Abstract
BACKGROUND
Estimates of retention in antiretroviral treatment (ART) programmes may be biased if patients who transfer to healthcare clinics are misclassified as lost to follow-up (LTFU) at their original clinic. In a large cohort, we estimated retention in care accounting for patient transfers using medical records.
METHODS
Using linked electronic medical records, we followed adults living with HIV (PLWH) in Cape Town, South Africa from ART initiation (2012–2016) through database closure at 36 months or 30 June 2016, whichever came first. Retention was defined as alive and with a healthcare visit in the 180 days between database closure and administrative censoring on 31 December 2016. Participants who died or did not have a healthcare visit in > 180 days were censored at their last healthcare visit. We estimated the cumulative incidence of retention using Kaplan–Meier methods considering (i) only records from a participant’s ART initiation clinic (not accounting for transfers) and (ii) all records (accounting for transfers), over time and by gender. We estimated risk differences and bootstrapped 95% confidence intervals to quantify misclassification in retention estimates due to patient transfers. results We included 3406 PLWH initiating ART. Retention through 36 months on ART rose from 45.4% (95% CI 43.6%, 47.2%) to 54.3% (95% CI 52.4%, 56.1%) after accounting for patient transfers. Overall, 8.9% (95% CI 8.1%, 9.7%) of participants were misclassified as LTFU due to patient transfers.
CONCLUSIONS
Patient transfers can appreciably bias estimates of retention in HIV care. Electronic medical records can help quantify patient transfers and improve retention estimates.
Keywords: retention in care, loss to follow-up, patient transfer, attrition, HIV, validation, engagement in HIV care
Sustainable Development Goals (SDGs): SDG 3 (good health and well-being); SDG 9 (industry, innovation and infrastructure); SDG 17 (partnerships for the goals)
Introduction
Retention in HIV care is critical to reaching UNAIDS’s targets of 90% of persons living with HIV (PLWH) aware of their status, 90% of those who know their status initiated on antiretroviral therapy (ART) and 90% of those initiating ART achieving viral suppression [1]. Accurate estimates of retention in HIV care are essential to identify patients who have become lost to follow-up (LTFU) from HIV care, evaluate HIV treatment programmes and plan resource allocation [2,3]. However, estimating retention in ART programmes is complicated by patient transfers – when a patient initiates treatment at one clinic and then subsequently moves to a different clinic. Estimates of retention in ART programmes may be biased if patients who transfer clinics or die are misclassified as LTFU at their original clinic [4].
A recent meta-analysis from sub-Saharan Africa estimated that as many as 36% of adults classified as LTFU had transferred to a new clinic [5,6]. In order to obtain unbiased estimates of retention in HIV care to inform programme evaluation and resource allocation, patient transfers need to be accounted for. However, ascertaining the status in routine clinical care of participants who are LTFU is often not straightforward. The lack of integrated medical records systems in many settings and difficulty in tracing patients who do not return for scheduled visits means that patients classified as LTFU at a clinic are typically a combination of those who have disengaged from care, died, or who are receiving care elsewhere [5,7]. As mortality among individuals initiating ART in low- and middle-income countries declines, undocumented patient transfers remain an important potential source of misclassification when estimating retention in HIV care [8,9].
Despite the importance of accounting for patient transfers when estimating retention in HIV care, few studies have evaluated the impact of patient transfers in retention estimates from large ART treatment programmes in sub-Saharan Africa using electronic medical records [3]. To fill this gap, we used linked electronic medical records data to estimate retention in care, with and without accounting for patient transfers. Over three years of follow-up in a large ART treatment programme in South Africa, we estimated misclassification in retention due to patient transfers overall, by gender, and by time since ART initiation using two different definitions of retention. Our goal was to examine how misclassification due to patient transfers impacted retention in care estimates from a large ART treatment programme in South Africa.
Methods
Data source and setting
As part of a data harmonisation effort to support HIV care, the Western Cape Department of Health links clinical, pharmacy, laboratory and available vital status data from all public clinics within the Western Cape Province via unique patient identifiers [10]. Data for the present analysis come from linked electronic medical record information used to identify a cohort of PLWH in a sub-district of Cape Town, South Africa. Electronic medical record information for participants in the sub-district was linked to all available public clinics in the Western Cape by the Western Cape Department of Health. The sub-district is one of 32 in South Africa’s Western Cape region. The overall prevalence of HIV in the Western Cape region is 6.6%, although considerable variation in HIV prevalence exists [11,12]. Between 2012 and 2015, PLWH in South Africa were eligible to initiate lifelong ART if they had a CD4 count < 350 cells/mm3. Starting in 2016, universal test and treat were rolled out, mandating access to lifelong ART for all PLWH [13].
