Abstract
We have established an in vitro model of long-term continuous Chlamydia pneumoniae infection in HEp-2 cells. Using transmission electron microscopy, we demonstrated the presence of spontaneous abnormal chlamydial inclusions similar in appearance to the persistent chlamydial forms induced in vitro by treatment with cytokines or antibiotics or by nutrient deprivation.
Chlamydia pneumoniae is a frequent cause of community-acquired pneumonia and bronchitis in adults and children. Like other chlamydial species, it can cause prolonged or chronic infections which may persist for months or years (11). These persistent infections have been implicated in the development of a number of chronic diseases, including chronic obstructive pulmonary disease, atherosclerosis, and asthma (20). However, whether persistent C. pneumoniae is a cause, a triggering cofactor, or an innocent bystander remains controversial.
Persistent chlamydial infections can be established in vitro using several methods, including treatment with cytokines (2, 4, 16, 18) or antibiotics (5, 6) or by deprivation of certain nutrients (13). In all cases they have been described as having morphologically abnormal reticulate bodies (RBs), which suggests that they are somehow altered during their otherwise normal development.
In the present study we describe the ultrastructural findings determined using an in vitro model of long-term continuous C. pneumoniae infection in HEp-2 cells, a respiratory epithelial cell line (14, 17).
Briefly, confluent HEp-2 cells were inoculated once with C. pneumoniae isolate TW-183 (ATCC VR2282) or CM-1 (ATCC VR1360) to achieve 100% infection. After 3 to 5 days, when lysis of most of the infected host cells was seen, the culture medium was replaced with fresh medium but without added cycloheximide. After 1 week of further incubation, growth of colonies of new host cells was observed. Since then, continuous C. pneumoniae cultures have been maintained for over 4 years by reseeding the infected host cells into new flasks to prevent overgrowth. No new cells or chlamydiae were added. C. pneumoniae remained viable and cultivable in this model.
Two days prior to sampling, the continuously infected cells were seeded onto six-well plates. Cell monolayers were trypsinized, and infected cells were collected into 1.5-ml centrifuge tubes and fixed with 3% glutaraldehyde in 0.1 M cacodylate buffer overnight at 4°C. Samples were prepared for transmission electron microscopy by standard procedures (9). Samples were postfixed in osmium tetroxide, followed by uranyl acetate. The cells were then dehydrated in increasing concentrations of ethanol (50, 70, and 90%) and acetone (90 and 100%) and subsequently embedded in Spurr's epoxy resin. Ultrathin sections (50 to 100 nm in thickness) were prepared and collected onto 200-mesh copper grids, contrasted with 1% uranyl acetate and Reynolds lead citrate before being examined, and photographed using a JEOL 1200EX transmission electron microscope.
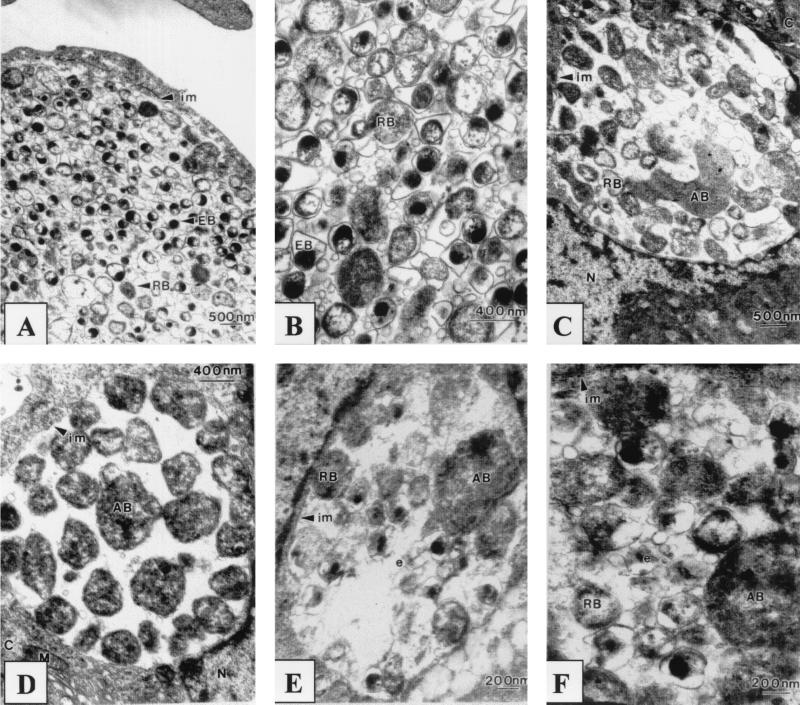
Three types of chlamydial inclusions were observed by transmission electron microscopy: typical, altered, and aberrant. Approximately 90% of the inclusions seen in the ultrathin sections were typical, with large inclusions ranging from approximately 5 to 12 μm in diameter. These typical inclusions contained an average of 350 tightly packed chlamydial bodies and some extracellular material as well as membranous material (Fig. 1A and B). Elementary bodies (EBs) were pear-shaped and electron opaque, with a large periplasmic space surrounded by an undulating cell membrane (Fig. 1B), and ranged from 200 to 400 nm in diameter. Larger RBs with electron-lucent nuclear centers were interspersed among the EBs; they were round and ranged in size from 400 to 800 nm. A number of RBs were also observed to be in the stage of binary fission. The inclusions were bound by a definite membrane and were closely apposed to the HEp-2 cell nuclei. Mitochondria were observed surrounding the inclusion but did not appear to be in close association with the inclusions.
