Abstract
Background
Dexamethasone decreases mortality in coronavirus disease 2019 (COVID-19) patients on intensive respiratory support (IRS) but is of uncertain benefit if less severely ill. We determined whether early (within 48 h) dexamethasone was associated with mortality in patients hospitalised with COVID-19 not on IRS.
Methods
We included patients admitted to US Veterans Affairs hospitals between 7 June 2020 and 31 May 2021 within 14 days after a positive test for severe acute respiratory syndrome coronavirus 2. Exclusions included recent prior corticosteroids and IRS within 48 h. We used inverse probability of treatment weighting (IPTW) to balance exposed and unexposed groups, and Cox proportional hazards models to determine 90-day all-cause mortality.
Results
Of 19 973 total patients (95% men, median age 71 years, 27% black), 15 404 (77%) were without IRS within 48 h. Of these, 3514 out of 9450 (34%) patients on no oxygen received dexamethasone and 1042 (11%) died; 4472 out of 5954 (75%) patients on low-flow nasal cannula (NC) only received dexamethasone and 857 (14%) died. In IPTW stratified models, patients on no oxygen who received dexamethasone experienced 76% increased risk for 90-day mortality (hazard ratio (HR) 1.76, 95% CI 1.47–2.12); there was no association with mortality among patients on NC only (HR 1.08, 95% CI 0.86–1.36).
Conclusions
In patients hospitalised with COVID-19, early initiation of dexamethasone was common and was associated with no mortality benefit among those on no oxygen or NC only in the first 48 h; instead, we found evidence of potential harm. These real-world findings do not support the use of early dexamethasone in hospitalised COVID-19 patients without IRS.
Short abstract
Although commonly used, dexamethasone within 48 h of admission was associated with increased 90-day mortality in patients hospitalised with COVID-19 not on oxygen and with no mortality benefit in patients on low-flow nasal cannula https://bit.ly/3l2aqjb
Introduction
Corticosteroids have emerged as an effective therapy for critically ill patients with coronavirus disease 2019 (COVID-19). The large UK RECOVERY randomised controlled trial of corticosteroids in COVID-19 patients demonstrated an overall 2.8% absolute decrease in mortality for patients treated with dexamethasone compared with usual care [1]. When stratified by respiratory support at randomisation, dexamethasone was associated with greater benefit among those on invasive mechanical ventilation (IMV) versus supplemental oxygen (inclusive of noninvasive mechanical ventilation (NIV)); dexamethasone was not significantly associated with mortality in those not on oxygen. Dissemination of these and other results led to rapid uptake in the use of corticosteroids for COVID-19 patients, particularly those receiving more intensive respiratory support (IRS) such as high-flow nasal cannula (HFNC), NIV and IMV [2–6].
However, whether corticosteroids are beneficial in all patients with COVID-19 remains uncertain. The association between corticosteroids and outcomes among a wider group of patients with COVID-19, including a larger proportion without IRS than in the RECOVERY trial, has been mixed [7–11]. Variability in the effect of corticosteroids may be due to numerous factors. A recent Cochrane review concluded that systemic corticosteroids “probably reduce all-cause mortality slightly” but that there is an “urgent need for good-quality evidence for specific subgroups of disease severity, for which we propose level of respiratory support at randomisation” [12].
We determined the association between corticosteroids and 90-day all-cause mortality using real-world clinical data from the Dept of Veterans Affairs (VA), the largest integrated healthcare system in the US. In a racially and geographically diverse, national cohort of hospitalised COVID-19 patients, we first assessed patterns of corticosteroid receipt. As nearly all patients on IRS received corticosteroids, mainly dexamethasone, we focused on those who were without IRS. We used propensity score weighting to account for confounding by indication. We hypothesised that dexamethasone would not be associated with mortality benefit in patients without IRS.
Methods
Study design and population
We conducted an observational cohort study of 27 168 patients admitted to a VA hospital within 14 days after a positive PCR or antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) between 7 June 2020 and 31 May 2021 (to allow 90-day follow-up on all) [13, 14]. Before 7 June 2020, corticosteroids were mostly initiated after 48 h. Index date was defined as date of presentation, including emergency room and time under observation status if not admitted directly. We determined length of stay by concatenating episodes of care separated by <24 h, with first episode on the index date as day 1. Due to changes in COVID-19 incidence and treatment protocols over time, we divided the observation period into seven time phases (table 1). Additional methodological details are provided in the supplementary material.
