Abstract
Preclinical drug studies routinely administer experimental compounds to animal models with the goal of minimizing potential adverse events from the procedure. In this study, we assessed the ability to train adult male Long Evans rats to accept daily voluntarily syringe feedings of l-3,4-dihydroxyphenylalanine (L-DOPA) compared to intraperitoneal (IP) injections. Rats were trained to become familiar with the syringe and then fed a training solution that did not contain the experimental compound. If the rat was compliant during the training phase, the dilution of training solution was continuously decreased and replaced with the experimental solution. Voluntary oral dosing compliance was recorded and quantified throughout the study. To assess drug activity within the drug-targeted tissues, the striatum and retina were collected and analyzed for L-DOPA, dopamine and DOPAC levels by high performance liquid chromatography (HPLC). Drug delivery efficiency by oral dosing was directly compared to IP injection by collecting plasma and analyzing L-DOPA levels with HPLC. Adult male rats had high compliance for voluntary oral dosing. HPLC showed that oral administration of the compound at the same dose as IP injection yielded significantly lower plasma levels, and that higher oral L-DOPA doses yield higher plasma L-DOPA content. This study describes detailed methodology to train adult rats to syringe feed experimental compounds and provides important preclinical research on drug dosing and drug delivery to the striatum and retina.
Keywords: Feeding, 3Rs, rodents, dosing/sampling, alternatives, refinement
Effective delivery of experimental compounds is a vital component for studies developing and investigating novel treatment methods in animal models of human diseases. The administration of these compounds is often a critical element of the experimental design1. Drug delivery methodology is fundamental because these substances may be repeatedly administered to the same and/or multiple animals throughout the experiment.
In laboratory animals, compound delivery usually requires some form of general anesthesia, sedation, or manual restraint. Any adverse effects related to substance administration should be carefully considered to avoid off-target effects, such as stress or trauma, that could interfere with research findings. One common practice in animal research that could benefit from refinement is the impact of restraint stress and pain induced by administration methods2–4. The 3Rs (Reduce, Refine and Replace) are an important consideration for all scientific experiments involving animals5.
There are several methods by which experimental compounds are administered to laboratory animals. One of the most common methods of systemically administrating substances to rodents is intraperitoneal (IP) injection6. IP injections are advantageous due to their reproducibility, precise dosing, and potential for high bioavailability of experimental compounds. However, a disadvantage of IP injections is that they require animals to be removed from their cages and restrained for the duration of the injection, which can elicit a stress response6, 7. IP injections have been associated with measurable signs of stress in mice, such as increased heartrate, blood temperature, and plasma corticosterone levels8. A more serious consequence associated with IP injections is the increased risk of puncturing vital organs, such as the cecum. Punctured organs are not only painful, but potentially result in peritonitis and mortality7, 9. Furthermore, IP injections are subject to first-pass metabolism in the liver, which can vary depending on the proximity of the injection to the mesenteric vessels and may lead to variation in bioavailability between injections. Thus, the common laboratory practice of performing IP injections as the primary technique for administering experimental compounds, although effective, represents a major animal welfare and scientific issue.
We began exploring alternatives to IP injections, such as oral gavage, to treat rats with precise doses of l-3,4-dihydroxyphenylalanine (L-DOPA), our compound of interest, on a daily basis10. Like the IP injection, rats must be manually restrained for oral gavage. A dull, curved needle is then inserted down the esophagus to inject directly into the stomach. Like the IP injection, this technique has also been associated with behavioral signs of stress in laboratory animals, with one study reporting a mortality rate of 32% due to asphyxia caused by impacted food and bedding material in the oropharynx7, 11, 12. Likewise, another study investigating the duration and intensity of stress caused by oral gavage in laboratory rats through implanted telemetry transponders found that the effects of the procedure can last up to an hour afterwards13. Oral gavage was not a feasible option given the frequency of drug delivery for our studies and the potential for stress-induced confounders.
Thus, given our need to administer long-term precise dosing of L-DOPA/carbidopa to rats safely with minimal invasiveness and limited stress to the animal, we explored oral administration options for systemic drug delivery. L-DOPA is an experimental therapeutic compound for diabetic eye disease14, 15, and carbidopa is a peripheral decarboxylase inhibitor that prevents premature oxidation of L-DOPA in the periphery before crossing the blood brain barrier and reaching its intended destination in the central nervous system. There are several published, experimentally validated techniques for administering compounds to rats voluntarily in controlled quantities. These techniques bypass the need for restraint and minimize pain, discomfort, and risk for trauma13, 16–18. Multiple studies have been shown to accurately deliver proper dosing of experimental compounds through mediums such as cookie dough, chocolate, and nut spreads (such as peanut butter) to animals voluntarily17–19. Although these voluntary oral delivery methods did not appear to interfere with experimental compound composition, several factors must be taken into consideration, such as drug stability and solubility. For delivery of L-DOPA/carbidopa, we sought a voluntary delivery method in which the amount of drug could be precisely administered, the drug could be suspended in solution due to insolubility issues, and the dose would be taken fairly quickly as L-DOPA can oxidize with light exposure.
Within the past fifteen years, syringe administration of experimental compounds to rats has had great success with minimal stress13, 20. These studies were able to dissolve their experimental compounds in a sucrose solution. Some compounds, such as L-DOPA, are sensitive to photo-oxidation and/or degradation at room temperature and therefore they must be administered quickly and under controlled conditions. L-DOPA also has very low solubility in aqueous, consumable solvents. Here, we developed and implemented a training and treatment paradigm for rats to voluntarily ingest controlled doses of L-DOPA/carbidopa as a drug cocktail with a homogenous suspension in an artificially sweetened, water-based mixture. We provide data on the reliability of this method for chronic drug delivery in male Long Evans rats, confirm that L-DOPA reaches its desired pharmacological destination, and experimentally validate it as a method to administer precise dosing of L-DOPA. Finally, we show that rats will comply with daily treatment using this method for four weeks, making it feasible for-long-term experiments that require daily treatment with minimal invasiveness and improved animal quality of life.