In South Africa, an estimated 3.4 million PLWH initiated ART between 2004 and 2015 [14]. The rapid scale-up of HIV treatment in South Africa has led to challenges in tracking patients’ engagement in ART treatment programmes [15]. Individuals testing positive for HIV and initiating ART are typically registered in a paper-based or electronic medical record system and expected to receive follow-up care at the clinic where they initiate ART. Once a patient is stable on ART, they are eligible to receive medication through decentralised adherence clubs that are facilitated by community health workers linked to a clinic [16]. When present, electronic medical record systems often differ across health facilities, making it infeasible to identify patient transfers in real time or close to real time for routine clinical care.
Study design and population
Data for the present analysis come from the cohort of all PLWH in the sub-district of the Western Cape region who were at least 15 years of age at their first positive HIV test in the study period, had a civil identification number, and a CD4 count measure at enrolment into the cohort between 2012 and 2013. Follow-up data for participants were available through the end of 2016. The primary purpose of this original cohort was to examine associations between gender, HIV testing, and subsequent HIV service uptake and outcomes [17]. South Africa has a national death registry; however, data for cohort members were not authorised for linkage to the national death registry.
Participants from the original cohort were included in our analysis if they initiated ART between 2012 and 2016 (n = 4184). To ensure all participants had sufficient person time available to meet outcome definitions, we excluded participants who did not initiate ART prior to 30 June 2016 (i.e. 6 months before the data were administratively censored; n = 9). In order to accurately determine time out of care, we also excluded participants whose ART initiation date did not match a visit encounter date (n = 499) or who had a record of initiating ART at more than one clinic (n = 270), for a total study population of 3406 patients (Figure 1). Ethical approval for the use of de-identified routine data was provided by the University of Cape Town (Protocol numbers 320/15 and 802/2014).
Figure 1.
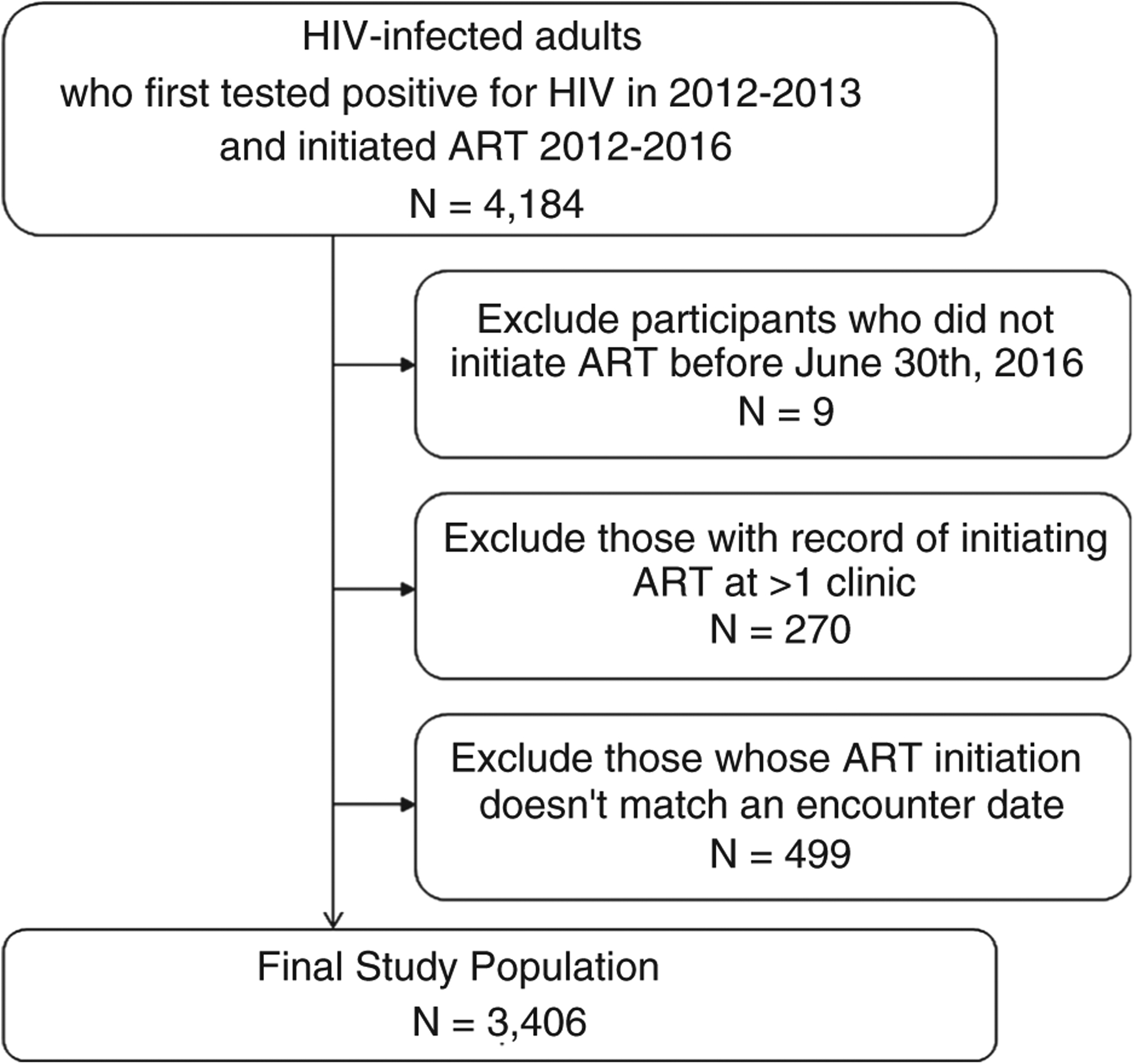
Study Population adults living with HIV and initiating antiretroviral therapy (ART) in a sub-district of Cape Town, South Africa 2012–2016.
Measures
