Abstract
Background
Mediastinal lymphadenopathy is a common finding in follow‐up after diagnosis of malignancy, and may represent recurrence of malignancy, or benign processes such as sarcoidosis. CT and PET‐CT are commonly used, despite limited ability to discriminate between benign and malignant disease, and although EBUS‐guided bronchoscopy is often performed, it is relatively invasive and may not always be safe in high‐risk patients. Clinical and radiological predictors for cancer recurrence could therefore be of value.
Methods
A retrospective cohort analysis of all patients with mediastinal lymphadenopathy and previous malignancy undergoing mediastinal lymph node sampling via bronchoscopy was undertaken. Demographics, smoking status, details of previous malignancy, time since cancer diagnosis, and imaging data were collected, as well as follow‐up in the years following the procedure. We then compared specific characteristics of patients with malignant and benign lymphadenopathy in order to identify predictors of malignant versus benign lymphadenopathy.
Results
A total of 113 patients were analyzed of which 63% had tumor recurrence, while the rest had benign disease including sarcoidosis. Smoking history and previous lung cancer were both correlated with lymph node malignancy, while symmetric hilar enlargement, and the presence of multiple pathological stations were correlated with benign outcome. Size, maximal SUV uptake or time interval since cancer diagnosis were not associated with the final diagnosis.
Conclusions
These findings may help in assessing the pretest probability of tumor recurrence in patients with mediastinal lymphadenopathy, thus aiding in the clinical decision‐making in such scenarios.
Keywords: bronchoscopy, cancer, endobronchial ultrasound, lymphadenopathy, sarcoidosis
Study aims and main results
INTRODUCTION
Mediastinal lymphadenopathy may occur in various infectious, inflammatory, or neoplastic conditions. The finding is common in patients with previous malignancy undergoing routine imaging follow‐up (either CT or PET‐CT). In these cases, the importance of differentiating malignant from benign causes of lymphadenopathy is crucial. In most cases, a histopathological diagnosis will be necessary, and a biopsy will be performed to distinguish malignancy from a benign lesion, such as sarcoidosis.
Sarcoidosis is a systemic inflammatory disease, characterized by the presence of noncaseating granulomas in involved tissues. 1 The most common expression of the disease is mediastinal lymphadenopathy, most commonly in the right paratracheal (4R), subcarinal (7), hilar (11, 12) and para‐aortic stations. 2
A new finding of mediastinal lymphadenopathy during surveillance of patients following malignancy may raise a diagnostic dilemma: any further treatment, as well as the prognosis, depends on whether the lesion is malignant or benign. Therefore, tissue examination is often sought. However, obtaining a tissue diagnosis is not always possible, either due to difficult access to the lesion, or due to other patient factors such as high procedural risk. Furthermore, false negative results in such procedures are not uncommon, especially in cases of lymphoma.
Mediastinal lymph node sampling is usually performed via endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA), which has mostly replaced the need for conventional TBNA (cTBNA) and mediastinoscopy.
PET‐CT is a commonly used modality to identify malignant disease. Uptake of the radioactive tracer is measured in standardized uptake values (SUV) units, and FDG uptake above 2.5 is considered pathological. However, despite the high sensitivity of this test, it lacks ideal specificity for malignancy, making discerning benign from malignant disease challenging in many cases. 3
In the current study, we aimed to find objective clinical and radiological characteristics predictive of malignancy or benign etiology in patients with mediastinal lymphadenopathy following a previous diagnosis of malignancy.
METHODS
We performed a retrospective analysis of all patients with a previous malignancy who underwent bronchoscopy for mediastinal lymph node sampling due to mediastinal lymphadenopathy that appeared during their follow‐up. All cases in which bronchoscopy was performed between the years 2004–2017 in Hadassah Medical Center (Jerusalem, Israel) were analyzed. The Hadassah Medical Center Research Ethics Board approved the study and waivered the need for patient informed consent due to the retrospective design of the study (no. 0297‐17‐HMO). All methods were carried out in accordance with the relevant guidelines and regulations.