Methods
Animals
Adult male Long Evans rats (2 months of age; Outbred, Strain 006, Charles River, Wilmington, MA) were used in this study. Male rats were used because they were originally intended for a study relying on penile vein injections before allocated to this study.
Rats were housed in circular racks with wedge-shaped cages (14 in. L × 19.1 in. W × 8.6 in. H; Optirat Gen II by Animal Care Systems, Inc, Centennial, CO) with corn-cob bedding and plastic tubes for enrichment. Two rats were housed per cage under normal 12:12 light:dark conditions (ambient lighting, approximately 40 lux), with chow and water provided ad libitum, and ambient room temperature maintained at approximately 22–25°C. Prior to handling by experimenters, rats were allowed to acclimate to our facility for 48 hours, placed in pairs into ventilated cages and then cages were changed every week thereafter by animal facility staff. All rat procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Atlanta VA Healthcare System and conformed to the Association for Research in Vision and Ophthalmology’s (ARVO’s) Statement for the Use of Animals in Ophthalmic and Visual Research and the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, United States, 8th edition).
Training and syringe feeding
Cohort 1 rats (n=13) were initially trained for two days for voluntarily feeding of the training solution from a 1 mL syringe (BD, Franklin Lakes, NJ), which consisted of 10% sucrose (Sigma-Alrich, St. Louis, MO) in water (Table 1). On day one, rats were introduced to the training solution and syringe. Rats were given the opportunity to smell and taste the training solution from the syringe while remaining in their cage. Some training solution was intentionally leaked into the cage so rats could taste it without interacting with the syringe. In most cases, rats were trained and syringe fed in their home cage with cage mates present. The experimenter would carefully place the syringe into the cage to favor the rat of interest. This was done to minimize disruptions to the rats’ environment during training and syringe feeding. Occasionally, if cage mates regularly competed for the same syringe, one rat would be isolated in a separate cage while the other was trained or syringe fed in their home cage. This was repeated again on day two, during which rats typically interacted with the syringe and began learning to consume training solution while the researcher slowly pushed sucrose water out of the syringe. Once trained to syringe-feed 10% training solution, sucrose concentration of the training solution was halved each day down to 2.5% sucrose. Each day of training, compliance was assessed in each rat. A rat was considered compliant if they were able to consume 2.0 ml/kg of the assigned training solution for that day. After a rat complied with the 2.5% training solution, rats were challenged to comply with vehicle solution (MediDrop Sucralose; Clear H2O, Portland, ME) for oral drug delivery. Rats that were non-compliant on the first try of receiving decreased sucrose in a training solution on the test day were recorded as “non-compliant” for that day and offered a higher concentration of sucrose training solution before being challenged with the lower sucrose training solution again that day. This training paradigm is outlined in Figure 1B. Compliance, henceforth defined as consuming 2 ml/kg of the training solution, was assessed at each of the five training days and reported as number of rats that were compliant/total rats per day.
Table 1:
Experimental design for experiments
| Cohort | Experiment | Groups | Outcomes | ||
|---|---|---|---|---|---|
| 1 | Training for syringe feeding | n=13 | NA | ||
| IP vs oral administration | IP L-DOPA (4) | Oral L-DOPA (5) | Control (4) | Blood draw after 15 min | |
| One-week washout | |||||
| Dose response | 20 mg/kg L-DOPA (5) | 10 mg/kg L-DOPA (4) | Control (4) | Blood draw after 15 min | |
| One-week washout | |||||
| Bioavailability | 20 mg/kg L-DOPA (6) | Control (6) | Tissue collection | ||
| 2 | Long duration compliance | L-DOPA (7) | Vehicle (6) | Compliance at 4 weeks | |
Figure 1:
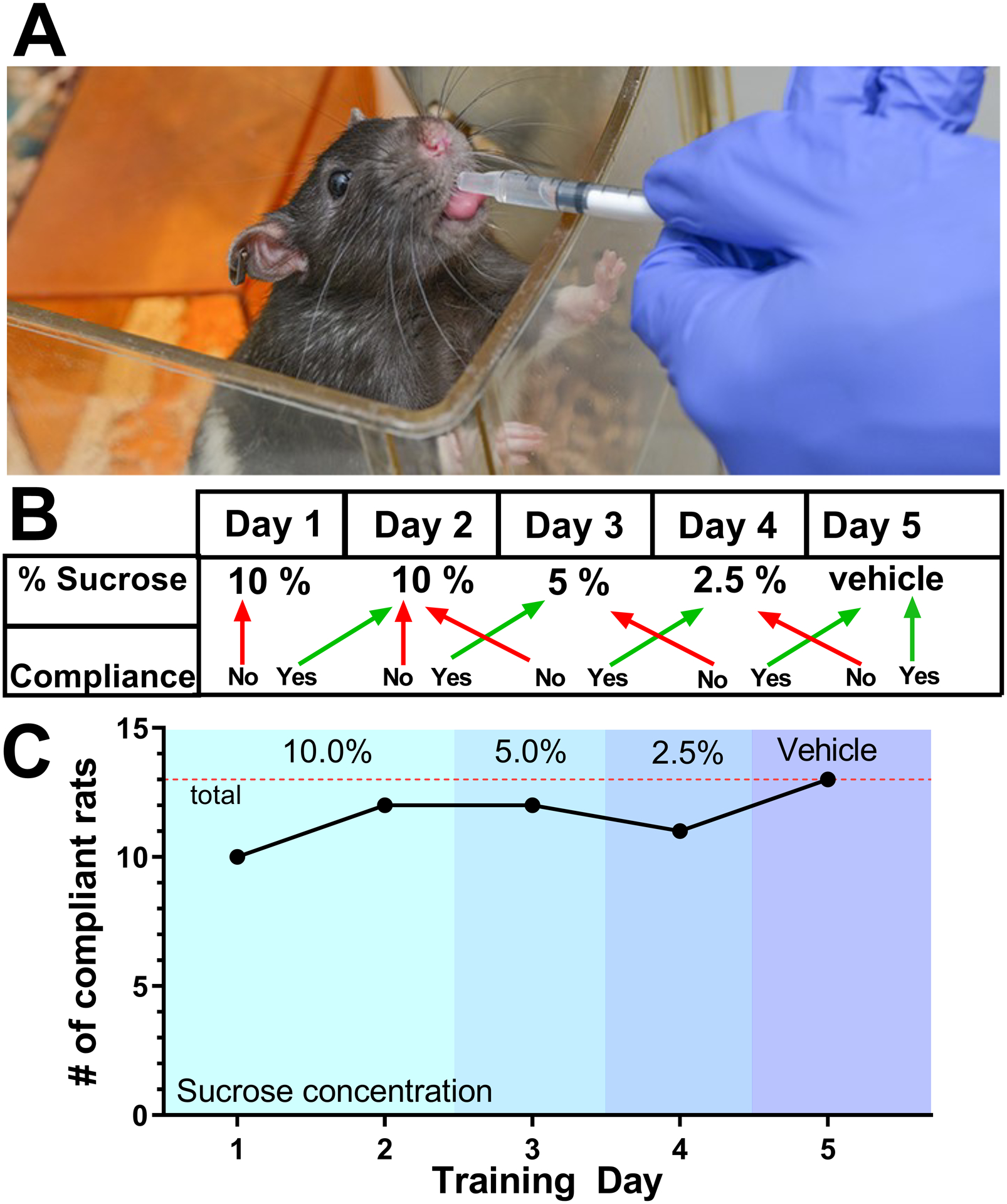