We defined a patient transfer as any switch from the original healthcare facility where a participant initiated ART to a different facility on a different day (i.e. patient transfers could not occur on the same day since patients may receive services at multiple facilities in one day). Our data did not contain information on the reason for transfer, thus we were unable to distinguish between ‘official’ transfers (e.g. when the original facility was aware of the transfer) and ‘silent’ transfers (e.g. when the facility was not aware of the transfer). Covariate information available in our data was limited to gender, age at ART initiation, year of ART initiation and CD4 count at enrolment, defined as a CD4 count within 3 months of first testing positive for HIV during the study period.
Retention definitions
The primary outcome was retention in care through up to 36 months after ART initiation. In South Africa, patients initiating ART are typically given a 30- to 90-day supply of drugs. South African HIV treatment guidelines define LTFU from HIV care as 90 days after one’s supply of ART drugs is exhausted [18]. Thus, we defined retention as alive and having had a healthcare visit in the previous 180 days. Retention in HIV care can be evaluated in multiple ways [19]. Retention at a given cross-section in time is often most helpful when evaluating a treatment programme or allocating resources, while estimates of time to first attrition can help researchers to identify participants at high risk for LTFU. Thus, we considered retention from two perspectives: at database closure and time to first attrition.
In the primary analysis, participants were followed from ART initiation until database closure. Database closure occurred after 36 months of follow-up or at 6 months prior to administrative censoring on 30 June 2016, whichever date came first. Participants were considered retained if they were alive and had a healthcare visit in the 180 days between database closure and administrative censoring (i.e. either 31 December 2016 or 42 months of follow-up). Implicit in this definition of retention is the possibility that a participant could become LTFU and return to care at any point prior to database closure and still be considered retained. Attrition was considered the inverse of retention (1-probability of retention). Due to the small number of deaths observed in our cohort (n = 98, 2.9%), we defined attrition as having died or not having had a healthcare visit for >180 days. Participants who met the definition of attrition were censored at their last recorded healthcare visit [19].
