Abstract
Nanotechnology in medical applications, especially in oncology as drug delivery systems, has recently shown promising results. However, although these advances have been promising in the pre-clinical stages, the clinical translation of this technology is challenging. To create drug delivery systems with increased treatment efficacy for clinical translation, the physicochemical characteristics of nanoparticles such as size, shape, elasticity (flexibility/rigidity), surface chemistry, and surface charge can be specified to optimize efficiency for a given application. Consequently, interdisciplinary researchers have focused on producing biocompatible materials, production technologies, or new formulations for efficient loading, and high stability. The effects of design parameters can be studied in vitro, in vivo, or using computational models, with the goal of understanding how they affect nanoparticle biophysics and their interactions with cells. The present review summarizes the advances and technologies in the production and design of cancer nanomedicines to achieve clinical translation and commercialization. We also highlight existing challenges and opportunities in the field.
Keywords: Tumor microenvironment, Nanomedicine, Drug delivery, Nanoparticle design, Drug loading, Clinical translation
Abbreviations: EPR, Permeability and retention; MDR, Multidrug resistance; TME, Tumor microenvironment; IFP, Interstitial fluid pressure; ECM, Extracellular matrix; TAF, Tumor-associated fibroblast; TAM, Tumor-associated macrophage; MPS, Mononuclear phagocyte system; RBC, Red blood cell; CFL, Cell-free layer; MMPs, Matrix metalloproteinases; TIMPs, Tissue inhibitor of metalloproteinases; DPD, Dissipative particle dynamic; CGMD, Coarse-grained molecular dynamic; MD, Molecular dynamic; MTA, Multi-tadpole assemblies; MEC, Minimum effective concentration; MTC, Minimum toxic concentration
Graphical abstract
1. Introduction
Nanotechnology has revolutionized drug design by introducing nanoparticles with many desirable and tunable characteristics. The growing interest in developing nanoparticles for cancer applications is largely due to their unique properties, which can be specified for each purpose, including diagnosis, immunotherapy, imaging and drug delivery (Box 1) [[1], [2], [3], [4], [5], [6]]. To date, some nanoparticles have been approved for clinical use, and others have shown great promise in preclinical studies but have not yet been approved. Safety and efficacy are the two important criteria for any new cancer nanomedicine that determine their failure or success. Improving the safety profile and increasing the accumulation of nanoparticles at the site of the cancer are the cornerstones of nanomedicine success in the clinic. However, the accumulation of nanoparticles at the site of the disease is the most important challenge for new nanoparticles, which is related to the biological barriers of nanoparticle transport [[7], [8], [9]]. It has been reported that 0.67–0.7% of the injected dose accumulates in the tumor and only 0.0014% eventually interacts with cancer cells [[10], [11], [12]].
Box 1. Attractive features of nanoparticles in oncological applications.
-
•
Reduced side effects and increased efficacy to improve therapeutic index;
-
•
Targeted drug delivery in a cell-, organelle-, and tissue-specific manner;
-
•
Potential to improve pharmaceutical properties of the drug such as tumor accumulation based on the enhanced permeability and retention (EPR) effect, circulating half-life, solubility, and stability;
-
•
Ability to produce sustained release of drug as well as stimulus-triggered drug release;
-
•
Facilitation of the biomacromolecular drug delivery to intracellular sites of action;
-
•
Co-delivery of a combination of drugs in one carrier to overcome multidrug resistance (MDR);
-
•
Adaptable to cancer diognostics;
-
•
Simultaneous combination of diagnosis and therapy, known as “theranostics”;
-
•
Possible to design synthetic vaccines using new approaches;
-
•
Protection of sensitive drugs against harsh environments such as low pH or high levels of proteases.
Alt-text: Box 1
Recent research on nanoparticles has resulted in better designs for therapeutic and diagnostic applications, thus enhancing clinical outcomes [13,14]. To fulfill the potential of nanoparticle technology, researchers and clinicians must be able to effectively monitor their transport and localization. Precise delivery requires complete control over the in vivo transport of nanoparticles, but precise control is a major challenge that requires a better understanding of how nanoparticles interact with biological systems [[15], [16], [17]]. Because these nano-bio interactions are complex, multiparametric, and dynamic, they present significant barriers to nanoparticle engineering [18]. Factors that exacerbate the complexity of these interactions are: the physicochemical properties of nanoparticles, the interactions between components of the biological and biochemical environments, and the kinetics of nano-bio interactions [19].
For cancer applications, penetration of the nanoparticle into the tumor is the most important consideration. The abnormal biology of the tumor microenvironment (TME) –including the abnormal vasculature, the elevated interstitial fluid pressure (IFP), and the dense extracellular matrix (ECM)– prevent the efficient delivery of nanoparticles [20,21]. Many approaches have been developed to target the TME to overcome these barriers and enhance penetration depth [22,23], including normalization of the vasculature [24,25], alleviation of mechanical stress [26], reduction of tumor hypoxia [27,28], reprogramming tumor-associated fibroblasts (TAFs) [29,30], and modulating tumor-associated macrophage (TAM) phenotype [31,32]. The success of such approaches depends on the type of tumor and the specific properties of the nanoparticles, so these methods are not effective in every clinical situation. In addition, these methods, which manipulate the characteristics of TME, can disrupt mechanical homeostasis of the TME, leading to additional problems such as metastasis [33]. Therefore, optimization of physicochemical properties the nanomedicine is often the best approach for increasing drug delivery and enhancing treatment.
Nanotechnology has struggled to improve the performance of delivery systems due to several limitations and barriers, including poor drug loading and unwanted drug release, which can cause toxicity and undesirable side effects. There is also sub-optimal distribution of nanoparticles in tissues, leading to insufficient drug concentration at the target site and premature drug release, which causes many side effects [34]. Hence, the need for advanced materials and efficient delivery systems is greater than ever. Best achieved through interdisciplinary partnerships, diverse research fields have focused on the development of nanomedicines including: (i) clinical chemists who specialize in drug delivery and clinical considerations; (ii) materials scientists in the production of new nanostructured materials; and (iii) engineers in manufacturing processes and innovation. But in general, these diverse groups have not formed multidisciplinary collaborations. This results in highly focused research and development pipelines that underestimate or do not recognize potential problems with the efficacy, implementation and robustness of the nanomedicine.
Various methods have been proposed to increase the accumulation of nanoparticles in the tissue. However, the complicated design of nanoparticles can make its clinical translation difficult. Here, we examine various effective and basic factors to accelerate the clinical translation of nanomedicine. We first discuss different physicochemical properties of nanoparticles as well as biological barriers against delivery of nanoparticles. Subsequently, the primary methods for drug loading and preparation, followed by stability tests, are examined in detail – as these steps are crucial for successful clinical translation. Finally, this review highlights the current status and common challenges, as well as future opportunities that may expedite the translation of nanoparticles from the bench to the clinic.
2. Biological barriers and the tumor microenvironment
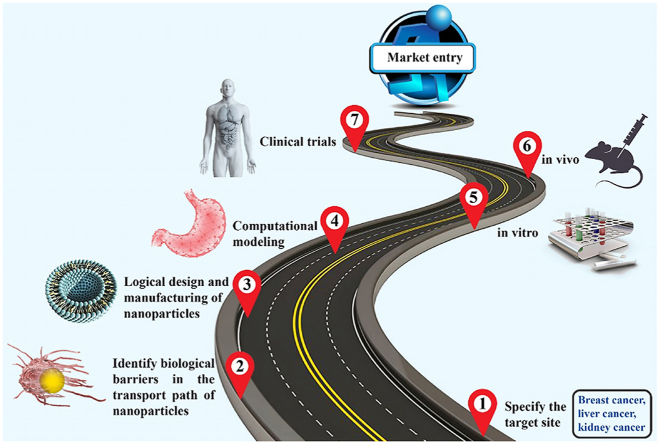
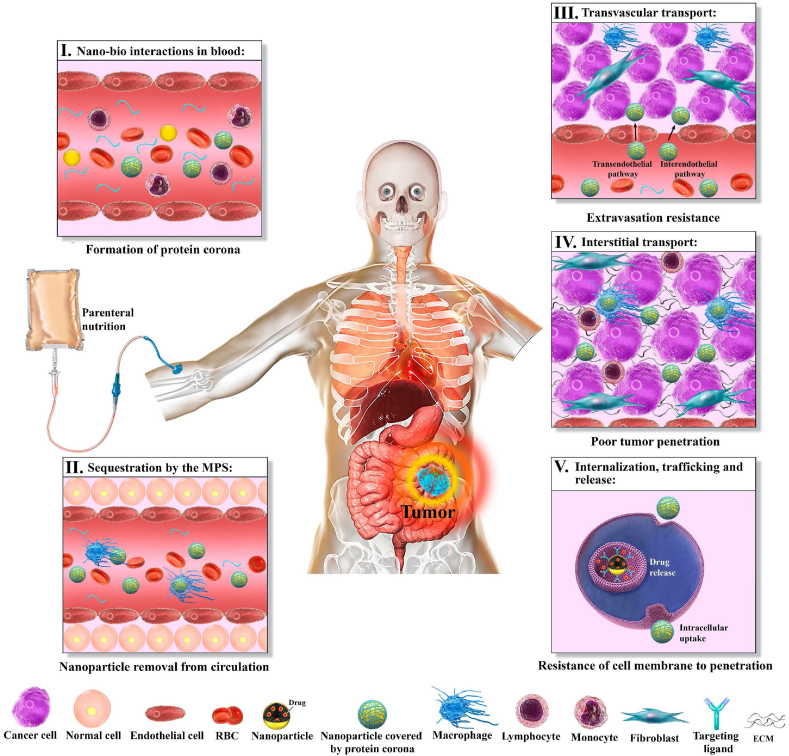
A useful drug delivery system requires the efficient circulation of nanoparticles through blood vessels, transvascular transport of nanoparticles into the interstitial space of the tumor, the uniform interstitial transport of the nanoparticles, binding to – or uptake by – the cells, and sufficient drug activity or release [35,36]. Encapsulation of drugs within nanoparticles can reduce their adverse effects and increase blood circulation time, leading to increased transvascular transport of nanoparticles into the tumor interstitium [[37], [38], [39]]. After injection, nanoparticles encounter various biological barriers that can greatly reduce therapeutic performance system (Fig. 1). Natural processes exist for clearance of foreign substances, which include organs such as the kidneys, spleen, and liver, as well as resident immune cells in various organs. Unfortunately, these processes also remove intravenously injected nanoparticles from the bloodstream. As a result, in addition to reducing circulation time, clearance leads to reduced delivery of nanoparticles and drug to the tumor [40,41].
Fig. 1.
Biological barriers faced by nanoparticles. (I) After injection, proteins in plasma accumulate on the surface of nanoparticles and form a protein corona that can change the interactions of nanoparticles with the biological environment. (II) Phagocytic cells detect nanoparticles because of the protein corona and remove them from the circulation. (III) Heterogeneous vascular leakage causes nonuniform distribution of nanoparticles in the tumor. In addition, high intra-tumor IFP reduces convection, limiting delivery into the tissue. (IV) After extravasation, dense ECM and high IFP are barriers against nanoparticle penetration. (V) Finally, cell membranes, which act as a shell to protect cellular organelles, resist the penetration of nanoparticles into the cell during the internalization process.
Clearance of nanoparticles from plasma is the first and most-important biological barrier; in fact, triggering endogenous defense systems may result in nanoparticle removal within hours or minutes [[42], [43], [44]]. Upon entering the plasma, the surface of the nanoparticles is coated with serum proteins such as opsonins in a process known as opsonization [45,46]. The formation of a protein corona changes the biological appearance of the nanoparticles, making them vulnerable to cells of the mononuclear phagocyte system (MPS): upon opsonization, the receptors of phagocytic cells recognize the nanoparticle surface or protein corona, bind to the surface, and initiate endocytosis, resulting in internalization and destruction of the nanoparticles [47].
In general, increasing the circulation time can increase extravasation and accumulation of injected species into the tumor. In blood vessels, red blood cells (RBCs) travel near the midline of the vessel. Affected by the presence of RBCs, particles generally migrate at a particular region of the vessels closer to the surface of the endothelial cells, called the cell-free layer (CFL) [48]. Under normal blood circulation conditions, different parameters of nanoparticles (geometry, size, stiffness, and surface charge) strongly affect the margination dynamics, which may limit the arrival of the nanoparticles at the endothelial surface [49]. For example, very small nanoparticles can intersperse within RBC clusters near the centerline, while larger nanoparticles tend to move in the CFL [50]. The lateral drift of nanoparticles toward endothelial walls allows interactions and adhesion between the nanoparticles and the surface of the vessel.
In some cases, the particle can extravasate from the vessel into the interstitium through passive mechanisms, without binding to the endothelium. The EPR effect, produced by the leaky endothelial junctions within tumors, can allow particles with specific dimensions and properties to exit the vasculature and enter the tumor tissue (Box 2). Although the EPR effect facilitates the accumulation of nanoparticles across the vessel: tissue boundary, penetration into the tumor is hindered due to the elevated IFP and low convection in the extravascular space [51]. The interactions between the nanoparticles and the vessel wall determine the extent of the EPR effect, and can be electrostatic, hydrodynamic, or steric [24,52,53]. Studies have shown that larger nanoparticle: pore size ratios hinder transvascular transport [37,49].
Box 2. Transvascular and interstitial transport of nanocarriers.
- Nanocarrier transport, accumulation and tumor penetration are influenced by the physicochemical characteristics or the particles and the biological properties of tumor tissue. The transport of nanocarriers is defined by specific and interrelated processes such as transvascular transport and penetration into the interstitium. These processes occur through mechanisms of diffusion and convection. Mathematical models have clarified the description and effect of each of these mechanisms. For example, Eq. (1) describes the process of transvascular transport [55]:
(1a) Diffusive processThis term originates from correction of Fick's first law to account for vessel wall permeability (P) and the surface area of the blood microvessels (A). This component also arises from the Brownian motion of nanoparticles, which, given the gradient of concentration between the microvessels () and the interstitial space (), drives particle motion in the direction towards the interstitial space.
Convective processThe fluid flux expressed in this term is based on Starling's law, in which is hydraulic conductivity of the wall of microvessels, and are hydrostatic pressures in the vascular and interstitial spaces, respectively; and are oncotic pressures in the vascular and interstitial spaces, respectively, and σ is the average osmotic reflection coefficient.
- We can also derive equations for the transport of nanocarriers in the interstitium. Like transvascular transport, interstitial transport occurs through both diffusion and convection. Other phenomena are also involved, such as metabolism or binding of nanocarriers to the ECM, their possible degradation, their capture by the mononuclear phagocytic system [18], and their uptake by tumor cells. Hence, the dynamics of drug concentration in the interstitium are affected by diffusion and convection through Equation (2):
(2a) Diffusive componentThis term is due to the effective coefficient of diffusion ( and variations of concentration in all directions ().
Convective componentThis term includes the direction and magnitude of fluid flow (), the spatial gradient of concentration (), and a coefficient () which is based on the fact that the colloid velocity in the ECM might be different from the velocity of the interstitial fluid (due to drag, adsorption or elimination effects).
The negative sign in the last term shows that interactions with the tumor lead to reduced interstitial transport.
Alt-text: Box 2
Upon entering the tumor interstitium, nanoparticles undergo cellular internalization where they can release their cargo. The cell plasma membrane is composed of a mixture of different lipids and proteins that creates a barrier with highly selective permeability that controls the movement of substances inside and outside of the cell [54]. Endocytosis is the major translocation pathway of nanoparticles across the cell membrane and is affected by the biophysical properties of the nanoparticles. Unfortunately, even if the nanoparticle enters the cell, it may not remain there long enough to be effective. This is largely due to multidrug resistance (MDR) – the active removal of foreign substances from the cells – which is a major mechanism of therapy evasion by tumors. Many foreign substances, including therapeutic agents, are expelled from the cell by MDR pumps, thus reducing therapeutic impact [49].
In addition to circulatory kinetics and clearance, the interactions of nanoparticles in the TME are very important. The TME contains cancer cells, stromal cells, fibroblasts, immune cells, and ECM [56]. Suppression of the immune system, overexpression of enzymes, the presence of acidic and hypoxic regions, as well as regions with overactivated redox processes are some of the features of tumor tissue that contribute to tumor progression and treatment resistance. The ECM is a particularly important barrier, as many tumors have excess, non-uniform ECM, due to inflammation, fibrosis, the activation of matrix metalloproteinases (MMPs) and MMP inhibitors, e.g., tissue inhibitor of metalloproteinases (TIMPs) [57,58].
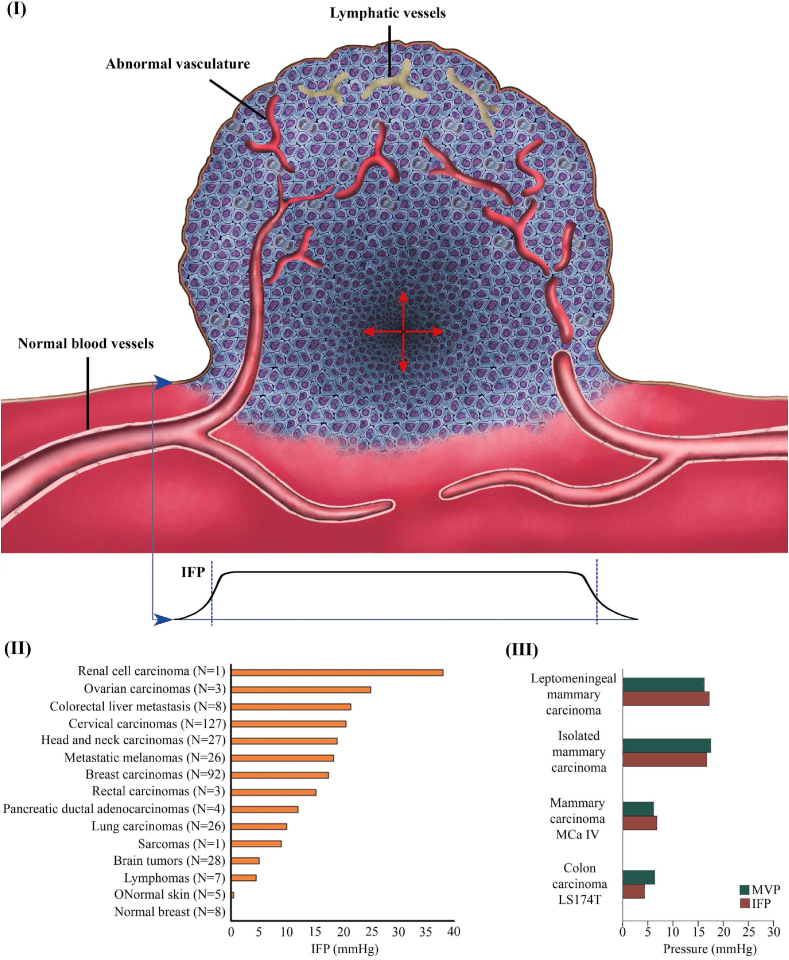
Because of chronic exposure to angiogenic growth factors, tumor blood vessels are abnormal in structure and function. The high permeability of tumor microvasculature (50–70 times that of normal vessels [59]) allows plasma to leak into the tumor interstitium and accumulate in the tumor tissue. In addition, the lymphatic vessels that normally remove fluid and macromolecules from the interstitium are absent from tumor tissue [60]. Because of this altered fluid homeostasis, interstitial fluid pressure (IFP) accumulates within tumors and decreases rapidly near the tumor boundary, where fluid is drained by nearby functional lymphatic vessels (Fig. 2I) [26,61,62]. High IFP is a prominent feature of tumor tissue and its presence has been confirmed in nearly all human tumors (Fig. 2II) [63,64]. IFP increases uniformly from tumor borders to the center and its value is almost equal to the microvascular pressure (Fig. 2III) [65]. High IFP and MPS are considered to be the most important barriers to the accumulation of nanoparticles in tumor tissue [65].
Fig. 2.
Alter fluid homeostasis in the tumor microenvironment. (I) Consistent features of tumor tissue are regions of high cancer cell density and abnormal microvascular networks; (II) Comparison of IFP level in normal and tumors tissues (reproduced from Ref. [26] with permission); (III) The IFP and microvascular pressure (MVP) for different tumor types (reproduced from Ref. [26] with permission).
3. Design principles
Cancer nanomedicine design, especially for solid tumors, is based on the EPR effect, known as passive targeting [66,67]. The EPR effect increases accumulation of nanoparticles at the target sites, resulting in less exposure to other organs. Although many studies have identified extravasation through gaps between endothelial cells as the cause of nanoparticle accumulation in the tumor, recent work suggests that other mechanisms are involved, including transport through transcellular channels or via endocytosis/transcytosis [68].
Systemic delivery of nanoparticles through the chaotic and dynamic environment in vivo is challenging. Blood flow is relatively rapid, so nanoparticles circulate throughout the human body once per minute. Blood flow velocity varies from 1.5 to 33 cm/s [69,70], with the lowest velocities in capillaries and venules. In addition, the total length of blood vessels in an adult human is ∼100,000 km [71]. Consequently, the particles are faced with a very long journey at a high speed, after which they may pass the tumor (at most a few centimeters in size) in seconds. Accordingly, long-lasting nanoparticles have significantly more opportunities to visit the tumor. Also, after extravasation, some nanoparticles take hours or even days to penetrate only 200 μm and reach the tumor core [72]. Hence, nanoparticles need a principled design, which is influenced by the physico-chemical characteristics of the nanoparticles such as size, shape, mechanical properties, surface chemistry, and surface charge. These properties can affect how the nanoparticles interact with cells and proteins [73], and can also determine their toxicity to normal tissues. Therefore, by optimally modifying these properties, nanoparticles can overcome biological barriers. Computer modeling techniques provide detailed information about physico-chemical features and molecular interactions of drugs or carriers of drugs or carriers, and have found broad applications in biology, biochemistry, and biophysics [[74], [75], [76], [77], [78]]. Many structural and physicochemical properties of nanomaterials can be examined using computational models prior to their synthesis and biological testing [79].
3.1. Size
Because nanoparticle size affects half-life, encapsulation efficiency, and cellular uptake, it is one of the most important design features [49,80]. Size also helps define the interaction of nanoparticles with biomolecules such as lipids, peptides, and DNA, thus critically influencing many aspects of nanoparticle function [81]. Studies illustrate that nanoparticles with sub-micron size have more advantages than microparticles for drug delivery system applications [44,82,83]. Similarly, it has been shown that the particle surface area affects drug release [84]. Smaller nanoparticles have a larger ratio of surface area to volume, so more of the associated drug is either at or near the surface of the particle. This results in faster drug release. On the other hand, larger nanoparticles have relatively more core volume and are capable of carrying more load, and the drug is released more slowly by diffusion [85]. Because size affects so many aspects or nanoparticle performance, it is challenging property to optimize.
For delivery to the tumor via the blood stream, the perfusion characteristics of normal and tumor vasculature –and the interactions between blood rheology and particle convection – need to be considered. Particles smaller than 500 nm tend to move parallel to the vessel wall and are minimally affected by the RBCs. Larger nanoparticles, on the other hand, experience lateral forces in the bloodstream that cause them to be “marginated” so they travel close to the vessel wall (Fig. 3I) [50]. Although large nanoparticles naturally travel closer to the vessel wall, they don't extravasate into tissue as easily as smaller particles. On the other hand, larger particles are not cleared as rapidly, so have longer circulation times [86,87], and they have less accumulation in normal tissues, thus producing fewer side effects [88]. Other factors, such as clearance by the kidneys and liver should also be considered to optimize the size. Renal clearance is very rapid for particles smaller than 6 nm in diameter, while larger particles are generally cleared by the liver reticuloendothelial system [11,87]. Uptake by the RES can be inhibited by PEGylation [87,89].
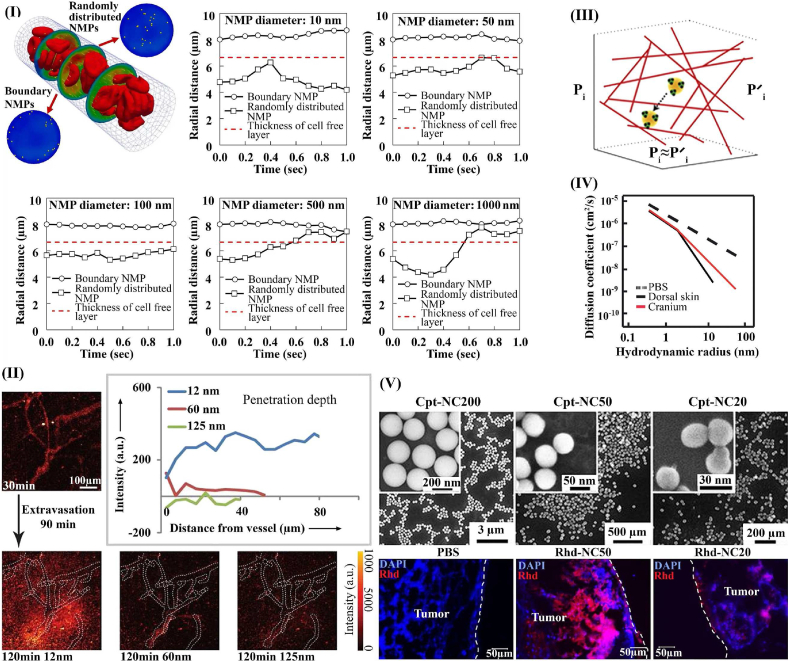
Fig. 3.
