels of IL‐6 increased significantly in the CA group and no change was observed in the SC group. Serum IL‐10 levels decreased significantly with SC treatment, and no change was observed for IL‐10 in the CA group.
Figure 3 shows the inflammatory profile between the CA and SC groups. In patients receiving the SC, serum IL‐8 and IL‐10 showed a significant decrease (Figure 3b,c). The serum TNF‐α levels were increased in the SC group (Figure 3e) and no change in IL‐6 and IFN‐ γ levels was observed (Figure 3a,d).
FIGURE 3.
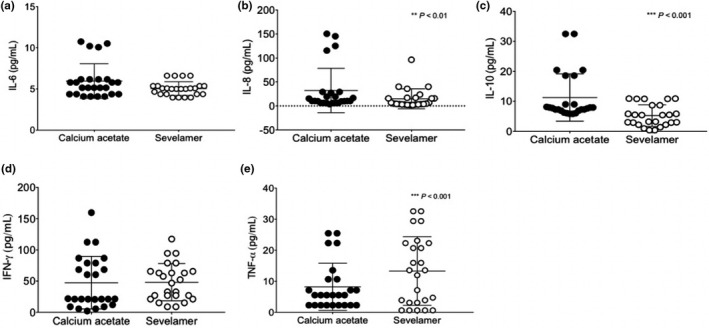
Changes in serum inflammatory markers after 12 weeks of treatment with sevelamer carbonate and calcium acetate. (a) Interleukin‐6, (b) Interleukin‐8, (c) Interleukin‐10, (d) IFN‐γ, (e) TNF‐α. Values expressed as mean ± SD **p < 0.01, ***p < 0.001
Vascular endothelial markers
Treatment with sevelamer carbonate at week 12 resulted in a significant increase in ICAM‐1, VCAM‐1, E‐selectin, and L‐selectin. Treatment with CA showed a significant decrease in E‐selectin levels and no change was observed with other endothelial markers. No statistical significance in the levels of P‐selectin was observed in either treatment group, Supplementary Table S2.
There were no significant differences observed in VCAM‐1, E‐selectin, and L‐selectin levels between the groups (Figure 4b,d,e). However, significant decreases in levels of ICAM‐1 (**p < 0.01) and P‐selectin (*p < 0.05) were observed when compared between treatment with CA and SC (Figure 4a,c).
FIGURE 4.
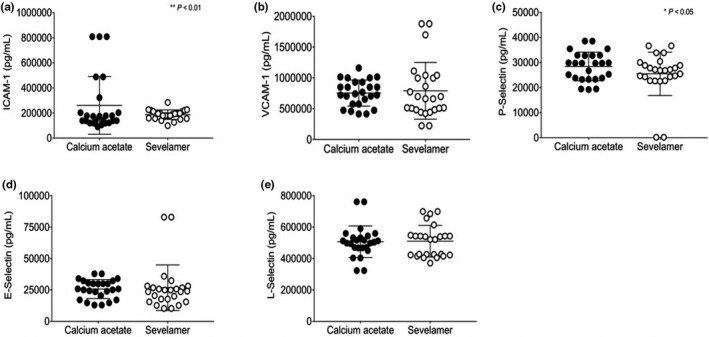
(a) ICAM‐1, (b) VCAM‐1, (c) P‐selectin, (d) E‐selectin, (e) L‐selectin. Values expressed as mean ± SD *p < 0.05, **p < 0.01
Vascular calcification markers
After 12 weeks of treatment with CA, there was a significant decrease in SOST, TRAcP5b, and alkaline phosphate Supplementary Table S3, whereas with SC, there was an increase in alkaline phosphate levels, a decrease in SOST levels, and no statistically significant decrease in TRAcP5b. No statistical changes in OPG were observed in either treatment group.
DISCUSSION
Despite the widespread use of phosphate binders in patients with end‐stage renal disease, relatively few studies have compared CA and SC with respect to their biomarkers of vascular calcification, inflammation, and endothelial dysfunction in non‐dialysis patients.