In a secondary analysis, we evaluated retention considering time to first attrition. Participants were followed from ART initiation until the earliest of the following: >180 days with no healthcare visits, death, 36 months of follow-up or administrative censoring on 31 December 2016. Retention was defined as alive and in care in the previous 180 days, and attrition was defined as the inverse of retention. Participants who met the definition of attrition were censored at their last recorded healthcare visit record if they died or at 181 days with no healthcare visits if they were LTFU.[20] Retention definitions are summarised in Table 1. In a sensitivity analysis, we evaluated both definitions of attrition as having died or >365 days with no healthcare visits.
Table 1.
Summary of retention and attrition definitions
| Retention definition | Attrition definition | Participants can return to care before the end of follow-up? | Follow-up | Censoring for participants who met attrition definition | |
|---|---|---|---|---|---|
| Retention at database closure | Alive and with a healthcare visit in the 180 days between database closure† and administrative censoring‡ | Dead or not having a healthcare visit for> 180 days | Yes | ART initiation until database closure six months prior to administrative censoring | Last recorded healthcare visit |
| First attrition | Alive and with a healthcare visit in the previous 180 days | Dead or not having a healthcare visit for> 180 days | No | ART initiation until the earliest of the following: >180 days with no healthcare visits, death or administrative censoring | Last recorded healthcare visit record if participant died or at 181 days with no healthcare visits if participant was lost to follow-up |
Database closure date: 30 June 2016.
Administrative censor date: 31 December 2016.
Statistical analysis
For all analyses, we estimated the cumulative incidence of retention using Kaplan–Meier methods. We first estimated retention considering only records from a participant’s original ART initiation clinic (i.e. not accounting for patient transfers) and, next, considering all records (i.e. accounting for patient transfers). We estimated risk differences and bootstrapped 95% confidence intervals (n = 1000 non-parametric bootstraps) to quantify misclassification in retention estimates due to patient transfers, over time and by gender. Analyses were conducted using Stata 13 (StataCorp, College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC).
Results
We included 3406 PLWH who initiated ART between 1 January 2012 and 30 June 2016. The majority of participants were women (66%), aged 20 to 39 years old (72%), and initiated ART prior to the rollout of universal ART in South Africa in 2016 (99%; Table 2). At enrolment into the cohort, over half of participants (55%) had a CD4 count ≤ 350, which was the threshold for ART initiation throughout much of the study period.
Table 2.
Demographic and clinical characteristics of 3406 adults living with HIV and initiating ART in Cape Town, South Africa
| Women 2248 (66.0) |
Men 1158 (34.0) |
Total 3406 |
|
|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) |
| CD4 count at enrolment† | |||
| ≤200 | 626 (30.7) | 296 (28.6) | 922 (30.0) |
| >200–350 | 521 (25.6) | 253 (24.5) | 774 (25.2) |
| >350–500 | 363 (17.8) | 199 (19.2) | 562 (18.3) |
| >500 | 528 (25.9) | 286 (27.7) | 814 (26.5) |
| Age at ART initiation, years | |||
| 15–19 | 70 (3.1) | 6 (0.5) | 76 (2.2) |
| 20–29 | 962 (43.0) | 214 (18.5) | 1176 (34.7) |
| 30–39 | 784 (35.1) | 497 (43.0) | 1281 (37.7) |
| 40–49 | 272 (12.2) | 302 (26.1) | 574 (16.9) |
| 50–59 | 125 (5.6) | 110 (9.5) | 235 (6.9) |
| 60+ | 24 (1.1) | 28 (2.4) | 52 (1.5) |
| ART initiation year | |||
| 2012 | 554 (24.6) | 381 (32.9) | 935 (27.5) |
| 2013 | 1015 (45.1) | 459 (39.6) | 1474 (43.2) |
| 2014 | 325 (14.5) | 181 (15.6) | 506 (14.9) |
| 2015 | 327 (14.6) | 117 (10.1) | 444 (13.0) |
| 2016 | 27 (1.2) | 20 (1.7) | 47 (1.4) |
Enrolment = within 3 months of the participant’s initial HIV test during the study period. Missing data: 12 observations missing for age at ART initiation, 334 missing for CD4 count at enrolment
Retention at database closure
Participants contributed a total of 83 580 person-months at risk over a median of 25–30 months of follow-up, depending on whether patient transfers were considered. When considering retention at database closure, retention through 36 months after ART initiation rose from 45.4% (95% CI 43.6%, 47.2%) to 54.3.2% (95% CI 52.4%,56.1%) after accounting for patient transfers (Figure 2). Overall, 8.9% (95% CI 8.1%, 9.7%) of participants were misclassified as not retained due to patient transfers (Figure 2; Table S1).