TABLE 1.
Characteristics of patients# stratified by highest oxygen support during the first 48 h of hospitalisation for COVID-19
| Overall | Oxygen support first 48 h | |||||
| None | NC only | Other/NIV | HFNC | IMV | ||
| Overall cohort | 19 973 | 9450 | 5954 | 2103 | 2051 | 415 |
| Demographics | ||||||
| Age, years | ||||||
| <50 | 1760 (9) | 1013 (11) | 459 (8) | 138 (7) | 120 (6) | 30 (7) |
| 50–59 | 2361 (12) | 1205 (13) | 691 (12) | 204 (10) | 216 (11) | 45 (11) |
| 60–69 | 4677 (23) | 2194 (23) | 1340 (23) | 490 (23) | 536 (26) | 117 (28) |
| 70–79 | 7230 (36) | 3155 (33) | 2235 (38) | 833 (40) | 837 (41) | 170 (41) |
| ≥80 | 3945 (20) | 1883 (20) | 1229 (21) | 438 (21) | 342 (17) | 53 (13) |
| Male | 18 993 (95) | 8948 (95) | 5651 (95) | 2002 (95) | 1991 (97) | 401 (97) |
| Race | ||||||
| White, non-Hispanic | 11 033 (55) | 5019 (53) | 3387 (57) | 1253 (60) | 1161 (57) | 213 (51) |
| Black, non-Hispanic | 5449 (27) | 2854 (30) | 1522 (26) | 498 (24) | 481 (23) | 94 (23) |
| Hispanic | 1738 (9) | 773 (8) | 526 (9) | 167 (8) | 216 (11) | 56 (13) |
| Other | 1144 (6) | 510 (5) | 330 (6) | 134 (6) | 134 (7) | 36 (9) |
| Unknown | 609 (3) | 294 (3) | 189 (3) | 51 (2) | 59 (3) | 16 (4) |
| Phase (admission date) | ||||||
| 1 (7 June–11 July 2020) | 1505 (8) | 590 (6) | 592 (10) | 115 (5) | 161 (8) | 47 (11) |
| 2 (12 July–15 Aug 2020) | 1688 (8) | 602 (6) | 714 (12) | 148 (7) | 190 (9) | 34 (8) |
| 3 (16 Aug–31 Oct 2020) | 2724 (14) | 931 (10) | 1145 (19) | 254 (12) | 319 (16) | 75 (18) |
| 4 (1 Nov–30 Nov 2020) | 2842 (14) | 891 (9) | 1215 (20) | 273 (13) | 405 (20) | 58 (14) |
| 5 (1 Dec–31 Dec 2020) | 4052 (20) | 2204 (23) | 866 (15) | 519 (25) | 387 (19) | 76 (18) |
| 6 (1 Jan–31 Jan 2021) | 3588 (18) | 2045 (22) | 749 (13) | 428 (20) | 300 (15) | 66 (16) |
| 7 (1 Feb–31 May 2021) | 3574 (18) | 2187 (23) | 673 (11) | 366 (17) | 289 (14) | 59 (14) |
| Selected conditions | ||||||
| Dementia | 2357 (12) | 1290 (14) | 659 (11) | 240 (11) | 137 (7) | 31 (7) |
| CHF | 4356 (22) | 1981 (21) | 1339 (22) | 555 (26) | 401 (20) | 80 (19) |
| COPD/asthma | 5903 (30) | 2433 (26) | 1978 (33) | 738 (35) | 636 (31) | 118 (28) |
| Charlson Comorbidity Index | ||||||
| 0 | 3718 (19) | 1885 (20) | 1034 (17) | 316 (15) | 407 (20) | 76 (18) |
| 1–2 | 6336 (32) | 2939 (31) | 1897 (32) | 627 (30) | 718 (35) | 155 (37) |
| 3–4 | 4622 (23) | 2102 (22) | 1457 (24) | 520 (25) | 457 (22) | 86 (21) |
| ≥5 | 5297 (27) | 2524 (27) | 1566 (26) | 640 (30) | 469 (23) | 98 (24) |
| Medication use | ||||||
| Corticosteroid, any systemic | ||||||
| First 48 h | 11 970 (60) | 3514 (37) | 4627 (78) | 1521 (72) | 1923 (94) | 385 (93) |
| Later than 48 h | 1607 (8) | 870 (9) | 467 (8) | 202 (10) | 47 (2) | 21 (5) |
| None | 6396 (32) | 5066 (54) | 860 (14) | 380 (18) | 81 (4) | 9 (2) |
| Dexamethasone | ||||||
| First 48 h | 11 361 (57) | 3198 (34) | 4472 (75) | 1447 (69) | 1871 (91) | 373 (90) |
| Later than 48 h | 1586 (8) | 833 (9) | 473 (8) | 201 (10) | 55 (3) | 24 (6) |
| None | 7026 (35) | 5419 (57) | 1009 (17) | 455 (22) | 125 (6) | 18 (4) |
| Remdesivir | ||||||
| First 48 h | 9533 (48) | 2716 (29) | 3706 (62) | 1197 (57) | 1637 (80) | 277 (67) |
| Later than 48 h | 1607 (8) | 768 (8) | 532 (9) | 188 (9) | 88 (4) | 31 (7) |
| None | 8833 (44) | 5966 (63) | 1716 (29) | 718 (34) | 326 (16) | 107 (26) |
| Prophylactic anticoagulant | ||||||
| First 48 h | 14 708 (74) | 6820 (72) | 4502 (76) | 1576 (75) | 1514 (74) | 296 (71) |
| Later than 48 h | 1752 (9) | 842 (9) | 463 (8) | 183 (9) | 212 (10) | 52 (13) |
| None | 3513 (18) | 1788 (19) | 989 (17) | 344 (16) | 325 (16) | 67 (16) |
| Therapeutic anticoagulant | ||||||
| First 48 h | 4550 (23) | 1815 (19) | 1446 (24) | 516 (25) | 647 (32) | 126 (30) |
| Later than 48 h | 13 921 (70) | 6627 (70) | 4220 (71) | 1478 (70) | 1333 (65) | 263 (63) |
| None | 1502 (8) | 1008 (11) | 288 (5) | 109 (5) | 71 (3) | 26 (6) |
| Vasopressors | ||||||
| First 48 h | 391 (2) | 55 (1) | 35 (1) | 22 (1) | 42 (2) | 237 (57) |
| Later than 48 h | 1197 (6) | 272 (3) | 272 (5) | 132 (6) | 417 (20) | 104 (25) |
| None | 18 385 (92) | 9123 (97) | 5647 (95) | 1949 (93) | 1592 (78) | 74 (18) |
| Intensive care | ||||||
| First 48 h | 4143 (21) | 1172 (12) | 884 (15) | 427 (20) | 1275 (62) | 385 (93) |
| Later than 48 h | 1468 (7) | 465 (5) | 554 (9) | 211 (10) | 221 (11) | 17 (4) |
| None | 14 362 (72) | 7813 (83) | 4516 (76) | 1465 (70) | 555 (27) | 13 (3) |
| Intubation | ||||||
| First 48 h | 415 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 415 (100) |
| Later than 48 h | 1078 (5) | 257 (3) | 259 (4) | 121 (6) | 441 (22) | 0 (0) |
| None | 18 480 (93) | 9193 (97) | 5695 (96) | 1982 (94) | 1610 (78) | 0 (0) |
| Hospital length of stay, days | ||||||