Effect of particle size on nanoparticle transport. I) Trajectory of nanoparticles inside the vessel (hematocrit:30%, number of NP: 100) [50] (reproduced from Ref. [50] with permission), II) Intravital imaging of size-dependent transvascular transport; small nanoparticles (<60 nm in diameter), extravasate most readily [82] (reproduced from Ref. [82] with permission). III) Diffusion through pores in the collagen network in the tumor ECM [91] (reproduced from Ref. [91] with permission). IV) The effect of hydrodynamic radius on diffusivity in PBS, the dorsal skin and the brain [90] (reproduced from Ref. [90] with permission). V) Accumulation and distribution of nanoparticles in tumor tissue; it is clear that nanoparticles with a diameter of 50 nm have a higher concentration in tumor tissue [98] (reproduced from Ref. [98] with permission).
Based on particle size to vessel wall pore size ratio and acceptably low clearance and extravasation in normal tissues, there is an optimal nanoparticle size range – 12 to 60 nm – that provides efficient transvascular transport in many tumors [88]. In tumors with large inter-endothelial junctions, particles as large as 100 nm may also be considered to increase the payload (Fig. 3II) [24,37]. Once they cross the vessel wall, nanoparticles generally need to penetrate into the target tissue to deliver the drug uniformly. For this step, the optimal range for nanoparticle diameter is 5–50 nm [86]. Interstitial convection of particles is driven by IFP gradients in the tumor, and is impeded by the electrostatic, hydrodynamic, and steric interactions in the interstitial space (Fig. 3III) [90,91]. Particle diffusion is inversely related to size and is also affected by interactions between the nanoparticles and extracellular fibers. Furthermore, because of steric hindrance, larger particles have very limited penetration into tumor tissue (Fig. 3IV) [92]. If sufficiently small compared to the pores in the interstitium, hydrodynamic and steric interaction become less important [93]. However, tumors are often fibrotic, with excess production of extracellular fibers, thus reducing penetration of nanoparticles. Because of these size-dependent differences in transvascular and interstitial transport, particles with hydrodynamic diameters larger than 50 nm may extravasate easily, but cannot effectively penetrate the interstitial space, causing them to accumulate around the vasculature [94]. Studies performed in vitro and in vivo have identified the optimal range of nanoparticle size for tissue penetration. The optimal size can vary for each nanoparticle system. For example, it is 2–6 nm for ultra-small Au nanoparticles [95], 30 nm for poly (ethylene glycol)-b-poly (lactic acid) (PEG-b-PLA) micelles [96], 50 nm for monodisperse drug−silica nanoconjugates (Fig. 3V) [97,98], and up to 70 nm for poly (lactic-co-glycolic acid) (PLGA) nanoparticles [99].
Nevertheless, given the large amount of heterogeneity that exists within a given tumor – and between tumor types – identifying a nanoparticle formulation optimal for all tumor applications has proved difficult [88]. Computational modeling can help predict the effect of nanoparticle size on the motion, margination, and nonspecific or specific adhesion of particles in a hemodynamic flow. For example, Gentile et al. [100] investigated the behavior of nanoparticles of different diameters (spherical particles 50, 100, 200, 500, and 750 nm in diameter, 1, 6, and 10 μm) in dynamic flows. This study showed that, for nanoparticles larger than 500 nm in diameter, gravitational forces influence the particle margination toward the wall. However, the migration of the particles toward the flow chamber wall is due to the Brownian motion for nanoparticles smaller than 500 nm in diameter [101]. Additionally, Decuzzi et al. used computational modeling to show that steric interactions, electrostatic interactions, van der Waals forces, steric interactions, and buoyancy parameters can lead to a ‘critical radius’ where the time for margination of particles toward a surface in shear flow will be the longest [102]. The predicted critical size is 50–200 nm, which is often in the range of nanoparticles such as micelles-liposomes-polymer particles. Examining the cellular uptake of particles with different sizes by Caco-2 cells, Desai MP et al. [103] found that nanoparticles with a diameter of 100 nm had a 2.5 and 6-fold uptake rate, respectively, compared with 1 μm and 10 μm microparticles. Mathematical modeling and experimental results using gold and silver nanoparticles in the size range of 2–100 nm demonstrate that particles of 40–50 nm more efficiently bind and induce receptor-mediated endocytic processes [104]. It has also been reported that nanoparticles larger than 50 nm fail to reach the cell nucleus [105]. Taking into account all the above-mentioned constraints, nanoparticles with diameter of 12–50 nm are most appropriate for transvascular and extravascular transport.
3.2. Shape
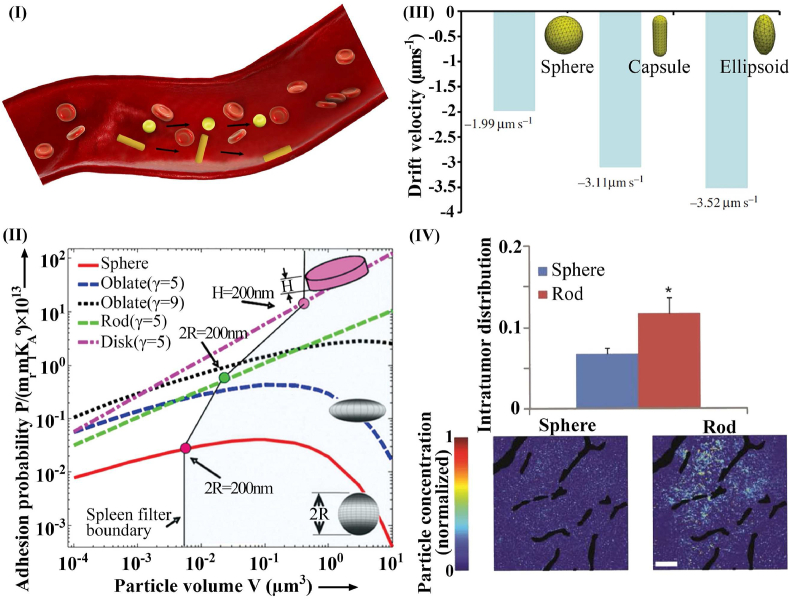
Shape is another important intrinsic property of nanoparticles that can affect their interactions with biological tissues. The shape of a nanoparticle influences how it circulates, penetrates the interstitium, gets internalized or cleared, and how it enters a target cell [1,106]. Although spherical nanoparticles have been studied more than non-spherical particles [96], mathematical modeling and recent experimental studies suggest that non-spherical particles may have advantages, especially in the blood circulation. For example, velocity gradients cause a heterogeneous distribution of forces along the axis of particle symmetry, thereby leading to particle tumbling and rotation (Fig. 4I). Shah et al. [107], and Tao et al. [108] found that anisotropic nanoparticles, such as rods and discs, have higher margination propensity in dynamic flow and higher wall adhesion than spherical nanoparticles, owing to biased hydrodynamic and Brownian forces (Fig. 4II). Geng et al. illustrated that filomicelles remain in the bloodstream for up to a week after injection – approximately ten times longer than spherical particles [109]. It has also been shown that lateral drifting velocities of ellipsoid-shaped nanoparticles are greater than spherical particles, facilitating their drift away from the core region of the vessel (Fig. 4III) [110].
Fig. 4.
Effect of particle size on nanoparticle transport. I) Nanoparticle margination: spherical nanoparticles tend to travel near the vessel axis, but hemodynamic forces cause non-spherical nanoparticles to migrate toward the vessel wall. II) Adhesion probabilities of nanoparticles are affected by shape and size [118] (reproduced from Ref. [118] with permission). III) Effect of nanoparticle shape on drift velocity in a capillary with 30% hematocrit [110] (reproduced from Ref. [110] with permission). IV) Interstitium distribution of rod and spherical-shape nanoparticles [112] (reproduced from Ref. [112] with permission).
In terms of the effect of transvascular flux, a comparison of spherical particles with elongated particles with equal hydrodynamic diameter demonstrated that elongated particles are superior to spherical ones [111]. This may be due to increased margination toward the vessel wall and a reduction of resistance to movement through the endothelial junctions [102]. Considering intratumoral distribution, aspherical nanoparticles generally distribute throughout the tumor interstitial space more efficiently [112]. Elongated particles exhibit lower steric and hydrodynamic interactions with collagen fibers and diffuse faster in the direction of their long axis, which leads to increased penetration depth and accumulation (Fig. 4IV) [113].
The shape of the nanoparticles also has important effects on cell uptake. Bartczak et al. created gold nanoparticles with four different forms – spherical, rod-shaped, hollow, and silica-gold core-shell particles. They found that spherical particle uptake was the highest and that of hollow particles was the lowest [114]. Indeed, geometric descriptions of shape such as the aspect ratio can be used to predict transport properties as well as the interaction between cells and particles. Chithrani et al. showed that spherical gold particles with size 14 or 7 nm have a higher cellular uptake (2.75–5 fold) than rod-shaped particles with dimensions of 14 × 40 nm and 14 × 74 nm [115]. For particles smaller than 100 nm, cellular internalization of spherical nanoparticles is more effective than rod-shaped particles, however, as size increases, higher aspect ratios result in better internalization [116]. Specifically, internalization is facilitated when rod-shaped particles align perpendicular to the cell membrane [37]. The endocytic kinetics of nanoparticles with different shapes (spherical, rod-like, cubic, and disk-like nanoparticles) were evaluated by Li et al. using a large scale dissipative particle dynamics (DPD) model [117]. Li et al. found using their model that spherical nanoparticles are efficiently accepted by cells, followed by cubic and rod-shaped, whereas disk-like nanoparticles are only found at the cell membrane surface [117]. To compare spherical and non-spherical nanoparticles, it is often useful to fix the diameter of the spherical particle and then compare with non-spherical particles that have the same surface-area-to-volume ratio and ligand-to-grafted chain ratio. Such analyses allow mechanistic studies of the effect of shape and are easily approached using computer simulations.
3.3. Surface charge
Nanoparticle surface charge is another important consideration for development of drug delivery systems. Surface charge influences the interactions between nanoparticles and microvessels or tumor interstitium, and thus their transport to – and within – the tumor. Surface charge affects various biophysical and biological functions including solubility, biological distribution, stability, cellular uptake, and cytotoxicity. Nanoparticles with surface charges (either negative or positive) tend to have minimal self–self interactions [119].
As described above, interactions between cells and nanoparticles play a key role in their physiological delivery and their biological functions. While travelling in the bloodstream, electrostatic interactions become important when the particles come into proximity to the vessel wall. Surface charge density can also affect nanoparticle clearance [87,120]. However, the positive charge of cationic particles attracts negatively-charged proteins in the bloodstream, causing them to adsorb to the particle surface. This facilitates their recognition by the immune system, and their subsequent removal [46,121]. Additionally, because endothelial basal lamina has a negative charge, positively charged nanoparticles are easily absorbed, limiting their circulation [24,52]. Conversely, negatively charged nanoparticles are repelled from the CFL, limiting their extravasation [122,123]. For these reasons, nanoparticles with neutral charge are better for vascular transport [1]. Nevertheless, it has been shown that interactions between cationic particles and the vessel wall can increase the chance of extravascular transport into tumors [49]. Furthermore, experimental and theoretical studies have shown that electrostatic interactions with the negatively charged glycocalyx of vascular endothelial cells can allow cationic nanoparticles to extravasate more readily than their anionic or neutral counterparts [122,123].
Because many charged species exist in the tumor environment, including cationic collagen and anionic hyaluronic acid, electrostatic interactions also affect how nanoparticles distribute in the tumor interstitium [124]. It has been shown that electrostatic repulsion hinders nanoparticle diffusion [91]. Since both electrostatic attraction and repulsion decrease particle transport, neutral nanoparticles disperse more efficiently in the interstitial space of the tumor than anionic and cationic particles [124,125]. Studies in vitro show that cationic nanoparticles are easily consumed by cells, but if the nanoparticles are coated with protein to decrease the positive charge, the difference in uptake of cationic and anionic particles is almost eliminated [92]. This may be due to the fact that cell membranes have negatively charged sulfate proteoglycans that attract cationic nanoparticles [126,127]. These properties of cationic nanoparticles can improve cellular uptake, payload delivery and cytotoxicity [128], although the increased uptake of cationic particles by cells may not be solely due to electrostatic adsorption to the plasma membrane surface [129,130].
Various mathematical and computational techniques and simulations have been performed to investigate the effect of surface charge on nanoparticle translocation. One of the most powerful methods for investigating the effect of the surface charge on pharmacokinetics is the Coarse-grained molecular dynamics (CGMD) method. Li and Gu applied the CGMD approach to investigate the effects of electrostatic attraction on the adhesion between nanoparticles and the membrane [131]. They showed that cationic particles have stronger adhesion than neutral particles. In addition, the adsorption of positively charged nanoparticles to the phosphate terminus of the lipids increases the tilt angle of lipids and thus enlarges the area of the head group, whereas negatively charged nanoparticles often induce the formation of highly ordered regions in fluid bilayers. Thus, surface charge greatly affects the adsorption mechanism [132].
3.4. Surface chemistry
In addition to electrostatic charge, other surface properties are important for the appropriate selection of nanocarriers. For example, an effective method for increasing the delivery of low solubility drugs to tumors is to encapsulate the drug within a nanocarrier that has a hydrophobic or amphiphilic surface [110,133]. Park et al. showed that drugs with low water solubility can be suspended in aqueous media using micelle nanoparticles based on amphiphilic block copolymers [134]. Similar advances have also been made using a liposome-based carriers [135]. Extensive research has been conducted on the efficiency of nanocarriers to overcome obstacles such as short circulation half-life, poor uptake by cells, and poor stability against hydrolytic and proteolytic degradation [136]. For example, it was found that nanoparticles can be engineered with surface chemistry that includes hydrophilic surface coatings to prevent formation of a protein corona and subsequent phagocytosis by macrophages [137]. Additionally, studies have shown that modifying the surface of nanoparticles with polysaccharides can improve cellular uptake [138]. Furthermore, hydrophilic polysaccharides can be grafted to hydrophobic molecules or to side chains of a hydrophobic polymer to produce surfaces with better performance [40]. This highlights the need for a better understanding of polysaccharide chemistry and how they can be leveraged to improve the biological properties of nanoparticles.
Some advances in this area have also come from computational modeling. By simulating the mechanisms of transmembrane osmosis, Zhang et al. found that penetration improves with an increasing number of hydrophilic surface species [139]. Additionally, Gupta et al. [140], used molecular dynamics (MD) simulations to determine the shape of AuNPs diffusion and the magnitude of the permeability as they pass through the skin lipid membrane. They reported a decrease in permeability with increased size of neutral hydrophobic AuNPs and found that the kinetics (release) and thermodynamics (free energy) play important roles in permeability. Specifically, free energy controls the shape of resistance and diffusion controls the magnitude of resistance.
To improve the delivery of nanoparticles to the tumor, it is important to prevent clearance by the MPS. It is possible to avoid the opsonization that encourages clearance by engineering the nanoparticle surface. The most common strategy for limiting organ uptake and immune system clearance is PEGylation of the nanoparticle surface – known as “stealthing” [141]. Coating the surface with PEG leads to increased time of particle circulation [142,143]. The functionalization with PEG depends on the length of the polymer chains and its density at the surface [86]. In clinical applications, it has been shown that administration of PEGylated particles can induce production of anti-PEG antibodies, further enhancing clearance [144,145]. This underscores the need to develop alternative methods for reducing the clearance of nanoparticles in vivo. Other coatings such as zwitterionic species and carbohydrates appear to be promising [146,147], as are engineered intralipid formulations [148] or liposomes, which have been used to deliver lipids [149]. As discussed previously, liposomes can overcome the effects of surface charge and block the uptake of gold nanorods by the MPS [150]. All these results suggest that the clearance and uptake of nanoparticles by the immune system is more complex than previously appreciated [151].
3.5. Elasticity
While many studies have focused on nanoparticle size, shape, and surface properties, less is known about how mechanical properties affect nanoparticle transport processes. It is reasonable to assume that nanoparticle rigidity or elasticity would affect their pharmacokinetics and pharmacodynamics, and indeed evidence suggests that (i) less rigid nanoparticles have the ability to stay in the circulation longer than their rigid counterparts, and (ii) in the circulatory system, less rigid nanoparticles are more resistant to phagocytosis than rigid nanoparticles [152]. In another approach, Kumar and Graham [153] used a non-Hookean capsule model to show that stiff nanoparticles tend to move toward the CFL while deformable nanoparticles accumulate at the center of the flow. This can increase the transvascular transport of stiffer nanoparticles. Deng et al. [154] quantified the effects of nanoparticles stiffness on tumor infiltration and found that stiffness can dramatically affect tumor penetration efficiency: nanoparticles with lower stiffness were able to move more easily through the ECM.
Limited nanoparticle penetration into tumor tissue is an important obstacle to the efficacy of cancer chemotherapy agents, and nanoparticle mechanics can affect this step. The Young's modulus– defined as how much force is required to deform a substance by a given amount– is used to describes the stiffness of a material, but is difficult to measure at the scale of nanoparticles [155]. However, computational studies have partially filled this gap. For example, the computational model of Yi and Gao, showed that soft nanoparticles engineered with specific binding receptors have a higher cellular uptake rate than their stiffer counterparts due to increased contact surface area with the cell membrane and more efficient receptor diffusion [156]. On the other hand, Anselmo et al. [152], found the opposite result in experimental studies, showing that at longer times (8 and 12h), stiffer nanoparticles are bound/internalized more readily than their softer counterparts; at shorter times (5 min–4 h), there were no statistical differences in cellular uptake. These disparate results highlight the need for more research on the impact of mechanical properties on nanoparticle performance. However, it is possible that the discrepancy between the studies of Yi and Gao [156] and Anselmo et al. [152] is due to the use of ligand coating in the study of Yi and Gao.
4. Transformable nanoparticles as advanced strategies for enhanced delivery efficiency
As previously discussed, optimizing the physical characteristics of nanoparticles requires compromise, because multiple factors affect nanoparticles in conflicting ways during the various stages of drug delivery, tissue penetration and cellular uptake. Optimal nanoparticle design needs to consider each of these steps. For example, surface modification of nanoparticles by PEGylation improves tumor penetration but hinders cellular internalization [157]. Additionally, cationic nanoparticles improve tumor penetration by facilitating the transcellular pathway but have a shorter circulation time in comparison with neutral or slightly negative nanoparticles [158]. Any drug delivery system that focuses on only one factor but fails on another cannot meet the requirements [159]. For this reason, transformable nanoparticles are being developed for advanced drug delivery. Transformable nanoparticles can integrate multiple ideal delivery properties into a single nanoplatform because they regulate their characteristics according to the requirements of various delivery stages [[160], [161], [162]].
4.1. Size/surface charge switch
Size and charge are the most widely-studied features for creating transformable nanoparticles. Size transitions allow large particles to be transformed into smaller species, while charge transitions use cationic nanoparticles to enhance tumor penetration [163]. It is also possible to combine size and charge transitions into a single delivery platform [164]. Importantly, endogenous characteristics of the tissue microenvironment, including overexpressed enzymes and mild acidity, can be used to trigger both size and charge transformations. Exogenous stimuli (e.g., magnetic field, light, and ultrasound) can also be used [165,166].
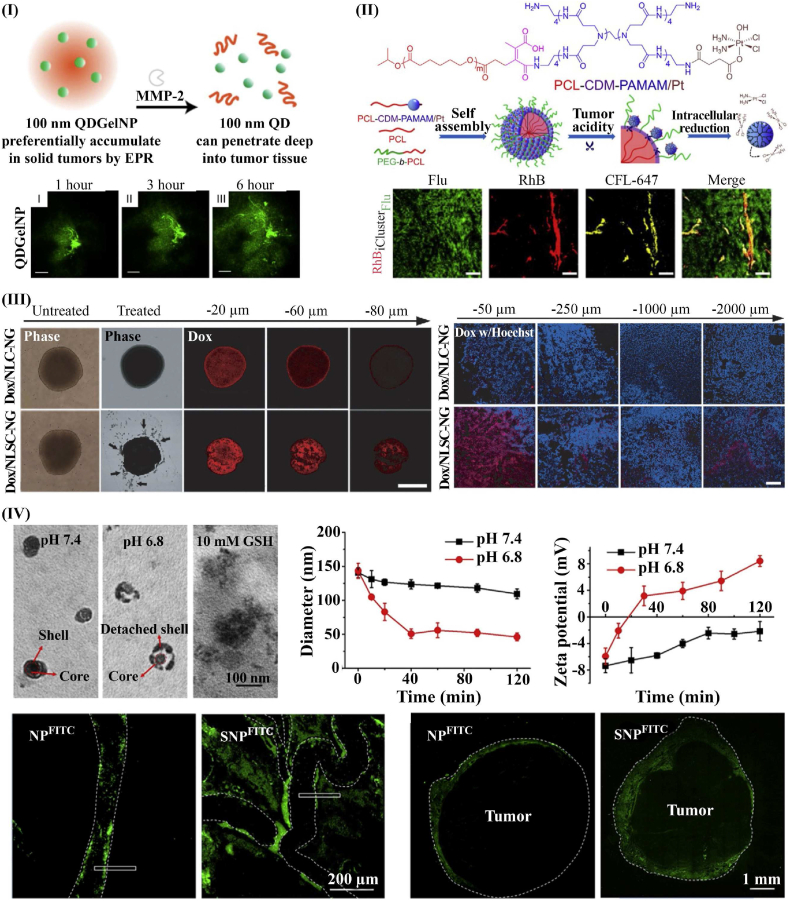
Hybrid large-small nanoparticles are composed of a large nanoparticle to facilitate circulation and accumulation and a smaller nanoparticle to improve penetration [167]. Hybrid nanoplatforms can be formed by encapsulating smaller nanoparticles within a larger particle, or by conjugating them onto the large nanoparticle. These nanoparticles accumulate at the tumor sites via the EPR effect, and then release smaller nanoparticles in response to stimuli. Fukumura et al. reported one example of size-transformable nanoparticle, with the nanoparticle formed by loading 10 nm quantum dots (QDs) into 100 nm gelatin nanoparticles (GNP) (Fig. 5I) [161]. At the tumor site, the gelatin scaffold of this nanoplatform is degraded by locally overexpressed MMP-2, which releases the smaller QDs. After release, further penetration of QDs into the tumor tissue was observed in vivo and in vitro. Additionally, studies have shown that many “small-in-large” hybrid nanoplatforms, such as poly amidoamine (PAMAM) dendrimer, polyplex micelles, dendrigraft poly (l-lysine) (PLL) in polymer micelles and liposomes improved tumor penetration [138]. Another typical design of hybrid nanoplatforms is small-on-large hybrid nanoparticles. An example of these nanoplatforms is the platinum prodrug-conjugated PAMAM (PAMAM/Pt) dendrimer, conjugated onto larger PEG-b-PCL nanoparticle (Fig. 5II) [168]. Within an acidic environment, PAMAM/Pt dendrimer detaches from the hybrid nanoparticle, improving the tumor penetration in vivo and in vitro. This hybrid nanoplatform also showed improved circulation behavior compared with free PAMAM/Pt dendrimer. Stylianopoulos et al. used a novel in silico approach to investigate multi-stage nanoparticles [169]. They simulated a multi-stage system in which 20 nm nanoparticles transform into 5 nm particles, demonstrating that tuning the drug release kinetics and binding affinities can optimize performance relative to traditional methods.
Fig. 5.
Size-charge switching of NPs. I) “small-in-large” hybrid nanoparticle enhanced tumor penetration. The small QDs were encapsulated into larger GNPs and MMP-2 catalyzes the size reduction [161] (reproduced from Ref. [161] with permission). II) “Small-on-large” hybrid nanoparticle converted by low pH. PAMAM nanoparticles with Flu-label were conjugated onto larger PEG-b-PCL nanoparticles with RhB-label. When exposed to tumor acidity, the small nanoparticle is released, enhancing penetration [168] (reproduced from Ref. [168] with permission). III) Charge-transformable nanogel increases in vitro penetration in a 3D tumor spheroid model (Left), and charge-transformable nanogel enhanced in vivo penetration of DOX in xenografted hepatocellular carcinoma (Right) [175] (reproduced from Ref. [175] with permission). IV) Integration of size and charge transition into a single platform; size and charge transition of SNP exposed to pH 6.8, enhanced extravasation of SNP from tumor microvessels (Bottom left), and increased penetration of SNP from the tumor border to center (Bottom right) [164] (reproduced from Ref. [164] with permission).