Flexible bronchoscopy was performed in all patients using intravenous sedation (using midazolam, fentanyl, pethidine, ketamine and propofol combinations), and local anesthesia with 2% lidocaine was delivered through the bronchoscope. Pulse rate, blood pressure, electrocardiogram, and oxygen saturation were continuously monitored. For each lymph node station biopsied, a minimum of three aspirates was performed and sent for both pathological and cytological analysis. Conventional TBNA (cTBNA) with a 19‐gauge WANG needle (Cook Medical Inc.) was performed in earlier cases. Following introduction of EBUS‐TBNA in our center, an Olympus endobronchial ultrasonography bronchoscope with 21‐gauge needles (Olympus Medical Systems Corporation) was used in subsequent cases. Patients were observed for at least 3 h after endoscopy.
We used computerized medical charts to collect individual patient data, including demographics (sex, age at biopsy, smoking status), details of previous malignancy (origin, date of diagnosis, treatment given), and symptoms and signs prior to bronchoscopy. Imaging data including size and uptake of the different lymph node stations, presence of symmetric hilar or para aortic nodes, biopsy results and clinical or subsequent diagnosis following the procedure was also obtained for all cases.
We excluded patients who underwent bronchoscopy as part of the primary malignancy diagnosis or workup, or who had known tumor recurrence in other sites at the time of bronchoscopy.
Pathological lymph nodes were defined as having a diameter of 11 mm (short axis measurement) or more, or an FDG uptake value of 2.5 SUV or more on FDG‐PET‐CT. Analysis included specific locations of pathological lymph nodes, number of pathological stations, maximal node size and maximal SUV uptake for each patient.
Following data extraction, we compared the two groups of patients (malignant vs. benign causes of lymphadenopathy). Benign lymphadenopathy was defined as any pathology results not indicating malignancy (e.g., sarcoid‐like granulomas, reactive or anthracotic lymphoid tissue or nondiagnostic sample). In addition, in order to confirm that negative results (no evidence of malignancy), were not false negatives, patients with a benign initial diagnosis were followed‐up for at least 12 months, and if cancer recurrence was diagnosed during this period, analysis of their data was performed as part of the malignant lymphadenopathy group.
We used Pearson's correlation (1‐tail) test and the Mantel–Haenszel common odds ratio estimate test (2‐tail) to assess the correlation between the different baseline characteristics and the biopsy results. p‐values of 0.05 or less were considered statistically significant. All statistical analyses were performed using IBM SPSS software.
RESULTS
We found 113 cases of patients with previous malignancy who had undergone bronchoscopic lymph node sampling due to mediastinal lymphadenopathy, which was identified during routine follow‐up. Seventy‐one biopsies (63%) showed recurrence of malignancy, and benign lymphadenopathy was discovered in 42 (37%), In half of these (21 patients), noncaseating granulomas suggestive of sarcoidosis were revealed.
Epidemiology
There were 64 females and 49 males with a mean age of 59 years. Sixty‐one patients (57%) were never smokers, 25 patients (23%) were current smokers at the time of the biopsy, and 21 (20%) were previous smokers. Age and gender (r = 0.11, p = 0.12; OR = 0.495, 95% CI: 0.221–1.107, p = 0.87, respectively) were not correlated with biopsy results, however previous and current smoking was significantly correlated with a recurrent malignancy (r = 0.203, p = 0.018, OR 2.5, 95% CI: 1.051–5.948, p = 0.038) compared to non‐smokers.
Other demographic parameters, including time interval since cancer diagnosis, presence of symptoms or weight loss, previous radiation or chemotherapy, were not correlated with outcome (Figure 1).
FIGURE 1.