| <7 | 10 604 (53) | 5633 (60) | 3316 (56) | 1098 (52) | 501 (24) | 56 (13) |
| 7–13 | 5365 (27) | 2248 (24) | 1613 (27) | 602 (29) | 788 (38) | 114 (27) |
| ≥14 | 4004 (20) | 1569 (17) | 1025 (17) | 403 (19) | 762 (37) | 245 (59) |
| Mortality (unadjusted, cumulative incidence) | ||||||
| 30 days | 2587 (13) | 724 (8) | 648 (11) | 351 (17) | 648 (32) | 210 (51) |
| 60 days | 3125 (16) | 939 (10) | 799 (13) | 421 (20) | 731 (36) | 234 (56) |
| 90 days | 3340 (17) | 1041 (11) | 857 (14) | 448 (21) | 752 (37) | 241 (58) |
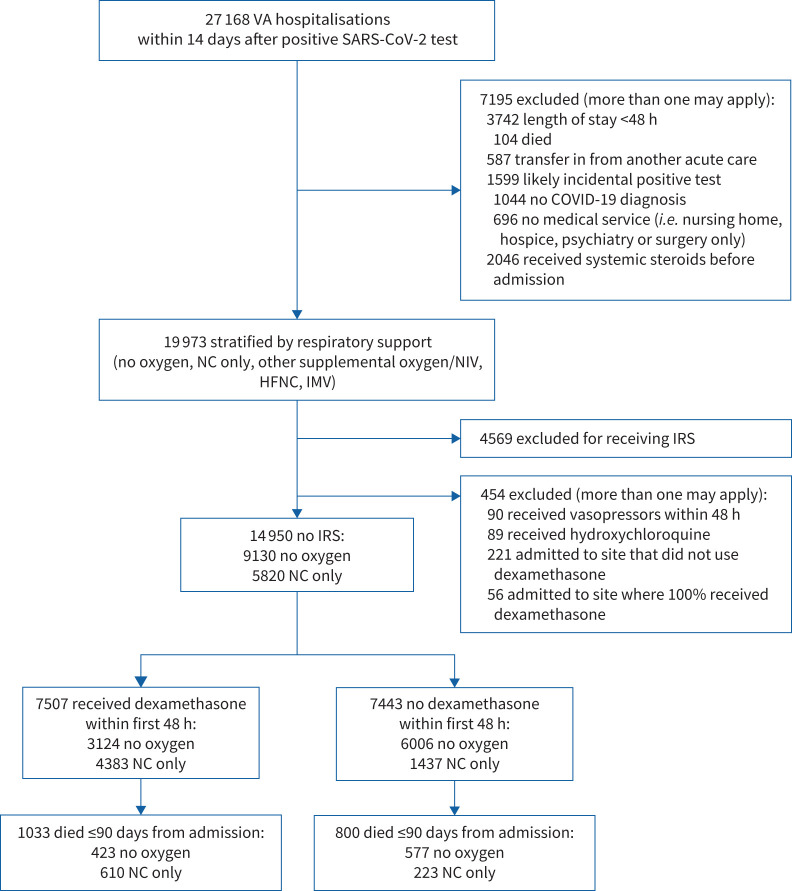
Data are presented as n or n (%). NC: nasal cannula; NIV: noninvasive ventilation; HFNC: high-flow nasal cannula; IMV: invasive mechanical ventilation; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease. #: the 19 973 patients stratified by respiratory support in figure 1, prior to additional exclusions for mortality analyses among those on no intensive respiratory support.
Our study was approved by the Institutional Review Boards of VA Puget Sound Health Care System (Seattle, WA, USA), and VA Connecticut Healthcare System and Yale University (New Haven, CT, USA), all of whom granted waivers of consent.
Exclusions
Of 27 168 patients, we excluded 7195, yielding a cohort of 19 973 patients (figure 1 and supplementary material). The most common exclusion was length of stay <48 h as these patients had insufficient time to receive dexamethasone, followed by any systemic corticosteroid exposure prior to the index date. This was defined as any corticosteroids within 14 days or receipt of corticosteroids for ≥14 days in the preceding 45 days. For mortality analyses, we further excluded 454 patients because they were at sites where no or all patients received dexamethasone (n=277), received hydroxychloroquine (n=89) or received vasopressors in the first 48 h (n=90), as these patients may have had an alternative indication for corticosteroids.
FIGURE 1.
Derivation of the study population. Time period of admission from 7 June 2020 through 31 May 2021. VA: Veterans Affairs; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; IRS: intensive respiratory support; NC: nasal cannula; NIV: noninvasive ventilation; HFNC: high-flow nasal cannula; IMV: invasive mechanical ventilation.
Exposures, outcomes and covariates
All data came from VA electronic health record extracts, which provide directly analysable demographics, comorbidities, medications, vital signs and laboratory results as well as notes that require text processing.
Dexamethasone exposure
Exposure was defined as at least one dose of oral or parenteral dexamethasone within 48 h after the index date as determined from barcode medication administration data. We also determined administration of other systemic corticosteroids (prednisone, prednisolone, methylprednisolone and/or hydrocortisone).
Outcome
The primary outcome was 90-day all-cause mortality, ascertained using inpatient records and VA death registry data to capture deaths outside of hospitalisation.
Respiratory support
We stratified patients by highest level of respiratory support during the initial 48 h of hospitalisation into the following categories: 1) no oxygen support; 2) low-flow oxygen via nasal cannula (NC) that was not identified as a high-flow or other delivery device; 3) other supplemental oxygen/NIV, including face mask, nonrebreather mask or other delivery not identifiable as low-flow NC or high-flow; 4) high-flow oxygen/HFNC; and 5) IMV. When no evidence of oxygen supplementation was found, patients were classified as without oxygen (category 1). IMV was identified by structured data sources (International Classification of Diseases, 10th Revision procedure and Current Procedural Terminology codes). Categories 2–4 were assessed from unstructured text notes using natural language processing, validated with manual chart review to identify key terms indicative of respiratory support (supplementary material).
Covariates
We obtained age, race, ethnicity, sex, comorbidities, additional medications, vital signs and laboratory results. We calculated the Charlson Comorbidity Index (CCI) [15] and the Veterans Health Administration COVID-19 (VACO) Index (table 2 and supplementary table E1) [16]. We focused on routinely collected laboratory tests that have been associated with increased mortality in COVID-19 [17], including albumin, liver function, lactate, white blood cell count and creatinine (table 2 and supplementary table E1). We selected the worst laboratory tests, temperature, blood pressure and pulse oximetry within the initial 48 h. To account for potential effects of co-prescribed medications, we included use of remdesivir and prophylactic anticoagulants within the initial 48 h [14]. Intensive care unit (ICU) admission was determined using VA bedsection codes [14, 18]. As there was generally very little missing data (<5%), an explicit level for missingness was used for selected covariates.
TABLE 2.