The beneficial effects of SC on serum lipids were expected from earlier studies in patients with CKD. 13 Significant decrease in total cholesterol and increase in HDL‐cholesterol was seen with SC but not with CA. In another study performed for 1 year in 200 patients with hemodialysis, SC improved the progression of coronary aortic calcification compared to calcium‐based binders. 14
In the present study, SC treatment demonstrated reductions in biomarkers related to endothelial function and inflammation. As expected from earlier studies, elevated levels of calcium were more common in CA‐treated patients. 15 As shown in previous studies, sevelamer effects on vascular calcification might be secondary to its lower calcium load versus lipid‐lowering properties. Further and consistent with earlier studies, SC significantly decreased the uric acid not observed with CA. 16 Johnson et al. showed that hyperuricemia is associated with insulin resistance, dyslipidemia, hypertension, and cardiovascular diseases in patients with CKD. 17
In patients with CKD, FGF‐23 concentrations constitutively elevate and increase progressively as kidney function worsens. 18 FGF‐23 is released in the face of phosphate overload in non‐CKD and patients with CKD. With reduced nephron mass, excessive FGF‐23 concentrations increase phosphorous elimination per nephron maintaining normal serum phosphorous concentrations. 19 This normalization is not without consequence; FGF‐23 is an inhibitor of 1,25‐dihydroxyvitamin D [1,25(OH)2D] synthesis and further aggravates the prevalent vitamin D deficiency seen in CKD. 20 Excessive levels of phosphorous and calcium are endogenous minerals capable of stimulating the phenotypic transformation of vascular smooth muscle cells into osteoblast‐like cells. 21 Despite normal values of calcium and phosphorous in patients with CKD, 40% of patients with CKD not receiving dialysis display evidence of calcification on imaging. 22 In the present study, SC significantly reduced the FGF‐23 levels at the end of 12 weeks of study. Experimental CKD models in animals showed that reducing intestinal phosphate absorption with sevelamer HCl lowered serum FGF‐23 and phosphorous concentrations. 23 Thus FGF‐23 is considered to be a logical target in early stages of CKD to slow the progression of calcification with phosphate binders. In a 6‐week period, Oliveria et al. targeted serum FGF‐23 in patients with stages 3 and 4 CKD with SC and CA, and at 6 weeks both phosphorous binders lowered FGF‐23 levels. 24 However, this short‐term study did not provide any evidence of reduction or progression of vascular calcification in response to FGF‐23 reduction.
Furthermore, vascular calcification causes hemodynamic alterations, such as reduced compliance of large conductance arteries and autonomic dysfunction. 25 Stiffening of the arterial media layer as a result of calcification manifests clinically via increased elevated pulse pressure. Moreover, higher FGF‐23 concentrations have been associated with increased arterial stiffness in patients with CKD. 26 , 27 Evidence suggests these homeostatic vascular protective mechanisms are deficient or nonfunctional in patients with CKD resulting in increased amounts of calcification in the CKD patient population. 28 , 29 A specific biomarker of interest is osteoprotegerin. Currently, there are few published clinical trials on the effect of sevelamer treatment on levels of calcification biomarkers, specifically feutin‐A in the non‐dialysis population. 30 , 31 However, this short‐term study did not provide any evidence of reduction or progression of vascular calcification in response to FGF‐23 reduction.
Additionally, inflammatory cytokines have been associated with the process of vascular calcification. Studies have demonstrated that treatment with sevelamer may have anti‐inflammatory effects in hemodialysis patients. 9 , 32 In the current study, SC significantly reduced the inflammatory markers IL‐6, IL‐8, IL‐10, CRP, TNFα, and IFN‐γ. All these inflammatory markers have been associated with adverse outcomes and complications in patients with CKD. Inflammatory cytokines also activate and upregulate the expression of adhesion molecules, intercellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1), E‐selectin, and P‐selectin, which play a fundamental role in endothelial dysfunction. Markers of endothelial function are associated with cardiovascular events. In the present study, there was a significant increase in circulating levels of ICAM‐1 and VCAM‐1 with SC compared with CA and these results are comparable with those reported in previous studies. 33 , 34 In accordance with the above speculation, chronic uremia is known to be associated with elevated pro‐inflammatory cytokine levels that can upregulate the expression and release of different adhesion molecules.