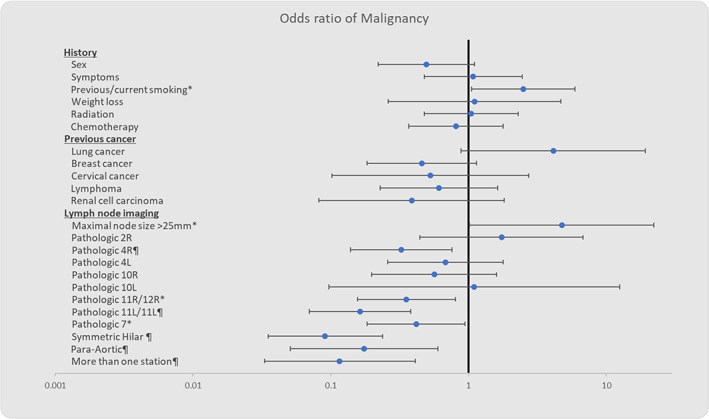
Odds ratios of a malignant outcome with different clinical and radiological characteristics. *p‐value <0.05, ¶p‐value <0.01
Characteristics of previous malignancy
Breast cancer, hematological malignancies and lung cancer were the most common tumors identified in our cohort (21, 17, and 13%, respectively). The median time interval between the diagnoses of cancer and bronchoscopic biopsy was 61.7 months (SD ± 61.38). Seventy percent of patients underwent surgical procedure as treatment for their malignancy, 41% received radiation therapy and 60% were given some form of chemotherapy.
Previous breast cancer was correlated with consequent benign lymphadenopathy (r = −0.159, p = 0.047) while lung cancer was associated with significantly more tumor recurrence in mediastinal lymph nodes (r = 0.18, p = 0.028). In five cases of melanoma, all had tumor recurrence, making the OR for malignancy infinite (r = 0.159, p = 0.046). Presence of other previous malignancies including lymphoma, cervical cancer and renal cell carcinoma was not correlated with the outcome. Table 1 summarizes the baseline patient characteristics.
TABLE 1.
Baseline characteristics
| Criteria | Number (%) |
|---|---|
| Sex | |
| Male | 49 (43.4) |
| Female | 64 (56.6) |
| Age at biopsy (mean ± SD) | 59.46 ± 14.26 |
| Smoking status (n = 107) | |
| Yes | 25 (23.4) |
| No | 61 (57) |
| Past | 21 (19.6) |
| Primary malignancy | |
| Breast | 24 (21.2) |
| Lymphoma | 20 (17.7) |
| Lung | 15 (13.3) |
| Renal cell carcinoma | 7 (6.2) |
| Cervix | 6 (5.3) |
| Melanoma | 5 (4.4) |
| Other a | 36 (31.9) |
| Time since first diagnosis (mean ± SD), months, (n = 111) | 61.77 ± 61.38 |
| Previous treatment | |
| Chemotherapy | 67 (59.3) |
| Radiation | 46 (40.7) |
| Surgical (including transplants) | 79 (69.9) |
| Biopsy results | |
| Recurrence of malignancy | 71 (62.8) |
| Benign lymphadenopathy | 42 (37.2) |
| Clinical symptoms prior to biopsy | |
| Systemic symptoms (cough, dyspnea, chest pain, weakness) | 38 (33.6) |
| Recurrent pneumonia | 6 (5.3) |
| Weight loss | 9 (8) |
Abbreviation: SD, standard deviation.
Other: thyroid, stomach, rectum, colon, liver, larynx, prostate, urinary bladder, carcinoid, esophagus, endometrium, pancreas.
Imaging
A significant correlation was found between maximal node size and a malignant outcome (r = 0.188, p = 0.027). Using specific cutoffs for dichotomization, the strongest correlation was found in maximal node size of >25 mm (r = 0.209, p = 0.016, OR = 4.755, 95% CI: 1.018–22.195, p = 0.0473). In contrast, no correlation was found between maximal mediastinal SUV uptake and biopsy results (r = 0.111, p = 0.21).
Presence of pathological nodes in the 4R, 11/12R, 11/12L, 7 and para‐aortic stations was strongly correlated with a benign result, as was presence of symmetrical hilar lymphadenopathy. Pathological 2R, 4 L, 10R, 10 L stations was not correlated with the outcome. No single pathological station was associated with malignancy.
A higher number of pathological lymph node stations was significantly correlated with a benign biopsy result (r = −0.422, p < 0.001). This was also the case in patients with more than one pathological station at presentation (r = −0.353, p < 0.001).
All patients with evidence of lymph node necrosis had malignant biopsy results (r = 0.159, p‐value = 0.046), making the OR infinite. See Table 2 for full imaging details.