Characteristics of the combined cohort of patients without oxygen or on nasal cannula only after inverse probability of treatment weighting for estimating the average treatment effect in the total population (ATE models)
| Dexamethasone | SMD | ||
| No | Yes | ||
| Cohort | 11 963.6 | 12 887.0 | |
| Age, years | |||
| <50 | 1082.7 (9.0) | 1142.0 (8.9) | −0.002 |
| 50–59 | 1367.3 (11.4) | 1551.5 (12.0) | 0.006 |
| 60–69 | 2800.3 (23.4) | 2959.5 (23.0) | −0.004 |
| 70–79 | 4206.0 (35.2) | 4662.9 (36.2) | 0.010 |
| ≥80 | 2507.4 (21.0) | 2571.2 (20.0) | −0.010 |
| Male | 11 323.9 (94.7) | 12 237.6 (95.0) | 0.003 |
| Race | |||
| White, non-Hispanic | 6430.7 (53.8) | 7170.0 (55.6) | 0.019 |
| Black, non-Hispanic | 3567.0 (29.8) | 3507.0 (27.2) | −0.026 |
| Hispanic | 940.7 (7.9) | 1120.5 (8.7) | 0.008 |
| Other | 666.5 (5.6) | 673.5 (5.2) | −0.003 |
| Unknown | 358.7 (3.0) | 416.1 (3.2) | 0.002 |
| Phase (admission date) | |||
| 1 (7 June–11 July 2020) | 1112.0 (9.3) | 865.6 (6.7) | −0.026 |
| 2 (12 July–15 Aug 2020) | 1063.1 (8.9) | 1074.1 (8.3) | −0.006 |
| 3 (16 Aug–31 Oct 2020) | 1764.3 (14.7) | 1784.1 (13.8) | −0.009 |
| 4 (1 Nov–30 Nov 2020) | 1567.9 (13.1) | 1894.9 (14.7) | 0.016 |
| 5 (1 Dec–31 Dec 2020) | 2347.1 (19.6) | 2693.3 (20.9) | 0.013 |
| 6 (1 Jan–31 Jan 2021) | 1998.7 (16.7) | 2315.6 (18.0) | 0.013 |
| 7 (1 Feb–31 May 2021) | 2110.5 (17.6) | 2259.4 (17.5) | −0.001 |
| Site dexamethasone prescribing | |||
| Low | 3173.2 (26.5) | 2421.9 (18.8) | −0.077 |
| Medium | 7115.5 (59.5) | 7480.7 (58.0) | −0.014 |
| High | 1674.9 (14.0) | 2984.5 (23.2) | 0.092 |
| Smoking status | |||
| Unknown | 384.8 (3.2) | 384.5 (3.0) | −0.002 |
| Never-smoker | 4080.9 (34.1) | 4491.1 (34.8) | 0.007 |
| Ex-smoker | 4576.0 (38.2) | 5277.6 (41.0) | 0.027 |
| Current smoker | 2921.8 (24.4) | 2734.0 (21.2) | −0.032 |
| AUDIT-C score | |||
| Unknown | 441.7 (3.7) | 431.4 (3.3) | −0.003 |
| 0 | 7978.6 (66.7) | 8489.5 (65.9) | −0.008 |
| 1–3 | 2456.4 (20.5) | 2962.0 (23.0) | 0.025 |
| 4–7 | 734.5 (6.1) | 719.6 (5.6) | −0.006 |
| ≥8 | 352.5 (2.9) | 284.6 (2.2) | −0.007 |
| Comorbidities | |||
| Myocardial infarction | 1078.8 (9.0) | 1096.0 (8.5) | −0.005 |
| CHF | 2678.1 (22.4) | 2865.7 (22.2) | −0.001 |
| CVD | 2113.7 (17.7) | 2094.2 (16.3) | −0.014 |
| Dementia | 1748.8 (14.6) | 1448.8 (11.2) | −0.034 |
| COPD | 3131.2 (26.2) | 3760.1 (29.2) | 0.030 |
| Rheumatoid arthritis | 186.2 (1.6) | 206.8 (1.6) | 0.000 |
| Peptic ulcer | 272.0 (2.3) | 270.9 (2.1) | −0.002 |
| Liver disease, mild | 1504.2 (12.6) | 1452.8 (11.3) | −0.013 |
| Diabetes, uncomplicated | 5683.8 (47.5) | 6182.6 (48.0) | 0.005 |
| Diabetes, complicated | 3873.3 (32.4) | 3969.4 (30.8) | −0.016 |
| Hemi- or paraplegia | 341.0 (2.9) | 285.3 (2.2) | −0.006 |
| Renal disease | 3228.2 (27.0) | 3422.0 (26.6) | −0.004 |
| Liver disease, moderate–severe | 226.5 (1.9) | 205.6 (1.6) | −0.003 |
| Metastatic cancer | 293.0 (2.4) | 256.2 (2.0) | −0.005 |
| HIV | 153.5 (1.3) | 150.8 (1.2) | −0.001 |
| Charlson Comorbidity Index | |||
| 0 | 2212.6 (18.5) | 2412.7 (18.7) | 0.002 |
| 1–2 | 3634.1 (30.4) | 4065.3 (31.5) | 0.012 |
| 3–4 | 2780.0 (23.2) | 3018.6 (23.4) | 0.002 |
| ≥5 | 3336.9 (27.9) | 3390.4 (26.3) | −0.016 |
| Number of doctors (prior year) | |||
| 0 | 4815.2 (40.2) | 5033.7 (39.1) | −0.012 |
| 1 | 3240.7 (27.1) | 3585.3 (27.8) | 0.007 |
| 2–4 | 3564.9 (29.8) | 3946.2 (30.6) | 0.008 |
| ≥5 | 342.8 (2.9) | 321.8 (2.5) | −0.004 |
| Speciality clinics attended | |||
| Cardiology | 3108.1 (26.0) | 3528.6 (27.4) | 0.014 |
| Coagulation | 192.2 (1.6) | 214.1 (1.7) | 0.001 |
| Pacemaker | 428.2 (3.6) | 424.7 (3.3) | −0.003 |
| Dialysis | 206.6 (1.7) | 218.6 (1.7) | 0.000 |
| Gastroenterology | 1147.9 (9.6) | 1277.5 (9.9) | 0.003 |
| Hepatology | 426.1 (3.6) | 359.0 (2.8) | −0.008 |
| Homeless | 817.4 (6.8) | 596.1 (4.6) | −0.022 |
| Co-medications | |||
| Prophylactic anticoagulants, first 48 h | 8665.8 (72.4) | 9589.0 (74.4) | 0.020 |
| Remdesivir, first 48 h | 3664.1 (30.6) | 6050.4 (46.9) | 0.163 |
| Laboratory values | |||
| Albumin, g·dL−1 | |||
| ≥3.5 | 4070.5 (34.0) | 3839.1 (29.8) | −0.042 |
| 3–3.49 | 3935.0 (32.9) | 4550.6 (35.3) | 0.024 |
| <3 | 3361.2 (28.1) | 3986.4 (30.9) | 0.028 |
| Missing | 596.9 (5.0) | 510.9 (4.0) | −0.010 |
| Alanine aminotransferase, IU·L−1 | |||
| <20 | 3461.5 (28.9) | 3151.6 (24.5) | −0.045 |
| 20–39 | 4786.1 (40.0) | 5418.4 (42.0) | 0.020 |
| ≥40 | 3195.2 (26.7) | 3995.4 (31.0) | 0.043 |
| Missing | 520.8 (4.4) | 321.6 (2.5) | −0.019 |
| Aspartate aminotransferase, IU·L−1 | |||
| <20 | 2285.0 (19.1) | 1586.6 (12.3) | −0.068 |
| 20–39 | 5309.3 (44.4) | 5767.2 (44.8) | 0.004 |
| ≥40 | 4369.3 (36.5) | 5533.2 (42.9) | 0.064 |
| Creatinine, mg·dL−1 | |||
| <1.2 | 5238.4 (43.8) | 5616.3 (43.6) | −0.002 |
| 1.2–1.99 | 4423.3 (37.0) | 4862.7 (37.7) | 0.008 |
| ≥2 | 2196.7 (18.4) | 2367.4 (18.4) | 0.000 |
| Missing | 105.2 (0.9) | 40.6 (0.3) | −0.006 |
| Fibrosis-4 index | |||
| <1.45 | 2289.3 (19.1) | 2087.1 (16.2) | −0.029 |
| 1.45–3.25 | 4791.3 (40.0) | 5341.2 (41.4) | 0.014 |