FIG. 1.
Continuous C. pneumoniae infection in HEp-2 cells inoculated with isolate CM-1 (A to D) or isolate TW-183 (E and F). (A and B) Typical inclusions; (C to F) altered inclusions; (D) aberrant inclusion. C, cytoplasm; N, nucleus; M, mitochondria; im, inclusion membrane; e, intrainclusional membranous material.
The second type of inclusion observed, altered inclusions, contained both normal EBs and RBs but in considerably lower numbers (approximately 70) compared to the normal population of 350 for the typical inclusion. These inclusions also contained pleomorphic aberrant bodies, which were 2.5 μm in diameter or up to four to five times the size of normal RBs; their cytoplasm was homogenous (Fig. 1C, E, and F). These aberrant bodies retained an identifiable small periplasmic space and outer membrane, characteristics of normal RBs. Altered inclusions were closely apposed to the nuclei of the HEp-2 cells. Host cell mitochondria were observed adjacent to the defined inclusion membrane.
The third type of inclusion observed was small and aberrant, averaging 4 μm in diameter. These inclusions contained about 60 aberrant bodies (ABs) which were similar in size to normal RBs but appeared electron dense and no longer retained a smooth spherical shape. The aberrant bodies were loosely arranged in the inclusion with a noticeable absence of any intrainclusional material and membranes (Fig. 1D). These dense ABs retained the characteristic chlamydial outer membrane structure, with very little periplasmic space and with the membranes more tightly bound to the chlamydial body, similar to normal RBs. No EBs were observed in these inclusions. Aberrant inclusions were often observed in HEp-2 cells with multiple inclusions. Some aberrant inclusions contained both ABs and intermediate bodies. The inclusions remained closely apposed to the HEp-2 cell nuclei and were surrounded by a defined inclusion membrane, with loosely associated host cell mitochondria, as had been observed for normal and altered inclusions.
The morphology of the persistent forms described here in our continuous in vitro model is similar to that previously described in several in vitro studies where persistent infection was induced by antimicrobial agents, cytokines, or media deprived of certain nutrients (2, 3, 4, 5, 6, 13, 16, 18). However, the persistent forms in this model occurred spontaneously, without the addition of any extraneous triggering agents.
Abnormal chlamydial development was first described in vitro after Chlamydia psittaci- and Chlamydia trachomatis-infected cells had been treated with penicillin (5, 8, 15), which induced the development of enlarged abnormal RBs, with formation of small daughter RBs budding from within the parent RBs. This effect was reversed following removal of the penicillin from the medium. Other antibiotics have also been reported to induce abnormal chlamydial development. Erythromycin, when used in subinhibitory concentrations, produced small inclusions of C. trachomatis, with RBs twice the size of typical RBs (6). Treatment of C. trachomatis with sulfonamides induced development of small inclusions containing irregular RBs with ruffled membranes, mini-RB-like forms, and numerous ghost particles (10, 12). Recently, Wolf et al. (19) described the aberrant development of C. pneumoniae AR-39 in HeLa 229 cells treated with 50 μg of ampicillin/ml. After 48 h of treatment, the RBs were abnormal and much larger and appeared to undergo little or no cell division, similar to the aberrant bodies observed in our continuous-infection model. Dreses-Werringloer et al. (7) investigated the effect of ciprofloxacin and ofloxacin on established C. trachomatis infection (2 to 3 days postinfection) in HEp-2 cells. They found that at the minimal bactericidal concentration, both drugs not only failed to eradicate chlamydiae from infected cells but also induced persistent infection that was characterized by a small number of small aberrant inclusions present through 20 days of culture. A similar observation has been previously reported; in that report, C. pneumoniae in a continuous infection remained viable after 6 days of treatment with ofloxacin at 4.0 μg/ml (4 times the MIC) (14). Dreses-Werringloer et al. (7) also noted a significant decrease in the expression of major outer membrane protein, while the production of hsp60 and chlamydial lipopolysaccharide was minimally affected. Removal of ciprofloxacin from the medium 10 or 14 days postinfection reversed the effect, allowing the persistent chlamydiae to differentiate into infectious EBs.