Systemic inflammation has been regarded as a cardiovascular risk factor. Our study demonstrated a negative correlation between serum VCAM‐1 and HDL levels and a highly significant correlation between both ICAM‐1 and VCAM‐1 and CRP levels; this is the first report of such a relationship in chronic hemodialysis patients. Moreover, compared with patients with normal CRP, patients with elevated CRP had significantly increased serum ICAM‐1. The above findings are particularly important because CRP is an accepted index of overall inflammatory activity and a surrogate of underlying cytokine stimulus. Based on the above results, an association among inflammation, adhesion molecule release, dyslipidemia, and atherosclerosis could be postulated.
Overall, SC versus CA resulted in significant reduction in either total cholesterol or LDL‐C, with p < 0.0 and <0.001, respectively. Both SC and CA resulted in distinct and statistically significant reduction in IL‐8 and TNF‐α as well as IFN‐γ a as compared to baselines, with greater impact by SC versus CA.
Limitations of the study
Baseline parameters are within same ranges between the two groups except for some parameters due to phosphate binder therapy that was discontinued in study subjects receiving phosphate binder therapy 3 weeks (washout) prior to starting of current study medications, which might not be enough duration.
CONFLICT OF INTEREST
Authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
D.M., D.N., S.A.M., and K.G. wrote the manuscript. S.A.M designed the research. D.M. and K.G. performed the research. D.M. analyzed the data. S.A.M. contributed new reagents/analytical tools.
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
This investigation would not have been possible without unrestricted funding from Sanofi/Genzyme. The authors have no conflicts of interest to declare.
Mason DL, Godugu K, Nnani D, Mousa SA. Effects of sevelamer carbonate versus calcium acetate on vascular calcification, inflammation, and endothelial dysfunction in chronic kidney disease. Clin Transl Sci. 2022;15:353–360. 10.1111/cts.13151
Darius L. Mason and Kavitha Godugu contributed equally to this work and should be considered co‐first authors.
Trial Registration: Registered at trial.com, registration number NCT01277497.
Funding information
Unrestricted funding was received from Sanofi/Genzyme.
REFERENCES
- 1. Viegas C, Araújo N, Marreiros C, Simes D. The interplay between mineral metabolism, vascular calcification and inflammation in chronic kidney disease (CKD): challenging old concepts with new facts. Aging (Albany NY). 2019;11:4274‐4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SJ, Lee I‐K, Jeon J‐H. Vascular calcification—new insights into its mechanism. Int J Mol Sci. 2020;21:2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vahed SZ, Mostafavi S, Khatibi SMH, Shoja MM, Ardalan M. Vascular calcification: an important understanding in nephrology. Vasc Health Risk Manag. 2020;16:167‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Affret A, Wagner S, El Fatouhi D, et al. Validity and reproducibility of a short food frequency questionnaire among patients with chronic kidney disease. BMC Nephrol. 2017;18:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Amm Intern Med. 2018;168:422‐430. [DOI] [PubMed] [Google Scholar]
- 6. Isakova T, Nickolas TL, Denburg M, et al. KDOQI us commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD‐MBD). Am J Kidney Dis. 2017;70:737‐751. [DOI] [PubMed] [Google Scholar]
- 7. Locatelli F, Del Vecchio L, Violo L, Pontoriero G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf. 2014;13:551‐561. [DOI] [PubMed] [Google Scholar]
- 8. Hutchison AJ. Oral phosphate binders. Kidney Int. 2009;75:906‐914. [DOI] [PubMed] [Google Scholar]
- 9. Ruospo M, Palmer SC, Natale P, et al. Phosphate binders for preventing and treating chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Cochrane Database Syst Rev. 2018;8:CD006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen NX, Duan D, O'Neill KD, et al. The mechanisms of uremic serum‐induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046‐1053. [DOI] [PubMed] [Google Scholar]