| >3.25 | 4308.2 (36.0) | 5107.3 (39.6) | 0.036 |
| Missing | 574.8 (4.8) | 351.5 (2.7) | −0.021 |
| Lactate, mmol·L−1 | |||
| <1.2 | 1764.8 (14.8) | 2009.6 (15.6) | 0.008 |
| 1.2– <2.0 | 3020.2 (25.2) | 3732.9 (29.0) | 0.037 |
| ≥2.0 | 1626.1 (13.6) | 2027.5 (15.7) | 0.021 |
| Missing | 5552.5 (46.4) | 5117.0 (39.7) | −0.067 |
| Platelet count, µL−1 | |||
| ≥150 | 7892.8 (66.0) | 8381.1 (65.0) | −0.009 |
| <150 | 3988.7 (33.3) | 4482.4 (34.8) | 0.014 |
| Missing | 82.1 (0.7) | 23.6 (0.2) | −0.005 |
| Total bilirubin, mg·dL−1 | |||
| <1 | 8779.4 (73.4) | 9524.9 (73.9) | 0.005 |
| 1–1.2 | 1004.1 (8.4) | 1187.0 (9.2) | 0.008 |
| >1.2 | 1664.4 (13.9) | 1857.2 (14.4) | 0.005 |
| Missing | 515.7 (4.3) | 318.0 (2.5) | −0.018 |
| White blood cell count, µL−1 | |||
| 4–10 | 6641.3 (55.5) | 6266.6 (48.6) | −0.069 |
| <4 | 3139.3 (26.2) | 3812.3 (29.6) | 0.033 |
| >10 | 2183.1 (18.2) | 2808.2 (21.8) | 0.035 |
| C-reactive protein measured | 6674.9 (55.8) | 7810.0 (60.6) | 0.048 |
| D-dimer measured | 8791.6 (73.5) | 10 350.8 (80.3) | 0.068 |
| Vital signs | |||
| Highest temperature, °F (°C) | |||
| <99 (<37.2) | 4466.1 (37.3) | 4443.3 (34.5) | −0.029 |
| 99–100 (37.2–37.8) | 2919.2 (24.4) | 3106.8 (24.1) | −0.003 |
| 100–102 (38.3–38.9) | 3077.0 (25.7) | 3567.9 (27.7) | 0.020 |
| >102 (>38.9) | 1459.9 (12.2) | 1719.3 (13.3) | 0.011 |
| Missing | 41.4 (0.3) | 49.7 (0.4) | 0.000 |
| Mean arterial pressure, mmHg | |||
| <60 | 320.4 (2.7) | 282.2 (2.2) | −0.005 |
| 60–69 | 1627.5 (13.6) | 1682.2 (13.1) | −0.006 |
| 70–89 | 7805.2 (65.2) | 8623.3 (66.9) | 0.017 |
| ≥90 | 2183.3 (18.2) | 2266.1 (17.6) | −0.007 |
| Missing | 27.2 (0.2) | 33.3 (0.3) | 0.000 |
| Lowest oxygen saturation, % | |||
| <88 | 643.0 (5.4) | 1099.6 (8.5) | 0.032 |
| 88–92 | 4961.0 (41.5) | 6515.0 (50.6) | 0.091 |
| 93–95 | 4459.7 (37.3) | 3888.3 (30.2) | −0.071 |
| ≥96 | 1651.6 (13.8) | 1106.9 (8.6) | −0.052 |
| Missing | 248.3 (2.1) | 277.2 (2.2) | 0.001 |
Data are presented as n or n (%) for the pseudo-populations. SMD: standardised mean difference; AUDIT-C: Alcohol Use Disorders Identification Test-Concise; CHF: congestive heart failure; CVD: cerebrovascular disease; COPD: chronic obstructive pulmonary disease.
Statistical analysis
We first compared COVID-19 patients by the five respiratory support categories using summary statistics (table 1). Because nearly all patients on IMV or HFNC received dexamethasone, there was insufficient variability to allow generation of propensity score weights. Category 3 (other/NIV) was heterogenous with respect to respiratory support used and illness severity. For these reasons, as well as the greater clinical equipoise, we restricted our analysis to patients without IRS (specifically, no oxygen or low-flow NC support only).
In those without IRS, we compared mortality by exposure to dexamethasone overall and stratified by NC. To account for confounding by indication, we generated propensity scores for the probability of receiving dexamethasone in the first 48 h using logistic regression. Models included covariates associated with dexamethasone exposure and mortality: comorbidities, laboratory results, vital signs, site utilisation patterns, co-medications and the time phases (table 2 and supplementary table E1). We constructed inverse probability of treatment weights from propensity scores for each patient to create pseudo-populations with balanced distributions of covariates [19]. In our primary analysis, we used average treatment effect (ATE) weights, reflecting the overall population from which the sample was taken. We used stabilised weights and trimmed from analysis the 10 patients with the most extreme high and low weights [20]. We calculated standardised mean differences between treatment groups and considered ≤0.2 as balanced. Using days since index date as the time scale, we compared differences in survival using weighted Kaplan–Meier plots [21] and estimated the ATE using Cox proportional hazards models to generate hazard ratios (HR) and 95% confidence limits using a robust variance estimator. We included the VACO Index in outcome models to further account for residual confounding [16].
Subgroup and sensitivity analyses
In subgroup analyses, we excluded patients admitted to the ICU within the first 48 h and restricted to those aged ≥70 years. In sensitivity analyses, we limited the window between a positive SARS-CoV-2 test to within 24 or 48 h of the index date. In addition, we considered exposure to any systemic corticosteroids within the first 48 h. We also evaluated the average treatment effect among the treated (ATT) population that received dexamethasone in weighted Cox proportional hazards models, and constructed unweighted, but multivariable adjusted models for all primary and subgroup analyses (supplementary tables E2 and E3).
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as p<0.05.
Study findings are reported as per the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (supplementary table E4).