Figure 2.
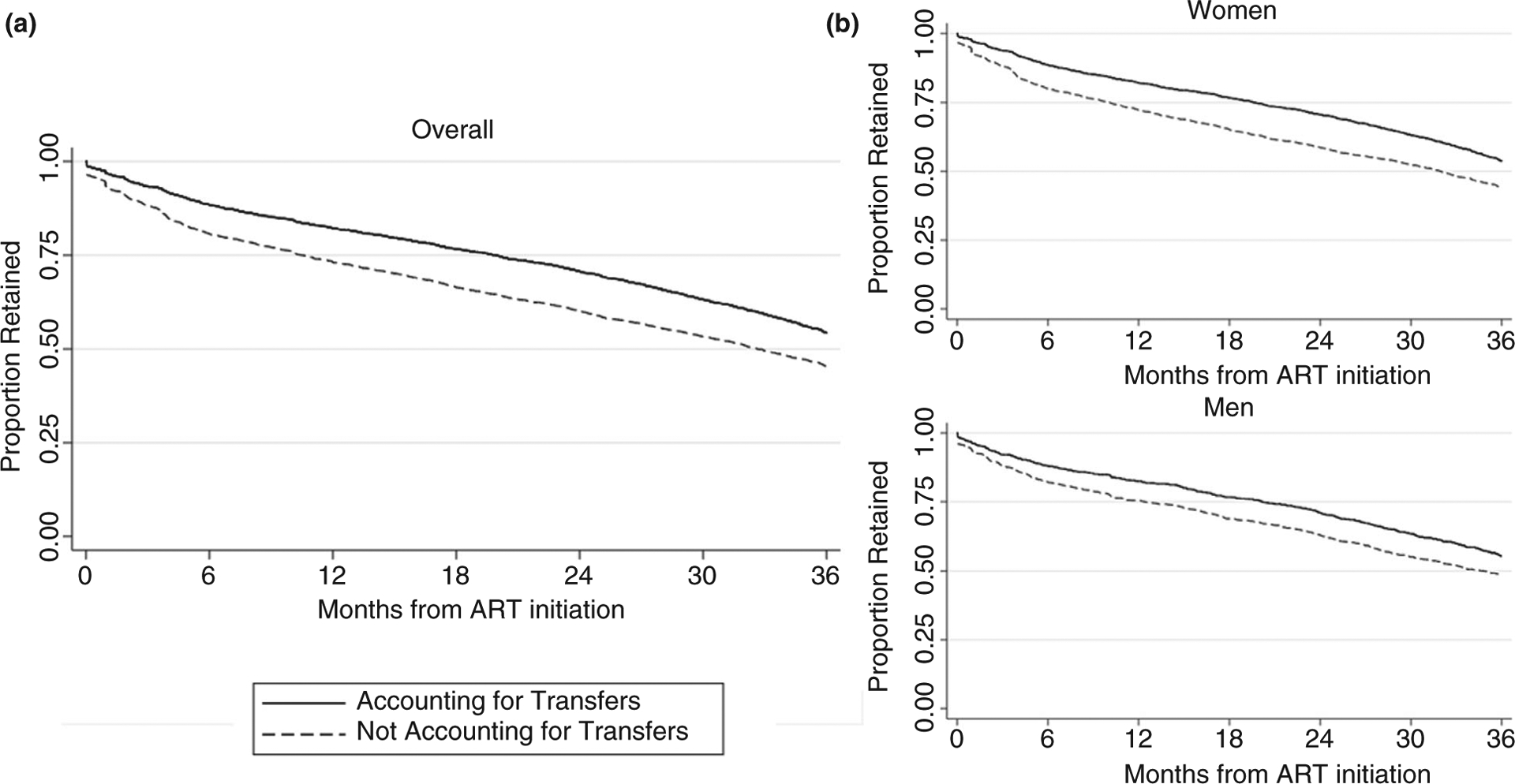
Retention at database closure through 36 months after ART initiation, not accounting for patient transfers and accounting for patient transfers, (A) overall and (B) by gender. Retention defined as having a healthcare visit in the 180 days between database closure and administrative censoring.