(A) A rat being syringe-fed sucrose water during the training period. (B) The five-day training paradigm. The green arrows indicate compliance and red arrows indicated non-compliance. Rats that refuse the dose on first try are considered non-compliant for that day and then immediately offered the sweeter concentration of training solution from the previous day. Once successful, the training concentration for that day is re-offered. (C) The number of rats (n=13) that were compliance on their first attempt for each of the five days of training. By day five, all 13 are compliant with the drug delivery vehicle solution. The dotted line indicates the total number of rats in the cohort.
Comparing oral dosing to IP injection:
Cohort 1 rats underwent training for syringe feeding and were randomly divided into IP L-DOPA (n=4), oral L-DOPA (n=5), or control (n=4) groups (Table 1). Both L-DOPA groups were treated with 10 mg/kg L-DOPA (Sigma-Aldrich, St. Louis, MO) and 2.5 mg/kg carbidopa monohydrate (Abcam, Cambridge, MA). Fifteen minutes after treatment, rats were anesthetized with isoflurane (5% induction in a gradual fill chamber, 1% maintenance delivered via facemask), and 180 μL of blood was drawn from lateral tail vein into cold EDTA coated tubes with 20 μL 0.1 N perchloric acid. Blood was centrifuged at 10,000 × g relative centrifugal force (Eppendorf Centrifuge 5804R, Eppendorf, Hauppauge, NY) to isolate plasma. Supernatant plasma was collected, and L-DOPA content was quantified using high performance liquid chromatography (HPLC). One blood draw from both the control group and the oral L-DOPA group were omitted due to a failure to collect 180 μL of blood.
Dose response:
After a one-week washout period, Cohort 1 rats were randomly assigned to one of three groups: 20 mg/kg oral L-DOPA co-administered with 5 mg/kg carbidopa (n=5), 10 mg/kg oral L-DOPA co-administered with 2.5 mg/kg carbidopa (n=4), or controls (n=4) with a one-week wash-out period between each treatment (Table 1). Fifteen minutes after treatment, rats were anesthetized with isoflurane (5% induction in a gradual fill chamber, 1% maintenance delivered via facemask), and 180 μL blood was drawn from lateral tail vein into EDTA coated tubes with 20 μL 0.1 N perchloric acid (Sigma-Aldrich, St. Louis, MO). Blood was centrifuged at 10,000 × g to isolate plasma. Supernatant plasma was collected, and L-DOPA content was quantified using HPLC. Note that data from one rat in 20 mg/kg L-DOPA group was not analyzed due to failure to draw the required 180 μL of blood.
Bioavailability of the drug in retina and brain tissue:
After a one-week washout period, Cohort 1 rats were randomly assigned into one of two groups: 20 mg/kg L-DOPA/5 mg/kg carbidopa (n=6) or controls (n=6). Fifteen minutes after treatment, rats were euthanized via decapitation, and retina and striatum were collected and immediately flash-frozen. Tissue was homogenized in 0.4 N perchloric acid to extract monoamines and then centrifuged at 10,000 × g. Supernatant was collected for HPLC assessment of DOPAC, dopamine, and L-DOPA and the remaining protein pellets were processed using the Pierce BCA assay (ThermoFisher Scientific, Waltham, MA) for total protein quantifications. Monoamine levels in the retina and striatum were normalized to total protein concentration of tissue to account for variability in the size of tissue collected from each rat. Note that one rat in Cohort 1 was lost to the study due to an unexpected death during the washout period before this experiment.
Long duration compliance testing:
In Cohort 2 rats (Table 1), we assessed compliance (voluntary consumption of 2 ml/kg rat) for four weekdays for rats treated with the vehicle solution only (Veh, n=6) and the L-DOPA/Carbidopa cocktail (20 mg/kg L-DOPA; 5 mg/kg carbidopa, n=7) and then repeated compliance testing four weeks after training. Initial training compliance data was not recorded for this cohort.
Oral drug cocktail preparation:
The L-DOPA/carbidopa cocktail was prepared with L-DOPA (Sigma-Aldrich, St. Louis, MO) and carbidopa monohydrate (Abcam, Cambridge, MA) to yield a 4:1 ratio of L-DOPA to carbidopa to emulate a commonly prescribed drug combination for Parkinson’s Disease21, 22. Once combined, solution was vortexed thoroughly in vehicle solution (MediDrop Sucralose; Clear H2O, Portland, ME) to create a homogenous suspension of L-DOPA/carbidopa. The L-DOPA/carbidopa cocktail was made fresh weekly and stored in a refrigerated, light-proof container. The cocktail was thoroughly re-mixed before use and kept on ice during use.