Results
Patient characteristics, dexamethasone exposure and respiratory support
Patients hospitalised during the seven phases (n=19 973) were mostly male (95%). Median (interquartile range) age was 71 (62–77) years; 55% were non-Hispanic White, 27% non-Hispanic Black and 9% Hispanic (table 1). Most patients (83%) were admitted within 1 day after a positive SARS-CoV-2 test. More than half overall (60%) received corticosteroids within 48 h, of whom 95% received dexamethasone. Concurrent remdesivir and prophylactic anticoagulants initiated within 48 h of admission were more common in those who received dexamethasone than in those who did not (remdesivir 43% versus 13% and anticoagulants 46% versus 10%, respectively).
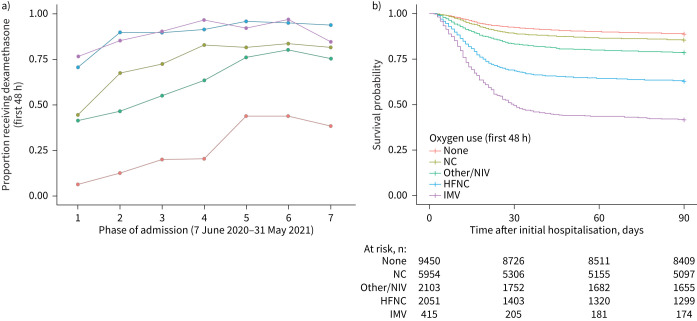
When stratified by highest level of respiratory support in the first 48 h of admission, 77% of patients were on either no oxygen (47%) or NC only (30%) (table 1). Dexamethasone was administered to 34% without oxygen, 75% on NC only, 69% on other supplemental oxygen/NIV, 91% on HFNC and 90% on IMV. Use of dexamethasone generally increased over time (figure 2a). Overall, unadjusted 90-day mortality was 17% and varied by respiratory support (figure 2b).
FIGURE 2.
a) Proportion of patients hospitalised for COVID-19 exposed to dexamethasone and b) unadjusted Kaplan–Meier survival curves for 90-day mortality according to respiratory support level. Phase 1: 7 June–11 July 2020; phase 2: 12 July–15 Aug 2020; phase 3: 16 Aug–31 Oct 2020; phase 4: 1 Nov–30 Nov 2020; phase 5: 1 Dec–31 Dec 2020; phase 6: 1 Jan–31 Jan 2021; phase 7: 1 Feb–31 May 2021. Note that the RECOVERY trial was halted on 8 June 2020, with a press release of results on 16 June 2020 (www.recoverytrial.net) [1]. NC: nasal cannula; NIV: noninvasive ventilation; HFNC: high-flow nasal cannula; IMV: invasive mechanical ventilation.
Dexamethasone and mortality in patients without IRS
Among patients without IRS, the median (IQR) duration of inpatient dexamethasone administration was 5 (3–8) days in patients without oxygen and 6 (4–9) days in patients on NC only. These were similar to hospital length of stay (table 1). Only 341 (3.6%) and 115 (1.9%) patients, respectively, received only 1 day of inpatient dexamethasone.
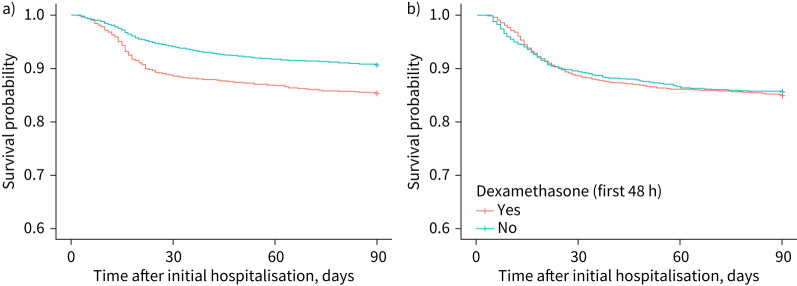
After propensity score weighting, our samples (pseudo-populations) were well balanced (table 2 and supplementary table E1). Among patients without oxygen, weighted Kaplan–Meier curves (figure 3a) show that those who received dexamethasone had higher mortality over 90 days than those who did not, with differences beginning to appear 10 days after the index date. In ATE estimates (table 3), patients without oxygen who received dexamethasone had a 76% increased hazard of 90-day mortality (HR 1.76, 95% CI 1.47–2.12).
FIGURE 3.
Inverse probability of treatment weighting Kaplan–Meier survival curves for 90-day mortality by dexamethasone use among patients hospitalised for COVID-19 on a) no oxygen or b) nasal cannula only in average treatment effect models.
TABLE 3.
Inverse probability of treatment weighting Cox proportional hazards models for 90-day mortality associated with early dexamethasone exposure in patients hospitalised for COVID-19 without intensive respiratory support
| No oxygen supplementation | Nasal cannula only | Combined group (no oxygen or nasal cannula only) | |
| Primary analysis | 1.76 (1.47–2.12) | 1.08 (0.86–1.36) | 1.59 (1.39–1.81) |
| Sensitivity and subgroup analyses | |||
| Restricted to positive SARS-CoV-2 test within 24 h | 1.94 (1.50–2.53) | 1.23 (0.93–1.64) | 1.54 (1.26–1.87) |
| Restricted to positive SARS-CoV-2 test within 48 h | 2.01 (1.55–2.60) | 1.27 (0.96–1.68) | 1.58 (1.30–1.92) |
| Any systemic corticosteroid | 1.77 (1.49–2.11) | 1.15 (0.92–1.43) | 1.61 (1.41–1.84) |
| Excluding patients admitted to the ICU within 48 h | 1.80 (1.36–1.74) | 1.18 (0.89–1.58) | 1.45 (1.19–1.67) |
| Restricted to patients aged ≥70 years | 1.76 (1.36–2.28) | 1.30 (0.99–1.72) | 1.53 (1.27–1.84) |
Data are presented as hazard ratio (95% CI). Models present the average treatment effect in the entire population (ATE models). SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit.
In patients on NC only, 90-day mortality was similar in those who did and did not receive dexamethasone, as shown in weighted Kaplan–Meier curves (figure 3b). ATE estimates demonstrated a nonsignificant 8% increased mortality risk (HR 1.08, 95% CI 0.86–1.37) in patients on NC only who received dexamethasone. When combining patients on no oxygen or NC only, dexamethasone was associated with ∼60% or more increased mortality risk.