TABLE 2.
Imaging characteristics
| Lymph node station | Enlarged nodes No. (%) | Size (mean ± SD), mm | Pathological FDG uptake. No. (%) | SUV (mean ± SD) |
|---|---|---|---|---|
| 2R | 12 (10.6) | 15.42 ± 5.02 | 5 (4.4) | 10.75 ± 4.20 |
| 4R | 66 (58.4) | 14.01 ± 5.72 | 42 (37.2) | 7.81 ± 4.32 |
| 4L | 21 (18.6) | 12.05 ± 3.62 | 12 (10.6) | 7.03 ± 2.83 |
| 10R | 17 (15) | 14.36 ± 6.56 | 14 (12.4) | 8.22 ± 3.79 |
| 10L | 3 (1.8) | 13.00 ± 6.24 | 4 (3.5) | 5 |
| 7 | 64 (54.9) | 16.88 ± 6.35 | 37 (32.7) | 11.41 ± 6.79 |
| 11/12R | 61 (54.0) | 17.24 ± 5.10 | 48 (42.5) | 8.53 ± 3.31 |
| 11/12L | 43 (38.1) | 16.44 ± 3.52 | 29 (25.7) | 8.34 ± 4.45 |
| Symmetrical hilar | 31 (27.4) | N/A | N/A | N/A |
| Necrotic areas | 5 (4.4) | N/A | N/A | N/A |
DISCUSSION
Our main goal was to find objective clinical and radiological characteristics predictive of malignancy or benign etiology in patients with mediastinal lymphadenopathy following malignancy. Such characteristics may aid physicians in decision‐making, regarding diagnostic procedures and follow‐up protocols.
Several reports show a correlation between specific imaging findings and biopsy results in patients presenting with mediastinal lymphadenopathy. A 2013 study indicates that specific anatomical distribution and morphological patterns of mediastinal lymph nodes, as demonstrated on spiral CT, can be useful in differentiating sarcoidosis from Hodgkin's lymphoma. The bilateral hilar zone (10 station) was involved more often in sarcoidosis than in Hodgkin's lymphoma. However, there was a higher tendency for presence of zone 1 (low cervical, supraclavicular and sternal notch) and zone 3 (prevascular and retrotracheal) lymphadenopathy in Hodgkin's lymphoma than in sarcoidosis (zones based on the IASLC map). 4
Another study from 2018 explored the advantages of differentiating inflammatory from malignant thoracic lymph nodes by integrating their features on PET and CT. SUV was shown to have high sensitivity but a low specificity, low positive and negative predictive values, and low diagnostic yield for differentiating benign from malignant disease. 5
With regard to the clinical characteristics of patients with mediastinal lymphadenopathy, a 2015 study attempted to describe the characteristics of benign and malignant mediastinal masses, especially those residing in histoplasmosis‐endemic regions. Several clinical characteristics, such as malaise, neck swelling, abnormal extra‐thoracic lymphadenopathy, lymphopenia, anterior mediastinal involvement and/or pleural effusion, were shown to predict a malignant etiology. The authors concluded that expectant management of patients lacking these characteristics might be safe and reduce unnecessary invasive testing. 6
Considering previously published data, we conducted a study to describe both clinical and radiological characteristics of patients with mediastinal lymphadenopathy, specifically in patients following malignancy. To our knowledge, this is the first study which has assessed the clinical and radiological risk factors for cancer recurrence in this scenario.
With regard to the clinical characteristics, we found a strong correlation between current and previous smoking with tumor recurrence. This is in keeping with previous studies showing a higher prevalence of cancer recurrence in smokers, as shown in the study by Tezel et al., in which patients who continue smoking following lung cancer surgery have a significant risk of tumor relapse or metastasis. 7 Another study which examined the role of smoking in patients with breast cancer, showed increased HER2 expression in recurrent breast tumors among smokers. 8
We found no correlation between the presence of symptoms and biopsy results. This is not surprising given the fact that these were mostly asymptomatic patients in whom imaging was performed as part of their routine oncological follow‐up, and also the fact that mediastinal lymphadenopathy is seldom symptomatic in itself.