IP injected drug cocktail preparation:
L-DOPA was dissolved in saline, while carbidopa was dissolved in DMSO (Sigma-Aldrich, St. Louis, MO). The final solution contained 2.5 mg/ml L-DOPA and 0.625 mg/ml Carbidopa in 10:1 saline-DMSO mixture prepared daily. The mixture was thoroughly vortexed, placed in a light-proof container, and kept on ice during use. The prescribed dose per animal treated with IP L-DOPA was 10 mg/kg L-DOPA and 2.5 mg/kg carbidopa.
HPLC:
Monoamines were examined by HPLC with electrochemical detection as described previously23. For HPLC, an ESA 5600A CoulArray detection system, equipped with an ESA Model 584 pump and an ESA 542 refrigerated autosampler was used. Separations were performed using an MD-150 × 3.2 mm C18 (3 μM) column at 23°C. The mobile phase consisted of 8% acetonitrile, 75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium and 0.025% trimethylamine at pH 3.0. A 25 μl of sample was injected. The samples were eluted isocratically at 0.4 mL/min and detected using a 6210 electrochemical cell (ESA, Bedford, MA) equipped with 5020 guard cell. Guard cell potential was set at 500 mV, while analytical cell potentials were −175, 150, 350 and 425 mV. The analytes were identified by the matching criteria of retention time to known standards (Sigma Chemical Co., St. Louis, MO.) of dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC). Compounds were quantified by comparing peak areas to those of standards on the dominant sensor.
Statistics:
Statistical analysis was performed using statistical software (GraphPad Prism version 8.0.0 for Mac, GraphPad Software, San Diego, California USA, www.graphpad.com.) Data was analyzed using one-way ANOVA with Holm-Sidak post-hoc analysis or Student’s t-test. All data is presented as mean with error bars representing standard error of the mean. For all data, statistical outliers were determined via Grubbs Outlier Test (GraphPad Prism version 8.0.0 for Mac, GraphPad Software, San Diego, California USA, www.graphpad.com) with a significance threshold of p < 0.05. Statistical outliers were excluded from statistical analyses and data presentation.
Results:
First, we showed that animals can be trained using serially diluted sucrose water to voluntarily consume the drug delivery vehicle from a syringe. By five days, all thirteen rats were trained to syringe-feed the drug delivery vehicle (Figure 1C). Rats show a high rate of initial compliance (10/13 compliant) with 10% sucrose water from a syringe during the first day of training. By Day 5 of training, all 13 rats complied with the drug delivery vehicle. (Figure 1C)
After training the rats to syringe-feed, it was important to experimentally validate that the drug was being effectively absorbed through the gastrointestinal pathway. It is generally accepted that oral administration of compounds offers less bioavailability than injection-based methods of systemic administration. Here, orally administered L-DOPA yielded a 35.05% decrease in average plasma L-DOPA levels compared to IP injection rats when given the same dose (10 mg/kg) (Figure 2A). Both