Subgroup and sensitivity analyses
Results were similar in subgroup analyses excluding patients admitted to the ICU within the first 48 h and limiting the sample to patients aged ≥70 years (table 3). Findings were also consistent considering SARS-CoV-2 testing within 24 or 48 h of the index date, exposure to all corticosteroids and when using ATT or multivariable Cox models (supplementary tables E2 and E3). Among patients on NC only, hazard ratios were similar using ATT estimates, but dexamethasone was associated with a statistically significant increase in mortality in multivariable Cox models (HR 1.31, 95% CI 1.08–1.60) (supplementary table E3).
Discussion
In this US national cohort of hospitalised patients with COVID-19, dexamethasone use was common and increased over time. Among patients on IRS, 90% received dexamethasone within 48 h of admission. Focusing on patients without IRS, where there is less evidence supporting corticosteroid use, we found that among patients without oxygen in the first 48 h, dexamethasone was administered in 34% and was associated with 76% increased 90-day mortality. Among those on NC only in the first 48 h, dexamethasone was administered in 75% and was associated with no mortality benefit. This real-world evidence does not support the use of dexamethasone in hospitalised COVID-19 patients without IRS in the first 48 h.
While we cannot rule out residual confounding, our findings were robust, employing several different approaches and in subgroup and sensitivity analyses, including limiting the time window for SARS-CoV-2 test result, exposure to any systemic corticosteroid, restricted to patients aged ≥70 years and excluding those who were admitted to the ICU within 48 h. Results were consistent using ATE, reflecting the overall population from which the sample was taken, and using ATT, reflecting the population who received dexamethasone. They were also consistent controlling for potential confounders such as demographics, phase of the pandemic, site prescribing patterns, comorbidities, vital signs, laboratory values and co-administration of medications, including remdesivir [16].
Importantly, patients without IRS in the initial 48 h represent the majority (77%) of COVID-19 admissions in the cohort; thus, our findings have important clinical implications on the potential unintended consequences of widespread dexamethasone adoption for COVID-19 among patients who are without IRS. We found that uptake of dexamethasone for COVID-19 patients hospitalised in the VA was rapid after release of the RECOVERY trial results in early June 2020 (www.recoverytrial.net) [1]. By mid-July 2020, most facilities had increased the proportion of patients administered dexamethasone to 90% of patients on HFNC or IMV within 48 h of admission, exceeding national estimates from other health systems [6]. However, sites also increased use of dexamethasone for patients with less severe COVID-19, including those not on oxygen or on NC only (figure 2), suggesting indication creep.
Our results provide real-world evidence of practice patterns and extend the findings from RECOVERY [1]. We provide clinically actionable evidence demonstrating significantly and substantially increased mortality in hospitalised COVID-19 patients not on oxygen who received early dexamethasone. Moreover, our results inform an area of significant clinical uncertainty, namely the use of dexamethasone in COVID-19 patients with less severe respiratory failure. Clinical guidelines issued by the US National Institutes of Health provide a moderate recommendation for corticosteroids in hospitalised COVID-19 patients “on supplemental oxygen” [22]. While dexamethasone was associated with improved outcomes in patients on oxygen support in RECOVERY, this category included all forms of oxygen, except for IMV, but inclusive of NIV. We further stratified patients by level of oxygen support during the initial 48 h of hospitalisation, addressing a significant knowledge gap [12]. We found a lack of benefit associated with dexamethasone in patients on only low-flow NC within 48 h of admission, suggesting that use of corticosteroids in this population should be re-considered and requires further prospective study.
Even before COVID-19, the impact of corticosteroids has been inconsistent in other causes of pneumonia, including influenza, community-acquired pneumonia and the original SARS [8, 23–26]. The impact of corticosteroids likely depends on multiple factors, including patient age and other characteristics, heterogeneity in host response to infection, aetiology of pneumonia, time since onset of infection, and presence and severity of acute respiratory distress syndrome [8, 10, 27–31]. While corticosteroids may decrease host inflammatory response, potentially modulating lung injury, they may also have harmful side-effects or unintended consequences on adaptive immune responses that may be important to resolution of infection and increase the risk of secondary infection.
There remain unanswered questions regarding the use of dexamethasone for patients hospitalised with COVID-19, particularly those without IRS. For some patients, initiation within 48 h of hospitalisation may be too early and could impair viral clearance [32]. While most patients in our cohort had positive SARS-CoV-2 testing within 1 day of hospitalisation, we do not know how long symptoms preceded seeking medical attention and testing. Corticosteroids may also have a differential effect depending on the degree of inflammation [33], but often extensive missing data and selection bias in obtaining tests such as C-reactive protein and interleukin-6 make this difficult to explore in real-world data. Furthermore, it is unclear whether the dexamethasone regimen used in RECOVERY is optimal or whether the formulation, dose and duration of corticosteroids should vary by factors such as patient age or severity of COVID-19 [2–4, 32]. While most corticosteroid use in our cohort was dexamethasone, we found consistent results when including all systemic corticosteroids and also when restricting to individuals aged ≥70 years, although other reports have found a potential for increased harm in older persons [31]. It is also unknown whether corticosteroids have a differential effect in breakthrough COVID-19 after vaccination or different variants of SARS-CoV-2.
There are several limitations to our study. First, the study was observational. While we used detailed clinical data that included measures reflecting illness severity and administration of co-medications in a large population well balanced by propensity for treatment, residual confounding for severity of illness could have contributed to greater mortality in those exposed to dexamethasone. Some laboratory results could have occurred after dexamethasone exposure, as both were ascertained within 48 h. However, the impact of dexamethasone on acute laboratory results is likely limited and this approach allowed an equal time window to detect the worst results in patients exposed and unexposed to dexamethasone. Although respiratory support algorithms were manually reviewed and validated, some misclassification may have occurred; however, substantial separation in Kaplan–Meier curves showing increasing mortality with greater respiratory support provides face validity. We also cannot rule out alternative indications for dexamethasone beyond COVID-19 in patients not on oxygen or on NC only. However, we excluded those on corticosteroids prior to admission and patients on vasopressors within the initial 48 h. Furthermore, we did not calculate dose and only assessed days of inpatient dexamethasone exposure. However, most patients had length of inpatient dexamethasone treatment equal to their hospital length of stay and very few received only one dose of dexamethasone (<4%). Finally, our cohort consisted predominantly of male Veterans, but had excellent racial and geographic variability.