The origin of the previous malignancy was found to be an important factor in predicting biopsy results in our cohort. Patients with previous lung or breast cancer were found to have a statistically significant correlation with malignant and benign biopsy results, respectively.
The recurrence dynamics of resected early‐stage NSCLC displays a “multi‐peak” pattern, with prominent similarities with the corresponding dynamics observed in early breast cancer. However, the timings of the peaks do not coincide. Peaks for breast cancer occur with a delay in comparison with the corresponding peaks for NSCLC. 9 Despite this, and given the long follow‐up interval in our cohort, including post‐bronchoscopy, we believe this is indeed an important factor in predicting the risk of cancer recurrence.
A common benign etiology of mediastinal lymphadenopathy is sarcoidosis, which was indeed diagnosed in a significant proportion of our cohort. Sarcoidosis was estimated to be up to 38 times more prevalent in patients with mediastinal lymphadenopathy following breast cancer than the general population, in a recent study by our group. 10 The lower likelihood of mediastinal cancer recurrence in patients with previous breast cancer in our cohort is therefore in keeping with this previous finding.
In addition, we found that all patients with previous melanoma had a malignant biopsy result with a statistical significance, although there were few cases. Malignant melanoma has the capacity to metastasize widely and quickly throughout the body. The lung is one of the most common sites for metastasis, with pulmonary involvement occurring in almost all cases of generalized disease. For example, among 1600 patients treated for malignant melanomas at Duke University Medical Center between 1970 and 1980, 16.3% developed thoracic metastasis. Isolated hilar and/or mediastinal adenopathy was present in nine patients. 11
Radiological characteristics were also found to be quite important in our cohort. We found a strong correlation between maximal node size and malignant biopsy result, in particular in nodes larger than 25 mm. Maximal SUV uptake, however, was not correlated with the final diagnosis. Our finding regarding lymph node size is in keeping with a previous meta‐analysis investigating the correlation between mediastinal lymph node size and malignancy. This analysis included studies published between 1985 and 2004, and found that lymph node diameter of 15 mm or more significantly raised the odds of malignancy. 12 We therefore show this conclusion is also true in cases with previous malignancy.
In another study by Prenzel et al., hilar and mediastinal lymph nodes from 256 patients with non‐small cell lung cancer (NSCLC) were sampled. The authors showed that although the benign nodes were smaller on average than the malignant nodes, lymph node size was not a reliable parameter prediction of metastatic involvement in those patients. 13 In our cohort, analysis was made using the largest node for each patient (rather than analyzing each node separately). In addition, our study included patients with several malignancy origins, possibly explaining the difference in outcomes.
Pathological nodes in stations 4R, 11/12R, 11/12L, 7 and the presence of symmetrical hilar and para‐aortic lymphadenopathy, were all found to be strongly correlated with a benign biopsy result. This finding is not surprising given 90% of patients with sarcoidosis have mediastinal lymphadenopathy, specifically in these stations. 2
Moreover, we found that patients with more than one pathological station had a greater likelihood of having a benign biopsy result, in contrast to patients with a solitary mediastinal mass who had a higher likelihood of malignancy recurrence. This finding may be plausible given the fact that benign mediastinal lymphadenopathy (either reactive lymphadenopathy or sarcoidosis) tends to be multifocal.
Another important finding was the radiological presence of necrotic areas within the enlarged lymph nodes, which unanimously favored a malignant process.
In conclusion, our findings highlight the important characteristics of patients with mediastinal lymphadenopathy following malignancy, facilitating risk stratification in this group of patients. We found that factors related to a malignant outcome are current or past smoking, previous lung cancer or melanoma, solitary station involvement, necrotic areas within lymph nodes, and maximal node size of 25 mm or more.
Furthermore, the parameters found to be related to a benign biopsy result are previous breast malignancy, lymphadenopathy in more than one station, pathological nodes in stations 4R, 11/12R, 11/12L and 7 and presence of symmetrical hilar and para‐aortic lymph node enlargement.
These findings may aid clinicians to predict benign versus malignant biopsy results in patients with mediastinal lymphadenopathy following malignancy, and possibly change patient management when contemplating invasive procedures.