the IP injected and orally administered L-DOPA groups showed increased plasma L-DOPA levels relative to Ctrl rats (one-way ANOVA, F (2, 8) = 6.219, p = 0.0235). Next, we doubled the dose of L-DOPA to show that the absorption of the experimental drug increased the plasma concentration of L-DOPA by 47.87% (Figure 2B). Plasma L-DOPA levels were significantly higher in the 10 mg/kg L-DOPA and 20 mg/kg L-DOPA groups compared to controls rats (one-way ANOVA, F(2, 9) = 11.71, p = 0.0031).
Figure 2:
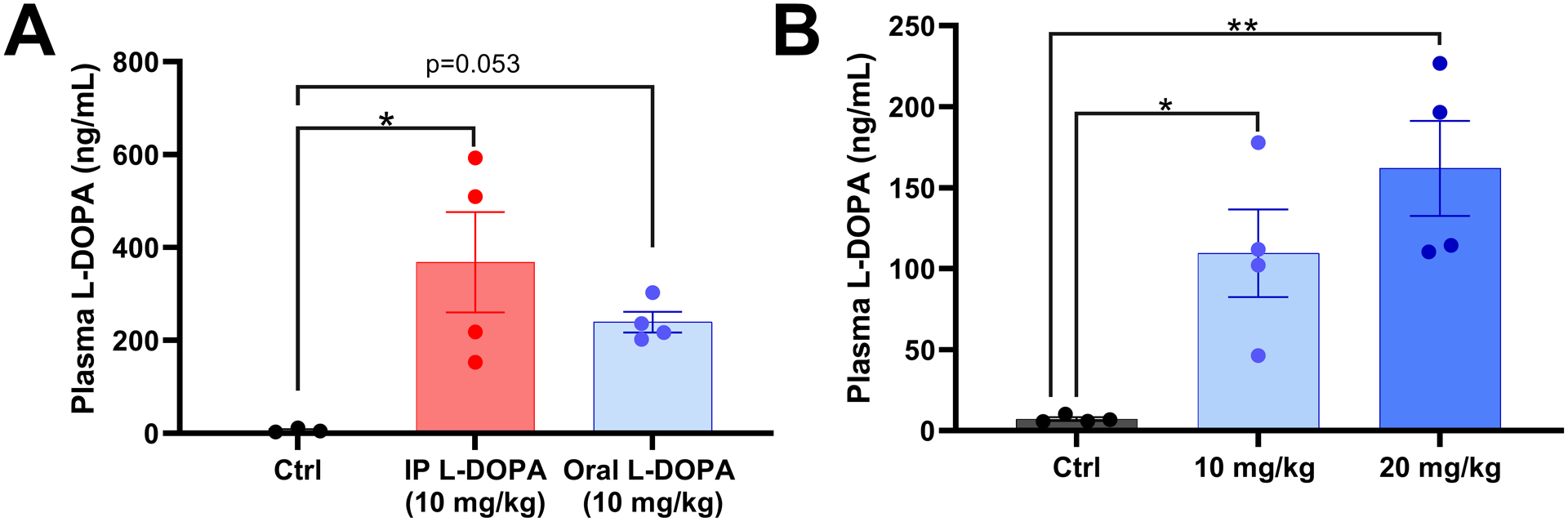
(A) Plasma L-DOPA levels in native control rats and rats treated with an IP injection of L-DOPA (10 mg/kg; n=4) or rats trained to orally consume L-DOPA (10 mg/kg; n=4). Both L-DOPA delivery routes significantly increased L-DOPA compared to native controls (n=3; one-way ANOVA F (2, 8) = 6.219, p = 0.0235, Holm Sidak post-hocs shown). (B) Plasma levels of L-DOPA for control rats and rats treated orally with 10 mg/kg (n=4) or 20 mg/kg of L-DOPA (n=4). The rats in both oral L-DOPA treatment groups has significantly increase plasma L-DOPA compared to controls (n=4; one-way ANOVA F (2, 9) = 11.71, p = 0.0031 Holm-Sidak post hoc analysis shown). Data shown is mean +/− SEM
Additionally, to show that the oral delivery of L-DOPA (20 mg/kg) reached the targeted tissue and potentially modulated dopaminergic activity, striatum and retina were analyzed for DOPAC, dopamine and L-DOPA content via HPLC. The retina and striatum are both tissues of interest for researchers trying to understand how the dopaminergic system is altered by diabetes and it’s complications to the nervous system. In the striatum, oral L-DOPA treatment led to significantly increased DOPAC (increased by 27.44%, Student’s t-test, t(20) = 2.342, p = 0.030), decreased dopamine (decreased by 11.17%, Student’s t-test, t(20) = 2.424, p = 0.025) and increased DOPAC/dopamine ratio (increased by 43.95%, Student’s t-test, t(10) = 3.719, p = 0.004) (Figure 3A,C). Oral L-DOPA treatment did not have a significant impact on L-DOPA content in the striatum (Figure 3E). Interestingly, the retinas of rats treated with oral L-DOPA did not show any significant changes in DOPAC, dopamine or the DOPAC/dopamine ratio, but instead showed significantly higher L-DOPA levels in the retina (increased by 918.10%, Student’s t-test, t(10) = 5.352, p = 0.0003), (Figure 3B, D, F).
Figure 3:
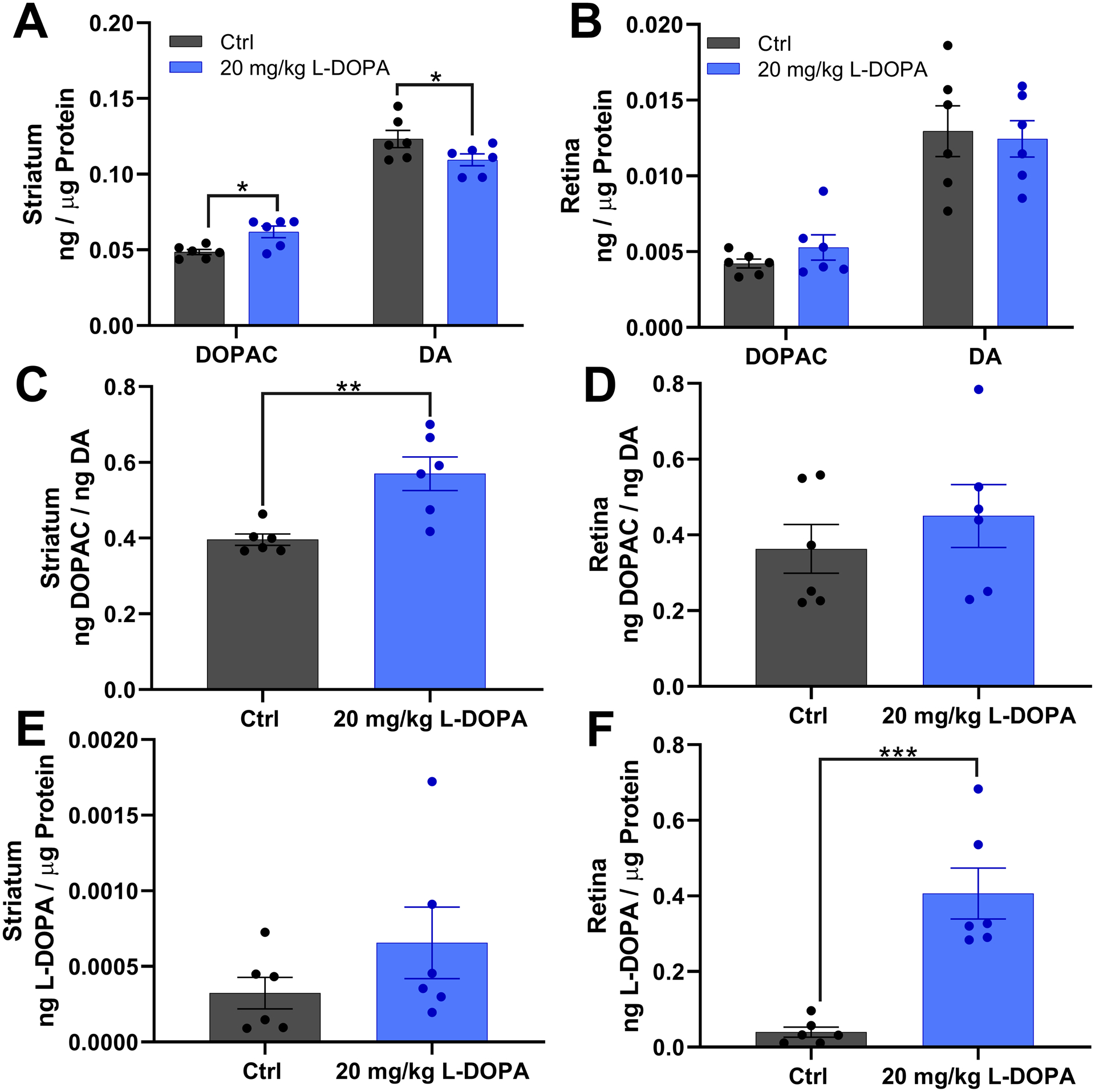
(A, B) Show dopamine (DA) and its metabolite DOPAC levels in the striatum and retina respectively. (C, D) shows the DOPAC/DA ratio, a metric for dopamine turnover, in the striatum and retina respectively. (E, F) show L-DOPA levels in the striatum and retina respectively. Striatum from rats treated with L-DOPA show significantly increased DOPAC (t(20) = 2.342, p = 0.030, t-test), significantly decreased dopamine (t(20) = 2.424, p = 0.025, t-test), and significantly increased DOPAC/DA ratio compared to controls (t(10) = 3.719, p = 0.004, t-test). Retinas from rats treated with L-DOPA (n=6) show significantly increased L-DOPA compared to controls (n=6; t(10) = 5.352, p < 0.001, t-test).