Size-switchable nanoparticles are another option for creating size-shrinkable nanosystems. In this case, enhanced tumor penetration is caused by nanoparticle shrinkage instead of the release of smaller nanoparticles [170]. For example, spiropyran-contained monodisperse nanoparticles exposed to ultraviolet (UV) light shrink in size from ∼150 to 40 nm; this process also accelerates the release of drugs loaded in the nanoparticles. These size-shrinkable nanoparticles exhibited better circulation behavior and improved penetration ability [171]. On the other hand, although smaller nanoparticles have better depth of tumor penetration, they are also more easily returned back into the bloodstream. Therefore, aggregation strategies have been proposed to prevent nanoparticles from re-entering the bloodstream by increasing the size of nanoparticles after extravasation. This leads to enhanced retention of nanoparticles in tumor sites and can increase anti-tumor efficacy [172]. Various studies have improved the therapeutic response based on this strategy. For example, using “Responsive Aggregation” strategies, Gao and co-workers were able to improve the retention of gold nanoparticles and therapeutic response in brain tumors [173]. This approach also obviated resistance to chemotherapy in breast cancer [174].
Charge-transformable nanosystems have been demonstrated with the use of pH-sensitive nanogels surrounding a polyelectrolyte core [175]. The nanogel is negatively charged during circulation and accumulates at the tumor site. After internalization into the tumor, the acidic environment triggers the nanogel to swell, thus enhancing local retention of the drug (Fig. 5III). Enzyme-activatable polymer−drug conjugates are another option for charge-transformable nanosystems [176]. This system enhances tumor penetration through transcellular transport via -glutamyl transpeptidase-activation. The improved tumor penetration in this system eradicated small solid tumors (∼100 mm3) and regressed larger tumors (∼500 mm3).
Moreover, combining tunable size and charge into one nanoplatform may enhance penetration of tumor via both paracellular and transcellular transport. Chen et al. examined a dual-transformable nanoparticle with both charge transformation and acid-activated shrinkage [164]. The shell of stacked nanoparticle (SNP) consists of a detachable shell with pH-sensitive bonds. This protects the cationic core from clearance during circulation but detaches in the low pH of the tumor environment. After the stacked shell detaches, the size of the nanoparticle decreases, and the surface charge reverses from −7.4 to 8.2 mV. The combination of small size and cationic charge provides ideal properties for extravasation of SNP from the tumor microvessels and enhances the penetration from the tumor vessels to deeper regions of the tissue (Fig. 5IV).
4.2. Shape switch
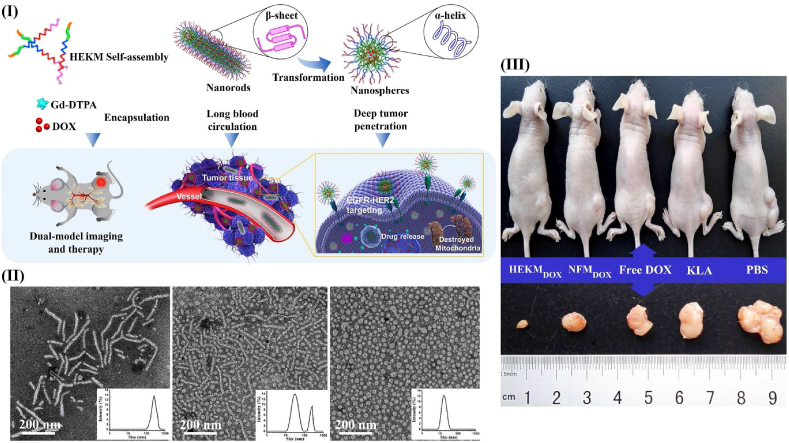
As mentioned previously, nanoparticle shape is an important design consideration that is difficult to optimize for all stages of the delivery process. Consequently, efforts have been made to develop nanoplatforms that can adopt different shapes depending on the environment [177]. For example, Wang et al. created shape-changing micelles that are responsive to MMP-2 [178]. These size-changing micelles, known as HEKMs, start as nanorod micelles (diameter: 20 nm, length: 50–300 nm), which enhances their circulation time and penetration into the tumor tissue. Once in the tumor, responsive linkers are cleaved by MMP-2, resulting in deformation of the rod-shaped structure into a sphere (diameter: 35 nm) (Fig. 6). This transformation increases cellular internalization.
Fig. 6.
Shape-switchable micelles. (I) Process of shape transformation by HEKMs [178] (reproduced from Ref. [178] with permission). (II) DLS and TEM images of HEKMs at 2 h and 4 h after exposure to MMP-2m [178] (reproduced from Ref. [178] with permission). (III) mouse tumor model, showing that delivering doxorubicin using HEKMs (HEKMDOX) is most efficient for tumor control [178] (reproduced from Ref. [178] with permission).
As for size changes, shape changes can also be induced using changes in pH. By incorporating pH-sensitive polymer chains, nanoparticles can reversibly alter their structure, mimicking the process of protein folding and self-assembly. For example, at pH 7.4, shape-changing single-chain polymers become deprotonated and hydrophobic, causing them to self-aggregate into large (68.7 ± 2.8 nm) spherical “multi-tadpole assemblies” (MTAs). When exposed to decreased pH (6.9), they disassemble into 9.6 nm rod-shaped nanoparticles, which then penetrate deeper into tissue [179]. Other studies have shown an improvement in therapeutic response through enhanced biocompatibility, biodistribution, and the induction of a potent antitumor immune response by transforming spherical nanoparticles into nanofibers in response to light and ROS stimuli [180,181].
5. Drug loading
Ideally, a nanoparticle drug should have high drug loading capacity, minimum toxicity to normal tissue, and require minimal polymer for drug loading. Specifically, the capacity for drug loading and determinants of drug efficacy depend on (1) the physicochemical characteristics of the nanocarriers; (2) the type of nanocarrier used, the methods of preparing the nanocarrier; (3) interactions between the carrier and drug (e.g., drug loading of therapeutics agents with high charge depends on electrostatic interactions with carriers); (4) the molecular weight; (5) conditions and duration of drug loading; and (6) biochemical conditions that can affect drug loading [182,183]. Therefore, each drug requires a specific method and custom carrier with optimized physicochemical properties for efficient drug loading. For example, drug loading capacity for solid lipid nanocarriers depends on physicochemical properties of the matrix, solubility of drug into excipient matrix [184,185].
5.1. Traditional strategies for drug loading
Traditionally, drug loading is performed using one of three strategies: pre-, co-, or post-loading. The optimal loading procedure depends on the specific structure and surface properties of the nanoparticle [186,187].
Pre-loading: In this method, drug clusters are formed first, and then these are surrounded by shells. By adjusting the thickness and diameter of the shell, the amount of drug loading can be specified [188,189]. This strategy, which is based on the core-shell structure, has several advantages, including preventing unwanted release of the drug as well as protecting the drug from the potentially destructive external environment. It is also possible to achieve controlled drug release and functional surface chemistry that responds to local tissue biochemistry through shell engineering [190]. Shell materials used in this strategy are usually polymers due to their biocompatibility, biodegradation kinetics, and ease of manufacturing [183,191], although other shell materials have been tested, including silica [192] and lipids [193]. The pre-loading method is applicable for loading materials with high molecular weight. The loading capacity of drugs varies from 12 to 78.5% depending on the pre-loading strategy [193,194]. Additionally, the size of nanocarriers produced based on this strategy varies from 40 to 984 nm [183,195].
Co-loading: This strategy encapsulates the drug during the formation of nanoparticles. There are various systems based on this process, including drug-polymer conjugates [196,197], drug-silsesquioxane conjugates [198], pure drugs [199,200], Metal-organic framework (MOF) with drug incorporated [201,202], proteins [203], solid-lipids [204], and polymers [205,206]. Covalent bonding is important for conjugate systems while π-π interactions, electrostatic, and hydrophobic interactions are important for proteins, polymers, etc. [186]. In this strategy, the loading capacity varies from 18.5% to 100% [207,208], and nanocarriers created are in the size range of 29–400 nm [197,209].
Post-loading: In this strategy, nanocarriers are first made and then the drug is loaded. Nanocarriers created for this drug loading strategy are porous structures such as carbon nanoparticles [210,211], silica nanoparticles [212,213], MOF nanoparticles [214,215], hydrogel nanoparticles [216], and iron nanoparticles [217]. The porous structure of nanoparticles provides features such as adjustable pore volume and size, high surface area, and straightforward chemistry for functionalization [218]. In the post-loading process, in order to encapsulate the drug in the nanocapsules, the carrier is incubated with the drug solution at a higher concentration, thus forcing the drug to accumulate in the capsule by diffusion [219,220]. Ultimately, drug-loaded carriers are separated by filtration or centrifugation. Other non-porous nanoparticles, such as polypeptides [221] and proteins [222], have also been investigated for post-loading strategies, in which drugs are loaded mainly through non-covalent hydrophobic interactions, π–π stacking, and hydrogen bonds [223,224]. Nanocarriers fabricated based on this strategy can achieve 11.8%–68% of the drug load [225,226], and no size limit has been reported.
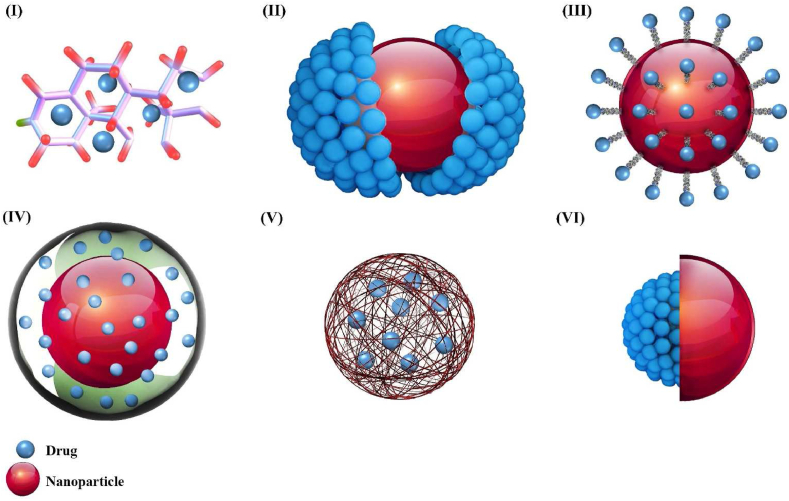
Considering pre-, co-, or post-drug loading strategies, the drug molecules can be coupled or complexed with the host molecule, linked on a surface, embedded in a matrix, or enclosed in the cavity of carriers (Fig. 7). Also, loading methods are mainly divided into active or passive loading. When the drug is entrapped before or during the manufacturing procedure, the method is so-called passive loading. When the drug is entrapped into the preformed nanoparticle, the method is known as active or remote loading [227]. Loading by these two methods can be based on pH gradient, temperature change, UV-induced crosslinking, etc. [228,229].
Fig. 7.
Methods for drug loading. (I) Molecular-level loading; The drug loading capacity of these polymers affects the structure of the polymers and the number of available active sites. (II) Direct link: the drug is confined in the surface of the nanocarrier mostly based on van der Waals forces. (III) Linker-mediated loading: binding of the drug to the carrier is performed via a coating, linker, or surfactant. (IV) Coating mediated loading: the drug is contained in a layer around the nanocarrier that is gel-like in nature. (V) Matrix loading system: a large amount of drug is loaded into the matrix, and only a small amount of drug is attached to the carrier surface. (VI) Cavity loading system; the drug is encapsulated in the inner cavity of a nanoparticle shell.
Molecular-level loading system: A popular method for attaching drugs to host molecules is known as the molecular-level loading system. In general, this approach attaches drugs with low molecular weight – such as anticancer drugs – to small molecules, peptides or polymers, [187,230]. The drug and carrier interactions can be classified into two types: physical or chemical, designated complex or prodrugs, respectively. Physical interactions or complexes are based on self-assembly and simple contact of host and guest molecules, which leads to the selective identification of these two molecules by size and shape [231]. Physical complexes have weak and nonspecific binding, so they are easily released into the TME by different stimuli (external/internal) such as changes in ionic strength, pH, or temperature [187]. In chemical interactions, drug molecules and nucleic acids bind to functional groups such as hydroxyl, thiol, carboxyl, and amine groups at the nanocarrier surface (end of polymer chains or branches) [232]. These active sites have a crucial role in the binding of drugs, improvement of the hydrophilicity of nanocarriers and their solubility in the cross-linked polymer. The drug loading capacity of these polymers is affected by the polymer structure as well as the number of active sites they have. Since the molecular weight of the loaded drug is much less than the polymer molecules, the loading capacity of the drug in this carrier is limited [187]. This strategy can use co- or post-loading to load the drug into nanocarriers.
Loading on the surface of the nanoparticle system: The amount of drug loaded onto the surface of a nanoparticle is determined by process of adsorption and desorption [187]. The overall amount that can be accommodated is affected by the surface-to-volume ratio (S/V) and the physicochemical properties of the nanoparticles. Higher S/V ratio means that there is more area to anchor drug molecules (i.e., more drug is placed on the nanoparticle surface) [187]. Surface properties such as charge, polarity, and chemical reactions also affect the drug loading. These properties determine the possibility of absorption but also the type of adsorbent guest molecule and the affinity between the drug molecule and the carrier [187,233]. In this loading system, drug molecules can be attached directly (directly adsorbed) or mediated (linker and coating) to the surface of nanoparticles. In drug loading with direct interaction, drug or contrast agent is loaded to the surface of nanocarrier mostly based on van der Waals forces, hydrogen/electrostatic/hydrophobic/π–π stacking interactions, and chemical bonds such as covalent and coordination bonds by designing disulfide, amide, ester and hydrazine linkers [217,234]. Drugs that are loaded on the surface of carriers have poor interactions and can be separated by simple desorption via change of pH or solvent in TME [234].
Drug binding to the carrier through a linker is performed in three different modes: (i) the linkers can be attached to the drug and then loaded onto the carrier surface; (ii) the linker can be attached to the carrier surface, followed by attachment of the drug to the linker [141]; and (iii) the drug is loaded to the carrier surface by the interaction between the two linkers [187]. A coating on the surface of the nanoparticles can also be used as an intermediate to improve the stability of nanocarriers or to aid drug loading. The coating can change the physicochemical properties of the surface and provide additional possibilities for drug absorption [187,235]. Most surface-loading processes use post-loading strategies to incorporate the drug.
Matrix Loading System: In the matrix drug loading system, a large amount of drug is loaded into the matrix and only a small amount of drug is attached to the carrier surface. As the material degrades, the loaded drug is gradually released from the carrier. The main disadvantage of this system is the large amount of material released as the matrix decomposes [187]. Structural properties and material composition of carriers can affect drug loading, drug release, and biochemical properties of carriers in in vivo environments. Based on structural properties, nanocarriers are classified into three types: compact solid nanoparticles, micelles, and nanogels [187]. Post-loading is used for nanogels because of the high porosity and high-water content. In this method, the drug incorporates into the network of the nanogels through diffusion [236,237]. On the other hand, pre-loading strategies are generally used for solid nanocarriers with low water content and low porosity [238]. Micelles are ordered aggregates self-assembled from amphiphilic molecules (small surfactants and amphiphilic polymers), and require a different strategy [239]: the drug is usually loaded with the amphiphilic polymer during micellar assembly, although hydrophilic drugs can still enter the hydrophobic core of the micelle after carrier formation (post-loading) [187].
Cavity Loading System: In this method, the drug is encapsulated in the inner cavity of the nanoparticle, so it is covered by a shell to prevent release. The drug loading in these systems depends on the nanoparticle structure, composition, properties, and preparation method. These nanoparticles can be made of organic compounds such as lipids and polymers or inorganic compounds including metal or oxides. They are also classified as vesicles or capsules, according to their structure. Capsules are nanocarriers whose inner cavities are coated by a single layer with numerous pores, while vesicles have impermeable coatings [187]. The capacity of drug loading in the vesicles is affected by numerous factors such as charge of the liposomes, lipid/cholesterol ratio, the lipid material, lipid to drug molar ratio, and different synthesis methods of nanocarriers that can enhance the capacity of the inner cavity and the drug loading content. The loading capacity in capsule systems depends on factors such as shell porosity, shell thickness and core size [187].
Some recent progresses in the area of traditional drug loading are summarized in Table 1.
Table 1.
A summary of recent efforts to load drugs based on traditional strategies.
| Loading strategy | Nanocarrier material | Size (nm) | Drug | Drug loading (%) | Ref. |
|---|---|---|---|---|---|
| Pre-loading | Polymer | 60–450 | Paclitaxel | 42.6 | [240] |
| 40–175 | Docetaxel; Paclitaxel; |
38–58.5 | [183] | ||
| 165–181 | Camptothecin | 29–52 | [241] | ||
| 83.2 | Curcumin | 78.5 | [194] | ||
| Silica | 58.6 | Paclitaxel | 59.2 | [192] | |
| Co-loading | Polymer | 70 | Curcumin | 49.5 | [242] |
| 98–110 | Paclitaxel | 8.67–28.32 | [205] | ||
| Protein | 217 | Paclitaxel | 27.2 | [203] | |
| Drug-drug interaction | 70 | Doxorubicin | 100 | [200] | |
| Drug-drug conjugate | 89 | Doxorubicin | Near 100 | [208] | |
| Drug-polymer conjugate | 100–200 | Doxorubicin | 18.5 | [207] | |
| 56.9–60.5 | Cabazitaxel | 29.5 | [196] | ||
| Drug-silsesquioxane conjugate | 62.3–105.2 | Cisplatin | 35–47 | [198] | |
| Post-loading | Polypeptide | 140 | Doxorubicin | 21.7–26.1 | [221] |
| Protein | 25.9–32.7 | Doxorubicin | 13.4 | [222] | |
| Hydrogel | 160–610 | Doxorubicin | 16.0–42.3 | [216] | |
| Magnesium silicate | 400 | Doxorubicin | 68.1 | [226] | |
| Calcium silicate hydrate | 30–50 | Docetaxel | 7.6 | [243] | |
| Carbon | 255 | Doxorubicin | 51.9 | [211] | |
| 200 | Camptothecin | 17 | [210] | ||
| 500–800 | Lovastatin | 25.63–36.26 | [244] | ||
| Iron | 209.6–601.8 | Doxorubicin | 209.6–601.8 | [217] | |
| 140–233 | Paclitaxel | 140–233 | [214] | ||
| MOF | 100 | Doxorubicin | 65.5 | [245] | |
| – | 5-Fluorouracil | 33.3 | [215] | ||
| Silica | 45–450 | Doxorubicin | 55 | [246] | |
| 200 | Doxorubicin | 4.1–38.5 | [247] | ||
| 241.5 | Doxorubicin | 12.3 | [248] | ||
| 200–400 | Doxorubicin | 32 | [249] | ||
| 130–190 | Doxorubicin; Paclitaxel; Curcumin; | Doxorubicin 5.7–51.5; Paclitaxel 5.7–51.9; Curcumin 3.8–51.9; |
[212] |
5.2. Microfluidics technology as an advanced strategy for drug loading
Importantly, many carriers have low drug loading capacity based on traditional strategies, so frequent dosing of these carriers are needed to treat patients with chemotherapy agents. This can increase non-specific accumulation, leading to side effects. New technologies such as microfluidic (or nanofluidic or lab-on-a-chip (LOC) technologies) have the potential to overcome problems caused by poor drug loading [250,251]. Microfluidics technology uses miniaturized systems and nanoliter volumes to pass fluids through small micro-chambers and micro-channels and can be used to mix small volumes of fluid and reduce waste [252]. Microfluidics systems have numerous advantages and applications in nanomedicine because they provide (i) control over temperature and other drug loading conditions (ii) specification of drug loading dynamics and throughput, and (iii) the ability to use small volumes of drug, limiting the consumption of materials (e.g., (picoliter) volumes).
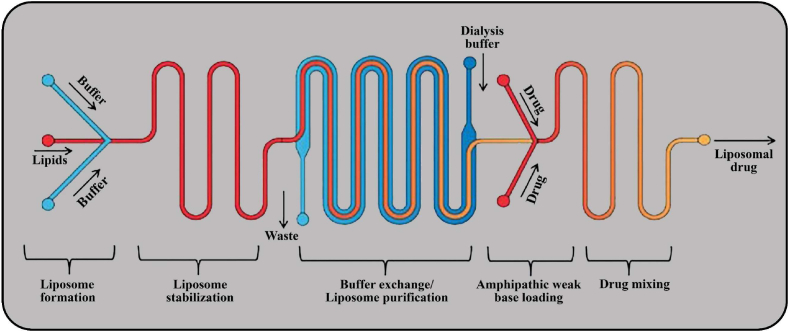
A key reason for using liposomes as a suitable carrier for drug delivery is the high loading capacity of vesicles. However, they have low efficiencies in drug loading, so advanced technology is needed to increase efficiencies. Drug loading using microfluidic technology provides a way to incorporate all stages of the loading process – e.g. liposome synthesis, buffer exchange, and drug/nanocarrier mixing – into a continuous and integrated process, decreasing processing time and costs (Fig. 8) [253]. For example, it is possible to create synthetic liposome membranes using a mixture of cholesterol and diacetyl phosphate in microfluidic systems, which allows rapid mixing of two miscible solutions and produces nanocarriers that are 40% smaller in size than traditional methods [253].
Fig. 8.
Microfluidic technology for liposomal drug loading operations: microfluidic hydrodynamic flow can be used to create drug-loaded liposomes. Prior to drug loading operations, there is a rapid application of a steep transmembrane ion gradients. Drug loading takes place in the “drug loading and incubation” zone, which includes most of the chip area. In this zone, micromixer structures have been installed to increase the interactions of amphipathic compounds and liposomes during the drug loading process, which leads to increased loading.
Microfluidics is an advanced technology that plays an important role in drug delivery strategy by aiding in the design and fabrication of nanocarriers that mimic and replicate biological and physiological systems. In addition, microfluidics technologies have great potential for high throughput production of pharmaceutical nanosystems and provide more precise control of the physico-chemical characteristics of a nanocarrier [187]. Table 2 summarizes recent drug loading efforts using microfluidic technology, which generally have a higher drug loading efficiency compared to traditional drug loading strategies (Table 1).
Table 2.
Representative examples of drug loading using microfluidics.
| Microfluidic technologies | Nanocarrier | Size (nm) | Drug | Drug loading (%) | Ref. |
|---|---|---|---|---|---|
| Herringbone pattern-based rapid mixing (HPRM) | Lipid-polymer hybrid nanoparticles | 136.4 | DOX and siRNA | 90 | [254] |
| 100–150 | Rifampicin | 60–70 | [255] | ||
| 39.4 | DOX | 90.8 | [256] | ||
| Biomimetic nanoparticles | 7.3–32 | Imaging agents | 70.1–94.2 | [257] | |
| Hydrodynamic flow focusing (HFF) | Liposomes | 106.8–334.2 | Bcl-2 antisense deoxyoligonucleotide | 71.3–74.8 | [258] |
| 190.9 | DOX | 71.8 | [259] | ||
| Polymeric nanoparticles (modified/unmodified natural polymer) | 60–220 | Paclitaxel (PTX) | 50–95 | [260] | |
| Polymeric nanoparticles (synthetic polymer) | 25–200 | Docetaxel | 10–98.19 | [261] | |
| 100 | Docetaxel | 95 | [262] | ||
| 50–200 | PTX | 69.1–99.1 | [263] | ||
| Silica-polymer hybrid nanoparticles | 150.6–206 | Sorafenib | – | [264] | |
| Hydrodynamic flow focusing combined with a spiral mixer (HFF-SM) | Lipid-polymer hybrid nanoparticles | 40 to 277 | DOX | – | [265] |
| 3D-HFF | Polymeric nanoparticles (synthetic polymer) | 100 | DOX | 50 | [266] |
| Microvortices-based mixer | Lipid-polymer-Au hybrid nanoparticles | 85.1 | DOX; sorafenib | 25.6, 66.0 | [267] |
| Mixing with a caterpillar micromixer | Iron oxide-polymer hybrid nanoparticles | 60–120 | PTX | 58–80 | [268] |
| 122–126 | Camptothecin | 73–100 | [269] | ||
| Spiral mixer | Silica-based nanoparticles | 150–400 | PTX | 30–90 | [217] |
| Gas-liquid microfluidic reactors | Polymeric nanoparticles (synthetic polymer) | 50–60 | 7-ethyl-10-hydroxycamptothecin | 4–10 | [270] |
| 10–100 | Curcumin | 30–60 | [271] | ||
| Microfluidic electroporation | Biomimetic nanoparticles | 80 | Imaging agents | 70.1–94.2 | [272] |
| Coaxial turbulent jet mixer | Polymeric nanoparticles (synthetic polymer) | 70–900 | Sorafenib | 5–60 | [273] |
Microfluidics technologies have shown superior performance for many aspects of drug loading. However, although they are relatively efficient at drug loading, the process is generally slow and low-throughput. Therefore, overall production efficiency can be low. To overcome this problem, it is possible to use parallel microchannels in a device [274,275], parallel devices [276,277], higher flow rates [240,278,279], or new fabrication paradigms [280,281] to achieve higher production rates (Table 3).
Table 3.