Beatty et al. (1, 2, 4) demonstrated that treatment with 0.2 ng of gamma interferon (IFN-γ)/ml inhibited intracellular growth of C. trachomatis in HeLa cells. IFN-γ restricted the division of RBs and interrupted their differentiation into infectious EBs. The development of large aberrant RB forms combined with the absence of EBs was characteristic of persistent C. trachomatis infection. The aberrant chlamydial development was also concomitant with a decrease in the levels of major outer membrane protein, the 60-kDa outer membrane protein, and lipopolysaccharide. Chlamydial growth was altered but not completely inhibited; infectious chlamydiae could be recovered after removal of IFN-γ from the media. In contrast to the other models of persistence, our continuously infected cells were productive at all time points, probably due to the fact that only 10% of inclusions were affected, with the majority of the inclusions undergoing typical development. The altered and aberrant inclusions observed in the continuous-infection model appeared to be morphologically similar to those observed in IFN-γ-treated C. trachomatis cultures (1).
Restriction of certain nutrients has also been demonstrated to induce persistence in chlamydiae. Harper et al. (13) described the development of large and distorted RBs of C. trachomatis in McCoy cells presented with decreasing concentrations of glucose and 13 amino acids. The very high infective load present in the continuously infected cells in our model may result in areas of local nutrient starvation, which may act as a trigger for the development of the persistent forms.
The results of our study demonstrate for the first time that the natural developmental cycle of C. pneumoniae may combine both the typical development phase and the persistent phase. The implications of the persistent phase in the C. pneumoniae developmental cycle are important for our understanding of the pathogenesis of C. pneumoniae infections and also for the diagnosis and treatment of associated diseases. It may be possible to develop specific agents to target the persistent phase of the development cycle, which should enable specific diagnosis of persistent C. pneumoniae infections.
Acknowledgments
Financial support for this work was provided by NHMRC grant no. 981383 to P. Timms and S. Mathews.
REFERENCES
- 1.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty W L, Morrison R P, Byrne G I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R B, Schatzki P F, Dalton H P. Ultrastructural effect of penicillin and cycloheximide on Chlamydia trachomatis strain HAR-13. Med Microbiol Immunol. 1982;171:151–159. doi: 10.1007/BF02123623. [DOI] [PubMed] [Google Scholar]
- 6.Clark R B, Schatzki P F, Dalton H P. Ultrastructural analysis of the effect of erythromycin on the morphology and developmental cycle of Chlamydia trachomatis HAR-13. Arch Microbiol. 1982;133:278–282. doi: 10.1007/BF00521290. [DOI] [PubMed] [Google Scholar]
- 7.Dreses-Werringloer, Padubrin U I, Jürgens-Saathoff B, Hudson A P, Zeidler H, Köhler L. Persistence of Chlamydia trachomatis is induced by ciprofloxacin and ofloxacin in vitro. Antimicrob Agents Chemother. 2000;44:3288–3297. doi: 10.1128/aac.44.12.3288-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galasso G J, Manire G P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1961;86:382–385. [PubMed] [Google Scholar]
- 9.Glauert A M, Lewis P R. Biological specimen preparation for transmission electron microscopy. Princeton, N.J: Princeton University Press; 1998. [Google Scholar]
- 10.Hammerschlag M R. Activity of trimethoprim-sulfamethoxazole against Chlamydia trachomatis in vitro. Rev Infect Dis. 1982;4:500–505. doi: 10.1093/clinids/4.2.500. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschlag M R, Chirgwin K, Roblin P M, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–182. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 12.Hammerschlag M R, Vuletin J C. Ultrastructural analysis of the effect of trimethoprim and sulphamethoxazole on the development of Chlamydia trachomatis in cell culture. J Antimicrob Chemother. 1985;15:209–217. doi: 10.1093/jac/15.2.209. [DOI] [PubMed] [Google Scholar]
- 13.Harper A, Pogson C I, Jones M L, Pearce J H. Chlamydial development is adversely affected by minor changes in amino acid supply, blood plasma amino acid levels, and glucose deprivation. Infect Immun. 2000;68:1457–1464. doi: 10.1128/iai.68.3.1457-1464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutlin A, Roblin P M, Hammerschlag M R. In vitro activities of azithromycin and ofloxacin against Chlamydia pneumoniae in a continuous-infection model. Antimicrob Agents Chemother. 1999;43:2268–2272. doi: 10.1128/aac.43.9.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto A, Manire G P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta S J, Miller R D, Ramirez J A, Summersgill J T. Inhibition of Chlamydia pneumoniae replication in HEp-2 cells by interferon-γ: role of tryptophan catabolism. J Infect Dis. 1998;177:1326–1331. doi: 10.1086/515287. [DOI] [PubMed] [Google Scholar]
- 17.Roblin P M, Dumornay W, Hammerschlag M R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summersgill J T, Sahney N N, Gaydos C A, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf K, Fischer E, Hackstadt T. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect Immun. 2000;68:2379–2385. doi: 10.1128/iai.68.4.2379-2385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong Y-K, Gallagher P J, Ward M E. Chlamydia pneumoniae and atherosclerosis. Heart. 1999;81:232–238. doi: 10.1136/hrt.81.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]