Abstract
Profound and durable responses to a single dose of pembrolizumab in lung cancer are rare. We encountered a non‐small cell lung cancer patient showing a deep and durable response with a single dose of pembrolizumab. A 79‐year‐old man reported bloody sputum for several weeks and visited a general physician. A chest x‐ray revealed a tumor shadow in the right middle lung field at that time, and the patient was referred to our hospital. He was diagnosed with adenocarcinoma of the lung by transbronchial biopsy. The expression of programmed death ligand 1 in tumor cells was 100% by immunostaining. Based on the above, immunotherapy with pembrolizumab was performed as first‐line therapy. Cancer cells had significantly shrunk at the end of the first cycle. The patient had grade‐3 immune‐related hepatitis at the end of the first cycle. Pembrolizumab treatment was stopped and prednisolone (80 mg/body) was initiated. Subsequently, liver function normalized, and prednisolone was tapered and discontinued. Since then, no tumor recurrence has been detected for 1.5 years without treatment. There have been few reports of profound and durable responses to a single dose of pembrolizumab in lung cancer. The results indicate that a single dose of pembrolizumab alone may be sufficient to cause durable response and serious immune‐related adverse events in some cases.
Keywords: durable response, immune‐related hepatitis, non‐small cell lung cancer, pembrolizumab, single dose
A single dose of pembrolizumab alone may be sufficient to cause durable response and serious immune‐related adverse events in some cases.

INTRODUCTION
Immune checkpoint inhibitors (ICI), including pembrolizumab, have caused a paradigm shift in treating various types of cancer. In patients with advanced non‐small cell lung cancer (NSCLC) and programmed death ligand 1 (PD‐L1) expression on at least 50% of tumor cells, pembrolizumab achieved significantly longer progression‐free and overall survival and fewer adverse events than platinum‐based chemotherapy (KEYNOTE‐024). 1 However, the administration must be discontinued in some cases because of serious immune‐related adverse events (irAEs). Nevertheless, deep and durable responses to a single dose of pembrolizumab in lung cancer are rare.
We encountered a NSCLC patient showing a profound and durable response to a single dose of pembrolizumab. We present and describe this case here.
CASE REPORT
A 79‐year‐old man reported bloody sputum for several weeks and visited a general physician. A chest x‐ray revealed a tumor shadow in the right middle lung field, and the patient was referred to our hospital. He had been smoking two packs of cigarettes per day for 40 years. He did not have a concomitant clinical or subclinical autoimmune disease. His Eastern Cooperative Oncology Group performance status (ECOG PS) was 1. Blood tests showed normal ranges for white blood cells (5470/μL), red blood cells (451 × 104/μL), platelets (22.3 × 104/μL), carcinoembryonic antigen (2.4 ng/mL), cytokeratin 19 fragments (2.9 ng/mL), pro‐gastrin‐releasing peptide (40.8 pg/mL). Neutrophil‐to‐lymphocyte ratio was 2.7. Chest x‐ray (Figure 1(a)) and contrast‐enhanced computed tomography (CT) (Figure 1(b)–(d)) showed tumors in the right middle and lower lobes and mediastinal lymph nodes swelling. Bronchoscopy revealed that a tumor was obstructing the right middle lobe. Transbronchial biopsies showed adenocarcinoma (Figure 2(a)). Tumor cells were positive for thyroid transcription factor 1 (TTF‐1) and negative for p40 by immunostaining. Further immunostaining revealed many CD138‐positive tumor‐associated plasma cells (Figure 2(b)). The number of CD4 (Figure 2(c)) and CD8 (Figure 2(d)) positive cells was enough. Expression of PD‐L1 in tumor cells accounted for 100% by immunostaining (22C3 clones) (Figure 2(e),(f). No other distant metastases were seen, and the patient was clinically diagnosed with right middle lobe adenocarcinoma cT4N3M0 stage IIIC. The genetic analysis of the original tumors revealed that epidermal growth factor receptor gene mutation, anaplastic lymphoma kinase gene fusion, and ROS‐1 gene fusion were negative. Based on the above, immunotherapy with pembrolizumab was performed as first‐line therapy. Cancer cells had significantly shrunk at the end of the first cycle. His laboratory data showed liver dysfunction before the second pembrolizumab treatment cycle. The patient was negative for markers of hepatitis virus (hepatitis B and hepatitis C). The anti‐mitochondrial antibody was negative. The anti‐nuclear antibody was positive (1:160) with a speckled pattern and elevated IgG (3158 mg/dL). Abdominal ultrasonography and abdominal contrast‐enhanced CT showed no apparent liver abnormalities. Although we did not perform a liver biopsy, the patient was clinically diagnosed with grade‐3 immune‐related hepatitis. Pembrolizumab treatment was stopped and prednisolone (80 mg/body) was initiated. Subsequently, liver function normalized, and prednisolone was tapered and discontinued (Figure 3). Since then, no tumor recurrence has been detected for 1.5 years without treatment (Figure 4).
FIGURE 1.