Representative examples of microfluidics technology with Increased production throughput.
| Methods | Nanomaterials | Size (nm) [15] | Nanocarrier | production rate | Ref. |
|---|---|---|---|---|---|
| Multiple systems in parallel | Polymeric nanoparticles | 50–200 | MPEG–PLGA (methoxyl poly-(ethylene glycol)–poly (lactic-co-glycolic acid)) | 25–100 | [277] |
| Multiple channels in parallel | Polymeric nanoparticles | 200 | DSPE-PEG (1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000]) | 4.5–84 | [282] |
| 13–150 | PLGA-PEG poly (lactide-co-glycolide)-b-poly (ethylene glycol) | 4.5–84 | [274] | ||
| 50–150 | PLGA-PEG | ∼ 10 | [275] | ||
| Using a coaxial turbulent jet mixer | Lipid-polymer-metal oxide nanoparticles | 25–100 | PLGA-PEG | 3.15 | [281] |
| Increasing flow rate | Polymeric nanoparticles | 100–240 for PLGA 100–240 for HPCS 70–280 for AcDX |
PLGA (poly (lactide-co-glycolide)); HPCS (hydrophobic chitosan); AcDX (acetalated dextran) |
45.6–242.8 | [283] |
| 112.2–570 | DSPE-PEG | 2.4–14.4 | [278] | ||
| 60–450 for PLGA 70–550 for AcDX |
PLGA AcDX |
∼ 700 | [240] | ||
| lipid-polymer hybrid nanoparticles |
30–170 | PLGA-PEG | ∼ 3 | [279] |
6. Drug release from nanocarriers
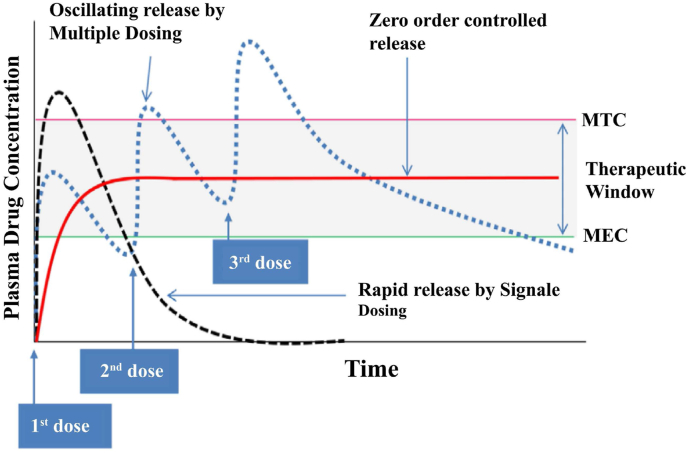
The mechanism of drug release from a nanocarrier is a crucial parameter in determining drug therapeutic efficacy. One of the main goals of release kinetics control is to maintain the level of drug in the blood between the minimum effective concentration (MEC -to provide effective dosing) and the minimum toxic concentration (MTC - to prevent causing toxic side effects) [284]. Once in the circulation, the plasma concentration of a free drug (such as doxorubicin) increases above the MTC and then rapidly drops below the MEC (Fig. 9). Large fluctuations in drug concentration can be reduced by using multiple doses, but this can also lead to patient non-compliance. Therefore, it is desirable to design nanocarriers that can deliver drugs with controlled release, avoiding the need for frequent dosing [285,286]. In general, release from nanocarriers can be tailored using both stable and controlled methods.
Fig. 9.
Concentration profiles of three different doses of a drug in plasma created by a single dose (black dotted line), multiple doses (blue dotted line), and zero-order controlled release (red solid line). For optimum treatment with minimal toxicity, the plasma concentration should remain between the MTC and the MEC [286] (reproduced from Ref. [286] with permission).
6.1. Sustained release
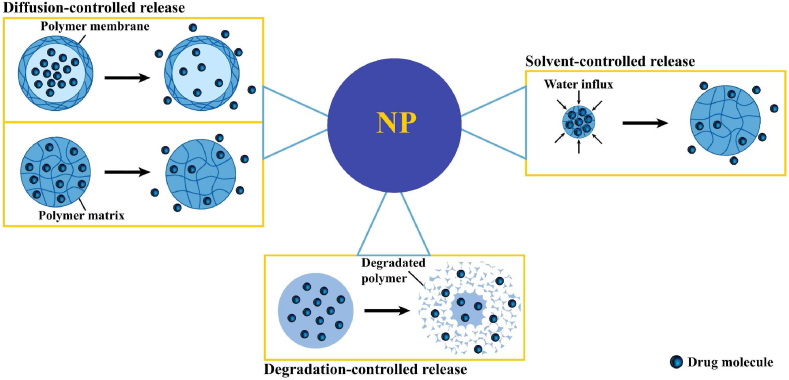
The release kinetics of nanocarriers are critically important for therapeutic efficacy. Many factors influence these kinetics, including the specific nanomedicine components (drugs, polymers, additives) and the chemical and physical interactions between these components. The preparation methods are also very important for controlling drug release and must be considered explicitly for each drug. Drugs that are rapidly metabolized and excreted benefit from sustained release over a long period of time. Sustained release can stabilize plasma concentrations of the drug at a constant level, thus reducing the need for higher doses, which also reduces side effects. The most important drug release mechanisms used for sustainable release are diffusion, solvent-mediated release, and degradation (Fig. 10) [40,55].
Fig. 10.
Sustained mechanisms of drug release from nanoparticles. Diffusion-controlled release: The diffusion process is based on mass transfer due to the concentration gradient, which is a kinetic process in non-equilibrium systems. In general, diffusion-controlled release is the primary mechanism in reservoir- or matrix-based systems. Solvent-controlled release: Solvent-controlled release is based on the transport of solvent into a carrier, which then affects drug release. In general, solvent-controlled release includes osmosis- and swelling-controlled release. Degradation-controlled release: Degradation can occur through surface or bulk mechanisms. Surface erosion occurs when the rate of polymer erosion is faster than the rate of water diffusion into the matrix.
Diffusion-controlled release: In general, diffusion-controlled release involves a reservoir- or matrix-based system and relies on mass transfer driven by a concentration gradient [287] (Box 3I). [288]. Reservoir-based systems consist of the main reservoir containing the drug, which is enclosed by a membrane. The release process is such that the drug first dissolves in the reservoir and then penetrates through the carrier membrane and into the surrounding environment due to the concentration gradient. The amount of loaded drug, the physicochemical properties of the membrane and its thickness are the most important factors in the release of the drug. The membrane is one of the most important factors in reservoir-based systems, but in matrix-based systems, there is no membrane and consequently no reservoir. In these systems, the drugs are placed on the surface of the matrix, so the drugs that are on the surface are released earlier than those in the center. The lag in the release of centrally-located drug is more evident for spherical nanoparticles because the drug molecules are farther away from the surface [289]. Drug release from the reservoir and matrix-based systems is an efficient, secure, and effective strategy for achieving sustainable release and has the ability to control multiple active agents. During the release process, a hydrodynamic layer may form on the outer surface of the nanocarrier, which inhibits diffusion and intensifies when it is saturated with the active substance. This boundary layer eventually leads to drug-release termination [55].
Box 3. Mathematical description of the release mechanisms.
-
I.
Diffusion-controlled release
Stefan–Maxwell equations or Fick's law of diffusion can describe drug diffusion.
Fick's first law of diffusion can be used to describe drug release through the membrane for reservoir systems [300]:
| (1b) |
: the flux of the drug,
D: the drug diffusion coefficient,
: drug concentration,
Generally, the drug diffusion coefficient is a function of drug concentration, but for simplifying modeling, it is considered constant [301].
For unsteady-state drug diffusion in a 1D slab-shaped matrix in which a drug is uniformly dispersed, Fick's second law of diffusion is suitable for describing this system [302]:
| (2b) |
Drug release via the edges of the thin slab-shaped can be ignored.
-
II.
Solvent-controlled release (Swelling based)
In swelling-controlled delivery systems, the time-scale for polymer relaxation (λ) is the rate-limiting step, and the rate-limiting step for drug diffusion time-scale is described by . Additionally, δ(t) is the time-dependent thickness of the swollen phase [303,304].
| (3) |
In swelling-controlled delivery systems, De is greater than one, but in diffusion-controlled delivery systems, the De is smaller than one.
The mathematical description of drug release in swelling controlled includes both drug diffusion and polymer relaxation [305]:
| (4) |
: ratio of the amount of molecule released at a given time;
: final amount of molecule released;
: constant
-
III.
Degradation-controlled release
Many mathematicians believe that the mechanisms of erosion and degradation are the same [55].
Carrier erosion model is, as the following equation, known as the mass increase of silicon content in the aqueous environment [306]:
| (5) |
where, and k are mass as and degradation rate, respectively. The rate of erosion is proportional to the pore volume created in the carrier.
Alt-text: Box 3
Solvent-controlled release: Solvent-controlled release is based on the transport of solvent into a drug carrier to enable drug release. In general, solvent-controlled release includes osmosis-controlled release and swelling-controlled release [290]. In the osmosis-controlled release, drug molecules are dispersed in a carrier covered with a semi-permeable polymer membrane (permeable for water and impermeable for drug); therefore, water can penetrate from outside of the carrier (low drug concentration) into the drug-loaded core (high drug concentration) and accumulate in the osmotic layers. In this system, osmotic pressure is used as a driving force of drug release. On the other hand, swelling-controlled release systems consist of polymeric materials such as hydrogels that can absorb large amounts of fluids. When these systems are placed in the body, polymer particles swell by absorbing water or other body fluids. The swelling increases the amount of aqueous solvent in the polymer formulation as well as the size of the meshes or pores, leading to drug penetration through the swollen polymer network to the outside environment. The drug release rate is influenced by the diffusion rate of water and the chain relaxation rate of polymers [291]. Factors such as hydrophobicity and the density of cross-links between the polymer chains affect the rate of swelling. Hydrophobicity prevents water from entering into the polymer and does not provide the necessary swelling to release the contents. However, swelling-controlled systems are mainly designed to rely on pH, because reducing pH leads to swelling (Box 3II) [292].
Degradation-controlled release: Recently, much research has focused on developing nanocarriers made of biodegradable polymers (e.g., polyesters, polyamides, polysaccharides, and amino acids). After complete release of the drug, these materials are disintegrated by natural biological processes, and do not need further manipulation [292]. The drug carriers are designed such that the polymer chains are degraded by hydrolysis and excreted from the body without causing long-term side effects. In these systems, the polymer matrix controls the rate of drug release [293]. When the effectiveness of a drug depends on the release of a high dose over a long time period, the use of degradation-controlled release systems is highly recommended [294].
Polymer erosion can occur through surface or bulk mechanisms [294]. Surface erosion occurs when the rate of polymer erosion is faster than the rate of water diffusion into the matrix [295,296]. Surface erosion over time reduces the system's dimensions. In bulk erosion, in contrast to surface erosion, water molecules penetrate the polymer more rapidly. Subsequently, chain breakage is initiated in the matrix, resulting in a complicated erosion/degradation process in the polymer [297]. In matrices made of polyesters such as poly-lactide, poly-glycolide and their copolymers, the polymer is degraded by hydrolytic cleavage of the ester bond, leading to disintegration into carbon dioxide and water, which are easily removed from the body. The kinetics of drug release are determined by the rate of polymer degradation, which depends on their molecular weight, monomer composition, end groups, and crystallinity [298]. The drug is first released from the surface and the pores connected to the surface; then, when the polymer is gradually degraded, the remaining drug is released at an intermediate rate. After complete destruction of the polymer matrix, the trapped drug is rapidly released (Box 3III) [299].
6.2. Controlled drug release
Although sustained release is appropriate for many clinical situations, there is also a need for more precise control of delivery. For example, slow release can enhance multidrug resistance (MDR), while rapid release can trigger side effects before the drug accumulates in the target tissue [55,307]. Therefore, in many cases, controlled drug release, guided by the drug pharmacokinetics and pharmacodynamics is required to optimize therapy. Consequently, creation of nanoparticles that respond to internal and external stimuli is an active area of research. When exposed to relevant stimuli, such nanoparticles undergo a physicochemical change such as swelling or erosion; subsequent disintegration of the nanoparticle structure results in drug release [308]. Such environment-responsive systems greatly reduce side effects by releasing the drug only within the target tissue, while also reducing the MDR that would otherwise be caused by rapid release of drug [309]. The potential for specific drug release based on local tissue conditions makes nanoparticles attractive for tumor -targeting applications. Dynamic stimuli for releasing drug from nanocarriers are divided into two categories – internal and external (Fig. 11).
Fig. 11.
Stimuli-responsive drug delivery system, Internal stimuli: Oxidative stress, Redox potential, Enzyme, pH; External stimuli: Thermal, Light, Magnetic, Ultrasound, Electric.
Internal stimuli: Internal stimuli are naturally-occurring, caused by physiological and pathological changes in the target tissue and cells. Evidence suggests that the biological properties of tumor tissue can be useful in developing effective delivery systems for therapeutic agents so that the carrier can selectively deliver the load to the tumor [[310], [311], [312], [313]]. The dysfunctional vascular network in tumor tissue, for example, can reduce oxygen delivery and create hypoxic regions with low partial oxygen pressure, which can increase tumor acidity and catalyze the production of bioreductive molecules [314]. As a result, tumor tissue is generally more acidic (pH = 6 to 7) than normal tissue (pH = 7.4) [315]. In addition, tumor tissue is enriched with angiogenic (e.g., MMP9) and proteolytic enzymes [316,317]. Moreover, reactive oxygen species [318] present in tumors can be used to release therapeutic agents at the tumor site by nanoparticles designed to be sensitive to ROS [319]. For intracellular drug release, differences in redox (reduction-oxidation) state in the cancer cell cytoplasm can also be used as a stimulant to release the drug inside the cell while extracellular drug remains stable [320].
External stimuli: The differences between normal and tumor tissue are small –and vary from patient to patient– so can't be used to design generalized nanoparticles based on internal stimuli release. Therefore, external stimuli are often more suitable for controlling drug release in the absence or poor performance of internal stimuli. Unlike internal stimuli, external stimuli allow precise spatial-temporal distribution of drug release, because the drug release is activated only if nanocarrier is exposed to stimuli [39,321]. Some of these stimuli are temperature, light, ultrasound, magnetic fields, and electric fields. Two or more of these stimuli can be used simultaneously in a multi-stimuli system. Different external sources are used to increase the temperature, depending on the conditions of each stimulus. Light activated transformation of the nanosystem at a particular wavelength causes the drug to be released from the carrier [322]. Ultrasound is of particular importance due to its non-invasive nature and its deeper penetration into tissue. Ultrasound-responsive nanocarriers must be stable so that the enclosure is stable before the arrival of ultrasonic waves but releases its payload upon exposure to the waves [323,324]. Magnetic fields have the ability to generate heat due to the alternating magnetic field for drug release and ablation, besides increasing the accumulation of nanoparticles in tumor tissue [325,326]. Finally, electric fields are one of the well-known stimuli that can be used by imposing mechanisms such as heat, electrochemical changes [327], disruption of carrier structure [328], and ROS production [329] to release drugs and treat cancer.
7. Nanoparticle stability
Despite many benefits of nanoparticles in therapeutic applications and drug delivery systems, problems such as complex manufacturing [330,331] and stability issues [332] pose challenges during their development. Stability is an important aspect of nanoparticle development to ensure the efficiency and safety of pharmaceutical products. For example, in intravenous injection, the accumulation of nanoparticles may cause capillary obstruction and embolism [333]. Additionally, high pressure and temperature can change the crystallization of the drug particles [334]. Therefore, nanoparticle-related stability issues require much attention when developing pharmaceutical products.
7.1. General issues of nanocarrier stability
Sedimentation/creaming and agglomeration are the most common causes of nanoparticle instability [335]. Depending on the density, the particles can “cream” (float to the surface) or separate and sediment to the bottom of the solution. The amount of sediment is determined by Stokes' law [336,337], indicating the importance of the effective particle size, viscosity of the environment, and density difference between the particles and medium in determining the amount of sediment. The most common way to reduce settling is to decrease the size of particles. Increasing the viscosity of the medium or matching the density of the particles and the medium are other strategies that are widely used to reduce settling [338]. Although sedimentation is one of the most important issues for colloids, it has less effects on nanoparticles, which are sufficiently small so that their rate of deposition is dramatically decreased.
Nanoparticles are intrinsically thermodynamically unstable due to their high surface energy, which can be exacerbated in designs with large surface area. These characteristics tend to cause nanoparticles to agglomerate to reduce surface energy [335]. Unwanted agglomeration causes problems such as increased settling/creaming rate and inconsistent dosing. To address these problems, it is possible to add stabilizers to the formulation. The choice of stabilizer requires consideration of safety profile, and various formulations can prevent the agglomeration of nanoparticles as well as cause wetting of the nanoparticle surface [339,340].
Improved stability of particles in an aqueous and non-aqueous medium is mainly achieved by enhancing electrostatic repulsion and steric stabilization [336,337] through the addition of ionic and non-ionic stabilizers. By combining these two stabilizing mechanisms, an effective stable dispersion of nanoparticle can be achieved. This compound approach decreases the self-repulsion between ionic surfactant molecules, resulting in closer packing of the stabilizer molecules [341].
Engineering the nanocarrier morphology is another method of enhancing stability that is gaining popularity. The promise of this strategy was demonstrated by Edwards et al. [342], who propose the conceptual benefits of various carrier properties, such as porous nanoparticles, aggregate particles, and hollow porous particles. In addition to providing short-term and long-term storage stability, stabilizers can be used to obtain successful formation and stabilization during the manufacturing process [102].
7.2. Characterizing stability of nanoparticles formulations
The choice of characterization techniques for nanoparticles stability depends on the nature of the stability issues as well as the dosage form of the drug product. The characterization techniques used to assess the stability properties of particles are presented in Table 4 [335,343].
Table 4.
Experimental methods to assess the nanoparticles stability.
| Properties | Technique |
|---|---|
| Particle size | Dynamic light scattering (DLS), Laser diffraction (LD), Photon correlation spectroscopy (PCS), Transmission electron microscopy (TEM), Scanning electron microscopy (SEM), and Atomic force microscopy (AFM), Brunauer-Emmett-Teller (BET) |
| Aggregation | DLS, LD, Inductively coupled plasma mass spectrometry (ICP-MS) |
| Dispersity | TEM, SEM, and AFM |
| Morphology | TEM, SEM, and AFM, X-ray diffraction (XRD), Brunauer-Emmett-Teller (BET) |
| Surface charge | Zeta potential |
| Encapsulation efficiency | Gel electrophoresis, Ultraviolet–visible light Spectroscopy (UV–vis) |
| Chemical connectivity | X-ray scattering (SAXS and WAXS), Nuclear magnetic resonance (NMR), High-performance liquid chromatography (HPLC), Raman spectroscopy, FT-IR, X-ray diffraction (XRD) |
| Phase transitions | Differential scanning calorimetry (DSC), Isothermal calorimetry (ITC) |
Average particle size and size distribution are the main metrics used to assess physical stability. Common techniques used to measure these parametes include PCS, DLS, LD, and Coulter counter. PCS/DLS is mainly employed to characterize the size and size distribution of small particles in the aqueous environment. In this method, the polydispersity index (PDI) is the mean of distribution size and particle size parameters, in which a PDI value greater than 0.5 refers to a broad size distribution and 0.1–0.25 corresponds to a narrow distribution [333]. However, the measurement range possible by this method is very narrow, so LD is used with PCS to overcome this limitation. LD has a wide range of detection, ranging from 20 nm to 2000 μm. The typical characterization parameters of LD are LD50, LD90, and LD99; corresponding to 50, 90, or 99% of the particles falling below a given size, respectively. The Coulter counter specifies the number of particles per unit volume for classes of different sizes, which is more accurate than LD. Although these techniques make it possible to measure particle size and size distribution, they do not have the ability to assess morphology. Therefore, direct imaging techniques such as TEM, SEM, and AFMare widely used to evaluate morphology of nanoparticles [335].
Particle surface charge is generally determined by measuring the zeta potential. Laser Doppler electrophoresis measures zeta by evaluating the electrophoretic mobility of particles in the environment. Particles with absolute zeta values above than 60 mV are extremely stable. As zeta decreases, stability decreases: zeta potentials of 30, 20 and less than 5 mV correspond to good stability, short-term, and rapid accumulation of aggregation, respectively. This is a general rule of thumb that is only valid for pure electrostatic stabilizers or combinations of low molecular weight surfactants [344].
In addition to surface charging, surface chemistry should also be evaluated. The most common technique used to assess chemical stability is HPLC, which provides a quantitatively accurate analysis on degradation impurities. Mass spectrometry is used with HPLC to determine the molecular structure of impurities. Other techniques (e.g., FTIR and NMR) are employed for chemical stability evaluation, but they are not widely used due to poor accuracy and sensitivity [335].
8. Clinical translation
Nanotechnology has made advancements in many fields including biology, chemistry, and engineering, but its potential for medical applications is vast and evolving. However, after two decades of nanomedicine research, it is still recognized as a new field of science and research, and many of its basic features are active areas of investigation. Nanomedicine will undoubtedly continue to be a major field of research and development for the next decade. Considerable research has been done on nano-based drug delivery to solid tumors resulting in functional nanoparticles, some of which have been approved and some of which are in the clinical trial and preclinical stages (Table 5).
Table 5.
Nanotechnology platforms, active pharmaceutical ingredients, and commercial status.