Bias in retention estimates due to patient transfers varied by gender and time since ART initiation. Compared to men, women were more likely to be misclassified as not retained due to patient transfers. For example, through 36 months on ART 9.8% (95% CI 8.8%, 10.9%) of women were misclassified as not retained, compared to 7.0% (95% CI 5.7%, 8.3%) of men (Table S1). For both men and women, the proportion of participants misclassified due to clinic transfers varied by 2–3% points over the follow-up period and was highest through 24 months after ART initiation (women: 12.0%, 95% CI 10.9%,13.0%; men: 8.1%, 95% CI 6.7%, 9.3%).
Retention considering time to first attrition.
When evaluating time to first attrition, estimates of retention and misclassification due to patient transfers were lower than when estimating retention at database closure. Retention through 36 months after ART initiation was 32.3% (95% CI 30.7%, 34.0%) not accounting for patient transfers and 37.2% (95% CI 35.4%, 38.9%) after accounting for patient transfers (Table S2). Overall, 4.8% (95% CI 4.3%, 5.4%) of participants were misclassified as not retained due to patient transfers through 36 months on ART. When evaluating time to first attrition, women were again more likely to be misclassified due to patient transfers than men. The proportion of participants misclassified due to patient transfers varied by time since ART initiation and peaked at approximately 12 months after ART initiation. For both definitions of attrition, estimates of misclassification due to patient transfers were similar when attrition was defined as death or> 365 days with no healthcare visits (Tables S3 and S4).
Discussion
In this study, we used routinely collected data from electronic medical records to estimate retention in care in a large ART programme in South Africa, accounting for patient transfers. In general, our results demonstrate that to obtain accurate estimates of retention in HIV care, patient transfers need to be taken into account and that traditional approaches, such as censoring participants when they transfer, may not be sufficient to accurately estimate retention in care. Not accounting for patient transfers biased estimates of retention at database closure by 9% and retention estimates considering time to first attrition by 5%. Misclassification due to patient transfers was higher among women than men.
We evaluated retention using two different definitions. Through 36 months on ART, estimates of retention at database closure were higher than when retention was evaluated using time to first attrition [3]. The higher estimated retention at database closure likely reflects the fact that participants could have been LTFU and returned to care at any point prior to database closure and still be considered retained. Conversely, when retention was evaluated using time to first attrition, participants were censored the first time they met the definition of attrition [20].
Similarly, estimates of misclassification due to patient transfers were also higher when retention was evaluated at database closure, compared to retention considering time to first attrition. When estimating time to first attrition, participants were considered misclassified if they did not return to their original ART clinic within 180 days but visited another health facility within 180 days. When estimating retention at database closure, participants were considered misclassified if they did not return to their original ART clinic during the six months between database closure and administrative censoring, but instead visited another health facility during that 6-month window. Thus, the higher level of misclassification due to patient transfers at database closure likely reflects the fact that, through up to 36 months after ART initiation, many participants had moved to a different health facility from where they initiated ART.
As access to lifelong ART has scaled-up throughout sub-Saharan Africa, people living with HIV are increasingly expected to maintain lifelong engagement in HIV care and treatment programmes. However, changes in residence, health status, migration, patient preferences and concerns about confidentiality and stigma may all result in frequent transfers between health facilities [3,21]. In this cohort, misclassification due to patient transfers was higher among women than men, likely due to the fact that women often are required to switch clinics during pregnancy and the postpartum period. While in principle, the need to account for patient transfers to obtain unbiased estimates of retention in ART treatment programmes is clear, in practice paper-based record keeping, incompatible data systems that are not linked or networked, and migration can make it difficult to account for patient movement between health facilities. The inability to accurately estimate retention in HIV care hampers efforts to evaluate HIV treatment programmes and may impact access to ongoing treatment for people living with HIV [22,23]. From this perspective, the unique ability to use linked electronic medical records to quantify misclassification due to patient transfers in a large HIV treatment programme in the Western Cape Region of South Africa provides policy makers and researchers with much-needed information on how patient transfers may affect estimates of retention in comparable ART treatment programmes.