In summary, we found no evidence of mortality benefit at 90 days associated with early initiation of dexamethasone in patients hospitalised with COVID-19 among those on no oxygen or NC only within the first 48 h of admission and instead found evidence of potential harm. These findings come from a large population with detailed clinical data providing real-world evidence that was consistent using different analytic approaches to control for confounding and remained robust in a variety of subgroup and sensitivity analyses. Given the frequent and continued administration of dexamethasone to a substantial proportion of patients who are not on oxygen or are on low-flow NC only, the real-world evidence presented here highlights the nonbeneficial and potentially harmful expansion in use of dexamethasone in hospitalised COVID-19 patients without IRS. Future work should also evaluate dexamethasone and associated outcomes among hospitalised patients with COVID-19 breakthrough infections and different SARS-CoV-2 variants.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02532-2021.Supplement (814.1KB, pdf)
Shareable PDF
Footnotes
Author contributions: K. Crothers, J. Tate, M.B. Goetz, B. Jones, V. Marconi, M.E. Ohl, C.T. Rentsch, M.C. Rodriguez-Barradas, A.C. Justice and K.M. Akgün conceived (formulated or helped in the evolution) of the study. J. Tate, R. DeFaccio, P.R. Alba and C.T. Rentsch curated the data. R. DeFaccio and J. Tate performed the formal analysis. K. Crothers, P.R. Alba, A.C. Justice and K.M. Akgün acquired funding. K. Crothers, R. DeFaccio, J. Tate, P.R. Alba, M.E. Ohl, C.T. Rentsch, A.C. Justice and K.M. Akgün designed the methodology. R. DeFaccio, J. Tate and S. Shahrir managed and coordinated the project. K. Crothers, J. Tate, P.R. Alba, B. Jones and K.M. Akgün contributed to validation. K. Crothers, R. DeFaccio, J. Tate and K.M. Akgün wrote the first draft of the manuscript. All authors agree to be accountable for the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. K. Crothers and K.M. Akgün are joint principal investigators. K. Crothers, R. DeFaccio, J. Tate and K.M. Akgün are guarantors.
This article has an editorial commentary: https://doi.org/10.1183/13993003.03222-2021This article has supplementary material available from erj.ersjournals.com
Data sharing: Owing to US Dept of Veterans Affairs (VA) regulations and our ethics agreements, the analytic datasets used for this study are not permitted to leave the VA firewall without a data use agreement. This limitation is consistent with other studies based on VA data. However, VA data are made freely available to researchers with an approved VA study protocol. For more information, please visit https://www.virec.research.va.gov or contact the VA Information Resource Center at VIReC@va.gov.
Conflict of interest: K. Crothers, R. DeFaccio, J. Tate, P.R. Alba, M.B. Goetz, B. Jones, J.T. King, M.E. Ohl, C.T. Rentsch, M.C. Rodriguez-Barradas, S. Shahrir, A.C. Justice and K.M. Akgün have no conflicts of interest to disclose. V. Marconi reports grant funding from Gilead, ViiV, CDC, NIH and VA; honoraria and travel support from Eli Lilly; and participation on a data safety monitoring board.
Support statement: VA/HSR&D, C19-20-406 (K. Crothers/K.M. Akgün) and 13-457 (P.R. Alba); VA/RR&D, 1I0IRX003666-01 (K. Crothers) and MVP000 (A.C. Justice). The Dept of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis or interpretation of data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Dept of Veterans Affairs or the US Government. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA 2020; 324: 1298–1306. doi: 10.1001/jama.2020.16761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 2020; 324: 1317–1329. doi: 10.1001/jama.2020.17022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324: 1307–1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324: 1330–1341. doi: 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta HB, An H, Andersen KM, et al. Use of hydroxychloroquine, remdesivir, and dexamethasone among adults hospitalized with COVID-19 in the United States: a retrospective cohort study. Ann Intern Med 2021; 174: 1395–1403. doi: 10.7326/M21-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest 2020; 130: 6417–6428. doi: 10.1172/JCI140617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Liao X, Zhou Y, et al. Comparison of associations between glucocorticoids treatment and mortality in COVID-19 patients and SARS patients: a systematic review and meta-analysis. Shock 2021; 56: 215–228. doi: 10.1097/SHK.0000000000001738 [DOI] [PubMed] [Google Scholar]
- 9.Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 2021; 72: e373–e381. doi: 10.1093/cid/ciaa1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartoletti M, Marconi L, Scudeller L, et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect 2021; 27: 105–111. doi: 10.1016/j.cmi.2020.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Li X, Fan G, et al. Low-to-moderate dose corticosteroids treatment in hospitalized adults with COVID-19. Clin Microbiol Infect 2021; 27: 112–117. doi: 10.1016/j.cmi.2020.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev 2021; 8: CD014963. doi: 10.1002/14651858.CD014963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PLoS Med 2020; 17: e1003379. doi: 10.1371/journal.pmed.1003379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ 2021; 372: n311. doi: 10.1136/bmj.n311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol 1999; 52: 137–142. doi: 10.1016/S0895-4356(98)00154-1 [DOI] [PubMed] [Google Scholar]
- 16.King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One 2020; 15: e0241825. doi: 10.1371/journal.pone.0241825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 2020; 3: e2022310. doi: 10.1001/jamanetworkopen.2020.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akgun KM, Tate JP, Pisani M, et al. Medical ICU admission diagnoses and outcomes in human immunodeficiency virus-infected and virus-uninfected veterans in the combination antiretroviral era. Crit Care Med 2013; 41: 1458–1467. doi: 10.1097/CCM.0b013e31827caa46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661–3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33: 1242–1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Shetterly S, Powers D, et al. Extension of Kaplan–Meier methods in observational studies with time-varying treatment. Value Health 2012; 15: 167–174. doi: 10.1016/j.jval.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines. 2021. www.covid19treatmentguidelines.nih.gov Date last accessed: 30 August 2021.
- 23.Moreno G, Rodriguez A, Reyes LF, et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 2018; 44: 1470–1482. doi: 10.1007/s00134-018-5332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197: 757–767. doi: 10.1164/rccm.201706-1172OC [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Liu J, Zhou Y, et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect 2020; 81: e13–e20. doi: 10.1016/j.jinf.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006; 129: 1441–1452. doi: 10.1378/chest.129.6.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–1684. doi: 10.1056/NEJMoa051693 [DOI] [PubMed] [Google Scholar]
- 28.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020; 8: 267–276. doi: 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 30.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015; 313: 677–686. doi: 10.1001/jama.2015.88 [DOI] [PubMed] [Google Scholar]
- 31.Jung C, Wernly B, Fjolner J, et al. Steroid use in elderly critically ill COVID-19 patients. Eur Respir J 2021; 58: 2100979. doi: 10.1183/13993003.00979-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Invest 2020; 130: 6218–6221. doi: 10.1172/JCI143331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha P, Furfaro D, Cummings MJ, et al. Latent class analysis reveals COVID-19-related ARDS subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med 2021; 204: 1274–1285. doi: 10.1164/rccm.202105-1302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02532-2021.Supplement (814.1KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02532-2021.Shareable (286KB, pdf)