| Status | Nanocarrier | Generic name and/or proprietary name | Drug | Advantages | Treatment/Disease | Approval status | Ref. |
|---|---|---|---|---|---|---|---|
| Approved | Liposome | DaunoXome | Daunorubicin | Increased drug delivery; Reduced drug toxicity | HIV-related Karposi's sarcoma | FDA (1996) | [347] |
| Doxil/Caelyx | Doxorubicin | Increased drug delivery; Reduced drug toxicity | Ovarian cancer, HIV- related karposi's Sarcoma and multiple myeloma | FDA (1995) EMA (1996) |
[348] | ||
| Marqibo | Vincristine | Improved delivery to tumor site, Decreased systemic toxicity; Solubilization and sustained release | Acute lymphoblastic leukemia | FDA (2012) | [349] | ||
| MEPACT | mifamurtide | Immuno-stimulatory effects, longer half-life in plasma | Osteosarcoma | EMA (2009) | [350] | ||
| Myocet | Doxorubicin | Reduced side effects, Decreased cardiotoxicity |
Metastatic breast cancer disease | EMA (2000) | [318] | ||
| Onivyde | Irinotecan | Increased drug delivery; Reduced drug toxicity | Pancreatic cancer | FDA (2015) | [351] | ||
| VYXEOS CPX-351 (Jazz Pharmaceuticals) | Cytarabine:daunorubicin (5:1 M ratio) | Combination therapy, Controlled release of the molecules Two molecules co-loaded with synergistic anti-tumor activity |
Acute myeloid leukemia (AML), AMLA with myelodysplasia-related changes (AML-MRC) | FDA (2017) EMA (2018) |
[352] | ||
| Albumin | Abraxane | Paclitaxel | Improved solubility; Improved delivery to tumor | Metastatic Pancreatic and breast cancer, Advanced NSCLC | FDA (2005) EMA (2008) |
[353] | |
| Hafnium oxide nanoparticles | NBTXR3 Hensify |
– | Increased tumor cell death | Locally advanced squamous cell carcinoma | CE Mark (2019) | [354] | |
| Undergoing clinical trials | Liposome | Atu027 | Small interfering RNA (siRNA) | Protection from degradation | Pancreatic cancer disease | Phase I/II | [355] |
| BP1001 | Growth factor receptor bound protein-2 (Grb-2) antisense oligonucleotide | Decreased the proliferation of gleevec-resistant CML cells | Leukemias | Phase II | [356] | ||
| Halaven E7389-LF | Eribulin Mesylate | A tubulin and microtubule inhibitor | Solid tumors | Phase I | [357] | ||
| JVRS-100 | Plasmid DNA |
Immunotherapy | Refractory/Relapsed leukemia | Phase I | [358] | ||
| LiPlaCis | Cisplatin | Specific degradation-controlled drug release via phospholipase A2 (PLA2) | Advanced or refractory tumors | Phase I | [359] | ||
| Lipocurc | Curcumin | Stable curcumin plasma concentrations during infusion | Solid tumors | Phase I/II | [360] | ||
| Lipusu | Paclitaxel | Solubilization, Sustained Release | Advanced solid tumors, or gastric, breast cancer | Phase IV | [361] | ||
| MM-302 | Doxorubicin | Chemotherapy, Targeted delivery |
Breast cancer | Phase II/III | [362] | ||
| MRX34 | Micro RNA (miRNA) | Protection from degradation | Liver cancer | Phase I | [363] | ||
| Mitoxantrone hydrochloride liposome | Mitoxantrone | Intercalating agent, a potent inhibitor of topoisomerase II | Lymphoma, Breast cancer | Phase II | [364] | ||
| Oncoprex | FUS1 (TUSC2) | Inhibit mechanisms that create drug resistance; Interrupts cancer cell signaling pathways | Lung cancer | Phase I/II | [365] | ||
| PROMITIL | Mitomycin-C | More effective and less toxic than conventional chemotherapy in various tumor models | Solid tumors | Phase I | [366] | ||
| Re-BMEDA-liposome | Re–N,N-bis (2-mercaptoethyl)-N′,N′-diethylethylenediamine | Better mean tumor growth inhibition rate and longer median survival time than chemotherapeutics | Advanced solid tumors | Phase I | [367] | ||
| SGT53 | Wild-type p53 sequence | Targeted drug delivery to metastatic lesions | Glioblastoma, or pancreatic cancer | Phase II | [368] | ||
| SGT94 | RB94 plasmid DNA | Marked cytotoxicity against tumor but not normal cells | Solid tumors | Phase I | [369] | ||
| siRNA-EphA2-DOPC | siRNA | Inhibited tumor growth | Solid tumors | Phase I | [74] | ||
| ThermoDox | Doxorubicin | Chemotherapy, Stimuli-responsive delivery | Hepatocellular carcinoma, Liver tumors (mild hypothermia) | Phase III | [370] | ||
| Lipid particle | DCR-MYC | siRNA | Protection from degradation | Advanced solid tumors | Phase I/II | [371] | |
| PNT2258 | Proprietary single-stranded DNAi (PNT100) | Safe and tolerable | Lymphomas | Phase II | [372] | ||
| TKM-080301 | siRNA | Favorable toxicity profile | Hepatocellular carcinoma | Phase III | [373] | ||
| Albumin | ABI-009 | Rapamycin | Antiangiogenic and antineoplastic activities | Bladder cancer, PEComa, or pulmonary arterial hypertension and advanced cancer with mTOR mutations 237,238 |
Phase II | [50] | |
| ABI-011 | Thiocolchicine analog | Effective in inhibiting the formation of novel microvessel and in disrupting established microvessels | Solid tumors or lymphomas | Phase I | [374] | ||
| Polymeric NP | AZD2811 | Aurora B kinase inhibitor | Increase biodistribution to tumor sites and provide extended release of encapsulated drug | Advanced solid tumors | Phase I/II | [375] | |
| BIND-014 | Docetaxel | Chemotherapy, Targeted delivery | Prostate, NSCLC, cervical, head and neck cancers | Phase II | [376] | ||
| CRLX101 | Camptothecin | Improved solubility in water, Inhibition of DNA topoisomerase I | NSCLC, Metastatic renal cell cancer, Ovarian and peritoneal cancer | Phase III | [377] | ||
| CRLX301 | Docetaxel | higher retention of drug in plasma, slower clearance | Advanced solid tumors | Phase I/II | [378] | ||
| Micelle | CriPec | Docetaxel | higher intratumor drug concentrations | Solid tumors, ovarian cancer | Phase II | [379] | |
| Docetaxel-PM DOPNP201 | Docetaxel | Enhance stability and improve delivery | Head and neck cancer and advanced solid tumors | Phase II | [51] | ||
| Genexol-PM | Paclitaxel | Controlled drug release platform | Breast and Head and neck cancer | Phase II | [380] | ||
| NC-6004 | Cisplatin | Improved circulation half-life, EPR phenomena | Lung, biliary, bladder, pancreatic cancers | Phase I/II | [381] | ||
| Anti-EGFR bispecific antibody minicells (bacteria derived nanoparticles) | TargomiRs | /miR-16 based microRNA | Intravenous administration avoiding the enzymatic degradation in the peripheral blood | Mesothelioma and NSCLC | Phase I | [382] | |
| Hafnium oxide nanoparticles | NBTXR3 PEP503 |
– | Antitumor effects with similar to standard radiation therapies | Locally advanced squamous cell carcinoma | Phase II | [383] | |
| Developing concepts | Liposome | Anti-EGFR-IL-dox | Doxorubicin | – | Breast cancer | Phase I/II | [52] |
| EGFR(V)-EDV-Dox | Doxorubicin | – | Recurrent glioblastoma | Phase I | [53] | ||
| FF-10831 | Gemcitabine | – | Advanced solid tumors | Phase I | [54] | ||
| IVAC_W_bre1_uID | RNA | – | Triple negative breast cancer | Phase I | [55] | ||
| Liposomal Annamycin | Annamycin | – | Acute myeloid leukemia | Phase II | [56] | ||
| Lipo-MERIT | RNA | – | advanced melanoma | Phase I | [57] | ||
| MM-310 | Docetaxel | – | Solid tumors | Phase I | [58] | ||
| MRT5201 | mRNA | – | Ornithine transcarbamylase deficiency | Phase I | [59] | ||
| TLD-1/Talidox | Doxorubicin | – | Advanced solid tumors | Phase I | [60] | ||
| Micelle | Imx-110 | Doxorubicin | – | Advanced solid tumors | Phase I | [61] | |
| IT-141 | SN-38 | – | Advanced cancer | Phase I | [62] | ||
| MTL-CEBPA | RNA | – | Advanced liver cancer | Phase I | [63] | ||
| Spherical gold nanoparticle | NU-0129 | Nucleic acid | – | Glioblastoma | Phase I | [64] |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration; CE Mark, European market approval.
In the field of nano-based drug delivery, the number of scientific publications is increasing exponentially, from fewer than 300 in 1999 to more than 10,000 per year [345]. These publications include studies that indirectly or directly address the optimization and scalability of nano-drug delivery systems. In the past two decades, the number of publications citing clinical trials that show clinical progress and development has been approximately 150 per year [345]. This number is nearly the same in the current decade, while non-clinical studies are increasing exponentially.
The stagnation of clinical progress versus the exponential increase in preclinical data is mainly due to challenges such as scalability, stability, and cost of materials. On the other hand, it takes several years for the results of clinical studies to be published, so only a few promising nanoparticles are in the process of clinical development and are being approved by regulatory agencies. However, many clinical trials also show unpredictable results that fail to achieve their goal. He et al. [346] calculated the success rate of nanoparticles for oncology applications in different clinical phases. They showed that the success rate is 94% in phase I, 48% in phase II, and 14% in phase III. It was also estimated that the chance of success from phase I to clinical approval is approximately 6%.
8.1. Obstacles and failures
While preclinical studies have consistently reported the promise of nanoparticles in cancer therapeutic applications, many candidate formulations fail in clinical trials. The failure of nanoparticles during clinical translation might be related to insufficient preclinical development (resulting in, for example, poor efficiency and toxicity) or unforeseen market challenges (due to, for example, high cost and production complexity). In the next section, we will further explore the reasons for the limited clinical success of nanoparticles.
8.1.1. Scientific considerations
The development of new nanoparticles for various applications, including nanocarriers and nanotheranostics, has highlighted the importance of potential adverse effects and toxicity. Nanomedicines can accumulate in various tissues, and their interactions with cells in vivo are not always predicted by benchtop experiments. Because of their unique formulations, nanoparticles can cause damage not seen with conventional medicines, including damage to cell organelles and unpredictable activation of blood clotting mechanisms [384]. Specific physicochemical properties contribute to toxicity, including size and shape. For example, investigating the toxicity of gold nanoparticles, Sun et al. found that rod-shaped nanoparticles were more toxic than cubic-shaped nanoparticles, while those with spherical shape had the best biocompatibility [385]. However, traces of gold nanoparticles were also found in the brain, heart, and lungs, which may result in long-term complications. Another study found that metallic nanoparticles of different sizes are toxic to sperm cells and have adverse effects through oxidative stress mechanisms [386,387]. The important conclusion is that any change in the physicochemical properties of a nanoparticle can affect toxicity and potential success in the clinic. For example, a phase I clinical trial for MRX34 was stopped in to severe immune-related toxicity in 20% of patients [388]. The development of MM-310 in Phase II trials was also terminated due to cumulative peripheral neuropathy [389]. Even after approval for clinical use, a drug may be pulled from the market due to safety concerns.
Because of the high attrition rate of cancer nanomedicines in clinical trials, we should re-evaluate the reasons for these failures. One of the candidates for cancer treatment was BIND-014, which, despite success in three other phase II trials, eventually failed in head and neck cancer. According to the former CEO of BIND Therapeutic, insufficient payload caused the failure of BIND-014 and he concluded that the choice of payload is a critical determinant of performance [390]. Other trials show that we need to be more selective of the patient population for admission to the trial. For example, the paclitaxel-polyglutamic acid conjugate Opaxio™ was evaluated in phase III clinical trials for the treatment of lung cancer. It was observed that it is appropriate only for women with specific estrogen levels, thus limiting demand for the drug [391].
A review of the literature suggests that the most important factor behind these clinical limitations and failures is our poor understanding of disease heterogeneity in patients. Therefore, fine-tuning a system based on patient biology is necessary, although it faces numerous challenges. Better computational models that can incorporate all aspects of malignancy, including proliferation, metastasis, and drug accumulation pathways, would help to resolve the discrepancies between preclinical and clinical efficacies.
8.1.2. Market
Large-scale and reproducible nanoparticle synthesis is crucial for clinical translation. However, this is not straightforward for many nanoparticles, and consequently, they never enter the market. Controlling and optimizing small batches is relatively easy, but large scale production and quality control are expensive and more challenging. In addition to manufacturing costs, the costs of preclinical development and running clinical trials are extremely high. It is also more difficult to obtain regulatory approval for new nanotherapeutics, especially when existing products on the market have the same target indication.
Nevertheless, commercialization of nanomedicine is expanding globally. At present, North America and Europe dominate the nanomedicine market, largely because these are regions of early nanomedicine development and regulatory frameworks are already in place. The industry in Asia is also growing due to increased funding for nanomedicine research and increased interest in nano-based therapies [392].
Considering the hurdles involved in taking a nanomedicine to market, it is not surprising that investors are often hesitant to enter this field. This, in addition to the high cost of developing nanomedicines, poses additional challenges to nanomedicine development through preclinical and clinical stages. Furthermore, because preclinical validation is not always robust, repeated clinical trials and additional financial investment are often required to optimize a formulation. For small biotechnology companies and academic laboratories, the cost of translating a potential nonomedicine to the clinic can be prohibitive. Even for large pharmaceutical companies, these costs can be a major barrier for investment. The cost of developing a new nanotherapy is estimated to be approximately $ 2.558 billion, according to the Tufts Center for Study of Drug Development [393]. In general, a biopharma startup company, unlike typical computer tech startups, requires years of sustained funding to move its product from discovery to clinical trials and toward regulatory approval. A failure at the clinical trial stage can cost tens to hundreds of millions of dollars; as a result, approved cancer nanomedicines often face termination threats during their development and testing [394].
Even if a nanomedicine manages to acquire funding and gets approval, they are usually more expensive than traditional chemotherapy drugs due to the high costs of development. And unfortunately, this higher cost often only translates into limited benefit to the patient, providing only a few weeks or months of survival or delayed progression. These cost/benefit considerations are another barrier to nanomedicine adoption and a concern for potential investors.
Financial challenges can be especially relevant to startup companies dedicated to nanomedicine development. For example, BIND Therapeutics was founded in 2007 to develop the Accurins® platform. The company raised a total of $ 877.7 M from capital markets, large Pharma partnerships, and venture capital [395]. An unsuccessful Phase II study led to plunging stock prices [396], and the company was eventually acquired by Pfizer for $40 M [397].
In contrast, providing a successful nanotherapy can attract capital. Celator Pharmaceuticals, for example, raised about $170 M from venture capital, which led to the final approval of Vyxeos [398]. Although it is too early to judge the commercial success of Vyxeos, it earned $75 M in the first year after its release (2018) [346]. It is also projected to reach $131 M by 2024 [399].
8.2. Standardization of preclinical research to enhance clinical translation
Some failures can be attributed to an inability of preclinical models to accurately predict the clinical performance of nanomedicines [400,401]. Mouse xenograft cancer models are the most common preclinical model, but they consistently overestimate the performance of nanomedicines [402,403]. This may be due to the critical importance of the EPR effect for delivery, which has been shown to be exaggerated in mouse xenograft models [404]. Nano-drugs accumulate in high levels in mouse xenograft tumors, whereas this effect is not consistent in human cancers. Existing biological differences not only confound clinical translation but, in some cases, result in nanomedicines being less effective than their traditional counterparts [405,406]. Indeed, problems with recapitulation of human disease in animal models are well-documented, especially with regards to mouse xenograft models. For example, orthopedic colorectal tumors in mice are typically localized on the outer serosa while in humans, colon cancer is more similar to polyps located in the lumen of the colon [407]. This difference can affect the penetration of tumor NPs and their interactions. This problem is also seen in other preclinical cancer models, including gallbladder cancer and ovarian cancer [408]. However, most nanomedicine development still relies on mouse xenograft models. This is because of the potential dangers of toxic side effects that need to be assessed before introduction into the clinic, and the many parameters that need to be measured to assess nanocarrier performance. To establish a robust nanomedicine, accumulation rate, release rate, drug metabolism, pharmacokinetic and pharmacodynamic evaluation, and treatment scheduling need to be considered and measured. If these parameters could be assessed directly in patients consistently, translation may be dramatically improved and accelerated.
The goal of preclinical experimentation is to evaluate the stability and performance of new nanomedicine technologies, identifying potential problems that might arise in later development. Therefore, the primary goal of preclinical testing should focus on therapeutic efficacy and the potential for adverse events. Translatable pre-clinical tests should be designed to provide detailed insights into key parameters affecting nano performance that do not create technical and costly challenges that delay investment.
In general, a common cause of clinical translation failure is our limited understanding of disease heterogeneity in each patient population and our inability to adjust the system according to specific patient characteristics. Therefore, another tool for preclinical development that can be used to complement mouse studies is computational modeling. Computational models can be used to predict optimal delivery, distribution, and treatment based on principles of linker design, nanoparticle structure and nano-bio interactions. In computational models, raw data extracted from clinical conditions are used as input, providing an important advantage over xenograft models when sufficient and accurate data are available [55,409]. With computational modeling, the selection of biological and physiological parameters is one of the most significant components affecting accuracy, and this is the most challenging step for model development. Therefore, a qualitative process for model validation is often implemented, in which one or more parameters are estimated, and then the model is compared with clinical data. In this way, parameter estimates are improved, and the model can be used to estimate nanoparticle accumulation, nano-bio interactions and tumor response. When successful, such models can reduce the need for animal testing and accelerate translation.
Organ-on-chip models are another approach that can potentially overcome some of the limitations of in vivo and in vitro models [410,411]. These platforms create multi-cellular tissues in vitro meant to recapitulate the important aspects of the disease, and can be used for drug testing. Depending on their complexity, these platforms may be used to investigate the effects of dose, particle extravasation and accumulation, the effect of physicochemical properties of the nanoparticles, and interstitial fluid flow rates [412,413]. These efforts have the potential to optimize nanomedicine design and implementation without the need for mouse studies or repeated clinical trials.
In summary, successful clinical translation of cancer nanomedicine projects can be improved by:
-
•
Building an accurate understanding of the interaction between nanomedicine behavior and human biology to improve the biodistribution, accumulation, and stability of nanoparticles through appropriate in vivo tests.
-
•
Transitioning from fundamental research to disease-driven rational research.
-
•
Development and utilization of more clinically relevant in vitro and in vivo models to optimize the physicochemical properties of nanoparticles, dosing schedules, and treatment combinations with a focus on how the disease develops in patients.
-
•
Pre-selecting patients who are likely to respond to nanomedicine-based therapies and developing formulations that can be adapted to specific patients.
9. Concluding remarks
Cancer nanomedicine research has recently become an interdisciplinary field by integrating different tools such as laboratory modeling of advanced diseases (such as on-chip tumors and 3D multicellular models), omics (such as nanoparticle barcoding), computational modeling, and artificial intelligence (for big data management, predicting nanomedical synthesis, and biological distribution). Such advances improve the relevance of fundamental and quantitative studies of nano-bio interactions. Consequently, it is possible to solve complex and multi-variate problems related to nanoparticle delivery that could not be solved with previous analytical tools. The definition of cancer nanomedicine now goes beyond a nano-sized vehicle for the delivery of chemotherapy drugs. Clinical and preclinical research have shown that nanomedicine also enables early detection and strengthens the immune system against tumors. These new roles will increase the likelihood that cancer nanomedicines will be successful in the clinic. In summary, recognizing important barriers to development and implementation can lead to new approaches that accelerate the transfer of nanomedicine from bench to the bedside. Recognizing, acknowledging and overcoming these barriers are the necessary steps for ensuring the success of nanomedicines.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
acknowledgement
Research was supported by NIH grants U01 CA261842 and R01 CA247441 (Lance L. Munn)
Contributor Information
Mohammad Souri, Email: souri1996m@gmail.com.
M. Soltani, Email: msoltani@uwaterloo.ca.
Farshad Moradi Kashkooli, Email: farshadmoradikashkooli@ymail.com.
Lance L. Munn, Email: munn@steele.mgh.harvard.edu.
References
- 1.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y., Feng X., Wan C., Lovell J.F., Jin H., Ding J. Role of nanoparticle-mediated immunogenic cell death in cancer immunotherapy. Asian J. Pharm. Sci. 2021;16:129–132. doi: 10.1016/j.ajps.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng X., Xu W., Xu X., Li G., Ding J., Chen X. Cystine proportion regulates fate of polypeptide nanogel as nanocarrier for chemotherapeutics. Sci. China Chem. 2021;64:293–301. [Google Scholar]
- 4.Feng X., Xu W., Li Z., Song W., Ding J., Chen X. Disease immunotherapy: immunomodulatory nanosystems (adv. Sci. 17/2019) Adv. Sci. 2019;6:1970100. doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Negro M., González-Rubio G., Aicart E., Landfester K., Guerrero-Martínez A., Junquera E. Insights into colloidal nanoparticle-protein corona interactions for nanomedicine applications. Adv. Colloid Interface Sci. 2021:102366. doi: 10.1016/j.cis.2021.102366. [DOI] [PubMed] [Google Scholar]
- 6.Khalifehzadeh R., Arami H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020;279:102157. doi: 10.1016/j.cis.2020.102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anchordoquy T.J., Barenholz Y., Boraschi D., Chorny M., Decuzzi P., Dobrovolskaia M.A., Farhangrazi Z.S., Farrell D., Gabizon A., Ghandehari H. ACS Publications; 2017. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D., Zhou S., Gao W. What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano. 2020;14:12281–12290. doi: 10.1021/acsnano.9b09713. [DOI] [PubMed] [Google Scholar]
- 9.Dutta B., Barick K., Hassan P. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021:102509. doi: 10.1016/j.cis.2021.102509. [DOI] [PubMed] [Google Scholar]
- 10.Elstad N.L., Fowers K.D. OncoGel (ReGel/paclitaxel)—clinical applications for a novel paclitaxel delivery system. Adv. Drug Deliv. Rev. 2009;61:785–794. doi: 10.1016/j.addr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Dai Q., Wilhelm S., Ding D., Syed A.M., Sindhwani S., Zhang Y., Chen Y.Y., MacMillan P., Chan W.C. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano. 2018;12:8423–8435. doi: 10.1021/acsnano.8b03900. [DOI] [PubMed] [Google Scholar]
- 12.Price L.S., Stern S.T., Deal A.M., Kabanov A.V., Zamboni W.C. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim B.Y., Rutka J.T., Chan W.C. Nanomed. New Engl. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 14.Pelaz B., Alexiou C., Alvarez-Puebla R.A., Alves F., Andrews A.M., Ashraf S., Balogh L.P., Ballerini L., Bestetti A., Brendel C. ACS Publications; 2017. Diverse Applications of Nanomedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Björnmalm M., Thurecht K.J., Michael M., Scott A.M., Caruso F. Bridging bio–nano science and cancer nanomedicine. ACS Nano. 2017;11:9594–9613. doi: 10.1021/acsnano.7b04855. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Roemeling C., Jiang W., Chan C.K., Weissman I.L., Kim B.Y. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017;35:159–171. doi: 10.1016/j.tibtech.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., Klaessig F., Castranova V., Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 19.Ke P.C., Lin S., Parak W.J., Davis T.P., Caruso F. A decade of the protein corona. ACS Nano. 2017;11:11773–11776. doi: 10.1021/acsnano.7b08008. [DOI] [PubMed] [Google Scholar]
- 20.Barua S., Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souri M., Soltani M., Kashkooli F.M., Shahvandi M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Contr. Release. 2022 doi: 10.1016/j.jconrel.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Hu J., Yuan X., Wang F., Gao H., Liu X., Zhang W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chin. Chem. Lett. 2021;32:1341–1347. [Google Scholar]
- 23.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan V.P., Stylianopoulos T., Martin J.D., Popović Z., Chen O., Kamoun W.S., Bawendi M.G., Fukumura D., Jain R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashkooli F.M., Soltani M., Rezaeian M., Meaney C., Hamedi M.-H., Kohandel M. Effect of vascular normalization on drug delivery to different stages of tumor progression: in-silico analysis. J. Drug Deliv. Sci. Technol. 2020;60:101989. [Google Scholar]