- 11. Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast‐specific proteins in vascular smooth muscle cells. Nephrol Dial Transplant. 2009;24:2051‐2058. [DOI] [PubMed] [Google Scholar]
- 12. Zheng CM, Lu KC, Wu CC, Hsu YH, Lin YF. Association of serum phosphate and related factors in ESRD‐related vascular calcification. Int J Nephrol. 2011;2011:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlassara H, Uribarri J, Cai W, et al. Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol. 2012;7:934‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chertow GM, Burke SK, Raggi P. Treat to Goal Working G: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245‐252. 10.1046/j.1523-1755.2002.00434.x [DOI] [PubMed] [Google Scholar]
- 15. Qunibi WY, Hootkins RE, McDowell LL, et al. Treatment of hyperphosphatemia in hemodialysis patients: the Calcium Acetate Renagel Evaluation (CARE study). Kidney Int. 2004;65:1914‐1926. [DOI] [PubMed] [Google Scholar]
- 16. Evenepoel P, Selgas R, Caputo F, et al. Efficacy and safety of sevelamer hydrochloride and calcium acetate in patients on peritoneal dialysis. Nephrol Dial Transplant. 2009;24:278‐285. [DOI] [PubMed] [Google Scholar]
- 17. Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB. Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis. 1999;33:225‐234. [DOI] [PubMed] [Google Scholar]
- 18. Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the mild to moderate kidney disease (MMKD) study. J Am Soc Nephrol. 2007;18:2600‐2608. [DOI] [PubMed] [Google Scholar]
- 19. Gutiérrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor‐23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor‐23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205‐2215. [DOI] [PubMed] [Google Scholar]
- 21. Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34‐S37. [DOI] [PubMed] [Google Scholar]
- 22. Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024‐1030. [DOI] [PubMed] [Google Scholar]
- 23. Nagano N, Miyata S, Abe M, et al. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531‐537. [DOI] [PubMed] [Google Scholar]
- 24. Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD‐MBD therapy? Clin J Am Soc Nephrol. 2010;5:286‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sigrist M, Bungay P, Taal MW, McIntyre CW. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707‐714. [DOI] [PubMed] [Google Scholar]
- 26. Jean G, Terrat JC, Vanel T, et al. High levels of serum fibroblast growth factor (FGF)‐23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792‐2796. [DOI] [PubMed] [Google Scholar]
- 27. Llauradó G, Megia A, Cano A, et al. FGF‐23/vitamin D axis in type 1 diabetes: the potential role of mineral metabolism in arterial stiffness. PLoS One. 2015;10:e0140222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moe SM, Reslerova M, Ketteler M, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int. 2005;67:2295‐2304. [DOI] [PubMed] [Google Scholar]
- 29. Brown RB, Razzaque MS. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. Bonekey Rep. 2015;4:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tousoulis D, Siasos G, Maniatis K, et al. Novel biomarkers assessing the calcium deposition in coronary artery disease. Curr Med Chem. 2012;19:901‐920. [DOI] [PubMed] [Google Scholar]
- 31. D’Marco L, Bellasi A, Raggi P. Cardiovascular biomarkers in chronic kidney disease: state of current research and clinical applicability. Dis Markers. 2015;2015:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang C, Liu X, Zhou Y, et al. New conclusions regarding comparison of sevelamer and calcium‐based phosphate binders in coronary‐artery calcification for dialysis patients: a meta‐analysis of randomized controlled trials. PLoS One. 2015;10:e0133938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arici M, Kahraman S, Gençtoy G, et al. Association of mineral metabolism with an increase in cellular adhesion molecules: another link to cardiovascular risk in maintenance haemodialysis? Nephrol Dial Transplant. 2006;21:999‐1005. [DOI] [PubMed] [Google Scholar]
- 34. Papayianni A, Alexopoulos E, Giamalis P, et al. Circulating levels of ICAM‐1, VCAM‐1, and MCP‐1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol Dial Transplant. 2002;17:435‐441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