Our study limitations include its retrospective nature, as well as the single center design. Furthermore, there was no reference to the staging of the previous malignancy due to lack of specific patient data. Therefore, the conclusions drawn from the analysis must be interpreted with caution and should be confirmed by further large‐scale prospective cohort studies.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors thank Professor Norman Grover, from the Hebrew university (Faculty of Medicine) for statistical analysis and guidance.
Fiterman N, Berkman N, Kuint R. Predictors of malignant lymph node involvement in patients with mediastinal lymphadenopathy and previous cancer: A cohort study. Thorac Cancer. 2022;13:631–636. 10.1111/1759-7714.14311
REFERENCES
- 1. Morgenthau AS, Iannuzzi MC. Recent advances in sarcoidosis. Chest. 2011;139(1):174–82. 10.1378/chest.10-0188 [DOI] [PubMed] [Google Scholar]
- 2. Criado E, Sánchez M, Ramírez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high‐resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567–86. 10.1148/rg.306105512 [DOI] [PubMed] [Google Scholar]
- 3. Fletcher JW, Kinahan PE. PET/CT standardized uptake values (SUVs) in clinical practice and assessing response to therapy. National Institute of Health. 2010;31(6):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehrian P, Ebrahimzadeh SA. Differentiation between sarcoidosis and Hodgkin's lymphoma based on mediastinal lymph node involvement pattern: evaluation using spiral CT scan. Pol J Radiol. 2013;78(3):15–20. 10.12659/PJR.889056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang H, Li QK, Auster M, Gong G. PET and CT features differentiating infectious/inflammatory from malignant mediastinal lymphadenopathy: a correlated study with endobronchial ultrasound‐guided transbronchial needle aspiration. Radiol Infect Dis. 2018;5(1):7–13. 10.1016/j.jrid.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naeem F, Metzger ML, Arnold SR, Adderson EE. Distinguishing benign Mediastinal masses from malignancy in a histoplasmosis‐endemic region. J Pediatr. 2015;167(2):409–15. 10.1016/j.jpeds.2015.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basbug Tezel Y, Akyil M, Tezel C, Tokgoz Akyil F, Evman S, Gürer D, et al. Impact of persistence of smoking on recurrence after early stage lung surgery. Eur Resp J. 2016;48(Suppl 60):PA4339. 10.1183/13993003.congress-2016.pa4339 [DOI] [Google Scholar]
- 8. Takada K, Kashiwagi S, Asano Y, Goto W, Kouhashi R, Yabumoto A, et al. The effect of smoking on biological change of recurrent breast cancer. J Transl Med. 2020;18(1):153. 10.1186/s12967-020-02307-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, et al. Recurrence dynamics for non‐small‐cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7(4):723–30. 10.1097/JTO.0b013e31824a9022 [DOI] [PubMed] [Google Scholar]
- 10. Arish N, Kuint R, Sapir E, Levy L, Abutbul A, Fridlender Z, et al. Characteristics of sarcoidosis in patients with previous malignancy: causality or coincidence? Respiration. 2017;93(4):247–52. 10.1159/000455877 [DOI] [PubMed] [Google Scholar]
- 11. Chen JTT, Dahmash NS, Ravin CE, Heaston DK, Putman CE, Seigler HF, et al. Metastatic melanoma to the thorax: report of 130 patients. Am J Roentgenol. 1981;137(2):293–8. 10.2214/ajr.137.2.293 [DOI] [PubMed] [Google Scholar]
- 12. de Langen AJ, Raijmakers P, Riphagen I, Paul MA, Hoekstra OS. The size of mediastinal lymph nodes and its relation with metastatic involvement: a meta‐analysis. Eur J Cardiothorac Surg. 2006;29(1):26–9. 10.1016/j.ejcts.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 13. Prenzel KL, Mönig SP, Sinning JM, Baldus SE, Brochhagen HG, Schneider PM, et al. Lymph node size and metastatic infiltration in non‐small cell lung cancer. Chest. 2003;123(2):463–7. 10.1378/chest.123.2.463 [DOI] [PubMed] [Google Scholar]