- 26.Stylianopoulos T., Munn L.L., Jain R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trend Canc. 2018;4:292–319. doi: 10.1016/j.trecan.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbasi A.Z., Gordijo C.R., Amini M.A., Maeda A., Rauth A.M., DaCosta R.S., Wu X.Y. Hybrid manganese dioxide nanoparticles potentiate radiation therapy by modulating tumor hypoxia. Cancer Res. 2016;76:6643–6656. doi: 10.1158/0008-5472.CAN-15-3475. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q., Feng L., Liu J., Zhu W., Dong Z., Wu Y., Liu Z. Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Advanced materials. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 29.Miao L., Newby J.M., Lin C.M., Zhang L., Xu F., Kim W.Y., Forest M.G., Lai S.K., Milowsky M.I., Wobker S.E. The binding site barrier elicited by tumor-associated fibroblasts interferes disposition of nanoparticles in stroma-vessel type tumors. ACS Nano. 2016;10:9243–9258. doi: 10.1021/acsnano.6b02776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ernsting M.J., Hoang B., Lohse I., Undzys E., Cao P., Do T., Gill B., Pintilie M., Hedley D., Li S.-D. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J. Contr. Release. 2015;206:122–130. doi: 10.1016/j.jconrel.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Lin Y.-X., Qiao S.-L., An H.-W., Ma Y., Qiao Z.-Y., Rajapaksha R.Y.J., Wang H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–163. doi: 10.1016/j.biomaterials.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 32.Su L., Zhang W., Wu X., Zhang Y., Chen X., Liu G., Chen G., Jiang M. Glycocalyx-mimicking nanoparticles for stimulation and polarization of macrophages via specific interactions. Small. 2015;11:4191–4200. doi: 10.1002/smll.201403838. [DOI] [PubMed] [Google Scholar]
- 33.Chen J., Jiang Z., Xu W., Sun T., Zhuang X., Ding J., Chen X. Spatiotemporally targeted nanomedicine overcomes hypoxia-induced drug resistance of tumor cells after disrupting neovasculature. Nano Lett. 2020;20:6191–6198. doi: 10.1021/acs.nanolett.0c02515. [DOI] [PubMed] [Google Scholar]
- 34.De Jong W.H., Borm P.J. Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomed. 2008;3:133. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan V.P., Stylianopoulos T., Boucher Y., Jain R.K. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Ann. Rev. Chem. Biomolecul. Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan V.P., Jain R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stylianopoulos T., Jain R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015;11:1893–1907. doi: 10.1016/j.nano.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X., Ni N., Ma Y., Wang Y., Leong D.T. Retooling cancer nanotherapeutics' entry into tumors to alleviate tumoral hypoxia. Small. 2020:2003000. doi: 10.1002/smll.202003000. [DOI] [PubMed] [Google Scholar]
- 39.Souri M., Soltani M., Kashkooli F.M. Scientific Reports; 2021. Computational Modeling of Thermal Combination Therapies by Magneto-Ultrasonic Heating to Enhance Drug Delivery to Solid Tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 41.Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 2010;5:523–528. doi: 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H., Hoang B., Fonge H., Reilly R.M., Allen C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharmaceut. Res. 2010;27:2343–2355. doi: 10.1007/s11095-010-0068-z. [DOI] [PubMed] [Google Scholar]
- 43.Litzinger D.C., Buiting A.M., van Rooijen N., Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly (ethylene glycol)-containing liposomes. Biochim. Biophys. Acta Biomembr. 1994;1190:99–107. doi: 10.1016/0005-2736(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 44.Singh R., Pantarotto D., Lacerda L., Pastorin G., Klumpp C., Prato M., Bianco A., Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson N.L., Polanski M., Pieper R., Gatlin T., Tirumalai R.S., Conrads T.P., Veenstra T.D., Adkins J.N., Pounds J.G., Fagan R. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol. Cell. Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- 46.Lane L.A. Physics in nanomedicine: phenomena governing the in vivo performance of nanoparticles. Appl. Phys. Rev. 2020;7 [Google Scholar]
- 47.Yoo J.-W., Chambers E., Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr. Pharmaceut. Des. 2010;16:2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 48.Mascheroni P., Schrefler B.A. In silico models for nanomedicine: recent developments. Curr. Med. Chem. 2018;25:4192–4207. doi: 10.2174/0929867324666170417120725. [DOI] [PubMed] [Google Scholar]
- 49.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015;33:941. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee T.-R., Choi M., Kopacz A.M., Yun S.-H., Liu W.K., Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci. Rep. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y., van der Meel R., Chen X., Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stylianopoulos T., Soteriou K., Fukumura D., Jain R.K. Cationic nanoparticles have superior transvascular flux into solid tumors: insights from a mathematical model. Ann. Biomed. Eng. 2013;41:68–77. doi: 10.1007/s10439-012-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiig H., Swartz M.A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 54.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Garland Publ, Inc.; London: 2002. The Molecular Biology of the Cell. [Google Scholar]
- 55.Kashkooli F.M., Soltani M., Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J. Contr. Release. 2020 doi: 10.1016/j.jconrel.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Kerkar S.P., Restifo N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng J., Yang Q., Shi K., Xiao Y., Wei X., Qian Z. Intratumoral fate of functional nanoparticles in response to microenvironment factor: implications on cancer diagnosis and therapy. Adv. Drug Deliv. Rev. 2019;143:37–67. doi: 10.1016/j.addr.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Nia H.T., Munn L.L., Jain R.K. Physical traits of cancer. Science. 2020:370. doi: 10.1126/science.aaz0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ud Din F., Aman W., Ullah I., Qureshi O.S., Mustapha O., Shafique S., Zeb A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017;12:7291. doi: 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira E.R., Kedrin D., Seano G., Gautier O., Meijer E.F., Jones D., Chin S.-M., Kitahara S., Bouta E.M., Chang J. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403–1407. doi: 10.1126/science.aal3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain R.K., Tong R.T., Munn L.L. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soltani M., Chen P. Numerical modeling of fluid flow in solid tumors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boucher Y., Baxter L.T., Jain R.K. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- 64.Baxter L.T., Jain R.K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 1989;37:77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 65.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7:653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youn Y.S., Bae Y.H. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018;130:3–11. doi: 10.1016/j.addr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Contr. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 68.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., MacMillan P., Zhang Y., Rajesh N.U., Hoang T. The entry of nanoparticles into solid tumours. Nat. Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 69.Young D.B. Control of cardiac output. Integr. Syst. Physiol.: Molecul. Funct. 2010;2:1–97. [Google Scholar]
- 70.Shargel L. Applied Biopharmaceutics and Pharmacokinetics; 1999. Physiologic Drug Distribution and Protein Binding. [Google Scholar]
- 71.Jones R.T. Blood flow. Annu. Rev. Fluid Mech. 1969;1:223–244. [Google Scholar]
- 72.Yuan F., Leunig M., Huang S.K., Berk D.A., Papahadjopoulos D., Jain R.K. Mirovascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 73.Cao J., Huang D., Peppas N.A. Advanced Drug Delivery Reviews; 2020. Advanced Engineered Nanoparticulate Platforms to Address Key Biological Barriers for Delivering Chemotherapeutic Agents to Target Sites. [DOI] [PubMed] [Google Scholar]
- 74.Soltani M., Moradi Kashkooli F., Souri M., Zare Harofte S., Harati T., Khadem A., Haeri Pour M., Raahemifar K. Enhancing clinical translation of cancer using nanoinformatics. Cancers. 2021;13:2481. doi: 10.3390/cancers13102481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dogra P., Butner J.D., Chuang Y.-l., Caserta S., Goel S., Brinker C.J., Cristini V., Wang Z. Mathematical modeling in cancer nanomedicine: a review. Biomed. Microdevices. 2019;21:1–23. doi: 10.1007/s10544-019-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shamsi M., Mohammadi A., Manshadi M.K., Sanati-Nezhad A. Mathematical and computational modeling of nano-engineered drug delivery systems. J. Contr. Release. 2019;307:150–165. doi: 10.1016/j.jconrel.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Stillman N.R., Kovacevic M., Igor B., Sabine H. In silico modelling of cancer nanomedicine, across scales and transport barriers. NPJ Comput. Mater. 2020;6 [Google Scholar]
- 78.Tehrani M.H., Soltani M., Moradi Kashkooli F., Mahmoudi M., Raahemifar K. Computational modeling of combination of magnetic hyperthermia and temperature-sensitive liposome for controlled drug release in solid tumor. Pharmaceutics. 2022;14:35. doi: 10.3390/pharmaceutics14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albanese A., Tang P.S., Chan W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 80.Kann B., Offerhaus H.L., Windbergs M., Otto C. Raman microscopy for cellular investigations—from single cell imaging to drug carrier uptake visualization. Adv. Drug Deliv. Rev. 2015;89:71–90. doi: 10.1016/j.addr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020:1–24. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popović Z., Liu W., Chauhan V.P., Lee J., Wong C., Greytak A.B., Insin N., Nocera D.G., Fukumura D., Jain R.K. A nanoparticle size series for in vivo fluorescence imaging. Angew. Chem. 2010;122:8831–8834. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi H.S., Liu W., Liu F., Nasr K., Misra P., Bawendi M.G., Frangioni J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 85.Kumar B., Jalodia K., Kumar P., Gautam H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017;41:260–268. [Google Scholar]
- 86.Perrault S.D., Walkey C., Jennings T., Fischer H.C., Chan W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 87.Choi H.S., Liu W., Misra P., Tanaka E., Zimmer J.P., Ipe B.I., Bawendi M.G., Frangioni J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stylianopoulos T. EPR-effect: utilizing size-dependent nanoparticle delivery to solid tumors. Ther. Deliv. 2013;4:421–423. doi: 10.4155/tde.13.8. [DOI] [PubMed] [Google Scholar]
- 89.Peracchia M., Fattal E., Desmaele D., Besnard M., Noel J., Gomis J., Appel M., d'Angelo J., Couvreur P. Stealth® PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Contr. Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 90.Pluen A., Boucher Y., Ramanujan S., McKee T.D., Gohongi T., di Tomaso E., Brown E.B., Izumi Y., Campbell R.B., Berk D.A. Role of tumor–host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stylianopoulos T., Diop-Frimpong B., Munn L.L., Jain R.K. Diffusion anisotropy in collagen gels and tumors: the effect of fiber network orientation. Biophys. J. 2010;99:3119–3128. doi: 10.1016/j.bpj.2010.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang J., Li L., Howard C.B., Mahler S.M., Huang L., Xu Z.P. Preparation of optimized lipid-coated calcium phosphate nanoparticles for enhanced in vitro gene delivery to breast cancer cells. J. Mater. Chem. B. 2015;3:6805–6812. doi: 10.1039/C5TB00912J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramanujan S., Pluen A., McKee T.D., Brown E.B., Boucher Y., Jain R.K. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diop-Frimpong B., Chauhan V.P., Krane S., Boucher Y., Jain R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang K., Ma H., Liu J., Huo S., Kumar A., Wei T., Zhang X., Jin S., Gan Y., Wang P.C. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M., Terada Y., Kano M., Miyazono K., Uesaka M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 97.Huo S., Ma H., Huang K., Liu J., Wei T., Jin S., Zhang J., He S., Liang X.-J. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013;73:319–330. doi: 10.1158/0008-5472.CAN-12-2071. [DOI] [PubMed] [Google Scholar]
- 98.Tang L., Yang X., Yin Q., Cai K., Wang H., Chaudhury I., Yao C., Zhou Q., Kwon M., Hartman J.A. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:15344–15349. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou J., Patel T.R., Sirianni R.W., Strohbehn G., Zheng M.-Q., Duong N., Schafbauer T., Huttner A.J., Huang Y., Carson R.E. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gentile F., Curcio A., Indolfi C., Ferrari M., Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J. Nanobiotechnol. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S.-Y., Ferrari M., Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009;20:495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 102.Decuzzi P., Lee S., Bhushan B., Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann. Biomed. Eng. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 103.Desai M.P., Labhasetwar V., Walter E., Levy R.J., Amidon G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharmaceut. Res. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 104.Jiang W., Kim B.Y., Rutka J.T., Chan W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 105.Rudolph C., Plank C., Lausier J., Schillinger U., Müller R.H., Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J. Biol. Chem. 2003;278:11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 106.Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., Discher D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2:249. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shah S., Liu Y., Hu W., Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J. Nanosci. Nanotechnol. 2011;11:919–928. doi: 10.1166/jnn.2011.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tao L., Hu W., Liu Y., Huang G., Sumer B.D., Gao J. Shape-specific polymeric nanomedicine: emerging opportunities and challenges. Exp. Biol. Med. 2011;236:20–29. doi: 10.1258/ebm.2010.010243. [DOI] [PubMed] [Google Scholar]
- 109.Geng Y.A.N., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., Discher D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Lian Y., Zhang L.T., Aldousari S.M., Hedia H.S., Asiri S.A., Liu W.K. Cell and nanoparticle transport in tumour microvasculature: the role of size, shape and surface functionality of nanoparticles. Interf. Focus. 2016;6:20150086. doi: 10.1098/rsfs.2015.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou Z., Ma X., Jin E., Tang J., Sui M., Shen Y., Van Kirk E.A., Murdoch W.J., Radosz M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials. 2013;34:5722–5735. doi: 10.1016/j.biomaterials.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 112.Chauhan V.P., Popović Z., Chen O., Cui J., Fukumura D., Bawendi M.G., Jain R.K. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. 2011;123:11619–11622. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pluen A., Netti P.A., Jain R.K., Berk D.A. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys. J. 1999;77:542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartczak D., Muskens O.L., Nitti S., Sanchez-Elsner T., Millar T.M., Kanaras A.G. Interactions of human endothelial cells with gold nanoparticles of different morphologies. Small. 2012;8:122–130. doi: 10.1002/smll.201101422. [DOI] [PubMed] [Google Scholar]
- 115.Chithrani B.D., Ghazani A.A., Chan W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 116.Huang X., Teng X., Chen D., Tang F., He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 117.Li Y., Kröger M., Liu W.K. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7:16631–16646. doi: 10.1039/c5nr02970h. [DOI] [PubMed] [Google Scholar]
- 118.Decuzzi P., Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 119.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., Chan W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:1–12. [Google Scholar]
- 120.Zamboni W.C., Torchilin V., Patri A.K., Hrkach J., Stern S., Lee R., Nel A., Panaro N.J., Grodzinski P. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin. Cancer Res. 2012;18:3229–3241. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zein R., Sharrouf W., Selting K. Physical properties of nanoparticles that result in improved cancer targeting. J. Oncol. 2020:2020. doi: 10.1155/2020/5194780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dellian M., Yuan F., Trubetskoy V., Torchilin V., Jain R. Vascular permeability in a human tumour xenograft: molecular charge dependence. Brit. J. Canc. 2000;82:1513–1518. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campbell R.B., Fukumura D., Brown E.B., Mazzola L.M., Izumi Y., Jain R.K., Torchilin V.P., Munn L.L. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 124.Lieleg O., Baumgärtel R.M., Bausch A.R. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys. J. 2009;97:1569–1577. doi: 10.1016/j.bpj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stylianopoulos T., Poh M.-Z., Insin N., Bawendi M.G., Fukumura D., Munn L.L., Jain R.K. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys. J. 2010;99:1342–1349. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belting M. Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem. Sci. 2003;28:145–151. doi: 10.1016/S0968-0004(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 127.Gratton S.E., Ropp P.A., Pohlhaus P.D., Luft J.C., Madden V.J., Napier M.E., DeSimone J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Donahue N.D., Acar H., Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019;143:68–96. doi: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 129.Chithrani B.D., Chan W.C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 130.Ritz S., Schöttler S., Kotman N., Baier G., Musyanovych A., Kuharev J.r., Landfester K., Schild H.r., Jahn O., Tenzer S. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16:1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 131.Li Y., Gu N. Thermodynamics of charged nanoparticle adsorption on charge-neutral membranes: a simulation study. J. Phys. Chem. B. 2010;114:2749–2754. doi: 10.1021/jp904550b. [DOI] [PubMed] [Google Scholar]
- 132.Harush-Frenkel O., Rozentur E., Benita S., Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–443. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- 133.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Sign. Transduct. Target. Therapy. 2018;3:1–19. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huh K.M., Lee S.C., Cho Y.W., Lee J., Jeong J.H., Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Contr. Release. 2005;101:59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 135.Liu J., Lee H., Huesca M., Young A., Allen C. Liposome formulation of a novel hydrophobic aryl-imidazole compound for anti-cancer therapy. Cancer Chemother. Pharmacol. 2006;58:306. doi: 10.1007/s00280-005-0161-x. [DOI] [PubMed] [Google Scholar]
- 136.Cai X., Liu X., Jiang J., Gao M., Wang W., Zheng H., Xu S., Li R. Molecular mechanisms, characterization methods, and utilities of nanoparticle biotransformation in nanosafety assessments. Small. 2020:1907663. doi: 10.1002/smll.201907663. [DOI] [PubMed] [Google Scholar]
- 137.Yang K., Ma Y.-Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010;5:579–583. doi: 10.1038/nnano.2010.141. [DOI] [PubMed] [Google Scholar]
- 138.Guardia P., Di Corato R., Lartigue L., Wilhelm C., Espinosa A., Garcia-Hernandez M., Gazeau F., Manna L., Pellegrino T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano. 2012;6:3080–3091. doi: 10.1021/nn2048137. [DOI] [PubMed] [Google Scholar]
- 139.Zhang H., Ji Q., Huang C., Zhang S., Yuan B., Yang K., Ma Y.-q. Cooperative transmembrane penetration of nanoparticles. Sci. Rep. 2015;5:10525. doi: 10.1038/srep10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta R., Rai B. Effect of size and surface charge of gold nanoparticles on their skin permeability: a molecular dynamics study. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep45292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Owens D.E., III, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharmaceut. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 142.Papahadjopoulos D., Allen T., Gabizon A., Mayhew E., Matthay K., Huang S., Lee K., Woodle M., Lasic D., Redemann C. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. Unit. States Am. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klibanov A.L., Maruyama K., Torchilin V.P., Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 144.Shimizu T., Ichihara M., Yoshioka Y., Ishida T., Nakagawa S., Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol. Pharm. Bull. 2012;35:1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 145.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Taylor & Francis; 2012. Antibodies against Polyethylene Glycol in Healthy Subjects and in Patients Treated with PEG-Conjugated Agents. [DOI] [PubMed] [Google Scholar]
- 146.García I., Marradi M., Penadés S. Glyconanoparticles: multifunctional nanomaterials for biomedical applications. Nanomedicine. 2010;5:777–792. doi: 10.2217/nnm.10.48. [DOI] [PubMed] [Google Scholar]
- 147.García K.P., Zarschler K., Barbaro L., Barreto J.A., O'Malley W., Spiccia L., Stephan H., Graham B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small. 2014;10:2516–2529. doi: 10.1002/smll.201303540. [DOI] [PubMed] [Google Scholar]
- 148.Liu L., Hitchens T.K., Ye Q., Wu Y., Barbe B., Prior D.E., Li W.F., Yeh F.-C., Foley L.M., Bain D.J. Decreased reticuloendothelial system clearance and increased blood half-life and immune cell labeling for nano-and micron-sized superparamagnetic iron-oxide particles upon pre-treatment with Intralipid. Biochim. Biophys. Acta Gen. Subj. 2013;1830:3447–3453. doi: 10.1016/j.bbagen.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jang D.-J., Moon C., Oh E. Improved tumor targeting and antitumor activity of camptothecin loaded solid lipid nanoparticles by preinjection of blank solid lipid nanoparticles. Biomed. Pharmacother. 2016;80:162–172. doi: 10.1016/j.biopha.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 150.Sun X., Yan X., Jacobson O., Sun W., Wang Z., Tong X., Xia Y., Ling D., Chen X. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 2017;7:319. doi: 10.7150/thno.18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alarcon E.I., Ahumada M. 2019. Nanoengineering Materials for Biomedical Uses. Springer. [Google Scholar]
- 152.Anselmo A.C., Zhang M., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E., Mitragotri S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9:3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 153.Kumar A., Graham M.D. Margination and segregation in confined flows of blood and other multicomponent suspensions. Soft Matter. 2012;8:10536–10548. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 154.Deng H., Song K., Zhang J., Deng L., Dong A., Qin Z. Modulating the rigidity of nanoparticles for tumor penetration. Chem. Commun. 2018;54:3014–3017. doi: 10.1039/c8cc00398j. [DOI] [PubMed] [Google Scholar]
- 155.Li Z., Xiao C., Yong T., Li Z., Gan L., Yang X. Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem. Soc. Rev. 2020;49:2273–2290. doi: 10.1039/c9cs00575g. [DOI] [PubMed] [Google Scholar]
- 156.Yi X., Gao H. Kinetics of receptor-mediated endocytosis of elastic nanoparticles. Nanoscale. 2017;9:454–463. doi: 10.1039/c6nr07179a. [DOI] [PubMed] [Google Scholar]
- 157.Li S.-D., Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Contr. Release: Off. J. Controll. Release Soc. 2010;145:178. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Levchenko T.S., Rammohan R., Lukyanov A.N., Whiteman K.R., Torchilin V.P. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharmaceut. 2002;240:95–102. doi: 10.1016/s0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 159.Zhang Y.R., Lin R., Li H.J., He W.l., Du J.Z., Wang J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2019;11:e1519. doi: 10.1002/wnan.1519. [DOI] [PubMed] [Google Scholar]
- 160.Schudel A., Chapman A.P., Yau M.-K., Higginson C.J., Francis D.M., Manspeaker M.P., Avecilla A.R.C., Rohner N.A., Finn M., Thomas S.N. Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol. 2020;15:491–499. doi: 10.1038/s41565-020-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wong C., Stylianopoulos T., Cui J., Martin J., Chauhan V.P., Jiang W., Popović Z., Jain R.K., Bawendi M.G., Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. Unit. States Am. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kashkooli F.M., Soltani M., Momeni M.M., rahmim A. Enhanced drug delivery to solid tumors via drug-loaded nanocarriers: an image-based computational framework. Front. Oncol. 2021 doi: 10.3389/fonc.2021.655781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Niu Y., Zhu J., Li Y., Shi H., Gong Y., Li R., Huo Q., Ma T., Liu Y. Size shrinkable drug delivery nanosystems and priming the tumor microenvironment for deep intratumoral penetration of nanoparticles. J. Contr. Release. 2018;277:35–47. doi: 10.1016/j.jconrel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 164.Chen J., Ding J., Wang Y., Cheng J., Ji S., Zhuang X., Chen X. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv. Mater. 2017;29:1701170. doi: 10.1002/adma.201701170. [DOI] [PubMed] [Google Scholar]
- 165.Helmlinger G., Yuan F., Dellian M., Jain R.K. Interstitial pH and pO 2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 166.Stylianopoulos T., Wong C., Bawendi M.G., Jain R.K., Fukumura D. Multistage nanoparticles for improved delivery into tumor tissue. Methods Enzymol. 2012;508:109–130. doi: 10.1016/B978-0-12-391860-4.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ding J., Chen J., Gao L., Jiang Z., Zhang Y., Li M., Xiao Q., Lee S.S., Chen X. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800. [Google Scholar]
- 168.Li H.-J., Du J.-Z., Du X.-J., Xu C.-F., Sun C.-Y., Wang H.-X., Cao Z.-T., Yang X.-Z., Zhu Y.-H., Nie S. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stylianopoulos T., Economides E.-A., Baish J.W., Fukumura D., Jain R.K. Towards optimal design of cancer nanomedicines: multi-stage nanoparticles for the treatment of solid tumors. Ann. Biomed. Eng. 2015;43:2291–2300. doi: 10.1007/s10439-015-1276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tong R., Chiang H.H., Kohane D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:19048–19053. doi: 10.1073/pnas.1315336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tong R., Hemmati H.D., Langer R., Kohane D.S. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J. Am. Chem. Soc. 2012;134:8848–8855. doi: 10.1021/ja211888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu W., Shevtsov M., Chen X., Gao H. Advances in aggregatable nanoparticles for tumor-targeted drug delivery. Chin. Chem. Lett. 2020;31:1366–1374. [Google Scholar]
- 173.Ruan S., Hu C., Tang X., Cun X., Xiao W., Shi K., He Q., Gao H. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano. 2016;10:10086–10098. doi: 10.1021/acsnano.6b05070. [DOI] [PubMed] [Google Scholar]
- 174.Xie R., Ruan S., Liu J., Qin L., Yang C., Tong F., Lei T., Shevtsov M., Gao H., Qin Y. Furin-instructed aggregated gold nanoparticles for re-educating tumor associated macrophages and overcoming breast cancer chemoresistance. Biomaterials. 2021;275:120891. doi: 10.1016/j.biomaterials.2021.120891. [DOI] [PubMed] [Google Scholar]
- 175.Ju C., Mo R., Xue J., Zhang L., Zhao Z., Xue L., Ping Q., Zhang C. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew. Chem. 2014;126:6367–6372. doi: 10.1002/anie.201311227. [DOI] [PubMed] [Google Scholar]
- 176.Zhou Q., Shao S., Wang J., Xu C., Xiang J., Piao Y., Zhou Z., Yu Q., Tang J., Liu X. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 177.Jia W., Wang Y., Liu R., Yu X., Gao H. Shape transformable strategies for drug delivery. Adv. Funct. Mater. 2021;31:2009765. [Google Scholar]