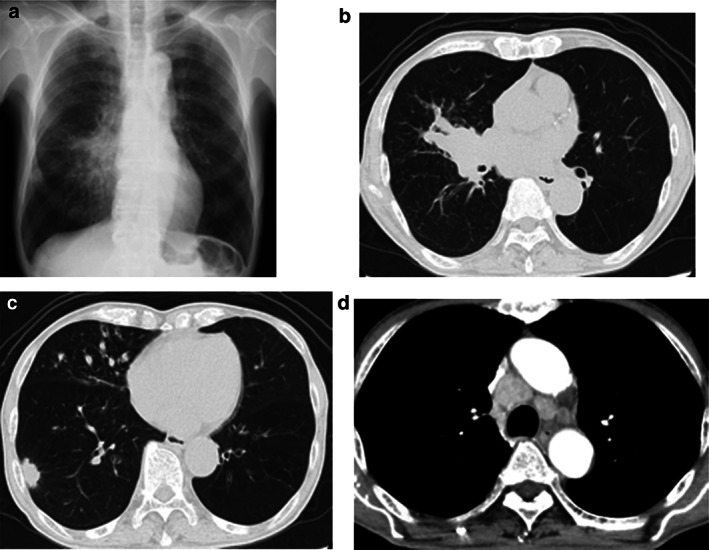
(a) Chest x‐ray and (b)–(d) contrast‐enhanced computed tomography at first admission
FIGURE 2.
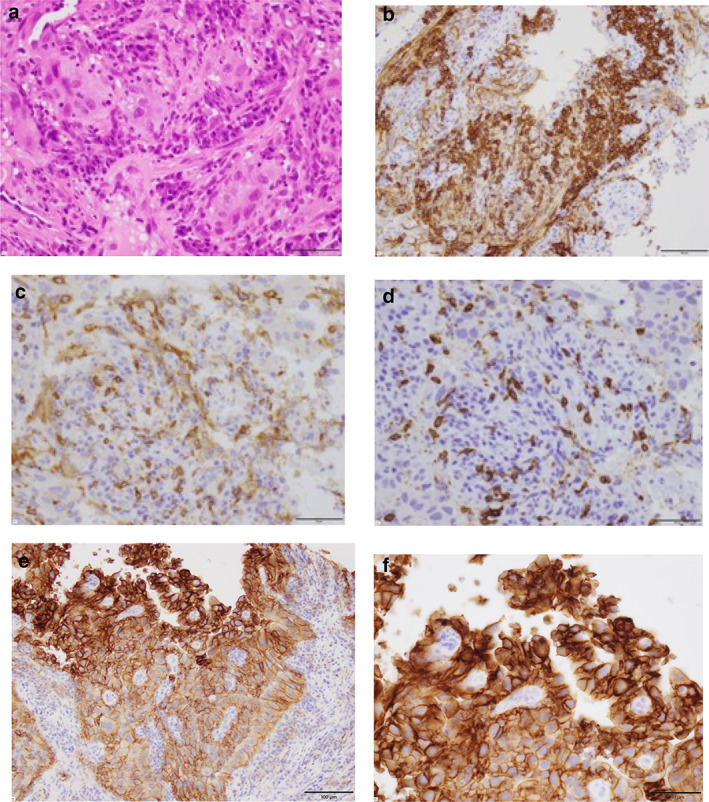
(a) Transbronchial biopsies revealed adenocarcinoma (hematoxylin–eosin staining, scale bar = 50 μm). (b)–(f) Immunostaining of the tumor cells. (b) A carcinoma with high density of CD138‐positive plasma cells in tumor stroma (scale bar = 100 μm). The number of CD4‐positive cells (scale bar = 50 μm) (c) and CD8‐positive cells (scale bar = 50 μm) (d) was enough. PD‐L1 was expressed on 100% of tumor cells (22C3 clones) (scale bar = 100 μm) (e) (scale bar = 50 μm) (f)
FIGURE 3.
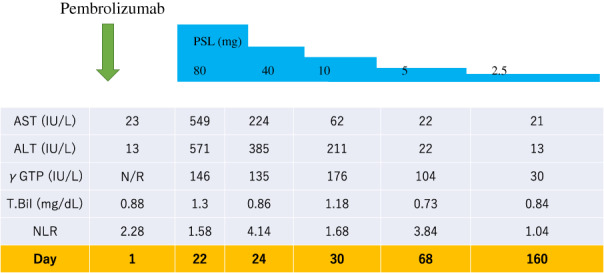
The clinical course of the present case. Immunotherapy with pembrolizumab was performed only at one cycle. The patient had grade‐3 immune‐related hepatitis at the end of the first cycle. Pembrolizumab treatment was stopped and prednisolone (80 mg/body) was initiated. Subsequently, liver function normalized, and prednisolone was tapered and discontinued. N/R = not recorded
FIGURE 4.