Finally, Vehicle and L-DOPA treated rats can remain trained 4 weeks after the initial training. Vehicle treated rats had 100% compliance for each day assessed 4 weeks after training, while L-DOPA treated rats had 85.71% and 71.43% compliance on days 1 and 3 despite having 100% percent compliance on all other days assessed (Figure 4).
Figure 4.
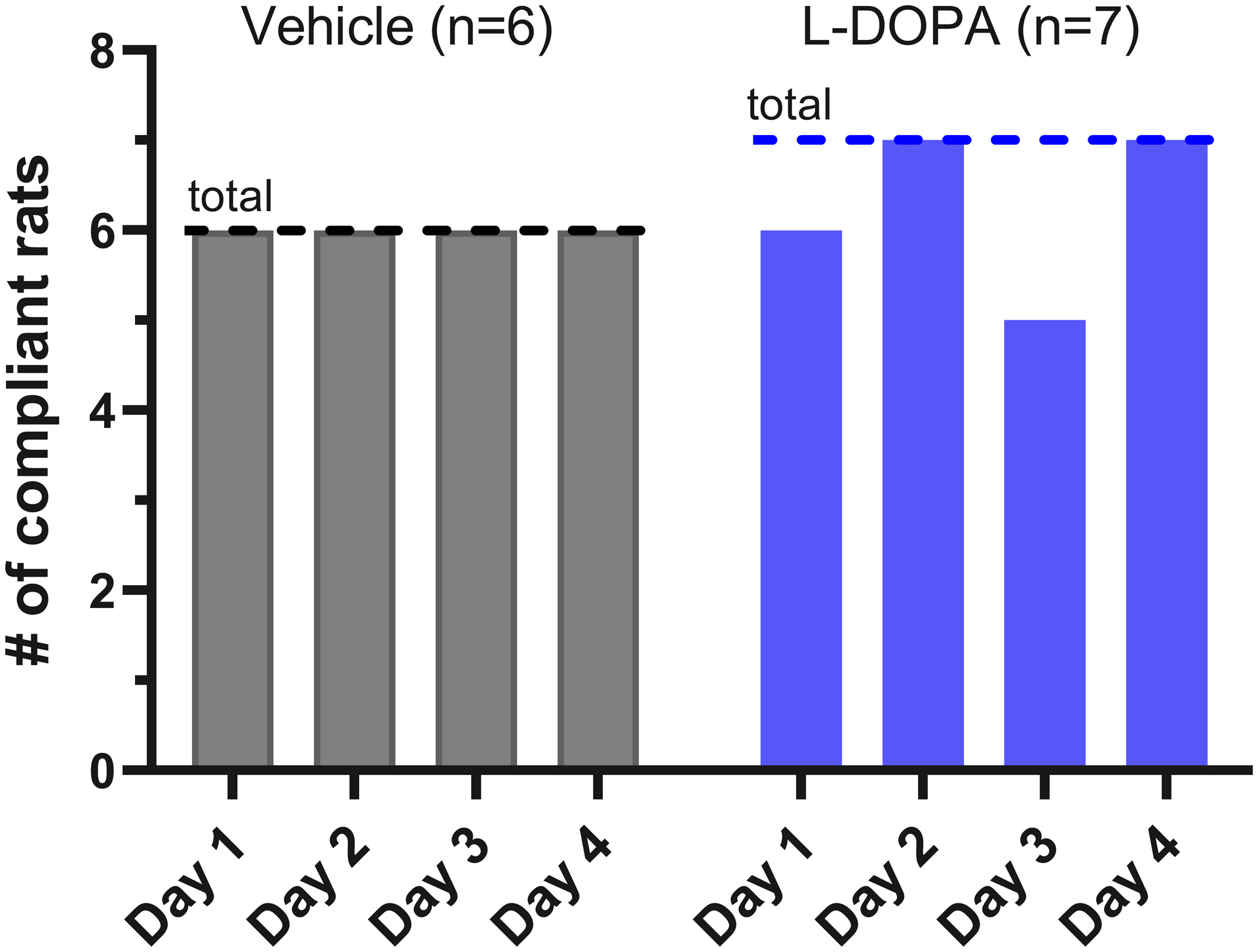
Average number of rats compliant for a daily dose of orally-delivered vehicle (n=6) or L-DOPA (n=7) four weeks after the initial training completion. L-DOPA treated rats showed a reduction in compliance relative to controls at days 1 and 3, while 100% of control animals are compliant on all four days. The dotted line indicates the total number of rats in the cohort.