- 178.Wang Z., Wang Y., Jia X., Han Q., Qian Y., Li Q., Xiang J., Wang Q., Hu Z., Wang W. MMP-2-controlled transforming micelles for heterogeneic targeting and programmable cancer therapy. Theranostics. 2019;9:1728. doi: 10.7150/thno.30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song C., Lin T., Zhang Q., Thayumanavan S., Ren L. pH-Sensitive morphological transitions in polymeric tadpole assemblies for programmed tumor therapy. J. Contr. Release. 2019;293:1–9. doi: 10.1016/j.jconrel.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qin Y., Tong F., Zhang W., Zhou Y., He S., Xie R., Lei T., Wang Y., Peng S., Li Z. Self-Delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv. Funct. Mater. 2021;31:2104645. [Google Scholar]
- 181.Liu R., Yu M., Yang X., Umeshappa C.S., Hu C., Yu W., Qin L., Huang Y., Gao H. Linear chimeric triblock molecules self-assembled micelles with controllably transformable property to enhance tumor retention for chemo-photodynamic therapy of breast cancer. Adv. Funct. Mater. 2019;29:1808462. [Google Scholar]
- 182.Lv S., Wu Y., Cai K., He H., Li Y., Lan M., Chen X., Cheng J., Yin L. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor–receptor coordination interactions. J. Am. Chem. Soc. 2018;140:1235–1238. doi: 10.1021/jacs.7b12776. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y., Yang G., Baby T., Chen D., Weitz D.A., Zhao C.X. Stable polymer nanoparticles with exceptionally high drug loading by sequential nanoprecipitation. Angew. Chem. 2020;132:4750–4758. [Google Scholar]
- 184.Jeong S.H., Park K. Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix. Int. J. Pharmaceut. 2008;361:26–32. doi: 10.1016/j.ijpharm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 185.Dang K.L., Matin N., Ho H. Google Patents; 2004. Increased Drug-Loading and Reduced Stress Drug Delivery Device. [Google Scholar]
- 186.Liu Y., Yang G., Jin S., Xu L., Zhao C.X. Development of high-drug-loading nanoparticles. ChemPlusChem. 2020;85:2143–2157. doi: 10.1002/cplu.202000496. [DOI] [PubMed] [Google Scholar]
- 187.Wang N., Cheng X., Li N., Wang H., Chen H. Nanocarriers and their loading strategies. Adv. Healthcare Mater. 2019;8:1801002. doi: 10.1002/adhm.201801002. [DOI] [PubMed] [Google Scholar]
- 188.Li Y., Shi J. Hollow-structured mesoporous materials: chemical synthesis, functionalization and applications. Adv. Mater. 2014;26:3176–3205. doi: 10.1002/adma.201305319. [DOI] [PubMed] [Google Scholar]
- 189.Lou X.W., Archer L.A., Yang Z. Hollow micro-/nanostructures: synthesis and applications. Adv. Mater. 2008;20:3987–4019. [Google Scholar]
- 190.Slowing I.I., Vivero-Escoto J.L., Wu C.-W., Lin V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 191.Han F.Y., Liu Y., Kumar V., Xu W., Yang G., Zhao C.-X., Woodruff T.M., Whittaker A.K., Smith M.T. Sustained-release ketamine-loaded nanoparticles fabricated by sequential nanoprecipitation. Int. J. Pharmaceut. 2020;581:119291. doi: 10.1016/j.ijpharm.2020.119291. [DOI] [PubMed] [Google Scholar]
- 192.Yang G., Liu Y., Wang H., Wilson R., Hui Y., Yu L., Wibowo D., Zhang C., Whittaker A.K., Middelberg A.P. Bioinspired core–shell nanoparticles for hydrophobic drug delivery. Angew. Chem. Int. Ed. 2019;58:14357–14364. doi: 10.1002/anie.201908357. [DOI] [PubMed] [Google Scholar]
- 193.Kheradmandnia S., Vasheghani-Farahani E., Nosrati M., Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol. Biol. Med. 2010;6:753–759. doi: 10.1016/j.nano.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 194.Zhang J., Li S., An F.-F., Liu J., Jin S., Zhang J.-C., Wang P.C., Zhang X., Lee C.-S., Liang X.-J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale. 2015;7:13503–13510. doi: 10.1039/c5nr03259h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Giardiello M., Liptrott N.J., McDonald T.O., Moss D., Siccardi M., Martin P., Smith D., Gurjar R., Rannard S.P., Owen A. Accelerated oral nanomedicine discovery from miniaturized screening to clinical production exemplified by paediatric HIV nanotherapies. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bteich J., McManus S.A., Ernsting M.J., Mohammed M.Z., Prud’homme R.K., Sokoll K.K. Using flash nanoprecipitation to produce highly potent and stable cellax nanoparticles from amphiphilic polymers derived from carboxymethyl cellulose, polyethylene glycol, and cabazitaxel. Mol. Pharm. 2017;14:3998–4007. doi: 10.1021/acs.molpharmaceut.7b00670. [DOI] [PubMed] [Google Scholar]
- 197.Zhang H., Wang J., Mao W., Huang J., Wu X., Shen Y., Sui M. Novel SN38 conjugate-forming nanoparticles as anticancer prodrug: in vitro and in vivo studies. J. Contr. Release. 2013;166:147–158. doi: 10.1016/j.jconrel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 198.Della Rocca J., Huxford R.C., Comstock-Duggan E., Lin W. Polysilsesquioxane nanoparticles for targeted platin-based cancer chemotherapy by triggered release. Angew. Chem. Int. Ed. 2011;50:10330–10334. doi: 10.1002/anie.201104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang P., Wang D., Su Y., Huang W., Zhou Y., Cui D., Zhu X., Yan D. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug–drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 200.Zhang R., Xing R., Jiao T., Ma K., Chen C., Ma G., Yan X. Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Appl. Mater. Interfaces. 2016;8:13262–13269. doi: 10.1021/acsami.6b02416. [DOI] [PubMed] [Google Scholar]
- 201.Liédana N., Galve A., Rubio C.s., Téllez C., Coronas J. CAF@ ZIF-8: one-step encapsulation of caffeine in MOF. ACS Appl. Mater. Interfaces. 2012;4:5016–5021. doi: 10.1021/am301365h. [DOI] [PubMed] [Google Scholar]
- 202.Miller S.R., Heurtaux D., Baati T., Horcajada P., Grenèche J.-M., Serre C. Biodegradable therapeutic MOFs for the delivery of bioactive molecules. Chem. Commun. 2010;46:4526–4528. doi: 10.1039/c001181a. [DOI] [PubMed] [Google Scholar]
- 203.Zhao D., Zhao X., Zu Y., Li J., Zhang Y., Jiang R., Zhang Z. Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int. J. Nanomed. 2010;5:669. doi: 10.2147/ijn.s12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huxford R.C., Dekrafft K.E., Boyle W.S., Liu D., Lin W. Lipid-coated nanoscale coordination polymers for targeted delivery of antifolates to cancer cells. Chem. Sci. 2012;3:198–204. doi: 10.1039/C1SC00499A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhu Z., Li Y., Li X., Li R., Jia Z., Liu B., Guo W., Wu W., Jiang X. Paclitaxel-loaded poly (N-vinylpyrrolidone)-b-poly (ε-caprolactone) nanoparticles: preparation and antitumor activity in vivo. J. Contr. Release. 2010;142:438–446. doi: 10.1016/j.jconrel.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 206.Chen K., Fu Z., Wang M., Lv Y., Wang C., Shen Y., Wang Y., Cui H., Guo X. Preparation and characterization of size-controlled nanoparticles for high-loading λ-cyhalothrin delivery through flash nanoprecipitation. J. Agric. Food Chem. 2018;66:8246–8252. doi: 10.1021/acs.jafc.8b02851. [DOI] [PubMed] [Google Scholar]
- 207.Shen Y., Jin E., Zhang B., Murphy C.J., Sui M., Zhao J., Wang J., Tang J., Fan M., Van Kirk E. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J. Am. Chem. Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 208.Song Q., Wang X., Wang Y., Liang Y., Zhou Y., Song X., He B., Zhang H., Dai W., Wang X. Reduction responsive self-assembled nanoparticles based on disulfide-linked drug–drug conjugate with high drug loading and antitumor efficacy. Mol. Pharm. 2016;13:190–201. doi: 10.1021/acs.molpharmaceut.5b00631. [DOI] [PubMed] [Google Scholar]
- 209.Cheow W.S., Hadinoto K. Self-assembled amorphous drug–polyelectrolyte nanoparticle complex with enhanced dissolution rate and saturation solubility. J. Colloid Interface Sci. 2012;367:518–526. doi: 10.1016/j.jcis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 210.Gu J., Su S., Li Y., He Q., Shi J. Hydrophilic mesoporous carbon nanoparticles as carriers for sustained release of hydrophobic anti-cancer drugs. Chem. Commun. 2011;47:2101–2103. doi: 10.1039/c0cc04598e. [DOI] [PubMed] [Google Scholar]
- 211.Li X., Liu C., Wang S., Jiao J., Di D., Jiang T., Zhao Q., Wang S. Poly (acrylic acid) conjugated hollow mesoporous carbon as a dual-stimuli triggered drug delivery system for chemo-photothermal synergistic therapy. Mater. Sci. Eng. C. 2017;71:594–603. doi: 10.1016/j.msec.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 212.Palanikumar L., Kim H.Y., Oh J.Y., Thomas A.P., Choi E.S., Jeena M., Joo S.H., Ryu J.-H. Noncovalent surface locking of mesoporous silica nanoparticles for exceptionally high hydrophobic drug loading and enhanced colloidal stability. Biomacromolecules. 2015;16:2701–2714. doi: 10.1021/acs.biomac.5b00589. [DOI] [PubMed] [Google Scholar]
- 213.Chen Y., Chen H., Zeng D., Tian Y., Chen F., Feng J., Shi J. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano. 2010;4:6001–6013. doi: 10.1021/nn1015117. [DOI] [PubMed] [Google Scholar]
- 214.Luo B., Xu S., Luo A., Wang W.-R., Wang S.-L., Guo J., Lin Y., Zhao D.-Y., Wang C.-C. Mesoporous biocompatible and acid-degradable magnetic colloidal nanocrystal clusters with sustainable stability and high hydrophobic drug loading capacity. ACS Nano. 2011;5:1428–1435. doi: 10.1021/nn103213y. [DOI] [PubMed] [Google Scholar]
- 215.Sun C.Y., Qin C., Wang C.G., Su Z.M., Wang S., Wang X.L., Yang G.S., Shao K.Z., Lan Y.Q., Wang E.B. Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Adv. Mater. 2011;23:5629–5632. doi: 10.1002/adma.201102538. [DOI] [PubMed] [Google Scholar]
- 216.Pan Y.-J., Chen Y.-Y., Wang D.-R., Wei C., Guo J., Lu D.-R., Chu C.-C., Wang C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials. 2012;33:6570–6579. doi: 10.1016/j.biomaterials.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 217.Wu M., Wang Q., Liu X., Liu J. Highly efficient loading of doxorubicin in Prussian Blue nanocages for combined photothermal/chemotherapy against hepatocellular carcinoma. RSC Adv. 2015;5:30970–30980. [Google Scholar]
- 218.Baek S., Singh R.K., Khanal D., Patel K.D., Lee E.-J., Leong K.W., Chrzanowski W., Kim H.-W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale. 2015;7:14191–14216. doi: 10.1039/c5nr02730f. [DOI] [PubMed] [Google Scholar]
- 219.Wong R.S.H., Dodou K. Effect of drug loading method and drug physicochemical properties on the material and drug release properties of poly (ethylene oxide) hydrogels for transdermal delivery. Polymers. 2017;9:286. doi: 10.3390/polym9070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del Pilar Rodriguez-Torres M., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16:1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lv S., Li M., Tang Z., Song W., Sun H., Liu H., Chen X. Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy. Acta Biomater. 2013;9:9330–9342. doi: 10.1016/j.actbio.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 222.Ren D., Dalmau M., Randall A., Shindel M.M., Baldi P., Wang S.W. Biomimetic design of protein nanomaterials for hydrophobic molecular transport. Adv. Funct. Mater. 2012;22:3170–3180. doi: 10.1002/adfm.201200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhuang W.-R., Wang Y., Cui P.-F., Xing L., Lee J., Kim D., Jiang H.-L., Oh Y.-K. Applications of π-π stacking interactions in the design of drug-delivery systems. J. Contr. Release. 2019;294:311–326. doi: 10.1016/j.jconrel.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 224.Wen J., Yang K., Liu F., Li H., Xu Y., Sun S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017;46:6024–6045. doi: 10.1039/c7cs00219j. [DOI] [PubMed] [Google Scholar]
- 225.Qu J.-B., Shao H.-H., Jing G.-L., Huang F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: preparation, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces. 2013;102:37–44. doi: 10.1016/j.colsurfb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 226.Wang B., Meng W., Bi M., Ni Y., Cai Q., Wang J. Uniform magnesium silicate hollow spheres as high drug-loading nanocarriers for cancer therapy with low systemic toxicity. Dalton Trans. 2013;42:8918–8925. doi: 10.1039/c3dt50659b. [DOI] [PubMed] [Google Scholar]
- 227.Kwon G.S., Okano T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996;21:107–116. [Google Scholar]
- 228.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012;64:223–236. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 229.Zhu Y., Yang B., Chen S., Du J. Polymer vesicles: mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017;64:1–22. [Google Scholar]
- 230.Wang Y., Cheetham A.G., Angacian G., Su H., Xie L., Cui H. Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017;110:112–126. doi: 10.1016/j.addr.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hu Q.-D., Tang G.-P., Chu P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: from design to applications. Acc. Chem. Res. 2014;47:2017–2025. doi: 10.1021/ar500055s. [DOI] [PubMed] [Google Scholar]
- 232.Seidi F., Jenjob R., Crespy D. Designing smart polymer conjugates for controlled release of payloads. Chem. Rev. 2018;118:3965–4036. doi: 10.1021/acs.chemrev.8b00006. [DOI] [PubMed] [Google Scholar]
- 233.Tagmatarchis N., Prato M. Functionalization of carbon nanotubes via 1, 3-dipolar cycloadditions. J. Mater. Chem. 2004;14:437–439. [Google Scholar]
- 234.Zhu J., Liao L., Bian X., Kong J., Yang P., Liu B. pH-Controlled delivery of doxorubicin to cancer cells, based on small mesoporous carbon nanospheres. Small. 2012;8:2715–2720. doi: 10.1002/smll.201200217. [DOI] [PubMed] [Google Scholar]
- 235.Yao X., Niu X., Ma K., Huang P., Grothe J., Kaskel S., Zhu Y. Graphene quantum dots-capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small. 2017;13:1602225. doi: 10.1002/smll.201602225. [DOI] [PubMed] [Google Scholar]
- 236.Buwalda S.J., Vermonden T., Hennink W.E. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules. 2017;18:316–330. doi: 10.1021/acs.biomac.6b01604. [DOI] [PubMed] [Google Scholar]
- 237.Li Y., Maciel D., Rodrigues J., Shi X., Tomas H. Biodegradable polymer nanogels for drug/nucleic acid delivery. Chem. Rev. 2015;115:8564–8608. doi: 10.1021/cr500131f. [DOI] [PubMed] [Google Scholar]
- 238.Ulbrich K., Hola K., Subr V., Bakandritsos A., Tucek J., Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016;116:5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 239.Wang Y., He J., Liu C., Chong W.H., Chen H. Thermodynamics versus kinetics in nanosynthesis. Angew. Chem. Int. Ed. 2015;54:2022–2051. doi: 10.1002/anie.201402986. [DOI] [PubMed] [Google Scholar]
- 240.Liu D., Zhang H., Cito S., Fan J., Mäkilä E., Salonen J., Hirvonen J., Sikanen T.M., Weitz D.A., Santos H.l.A. Core/shell nanocomposites produced by superfast sequential microfluidic nanoprecipitation. Nano Lett. 2017;17:606–614. doi: 10.1021/acs.nanolett.6b03251. [DOI] [PubMed] [Google Scholar]
- 241.Cai K., He X., Song Z., Yin Q., Zhang Y., Uckun F.M., Jiang C., Cheng J. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J. Am. Chem. Soc. 2015;137:3458–3461. doi: 10.1021/ja513034e. [DOI] [PubMed] [Google Scholar]
- 242.Chow S.F., Wan K.Y., Cheng K.K., Wong K.W., Sun C.C., Baum L., Chow A.H.L. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur. J. Pharm. Biopharm. 2015;94:436–449. doi: 10.1016/j.ejpb.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 243.Wu J., Zhu Y.-J., Chen F., Zhao X.-Y., Zhao J., Qi C. Amorphous calcium silicate hydrate/block copolymer hybrid nanoparticles: synthesis and application as drug carriers. Dalton Trans. 2013;42:7032–7040. doi: 10.1039/c3dt50143d. [DOI] [PubMed] [Google Scholar]
- 244.Zhao P., Wang L., Sun C., Jiang T., Zhang J., Zhang Q., Sun J., Deng Y., Wang S. Uniform mesoporous carbon as a carrier for poorly water soluble drug and its cytotoxicity study. Eur. J. Pharm. Biopharm. 2012;80:535–543. doi: 10.1016/j.ejpb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 245.Ren H., Zhang L., An J., Wang T., Li L., Si X., He L., Wu X., Wang C., Su Z. Polyacrylic acid@ zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014;50:1000–1002. doi: 10.1039/c3cc47666a. [DOI] [PubMed] [Google Scholar]
- 246.Chen Y., Chen H., Guo L., He Q., Chen F., Zhou J., Feng J., Shi J. Hollow/rattle-type mesoporous nanostructures by a structural difference-based selective etching strategy. ACS Nano. 2010;4:529–539. doi: 10.1021/nn901398j. [DOI] [PubMed] [Google Scholar]
- 247.Yang S., Chen D., Li N., Mei X., Qi X., Li H., Xu Q., Lu J. A facile preparation of targetable pH-sensitive polymeric nanocarriers with encapsulated magnetic nanoparticles for controlled drug release. J. Mater. Chem. 2012;22:25354–25361. [Google Scholar]
- 248.Gong H., Xie Z., Liu M., Zhu H., Sun H. Redox-sensitive mesoporous silica nanoparticles functionalized with PEG through a disulfide bond linker for potential anticancer drug delivery. RSC Adv. 2015;5:59576–59582. [Google Scholar]
- 249.Croissant J.G., Zhang D., Alsaiari S., Lu J., Deng L., Tamanoi F., AlMalik A.M., Zink J.I., Khashab N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Contr. Release. 2016;229:183–191. doi: 10.1016/j.jconrel.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 250.Zhang L., Chen Q., Ma Y., Sun J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 2019;3:107–120. doi: 10.1021/acsabm.9b00853. [DOI] [PubMed] [Google Scholar]
- 251.Damiati S., Kompella U.B., Damiati S.A., Kodzius R. Microfluidic devices for drug delivery systems and drug screening. Genes. 2018;9:103. doi: 10.3390/genes9020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sohrabi S., Moraveji M.K. Droplet microfluidics: fundamentals and its advanced applications. RSC Adv. 2020;10:27560–27574. doi: 10.1039/d0ra04566g. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 253.Hood R., Vreeland W., DeVoe D.L. Microfluidic remote loading for rapid single-step liposomal drug preparation. Lab Chip. 2014;14:3359–3367. doi: 10.1039/c4lc00390j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang L., Feng Q., Wang J., Sun J., Shi X., Jiang X. Microfluidic synthesis of rigid nanovesicles for hydrophilic reagents delivery. Angew. Chem. 2015;127:4024–4028. doi: 10.1002/anie.201500096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bokare A., Takami A., Kim J.H., Dong A., Chen A., Valerio R., Gunn S., Erogbogbo F. Herringbone-patterned 3D-printed devices as alternatives to microfluidics for reproducible production of lipid polymer hybrid nanoparticles. ACS Omega. 2019;4:4650–4657. doi: 10.1021/acsomega.9b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sun J., Zhang L., Wang J., Feng Q., Liu D., Yin Q., Xu D., Wei Y., Ding B., Shi X. Tunable rigidity of (polymeric core)–(lipid shell) nanoparticles for regulated cellular uptake. Advanced materials. 2015;27:1402–1407. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 257.Kim Y., Fay F., Cormode D.P., Sanchez-Gaytan B.L., Tang J., Hennessy E.J., Ma M., Moore K., Farokhzad O.C., Fisher E.A. Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics. ACS Nano. 2013;7:9975–9983. doi: 10.1021/nn4039063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Koh C.G., Zhang X., Liu S., Golan S., Yu B., Yang X., Guan J., Jin Y., Talmon Y., Muthusamy N. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J. Contr. Release. 2010;141:62–69. doi: 10.1016/j.jconrel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bose R.J., Paulmurugan R., Moon J., Lee S.-H., Park H. Cell membrane-coated nanocarriers: the emerging targeted delivery system for cancer theranostics. Drug Discov. Today. 2018;23:891–899. doi: 10.1016/j.drudis.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 260.Majedi F.S., Hasani-Sadrabadi M.M., VanDersarl J.J., Mokarram N., Hojjati-Emami S., Dashtimoghadam E., Bonakdar S., Shokrgozar M.A., Bertsch A., Renaud P. On-chip fabrication of paclitaxel-loaded chitosan nanoparticles for cancer therapeutics. Adv. Funct. Mater. 2014;24:432–441. [Google Scholar]
- 261.Valencia P.M., Pridgen E.M., Rhee M., Langer R., Farokhzad O.C., Karnik R. Microfluidic platform for combinatorial synthesis and optimization of targeted nanoparticles for cancer therapy. ACS Nano. 2013;7:10671–10680. doi: 10.1021/nn403370e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kolishetti N., Dhar S., Valencia P.M., Lin L.Q., Karnik R., Lippard S.J., Langer R., Farokhzad O.C. Engineering of self-assembled nanoparticle platform for precisely controlled combination drug therapy. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:17939–17944. doi: 10.1073/pnas.1011368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hasani-Sadrabadi M.M., Karimkhani V., Majedi F.S., Van Dersarl J.J., Dashtimoghadam E., Afshar-Taromi F., Mirzadeh H., Bertsch A., Jacob K.I., Renaud P. Microfluidic-assisted self-assembly of complex dendritic polyethylene drug delivery nanocapsules. Adv. Mater. 2014;26:3118–3123. doi: 10.1002/adma.201305753. [DOI] [PubMed] [Google Scholar]
- 264.Herranz-Blanco B., Liu D., Mäkilä E., Shahbazi M.A., Ginestar E., Zhang H., Aseyev V., Balasubramanian V., Salonen J., Hirvonen J. On-chip self-assembly of a smart hybrid nanocomposite for antitumoral applications. Adv. Funct. Mater. 2015;25:1488–1497. [Google Scholar]
- 265.Feng Q., Zhang L., Liu C., Li X., Hu G., Sun J., Jiang X. Microfluidic based high throughput synthesis of lipid-polymer hybrid nanoparticles with tunable diameters. Biomicrofluidics. 2015;9 doi: 10.1063/1.4922957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sun J., Xianyu Y., Li M., Liu W., Zhang L., Liu D., Liu C., Hu G., Jiang X. A microfluidic origami chip for synthesis of functionalized polymeric nanoparticles. Nanoscale. 2013;5:5262–5265. doi: 10.1039/c3nr01289a. [DOI] [PubMed] [Google Scholar]
- 267.Mieszawska A.J., Kim Y., Gianella A., van Rooy I., Priem B., Labarre M.P., Ozcan C., Cormode D.P., Petrov A., Langer R. Synthesis of polymer–lipid nanoparticles for image-guided delivery of dual modality therapy. Bioconjugate Chem. 2013;24:1429–1434. doi: 10.1021/bc400166j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hasani-Sadrabadi M.M., Dashtimoghadam E., Bahlakeh G., Majedi F.S., Keshvari H., Van Dersarl J.J., Bertsch A., Panahifar A., Renaud P., Tayebi L. On-chip synthesis of fine-tuned bone-seeking hybrid nanoparticles. Nanomedicine. 2015;10:3431–3449. doi: 10.2217/nnm.15.162. [DOI] [PubMed] [Google Scholar]
- 269.Bleul R., Thiermann R., Marten G.U., House M.J., Pierre T.G.S., Häfeli U.O., Maskos M. Continuously manufactured magnetic polymersomes–a versatile tool (not only) for targeted cancer therapy. Nanoscale. 2013;5:11385–11393. doi: 10.1039/c3nr02190d. [DOI] [PubMed] [Google Scholar]
- 270.Cao Y., Silverman L., Lu C., Hof R., Wulff J.E., Moffitt M.G. Microfluidic manufacturing of SN-38-loaded polymer nanoparticles with shear processing control of drug delivery properties. Mol. Pharm. 2018;16:96–107. doi: 10.1021/acs.molpharmaceut.8b00874. [DOI] [PubMed] [Google Scholar]
- 271.Chen R., Wulff J.E., Moffitt M.G. Microfluidic processing approach to controlling drug delivery properties of curcumin-loaded block copolymer nanoparticles. Mol. Pharm. 2018;15:4517–4528. doi: 10.1021/acs.molpharmaceut.8b00529. [DOI] [PubMed] [Google Scholar]
- 272.Rao L., Cai B., Bu L.-L., Liao Q.-Q., Guo S.-S., Zhao X.-Z., Dong W.-F., Liu W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–3505. doi: 10.1021/acsnano.7b00133. [DOI] [PubMed] [Google Scholar]
- 273.Liu D., Bernuz C.R., Fan J., Li W., Correia A., Hirvonen J., Santos H.A. A nano-in-nano vector: merging the best of polymeric nanoparticles and drug nanocrystals. Adv. Funct. Mater. 2017;27:1604508. [Google Scholar]
- 274.Lim J.-M., Bertrand N., Valencia P.M., Rhee M., Langer R., Jon S., Farokhzad O.C., Karnik R. Parallel microfluidic synthesis of size-tunable polymeric nanoparticles using 3D flow focusing towards in vivo study. Nanomed. Nanotechnol. Biol. Med. 2014;10:401–409. doi: 10.1016/j.nano.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Min K.-I., Lee H.-J., Kim D.-P. Three-dimensional flash flow microreactor for scale-up production of monodisperse PEG–PLGA nanoparticles. Lab Chip. 2014;14:3987–3992. doi: 10.1039/c4lc00700j. [DOI] [PubMed] [Google Scholar]
- 276.Romanowsky M.B., Abate A.R., Rotem A., Holtze C., Weitz D.A. High throughput production of single core double emulsions in a parallelized microfluidic device. Lab Chip. 2012;12:802–807. doi: 10.1039/c2lc21033a. [DOI] [PubMed] [Google Scholar]
- 277.Kang X., Luo C., Wei Q., Xiong C., Chen Q., Chen Y., Ouyang Q. Mass production of highly monodisperse polymeric nanoparticles by parallel flow focusing system. Microfluid. Nanofluidics. 2013;15:337–345. [Google Scholar]
- 278.Gdowski A., Johnson K., Shah S., Gryczynski I., Vishwanatha J., Ranjan A. Optimization and scale up of microfluidic nanolipomer production method for preclinical and potential clinical trials. J. Nanobiotechnol. 2018;16:1–10. doi: 10.1186/s12951-018-0339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kim Y., Lee Chung B., Ma M., Mulder W.J., Fayad Z.A., Farokhzad O.C., Langer R. Mass production and size control of lipid–polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012;12:3587–3591. doi: 10.1021/nl301253v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Eggersdorfer M., Zheng W., Nawar S., Mercandetti C., Ofner A., Leibacher I., Koehler S., Weitz D. Tandem emulsification for high-throughput production of double emulsions. Lab Chip. 2017;17:936–942. doi: 10.1039/c6lc01553k. [DOI] [PubMed] [Google Scholar]
- 281.Lim J.-M., Swami A., Gilson L.M., Chopra S., Choi S., Wu J., Langer R., Karnik R., Farokhzad O.C. Ultra-high throughput synthesis of nanoparticles with homogeneous size distribution using a coaxial turbulent jet mixer. ACS Nano. 2014;8:6056–6065. doi: 10.1021/nn501371n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kitazoe K., Wang J., Kaji N., Okamoto Y., Tokeshi M., Kogure K., Harashima H., Baba Y. A touch-and-go lipid wrapping technique in microfluidic channels for rapid fabrication of multifunctional envelope-type gene delivery nanodevices. Lab Chip. 2011;11:3256–3262. doi: 10.1039/c1lc20392d. [DOI] [PubMed] [Google Scholar]
- 283.Liu D., Cito S., Zhang Y., Wang C.F., Sikanen T.M., Santos H.A. A versatile and robust microfluidic platform toward high throughput synthesis of homogeneous nanoparticles with tunable properties. Adv. Mater. 2015;27:2298–2304. doi: 10.1002/adma.201405408. [DOI] [PubMed] [Google Scholar]
- 284.Siegel R.A., Rathbone M.J. In: Advances in Delivery Science and Technology. Siepmann J., et al., editors. Controlled Release Society; 2012. Fundamentals and applications of controlled release drug delivery. [Google Scholar]
- 285.Bajpai A.K., Shukla S.K., Bhanu S., Kankane S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008;33:1088–1118. [Google Scholar]
- 286.Lee J.H., Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015;125:75–84. doi: 10.1016/j.ces.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Williams M. In: Crank J., editor. vol. 50. Clarendon Press; Oxford: 1975. p. 414. (The Mathematics of Diffusion). £ 13. Pergamon, 1977. [Google Scholar]
- 288.Pundir S., Badola A., Sharma D. Sustained release matrix technology and recent advance in matrix drug delivery system: a review. Int. J. Drug Res. Tech. 2013;3:12–20. [Google Scholar]
- 289.Siepmann J., Siegel R.A., Rathbone M.J. 2012. Fundamentals and Applications of Controlled Release Drug Delivery. Springer. [Google Scholar]
- 290.Langer R., Peppas N. Chemical and physical structure of polymers as carriers for controlled release of bioactive agents: a review. J. Macromol. Sci.-Rev. Macromol. Chem. Phys. 1983;23:61–126. [Google Scholar]
- 291.Peppas N., Bures P., Leobandung W., Ichikawa H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 292.Ma Y., Zheng Y., Zeng X., Jiang L., Chen H., Liu R., Huang L., Mei L. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int. J. Nanomed. 2011;6:2679. doi: 10.2147/IJN.S25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.C.D.I. Poliméricos, B.C.S.E.A. de, Michele Soares Tacchi Campos.