Several methods have been proposed to account for misclassification due to attrition when estimating mortality in HIV treatment programmes and could be readily applied to estimates of retention in care [24,25]. Such methods typically rely on tracing a subset of patients to ascertain vital status or retention information and use inverse probability weighting to correct mortality estimates [5,7,25–29]. In order to account for potential selection bias in which participants can be traced, information on factors associated with mortality or attrition and being traced is needed to construct inverse probably weights and obtain unbiased estimates of mortality or retention in care [25]. In practice, tracing participants is not always feasible. Thus, covariate information on factors associated with being both LTFU and being traced may be poorly measured or missing entirely in large HIV treatment programmes [25].
In our analysis, we estimated misclassification due to patient transfers using linked medical record information. Such an approach allowed us to measure patient transfers directly, without having to trace patients or gather information on factors associated with attrition or being traced [3,30]. However, data linkage approaches to validate retention estimates have limitations. In our data, we were not able to distinguish between ‘official’ transfers and ‘silent’ transfers. The higher levels of misclassification due to patient transfers among women observed in our analysis likely reflect mandated transfers from an antenatal clinic to an ART clinic for women after a pregnancy. Additionally, we also were not able to isolate healthcare visits made exclusively for HIV-related care from other regular healthcare visits, which may have over-estimated transfers and attrition. Finally, our data were not authorised by authorities for linkage to South Africa’s national death registry, and therefore, mortality in our data is likely underestimated. Finally, while our data covered all public healthcare facilities in the Western Cape province, it is possible that participants identified as LTFU could have transferred to healthcare facilities outside of the Province or to private clinics within the Western Cape, which we were unable to measure. Given these limitations, our estimates of misclassification due to patient transfers can be viewed as an upper limit of potential bias.
Conclusions
As access to lifelong ART has scaled-up throughout sub-Saharan Africa, people living with HIV are increasingly expected to maintain lifelong engagement in ART treatment programmes. Our analysis demonstrates that patient transfers among PLWH on ART are common and can appreciably bias estimates of retention in care. When tracing patients to ascertain engagement in care status is not feasible, estimates of misclassification due to patient transfers from routinely collected medical record data may help to inform programme evaluation, resource allocation and researchers estimating retention in HIV care.
Supplementary Material
Table S1. Retention at database closure1: Estimates of retention in antiretroviral therapy (ART) treatment (A) overall and (B) by gender, not accounting for patient transfers and accounting for patient transfers.
Table S2. Time to first attrition1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers.
Table S3. Retention at database closure1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers when attrition is defined as died or our of care> 365 days.
Table S4. Time to first attrition1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers when attrition is defined as died or our of care> 365 days.
Acknowledgements
We thank and acknowledge the Provincial Health Data Centre of the Western Cape Department of Health, and Mariette Smith in particular, for the data linkage, preparation and anonymisation for the parent study, and Andrew Boulle for assistance in understanding and interpreting the various data sources. We also thank the South African Medical Research Council, the National Institute of Mental Health (R01MH106600, D43 TW011308 and R00MH112413), the Fogarty International Center (D43 TW011308) and the National Institute of Child Health and Development (1R24HD077976) for grant support. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health or the South African Medical Research Council. A portion of this was presented at the Society for Epidemiological Research meeting in Minneapolis, MN on June 18-21, 2019. This work was supported by grants awarded jointly by the National Institute of Mental Health and the South African Medical Research Council (grant number R01 MH106600), the National Institute of Mental Health (R00MH112413), the Fogarty International Center and the National Institute of Mental Health (D43 TW011308) and the National Institute of Child Health and Development (1R24HD077976). Data for the present analysis come from the Provincial Health Data Centre of the Western Cape Department of Health and cannot be passed on to third parties without prior approval from the Provincial Health Data Center (https://www.westerncape.gov.za/general-publication/provincial-health-data-centre). Code for all analyses is available from Angela Bengtson (angela_bengtson@brown.edu).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.UNAIDS. 90-90-90: Treatment for All 2018. (Available from: http://www.unaids.org/en/resources/909090.) [5 January 2018]