Discussion:
Here, we developed and experimentally validated a method to train rats to syringe feed voluntarily as a means to administer precise doses of experimental compounds. We verified that rats can be trained within five days using sucrose water to comply with voluntary oral dosing of L-DOPA via syringe-feeding with an artificially sweetened (sucralose) solution. Administering L-DOPA through voluntary syringe feeding allowed for sufficient absorption to target regions: the blood in a dose dependent fashion (Figure 2) and the retina (Figure 3). Additionally, L-DOPA exerted a pharmaceutical effect on dopaminergic activity in the striatum, which showed increased dopamine turnover via the DOPAC/dopamine ratio (Figure 3). Previous studies by Aung et al, which employed IP injections of L-DOPA (without carbidopa co-administration) in diabetic rats, did not show altered dopamine or DOPAC in response to L-DOPA administration and did not attempt to measure retinal L-DOPA content.14
This method successfully circumvents some of the well-cited drawbacks associated with conventional IP injections and offers several advantages over IP injections while retaining the capacity for precise dosing. Syringe-feeding rats voluntarily negated the need to restrain rats with force, resulting in a lower stress method to administer experimental compounds to rats on a daily basis. Importantly, potential injuries caused by needle insertion within the peritoneal cavity and/or premature euthanization were avoided. Additionally, this technique for drug administration does not demand the special skill set or advanced training that other methods require, like oral gavage. Thus, we consider this technique to be more accessible to researchers with less experience handling and administering drugs to rodent models, while also providing precise dosing. This technique could also be implemented with drugs that are affected by stress hormones. Additionally, since this technique employs an artificially sweetened, water-based gel matrix, it can mask the taste of mildly bitter medications and reinforce compliance with ingestion of experimental compounds. The viscous, yet liquid, gel matrix suspends drug particles homogeneously after vertexing, allowing for consistent dosing between animals and experiments. Taken together, our data suggest that oral drug delivery is a suitable replacement for conventional IP injections of L-DOPA that have been traditionally implemented in rat experiments.
Another benefit to this approach for delivering experimental compounds is that it mirrors how drugs are taken in the clinic. Most human medications are administered orally. Combinations of drugs, such as L-DOPA/carbidopa used here, are difficult to combine into a single injectable solution due to different solubility profiles in injection-safe solvents. Because this method administers a homogenous suspension to accomplish precise dosing, this method is ideal for administering drug mixtures that are otherwise hard to combine into a solvent. Additionally, we are able to quantify how much drug delivery reaches our target tissues of interest.
While these experiments lay the foundations for a reliable and voluntary method to administer experimental compounds to laboratory rats, there are some limitations in the utility of this technique depending on the needs of the researcher. First is the lack of 100% compliance in L-DOPA treated rats at four weeks post-training, while Vehicle treated animals had 100% compliance each day (Figure 4). These data suggest that adding L-DOPA to the mixture decreased compliance, perhaps by decreasing the palatability of the mixture. For the purposes of our experiments, these non-compliant animals would comply with their prescribed doses if the mixture was supplemented with 10% sucrose. Still, this represents a limitation that must be taken into account, and a researcher may have to modify this method to administer different compounds depending on the dose and palatability.
Second, our HPLC results are consistent with the evidence that ingestion of experimental compounds is a less efficient administration strategy than IP or IV injection (Figure 2A). Researchers looking to maximize efficacy using expensive experimental compounds may opt for the IP or IV injection to maximize bioavailability for a given dose.
Third, we re-used the first cohort of rats for three experiments which included repeated exposure to isoflurane anesthetic for blood draw from the tail vein. These experiments were done one week apart which should have been sufficient time for the L-DOPA to clear (half-life of 0.9 hours)24 and also long enough to not produce a stressful aversion to repeated isoflurane exposures25. However, these could be potential confounders in our results. Finally, this study was performed on all male rats, and is therefore limited in its generalizability to studies using both male and female rats.
Factors that may influence success with this technique include the palatability of the experimental compound being administered. Unpleasant tasting or unpalatable compounds may be more suitable as an injection if rats cannot be trained to consume them. Similarly, compounds that exert an unpleasant or toxic effect on the rat may ultimately be avoided by the rat and would be more suitable as an injectable for routine treatment. It is worth noting that the drug delivery vehicle can easily be sweetened or supplemented to further mask the taste of an experimental compound. For example, non-compliant rats for these experiments would still consume their dose of L-DOPA if it was sweetened with 10% sucrose. Furthermore, some drugs, such as insulin, are subject to degradation from stomach and bile acids, making them unsuitable for ingestion and administration with this method. It also remains unclear whether the age of the rats affects their compliance or capacity to be trained.
Our study describes compliance measured out to 4 weeks (Figure 4), yet many experimental designs require rats to be treated daily for several months. While not studied here, we expect the oral dosing behavior to persist across several months, particularly if performed routinely. Rat strains other than Long Evans rats are commonly used for experiments requiring routine administration of experimental compounds, and it is not known if all strains will respond as favorably to this treatment paradigm. Additional studies will need to be conducted to monitor compliance in other rodents as well as in long-term dosing.
Taken together, these data suggest that laboratory rodents may be trained to voluntarily ingest palatable drug combinations that are otherwise difficult to administer using a single injection. Voluntary oral dosing may be a suitable replacement to conventional methods to administer compounds, such as IP injection or oral gavage, and may serve as a refinement that improves animal well-being in laboratories.
Acknowledgements
This work was supported by the Department of Veterans Affairs Rehab R&D Service Career Development Awards (CDA-2; RX002928) to RSA, Merit Award (RX002615) and Research Career Scientist Award (RX003134) to MTP. This study was supported in part by the Emory HPLC Bioanalytical Core (EHBC), which was supported by the Department of Pharmacology, Emory University School of Medicine and the Georgia Clinical & Translational Science Alliance of the National Institutes of Health under Award Number UL1TR002378.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
Research Data Availability Statement
All data used in this manuscript have been reported. The authors will freely share any further information upon request.