- 294.Zuleger S., Lippold B.C. Polymer particle erosion controlling drug release. I. Factors influencing drug release and characterization of the release mechanism. Int. J. Pharm. 2001;217:139–152. doi: 10.1016/s0378-5173(01)00596-8. [DOI] [PubMed] [Google Scholar]
- 295.Middleton J.C., Tipton A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 296.von Burkersroda F., Schedl L., Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–4231. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 297.Uhrich K.E., Cannizzaro S.M., Langer R.S., Shakesheff K.M. Polymeric systems for controlled drug release. Chem. Rev. Columbus. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 298.Fredenberg S., Wahlgren M., Reslow M., Axelsson A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review. Int. J. Pharmaceut. 2011;415:34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 299.Versypt A.N.F., Pack D.W., Braatz R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—a review. J. Contr. Release. 2013;165:29–37. doi: 10.1016/j.jconrel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Kashkooli F.M., Soltani M., Hamedi M.-H. European Journal of Pharmaceutical Sciences; 2020. Drug Delivery to Solid Tumors with Heterogeneous Microvascular Networks: Novel Insights from Image-Based Numerical Modeling; p. 105399. [DOI] [PubMed] [Google Scholar]
- 301.Lin C.-C., Metters A.T. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 302.Korsmeyer R., Gurny R., Doelker E., Buri P., Peppas N. Mechanisms of potassium chloride release from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J. Pharmaceut. Sci. 1983;72:1189–1191. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 303.Brazel C.S., Peppas N.A. Dimensionless analysis of swelling of hydrophilic glassy polymers with subsequent drug release from relaxing structures. Biomaterials. 1999;20:721–732. doi: 10.1016/s0142-9612(98)00215-4. [DOI] [PubMed] [Google Scholar]
- 304.Yoshida R., Sakai K., Okano T., Sakurai Y. Pulsatile drug delivery systems using hydrogels. Adv. Drug Deliv. Rev. 1993;11:85–108. [Google Scholar]
- 305.Peppas N.A., Sahlin J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharmaceut. 1989;57:169–172. [Google Scholar]
- 306.Tzur-Balter A., Young J.M., Bonanno-Young L.M., Segal E. Mathematical modeling of drug release from nanostructured porous Si: combining carrier erosion and hindered drug diffusion for predicting release kinetics. Acta Biomater. 2013;9:8346–8353. doi: 10.1016/j.actbio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 307.Kashkooli F.M., Soltani M., Souri M., Meaney C., Kohandel M. Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today. 2021;36:101057. [Google Scholar]
- 308.Liu J., Chen Q., Feng L., Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55–73. [Google Scholar]
- 309.Cheng R., Meng F., Deng C., Klok H.-A., Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 310.Zhang M., Dai Z., Theivendran S., Gu Z., Zhao L., Song H., Yang Y., Yu C. Nanotechnology enabled reactive species regulation in biosystems for boosting cancer immunotherapy. Nano Today. 2021;36:101035. [Google Scholar]
- 311.Fu Q., Li Z., Fu F., Chen X., Song J., Yang H. Stimuli-responsive plasmonic assemblies and their biomedical applications. Nano Today. 2021;36:101014. doi: 10.1016/j.nantod.2020.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wei L., Chen J., Ding J. Sequentially stimuli-responsive anticancer nanomedicines. Future Med. 2021 doi: 10.2217/nnm-2021-0019. [DOI] [PubMed] [Google Scholar]
- 313.Soltani M., Souri M., Kashkooli F.M. Scientific Reports; 2021. Effects of Hypoxia and Nanocarrier Size on pH-Responsive Nano-Delivery System to Solid Tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pouysségur J., Dayan F., Mazure N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 315.Gatenby R.A., Gillies R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 316.Crowther M., Brown N., Bishop E., Lewis C. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J. Leukoc. Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 317.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Leonard R., Williams S., Tulpule A., Levine A., Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet™) Breast. 2009;18:218–224. doi: 10.1016/j.breast.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 319.Block M.L., Zecca L., Hong J.-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 320.Saito G., Swanson J.A., Lee K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 321.Hoare T., Santamaria J., Goya G.F., Irusta S., Lin D., Lau S., Padera R., Langer R., Kohane D.S. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009;9:3651–3657. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lu J., Choi E., Tamanoi F., Zink J.I. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4:421. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rezaeian M., Sedaghatkish A., Soltani M. Numerical modeling of high-intensity focused ultrasound-mediated intraperitoneal delivery of thermosensitive liposomal doxorubicin for cancer chemotherapy. Drug Deliv. 2019;26:898–917. doi: 10.1080/10717544.2019.1660435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sedaghatkish A., Rezaeian M., Heydari H., Ranjbar A.M., Soltani M. Acoustic streaming and thermosensitive liposomes for drug delivery into hepatocellular carcinoma tumor adjacent to major hepatic veins; an acoustics–thermal–fluid-mass transport coupling model. Int. J. Therm. Sci. 2020;158:106540. [Google Scholar]
- 325.Davoodi P., Lee L.Y., Xu Q., Sunil V., Sun Y., Soh S., Wang C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018;132:104–138. doi: 10.1016/j.addr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 326.Soltani M., Tehrani M.H., Kashkooli F.M., Rezaeian M. Effects of magnetic nanoparticle diffusion on microwave ablation treatment: a numerical approach. J. Magn. Magn Mater. 2020;514:167196. [Google Scholar]
- 327.Dixit C.K., Kadimisetty K., Otieno B.A., Tang C., Malla S., Krause C.E., Rusling J.F. Electrochemistry-based approaches to low cost, high sensitivity, automated, multiplexed protein immunoassays for cancer diagnostics. Analyst. 2016;141:536–547. doi: 10.1039/c5an01829c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Servant A., Bussy C., Al-Jamal K., Kostarelos K. Design, engineering and structural integrity of electro-responsive carbon nanotube-based hydrogels for pulsatile drug release. J. Mater. Chem. B. 2013;1:4593–4600. doi: 10.1039/c3tb20614a. [DOI] [PubMed] [Google Scholar]
- 329.Jeon G., Yang S.Y., Byun J., Kim J.K. Electrically actuatable smart nanoporous membrane for pulsatile drug release. Nano Lett. 2011;11:1284–1288. doi: 10.1021/nl104329y. [DOI] [PubMed] [Google Scholar]
- 330.Abdelwahed W., Degobert G., Stainmesse S., Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 331.Kipp J. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharmaceut. 2004;284:109–122. doi: 10.1016/j.ijpharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 332.Rabinow B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 333.Patravale V., Date A.A., Kulkarni R. Nanosuspensions: a promising drug delivery strategy. J. Pharm. Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 334.Radtke M. 2001. Pure Drug Nanoparticles for the Formulation of Poorly Soluble Drugs; p. 3. New drugs. [Google Scholar]
- 335.Wu L., Zhang J., Watanabe W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011;63:456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 336.C.-j. Kim, Advanced Pharmaceutics: Physicochemical Principles, CRC Press2004.
- 337.M.T. Nutan, I.K. Reddy, General Principles of Suspensions, Pharmaceutical suspensions, Springer2010, pp. 39-65.
- 338.Ali Y., Lehmussaari K. Industrial perspective in ocular drug delivery. Adv. Drug Deliv. Rev. 2006;58:1258–1268. doi: 10.1016/j.addr.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 339.Gao L., Zhang D., Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res. 2008;10:845–862. [Google Scholar]
- 340.Van Eerdenbrugh B., Van den Mooter G., Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharmaceut. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 341.M.J. Rosen, J.T. Kunjappu, Surfactants and Interfacial Phenomena, John Wiley & Sons2012.
- 342.Edwards D.A., Hanes J., Caponetti G., Hrkach J., Ben-Jebria A., Eskew M.L., Mintzes J., Deaver D., Lotan N., Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276:1868–1872. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 343.Ramezanpour M., Leung S., Delgado-Magnero K., Bashe B., Thewalt J., Tieleman D. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim. Biophys. Acta Biomembr. 2016;1858:1688–1709. doi: 10.1016/j.bbamem.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 344.Mishra P.R., Al Shaal L., Müller R.H., Keck C.M. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int. J. Pharmaceut. 2009;371:182–189. doi: 10.1016/j.ijpharm.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 345.Mu Q., Yu J., McConnachie L.A., Kraft J.C., Gao Y., Gulati G.K., Ho R.J. Translation of combination nanodrugs into nanomedicines: lessons learned and future outlook. J. Drug Target. 2018;26:435–447. doi: 10.1080/1061186X.2017.1419363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.He H., Liu L., Morin E.E., Liu M., Schwendeman A. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Acc. Chem. Res. 2019;52:2445–2461. doi: 10.1021/acs.accounts.9b00228. [DOI] [PubMed] [Google Scholar]
- 347.Fox J. NATURE PUBLISHING CO 345 PARK AVE SOUTH; NEW YORK, NY: 1995. FDA Advisers Okay NeXstars DaunoXome; pp. 10010–11707. [Google Scholar]
- 348.Wibroe P.P., Ahmadvand D., Oghabian M.A., Yaghmur A., Moghimi S.M. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil®, Caelyx®, DOXOrubicin, and SinaDoxosome. J. Contr. Release. 2016;221:1–8. doi: 10.1016/j.jconrel.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 349.Silverman J.A., Deitcher S.R. Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013;71:555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ando K., Mori K., Corradini N., Redini F., Heymann D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expet Opin. Pharmacother. 2011;12:285–292. doi: 10.1517/14656566.2011.543129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Carnevale J., Ko A.H. MM-398 (nanoliposomal irinotecan): emergence of a novel therapy for the treatment of advanced pancreatic cancer. Future Oncol. 2016;12:453–464. doi: 10.2217/fon.15.333. [DOI] [PubMed] [Google Scholar]
- 352.Alfayez M., Kantarjian H., Kadia T., Ravandi-Kashani F., Daver N. CPX-351 (vyxeos) in AML. Leuk. Lymphoma. 2020;61:288–297. doi: 10.1080/10428194.2019.1660970. [DOI] [PubMed] [Google Scholar]
- 353.Miele E., Spinelli G.P., Miele E., Tomao F., Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009;4:99. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.-P., Agoston P., Hong A., Mervoyer A., Rastrelli M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 355.Strumberg D., Schultheis B., Traugott U., Vank C., Santel A., Keil O., Giese K., Kaufmann J., Drevs J. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int. J. Clin. Pharm. Ther. 2012;50:76. doi: 10.5414/cpp50076. [DOI] [PubMed] [Google Scholar]
- 356.Ohanian M., Ashizawa A.T., Garcia-Manero G., Pemmaraju N., Kadia T., Jabbour E., Ravandi F., Borthakur G., Andreeff M., Konopleva M. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: a single-centre, open-label, dose-escalation, phase 1/1b trial. Lanc. Haematol. 2018;5:e136–e146. doi: 10.1016/S2352-3026(18)30021-8. [DOI] [PubMed] [Google Scholar]
- 357.Yamamoto N., Sato J., Koyama T., Iwasa S., Shimomura A., Kondo S., Kitano S., Yonemori K., Fujiwara Y., Tamura K. Phase I study of liposomal formulation of eribulin (E7389-LF) in patients (pts) with advanced solid tumours: primary results of dose-escalation part. Ann. Oncol. 2019;30:v125. [Google Scholar]
- 358.Chang S., Warner J., Liang L., Fairman J. A novel vaccine adjuvant for recombinant flu antigens. Biologicals. 2009;37:141–147. doi: 10.1016/j.biologicals.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.de Jonge M.J., Slingerland M., Loos W.J., Wiemer E.A., Burger H., Mathijssen R.H., Kroep J.R., den Hollander M.A., van der Biessen D., Lam M.-H. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur. J. Cancer. 2010;46:3016–3021. doi: 10.1016/j.ejca.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 360.Greil R., Greil-Ressler S., Weiss L., Schönlieb C., Magnes T., Radl B., Bolger G.T., Vcelar B., Sordillo P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018;82:695–706. doi: 10.1007/s00280-018-3654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhou H., Yan J., Chen W., Yang J., Liu M., Zhang Y., Shen X., Ma Y., Hu X., Wang Y. Population pharmacokinetics and exposure–safety relationship of paclitaxel liposome in patients with non-small cell lung cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Wickham T., Futch K. AACR; 2012. Abstract P5-18-09: a Phase I Study of MM-302, a HER2-Targeted Liposomal Doxorubicin, in Patients with Advanced, HER2-Positive Breast Cancer. [Google Scholar]
- 363.Hong D.S., Kang Y.-K., Borad M., Sachdev J., Ejadi S., Lim H.Y., Brenner A.J., Park K., Lee J.-L., Kim T.-Y. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Brit. J. Canc. 2020;122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wang L., Zhao Y., Li T., Cao J., Du Y., Tao Z., Peng W., Wang B., Zhang J., Wang Z. American Society of Clinical Oncology; 2020. Efficacy and Safety of Liposomal Mitoxantrone (Lipo-MIT) in Advanced Breast Cancer (ABC): A Randomized, Open Label, Active-Controlled, Single-Center, Phase II Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rimkus T., Sirkisoon S., Harrison A., Lo H.-W. Tumor suppressor candidate 2 (TUSC2; FUS-1) and human cancers. Discov. Med. 2017;23:325. [PMC free article] [PubMed] [Google Scholar]
- 366.Gabizon A., Grenader T., Tahover E., Shmeeda H., Golan T., Berger R., Geva R., Wolf I., Perets R., Amitay Y. A phase 1B study of pegylated liposomal mitomycin-C prodrug with or without capecitabine and bevacizumab in third line chemotherapy of colorectal cancer. Ann. Oncol. 2016;27:vi202. [Google Scholar]
- 367.Chang Y.-J., Chang C.-H., Yu C.-Y., Chang T.-J., Chen L.-C., Chen M.-H., Lee T.-W., Ting G. Therapeutic efficacy and microSPECT/CT imaging of 188Re-DXR-liposome in a C26 murine colon carcinoma solid tumor model. Nucl. Med. Biol. 2010;37:95–104. doi: 10.1016/j.nucmedbio.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 368.Senzer N., Nemunaitis J., Nemunaitis D., Bedell C., Edelman G., Barve M., Nunan R., Pirollo K.F., Rait A., Chang E.H. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol. Ther. 2013;21:1096–1103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Siefker-Radtke A., Zhang X.-q., Guo C.C., Shen Y., Pirollo K.F., Sabir S., Leung C., Leong-Wu C., Ling C.-M., Chang E.H. A phase l study of a tumor-targeted systemic nanodelivery system, SGT-94, in genitourinary cancers. Mol. Ther. 2016;24:1484–1491. doi: 10.1038/mt.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zagar T.M., Vujaskovic Z., Formenti S., Rugo H., Muggia F., O'Connor B., Myerson R., Stauffer P., Hsu I.-C., Diederich C. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperther. 2014;30:285–294. doi: 10.3109/02656736.2014.936049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Tolcher A.W., Papadopoulos K.P., Patnaik A., Rasco D.W., Martinez D., Wood D.L., Fielman B., Sharma M., Janisch L.A., Brown B.D. A Phase I Study in Patients with Advanced Solid Tumors. American Society of Clinical Oncology; 2015. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC. [Google Scholar]
- 372.Tolcher A.W., Rodrigueza W.V., Rasco D.W., Patnaik A., Papadopoulos K.P., Amaya A., Moore T.D., Gaylor S.K., Bisgaier C.L., Sooch M.P. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014;73:363–371. doi: 10.1007/s00280-013-2361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.El Dika I., Lim H.Y., Yong W.P., Lin C.C., Yoon J.H., Modiano M., Freilich B., Choi H.J., Chao T.Y., Kelley R.K. An open-label, multicenter, phase I, dose escalation study with phase II expansion cohort to determine the safety, pharmacokinetics, and preliminary antitumor activity of intravenous TKM-080301 in subjects with advanced hepatocellular carcinoma. Oncol. 2019;24:747. doi: 10.1634/theoncologist.2018-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Raspaglio G., Ferlini C., Mozzetti S., Prislei S., Gallo D., Das N., Scambia G. Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors. Biochem. Pharmacol. 2005;69:113–121. doi: 10.1016/j.bcp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 375.Ashton S., Song Y.H., Nolan J., Cadogan E., Murray J., Odedra R., Foster J., Hall P.A., Low S., Taylor P. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad2355. 325ra317-325ra317. [DOI] [PubMed] [Google Scholar]
- 376.Hrkach J., Von Hoff D., Ali M.M., Andrianova E., Auer J., Campbell T., De Witt D., Figa M., Figueiredo M., Horhota A. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003651. 128ra139-128ra139. [DOI] [PubMed] [Google Scholar]
- 377.Weiss G.J., Chao J., Neidhart J.D., Ramanathan R.K., Bassett D., Neidhart J.A., Choi C.H.J., Chow W., Chung V., Forman S.J. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest. N. Drugs. 2013;31:986–1000. doi: 10.1007/s10637-012-9921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Piha-Paul S.A., Thein K.Z., De Souza P., Kefford R., Gangadhar T., Smith C., Schuster S., Zamboni W.C., Dees C.E., Markman B. First-in-human, phase I/IIa study of CRLX301, a nanoparticle drug conjugate containing docetaxel, in patients with advanced or metastatic solid malignancies. Invest. N. Drugs. 2021:1–10. doi: 10.1007/s10637-021-01081-x. [DOI] [PubMed] [Google Scholar]
- 379.Braal C., de Bruijn P., Atrafi F., van Geijn M., Rijcken C., Mathijssen R., Koolen S. A new method for the determination of total and released docetaxel from docetaxel-entrapped core-crosslinked polymeric micelles (CriPec®) by LC–MS/MS and its clinical application in plasma and tissues in patients with various tumours. J. Pharmaceut. Biomed. Anal. 2018;161:168–174. doi: 10.1016/j.jpba.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 380.Park I.H., Sohn J.H., Kim S.B., Lee K.S., Chung J.S., Lee S.H., Kim T.Y., Jung K.H., Cho E.K., Kim Y.S. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer, Cancer research and treatment. Off. J. Kor. Canc. Assoc. 2017;49:569. doi: 10.4143/crt.2016.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Plummer R., Wilson R., Calvert H., Boddy A., Griffin M., Sludden J., Tilby M., Eatock M., Pearson D., Ottley C. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Brit. J. Canc. 2011;104:593–598. doi: 10.1038/bjc.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.van Zandwijk N., Pavlakis N., Kao S., Clarke S., Lee A., Brahmbhatt H., MacDiarmid J., Pattison S., Leslie F., Huynh Y. MesomiR 1: a Phase I study of TargomiRs in patients with refractory malignant pleural mesothelioma (MPM) and lung cancer (NSCLC) Ann. Oncol. 2015;26:ii16. [Google Scholar]
- 383.Maggiorella L., Barouch G., Devaux C., Pottier A., Deutsch E., Bourhis J., Borghi E., Levy L. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8:1167–1181. doi: 10.2217/fon.12.96. [DOI] [PubMed] [Google Scholar]
- 384.Sahu T., Ratre Y.K., Chauhan S., Bhaskar L., Nair M.P., Verma H.K. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021;63:102487. [Google Scholar]
- 385.Sun Y.-N., Wang C.-D., Zhang X.-M., Ren L., Tian X.-H. Shape dependence of gold nanoparticles on in vivo acute toxicological effects and biodistribution. J. Nanosci. Nanotechnol. 2011;11:1210–1216. doi: 10.1166/jnn.2011.3094. [DOI] [PubMed] [Google Scholar]
- 386.Hillyer J.F., Albrecht R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharmaceut. Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 387.Qiu Y., Liu Y., Wang L., Xu L., Bai R., Ji Y., Wu X., Zhao Y., Li Y., Chen C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials. 2010;31:7606–7619. doi: 10.1016/j.biomaterials.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 388.Mirna Therapeutics Halts Phase 1 Clinical Study of MRX34.https://www.businesswire.com/news/home/20160920006814/en/Mirna-Therapeutics-Halts-Phase-1-Clinical-Study(accessed July 1.
- 389.Merrimack Discontinues Development of MM-310.http://investors.merrimack.com/news-releases/news-release-details/merrimack-discontinues-development-mm-310(accessed July 1.
- 390.Wong C.H., Siah K.W., Lo A.W. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273–286. doi: 10.1093/biostatistics/kxx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Langer C.J., O'Byrne K.J., Socinski M.A., Mikhailov S.M., Leśniewski-Kmak K., Smakal M., Ciuleanu T.E., Orlov S.V., Dediu M., Heigener D. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J. Thorac. Oncol. 2008;3:623–630. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 392.Zhang C., Yan L., Wang X., Zhu S., Chen C., Gu Z., Zhao Y. Progress, challenges, and future of nanomedicine. Nano Today. 2020;35:101008. [Google Scholar]
- 393.EI comment on the new Tufts Study on Drug DevelopmentCosts.https://www.keionline.org/22646(accessed July 1.
- 394.Moore T.J., Zhang H., Anderson G., Alexander G.C. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration. 2015-2016, JAMA internal medicine. 2018;178:1451–1457. doi: 10.1001/jamainternmed.2018.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.h.w.s.g.A.e.d.b.-k.h.a.J . 2019. United States Securities and Exchange Commission. [Google Scholar]
- 396.Trouble brewing at Bind Therapeutics as major cuts announced.https://www.fiercebiotech.com/r-d/trouble-brewing-at-bind-therapeutics-as-major-cuts-announced(accessed July 1.
- 397.P.W.B.B.B.T.B.A.f.M.h.w.b.c.a.p.-w.-b.-b.-b.-t.-b.-a.-f.-.-m.-a. July.
- 398.Lancet J.E., Uy G.L., Cortes J.E., Newell L.F., Lin T.L., Ritchie E.K., Stuart R.K., Strickland S.A., Hogge D., Solomon S.R. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol. 2018;36:2684. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.V.t.b.t.b.A.t.m. leader https://www.europeanpharmaceuticalreview.com/news/37150/aml-treatment-market-reach-932-6-million-2024/
- 400.Park K. 2019. The Beginning of the End of the Nanomedicine Hype. [DOI] [PubMed] [Google Scholar]
- 401.Yang V.C. Personal perspectives and concerns over the so-called nanomedicine. J. Contr. Release: Off. J. Controll. Release Soc. 2019;311:322. doi: 10.1016/j.jconrel.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 402.Tran S., DeGiovanni P.-J., Piel B., Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 2017;6:44. doi: 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 404.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 405.Weissig V., Pettinger T.K., Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014;9:4357. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Caster J.M., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2017;9:e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 407.Heasley L.E. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–1569. doi: 10.1038/sj.onc.1204183. [DOI] [PubMed] [Google Scholar]
- 408.Adityan S., Tran M., Bhavsar C., Wu S.Y. Journal of Controlled Release; 2020. Nano-therapeutics for Modulating the Tumour Microenvironment: Design, Development, and Clinical Translation. [DOI] [PubMed] [Google Scholar]
- 409.Combest A.J., Roberts P.J., Dillon P.M., Sandison K., Hanna S.K., Ross C., Habibi S., Zamboni B., Müller M., Brunner M. Genetically engineered cancer models, but not xenografts, faithfully predict anticancer drug exposure in melanoma tumors. Oncol. 2012;17:1303. doi: 10.1634/theoncologist.2012-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Bazou D., Maimon N., Gruionu G., Munn L.L. Self-assembly of vascularized tissue to support tumor explants in vitro. Integr. Biol. 2016;8:1301–1311. doi: 10.1039/c6ib00108d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Bazou D., Maimon N., Gruionu G., Grahovac J., Seano G., Liu H., Evans C.L., Munn L.L. Vascular beds maintain pancreatic tumour explants for ex vivo drug screening. J. Tissue Eng. Regenerat. Med. 2018;12:e318–e322. doi: 10.1002/term.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kwak B., Ozcelikkale A., Shin C.S., Park K., Han B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J. Contr. Release. 2014;194:157–167. doi: 10.1016/j.jconrel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Albanese A., Lam A.K., Sykes E.A., Rocheleau J.V., Chan W.C. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 2013;4:1–8. doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]