- 2.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011: 52: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox MP, Bor J, Brennan AT et al. Estimating retention in HIV care accounting for patient transfers: A national laboratory cohort study in South Africa. PLoS Medicine 2018: 15: e1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etoori D, Wringe A, Kabudula CW et al. Misreporting of patient outcomes in the south African national HIV treatment database: consequences for programme planning, monitoring, and evaluation. Front Public Health 2020: 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zurcher K, Mooser A, Anderegg N et al. Outcomes of HIV positive patients lost to follow-up in African treatment programmes. Trop Med Int Health. 2017: 22: 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas AD, Zaniewski E, Anderegg N et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc 2018: 21: e25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chammartin F, Zurcher K, Keiser O et al. Outcomes of Patients Lost to Follow-up in African Antiretroviral Therapy Programs: Individual Patient Data Meta-analysis. Clin Infect Dis 2018: 67: 1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulle A, Schomaker M, May MT et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med 2014: 11: e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornell M, Johnson LF, Wood R et al. Twelve-year mortality in adults initiating antiretroviral therapy in South Africa. J Int AIDS Soc 2017: 20 21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck EJ, Shields JM, Tanna G et al. Developing and implementing national health identifiers in resource limited countries: why, what, who, when and how? Global health action. 2018: 11: 1440782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Statistics South Africa. Mid-Year Population Estimates: 2017. Pretoria, South Africa: Statistics South Africa; 2017. [Google Scholar]
- 12.Poolman M, Nvd Walt, Luwaca B. Annual Progress Report 2015/16. Western Cape, South Africa: Western Cape Provid-incial Council on AIDS; 2017. [Google Scholar]
- 13.Meintjes G, Black J, Conradie F et al. Southern African HIV Clinicians Society adult antiretroviral therapy guidelines: Update on when to initiate antiretroviral therapy. South Afr J HIV Med 2015: 16: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med 2017: 18: 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornell M, Technau K, Fairall L et al. Monitoring the South African National Antiretroviral Treatment Programme, 2003–2007: the IeDEA Southern Africa collaboration. South Afr Med J 2009: 99: 653–660. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson L, Harley B, Sharp J et al. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Trop Med Int Health 2016: 21: 743–749. [DOI] [PubMed] [Google Scholar]
- 17.Lurie MN, Kirwa K, Callaway J et al. Quantifying the HIV treatment cascade in a South African health sub-district by gender: retrospective cohort study. Trop Med Int Health 2019: 25: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health SA. National Consolidated Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: Department of Health; 2015. [Google Scholar]
- 19.Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol 2013: 66: 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesko CR, Edwards JK, Cole SR, Moore RD, Lau B. When to Censor? Am J Epidemiol 2018: 187(3): 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS 2015: 10: 430–438. [DOI] [PubMed] [Google Scholar]
- 22.Bengtson AM, Go V, Kumwenda W et al. “A way of escaping”: a qualitative study exploring reasons for clinic transferring and its impact on engagement in care among women in Option B+. AIDS Care 2019: 32: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bengtson AM, Kumwenda W, Lurie M et al. Improving Monitoring of Engagement in HIV Care for Women in Option B+: A Pilot Test of Biometric Fingerprint Scanning in Lilongwe, Malawi. AIDS Behav 2019: 24: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng EH, Odeny TA, Lyamuya R et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in eastern Africa: application of a sampling-based approach. Clin Infect Dis 2016: 62: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schomaker M, Gsponer T, Estill J, Fox M, Boulle A. Non-ignorable loss to follow-up: correcting mortality estimates based on additional outcome ascertainment. Stat Med 2014: 33: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkhof MW, Spycher BD, Yiannoutsos C et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One 2010: 5: e14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Spycher BD, Sidle J et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Medicine 2011: 8: e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiragga AN, Castelnuovo B, Musomba R et al. Comparison of methods for correction of mortality estimates for loss to follow-up after ART initiation: a case of the Infectious Diseases Institute, Uganda. PLoS One 2013: 8: e83524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA 2008: 300: 506–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan SR, Oosthuizen C, Stinson K et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study. PLoS Medicine 2017: 14: e1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Retention at database closure1: Estimates of retention in antiretroviral therapy (ART) treatment (A) overall and (B) by gender, not accounting for patient transfers and accounting for patient transfers.
Table S2. Time to first attrition1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers.
Table S3. Retention at database closure1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers when attrition is defined as died or our of care> 365 days.
Table S4. Time to first attrition1: Estimates of retention in antiretroviral therapy (ART) treatment A) overall and B) by gender, not accounting for patient transfers and accounting for patient transfers when attrition is defined as died or our of care> 365 days.