References
- 1.Turner PV, Pekow C, Vasbinder MA, et al. Administration of substances to laboratory animals: equipment considerations, vehicle selection, and solute preparation. J Am Assoc Lab Anim Sci 2011; 50: 614–627. 2012/02/15. [PMC free article] [PubMed] [Google Scholar]
- 2.Gärtner DBKD K. Stress response of rats to handling and experimental procedures. Laboratory Animals 1980; 14: 267–274. DOI: 10.1258/002367780780937454. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt U and Hiemke C. Strain Differences in Open-Field and Elevated Plus-Maze Behavior of Rats Without and With Pretest Handling. Pharmacology Biochemistry and Behavior 1998; 59: 807–811. DOI: 10.1016/S0091-3057(97)00502-9. [DOI] [PubMed] [Google Scholar]
- 4.Balcombe JP, Barnard ND and Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 2004; 43: 42–51. 2005/01/27. [PubMed] [Google Scholar]
- 5.Tannenbaum J and Bennett BT. Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. Journal of the American Association for Laboratory Animal Science : JAALAS 2015; 54: 120–132. [PMC free article] [PubMed] [Google Scholar]
- 6.Turner PV, Brabb T, Pekow C, et al. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 2011; 50: 600–613. 2012/02/15. [PMC free article] [PubMed] [Google Scholar]
- 7.Turner PV, Brabb T, Pekow C, et al. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 2011; 50: 600–613. [PMC free article] [PubMed] [Google Scholar]
- 8.Meijer MK SB, van Zutphen LF, Baumans V. Effect of restraint and injection methods on heart rate and body temperature in mice. Laboratory Animals 2006; 40: 382–391. DOI: 10.1258/002367706778476370. [DOI] [PubMed] [Google Scholar]
- 9.Coria-Avila GA, Gavrila AM, Ménard S, et al. Cecum location in rats and the implications for intraperitoneal injections. Lab Animal 2007; 36: 25–30. DOI: 10.1038/laban0707-25. [DOI] [PubMed] [Google Scholar]
- 10.Turner PV, Vaughn E, Sunohara-Neilson J, et al. Oral gavage in rats: animal welfare evaluation. J Am Assoc Lab Anim Sci 2012; 51: 25–30. 2012/02/15. [PMC free article] [PubMed] [Google Scholar]
- 11.Arantes-Rodrigues R, Henriques A, Pinto-Leite R, et al. The effects of repeated oral gavage on the health of male CD-1 mice. Lab Animal 2012; 41: 129–134. DOI: 10.1038/laban0512-129. [DOI] [PubMed] [Google Scholar]
- 12.Germann PG and Ockert D. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a Fischer 344 rat carcinogenicity study. Lab Anim Sci 1994; 44: 338–343. 1994/08/01. [PubMed] [Google Scholar]
- 13.Atcha Z, Rourke C, Neo AH, et al. Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci 2010; 49: 335–343. 2010/07/01. [PMC free article] [PubMed] [Google Scholar]
- 14.Aung MH, Park HN, Han MK, et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci 2014; 34: 726–736. 2014/01/17. DOI: 10.1523/jneurosci.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MK, Aung MH, Mees L, et al. Dopamine Deficiency Mediates Early Rod-Driven Inner Retinal Dysfunction in Diabetic Mice. Invest Ophthalmol Vis Sci 2018; 59: 572–581. 2018/01/27. DOI: 10.1167/iovs.17-22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillmann S and Wegener G. Syringe-feeding as a novel delivery method for accurate individual dosing of probiotics in rats. Benef Microbes 2018; 9: 311–315. 2017/12/22. DOI: 10.3920/BM2017.0127. [DOI] [PubMed] [Google Scholar]
- 17.Corbett A, McGowin A, Sieber S, et al. A method for reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Laboratory Animals 2012; 46: 318–324. DOI: 10.1258/la.2012.012018. [DOI] [PubMed] [Google Scholar]
- 18.Diogo LN, Faustino IV, Afonso RA, et al. Voluntary Oral Administration of Losartan in Rats. Journal of the American Association for Laboratory Animal Science : JAALAS 2015; 54: 549–556. [PMC free article] [PubMed] [Google Scholar]
- 19.Huang-Brown KM and Guhad FA. Chocolate, an effective means of oral drug delivery in rats. Lab Anim (NY) 2002; 31: 34–36. 2002/10/31. DOI: 10.1038/5000197. [DOI] [PubMed] [Google Scholar]
- 20.Schleimer SB, Johnston GA and Henderson JM. Novel oral drug administration in an animal model of neuroleptic therapy. J Neurosci Methods 2005; 146: 159–164. 2005/08/02. DOI: 10.1016/j.jneumeth.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Kaakkola S, Männistö PT, Nissinen E, et al. The effect of an increased ratio of carbidopa to levodopa on the pharmacokinetics of levodopa. Acta Neurologica Scandinavica 1985; 72: 385–391. DOI: 10.1111/j.1600-0404.1985.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 22.Tambasco N, Romoli M and Calabresi P. Levodopa in Parkinson’s Disease: Current Status and Future Developments. Curr Neuropharmacol 2018; 16: 1239–1252. DOI: 10.2174/1570159X15666170510143821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song CH, Fan X, Exeter CJ, et al. Functional analysis of dopaminergic systems in a DYT1 knock-in mouse model of dystonia. Neurobiology of disease 2012; 48: 66–78. 2012/06/05. DOI: 10.1016/j.nbd.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huebert ND, Palfreyman MG and Haegele KD. A comparison of the effects of reversible and irreversible inhibitors of aromatic L-amino acid decarboxylase on the half-life and other pharmacokinetic parameters of oral L-3,4-dihydroxyphenylalanine. Drug Metab Dispos 1983; 11: 195–200. 1983/05/01. [PubMed] [Google Scholar]
- 25.Wong D, Makowska IJ and Weary DM. Rat aversion to isoflurane versus carbon dioxide. Biol Lett 2013; 9: 20121000. 2012/12/21. DOI: 10.1098/rsbl.2012.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this manuscript have been reported. The authors will freely share any further information upon request.