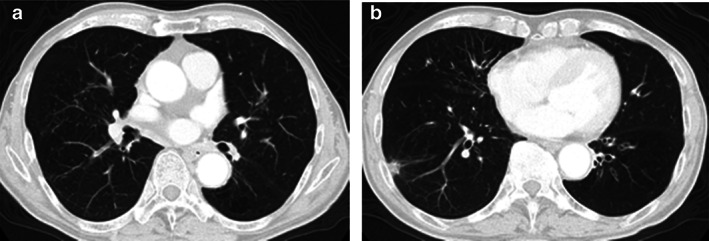
(a),(b) Computed tomography 12 months after pembrolizumab treatment. No tumor recurrence has been detected for 18 months without treatment
DISCUSSION
We encountered a NSCLC patient showing a profound and durable response to a single dose of pembrolizumab. The response was maintained for 1.5 years without treatment.
To the best of our knowledge, a profound and durable response with a single dose of pembrolizumab in lung cancer is extremely rare. We could find only two previous case reports. 2 , 3 In each case, treatment was discontinued at one time because of a severe irAE. Our case was grade‐3 immune‐related hepatitis. One case was a myasthenic crisis. 2 Another was pneumonitis. 3 One patient received nivolumab and another patient received pembrolizumab. Similar cases have been reported in other cancers. 4 , 5 , 6 These findings indicate that even a single dose of ICI can cause serious irAEs. Furthermore, these findings suggest that self‐reactive lymphocytes exist in humans by nature. 4 , 7 On the other hand, irAE is thought to represent bystander effects from activated T‐cells. It is plausible that patients responding to ICIs would have a greater likelihood of autoimmune toxicities. 8
In the present case, the primary cells infiltrating the tumor were CD138‐positive plasma cells. The same case report has been made for lung cancer. 9 , 10 Tumor‐infiltrating plasma cells are a favorable prognostic factor in lung cancer. 11 , 12 In various cancer types, the presence of B cells and tertiary lymphoid structures is associated with longer disease‐free and overall survival in patients treated with ICI. 13 , 14 , 15 B cells might contribute to antitumor immunity by interacting with and activating CD4‐CXCL13 that could help prime CD8‐CXCL13 through conventional type 1 dendritic cells. 16 Further studies are needed to identify the predictive value of B cells and plasma cells. Furthermore, in this case, the effect of a single dose of pembrolizumab was maintained for a long time, and the impact of immunotherapy may last longer with just one dose.
CONCLUSION
In conclusion, we present a case of NSCLC patient showing a profound and durable response to a single dose of pembrolizumab. The response was maintained for 1.5 years without treatment. The results indicate that a single dose of pembrolizumab alone may be sufficient to cause durable response and serious irAEs in some cases.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Kondo Y, Kunishige M, Kadota N, Okano Y, Machida H, Hatakeyama N, et al. A single dose of pembrolizumab treatment causing a profound and durable response in lung cancer. Thorac Cancer. 2022;13:648–652. 10.1111/1759-7714.14314
REFERENCES
- 1. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 2. Tan RYC, Toh CK, Takano A. Continued response to one dose of Nivolumab complicated by Myasthenic crisis and myositis. J Thorac Oncol. 2017;12:e90–e1. [DOI] [PubMed] [Google Scholar]
- 3. Tokuyasu H, Ishikawa S, Sakai H, Ikeuchi T, Miura H. Single pembrolizumab treatment causing profound durable response in a patient with pulmonary pleomorphic carcinoma. Respir Med Case Rep. 2019;28:100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kimura T, Fukushima S, Miyashita A, Aoi J, Jinnin M, Kosaka T, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016;107:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maamari J, Yeung SJ, Chaftari PS. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am J Emerg Med. 2019;37:376.e1. [DOI] [PubMed] [Google Scholar]
- 6. Shen L, Chen H, Wei Q. Immune‐therapy‐related toxicity events and dramatic remission after a single dose of Pembrolizumab treatment in metastatic Thymoma: a case report. Front Immunol. 2021;12:621858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shirai T, Sano T, Kamijo F, Saito N, Miyake T, Kodaira M, et al. Acetylcholine receptor binding antibody‐associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol. 2016;46:86–8. [DOI] [PubMed] [Google Scholar]
- 8. Das S, Johnson DB. Immune‐related adverse events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suyama T, Fukuda Y, Soda H, Ogawara D, Iwasaki K, Hara T, et al. Successful treatment with nivolumab for lung cancer with low expression of PD‐L1 and prominent tumor‐infiltrating B cells and immunoglobulin G. Thorac Cancer. 2018;9:750–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadota N, Hatakeyama N, Hino H, Kunishige M, Kondo Y, Okano Y, et al. Complete and durable response of pulmonary large‐cell neuroendocrine carcinoma to pembrolizumab. Cancer Rep. 2021. 10.1002/cnr2.1589. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lohr M, Edlund K, Botling J, Hammad S, Hellwig B, Othman A, et al. The prognostic relevance of tumor‐infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non‐small cell lung cancer. Cancer Lett. 2013;333:222–8. [DOI] [PubMed] [Google Scholar]
- 12. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5. [DOI] [PubMed] [Google Scholar]
- 15. Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–60. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Chen H, Mo H, Hu X, Gao R, Zhao Y, et al. Single‐cell analyses reveal key immune cell subsets associated with response to PD‐L1 blockade in triple‐negative breast cancer. Cancer Cell. 2021;39:1578–93. [DOI] [PubMed] [Google Scholar]


