Abstract
A total of 3,051 methicillin-susceptible Staphylococcus aureus (MSSA) isolates and methicillin-resistant S. aureus (MRSA) isolates in Europe were compared. MRSA isolates constituted 25% of all isolates and were more prevalent in southern Europe. MRSA isolates appeared to be more prevalent in intensive care units than in outpatient departments. Only a small minority of MSSA isolates were multidrug resistant, whereas the majority of MRSA isolates were multidrug resistant.
Methicillin resistance in Staphylococcus aureus is now common in many areas of the world. The frequencies of infections and outbreaks due to methicillin-resistant S. aureus (MRSA) have continued to increase (7, 11, 12). It is noteworthy that the prevalence of MRSA varies from one geographic region to another and between different institutions in a given area. The prevalence of MRSA differs markedly among European countries (18). MRSA is an increasingly important clinical problem since MRSA is often multidrug resistant and therapeutic options are limited.
The aim of the present study was to analyze recent data on the epidemiologies and susceptibilities of 3,051 S. aureus isolates from 25 university hospitals participating in the European SENTRY Antimicrobial Surveillance Program from April 1997 through February 1999 (6). The epidemiologies of methicillin-susceptible S. aureus (MSSA) and MRSA isolates were studied by determining their prevalences in different specimens, on various wards, and in different age groups. The in vitro activities of 21 various antibiotic compounds were tested, and additionally, the percentage of multidrug-resistant isolates was determined for MSSA and MRSA isolates.
The species of the isolates (only one isolate per patient was allowed) were determined at the source and when deemed clinically significant by local criteria and were sent to the Eijkman-Winkler Institute (the European reference center for the SENTRY Antimicrobial Surveillance Program), together with relevant information for the isolate. The MICs of a range of antibiotics were determined by a broth microdilution (Sensititre, Westlake, Ohio) method by standard methods defined by the National Committee for Clinical Laboratory Standards (10). The origins of the S. aureus isolates tested are shown in Table 1. The presence of the mecA gene was determined by PCR with primers whose sequences were 5′-GTTGTAGTTGTCGGGTTTGG and 5′-CTTCCACATACCATCTTCTTTAAC.
TABLE 1.
Origins of S. aureus isolates
| City | Country | No. of isolates | % MRSA |
|---|---|---|---|
| London | England | 131 | 28 |
| Utrecht | The Netherlands | 147 | 2 |
| Brussels | Belgium | 82 | 25 |
| Düsseldorf | Germany | 215 | 5 |
| Freiburg | Germany | 132 | 4 |
| Lausanne | Switzerland | 114 | 2 |
| Linz | Autstria | 117 | 9 |
| Paris I | France | 219 | 25 |
| Paris II | France | 119 | 20 |
| Lille | France | 188 | 12 |
| Lyon | France | 192 | 18 |
| Warsaw | Poland | 58 | 33 |
| Cracow | Poland | 101 | 23 |
| Coimbra | Portugal | 318 | 54 |
| Madrid | Spain | 113 | 12 |
| Seville | Spain | 132 | 34 |
| Barcelona | Spain | 107 | 9 |
| Rome | Italy | 145 | 58 |
| Genoa | Italy | 152 | 43 |
| Tirana | Albania | 23 | 17 |
| Athens | Greece | 128 | 34 |
| Ankara I | Turkey | 24 | 21 |
| Ankara II | Turkey | 77 | 44 |
| Istanbul | Turkey | 4 | 0 |
| Hash Homer | Israel | 13 | 31 |
Twenty-five percent of the isolates were methicillin resistant. The prevalence of MRSA is comparable to that found in recent U.S. studies (7, 12), but the percentage of MRSA isolates is less than half of the percentage reported from Japan (4). The prevalence of MRSA was confirmed to vary considerably between different European countries and also between hospitals within a country (Table 1) (18). In general, the highest prevalence of MRSA isolates was seen in hospitals in Portugal (54%) and Italy (43 to 58%). In contrast, the prevalence of MRSA was lowest in participating hospitals in Switzerland and The Netherlands (2%). However, only a few hospitals per country participated in the European SENTRY Antimicrobial Surveillance Program study. In addition, large differences in a country may occur; e.g., the proportion of MRSA isolates was 34% for the hospital in Seville, Spain, whereas it was 9% for the hospital in Barcelona, Spain. Similar observations were reported in recent U.S. studies of the prevalence of MRSA (2). The reason for the low prevalence in some university hospitals may be related to the rapid identification and strict policies of isolation of patients with MRSA colonization or infection, combined with the restricted use of antibiotics.
The prevalence of methicillin resistance was highest among S. aureus isolates deemed responsible for nosocomial pneumonia (34.4%); the prevalence of methicillin resistance was 28.3% among urinary tract infection isolates and 23.8% among blood isolates and was lowest among isolates associated with skin and soft tissue infections (22.4%). These differences might be due to prolonged antibiotic treatment of severely sick patients, which generally have longer hospital stays, resulting in enhanced selection pressure. However, U.S. SENTRY Antimicrobial Surveillance Program staphylococcal isolates from different sources displayed rates of resistance comparable to those described above (12).
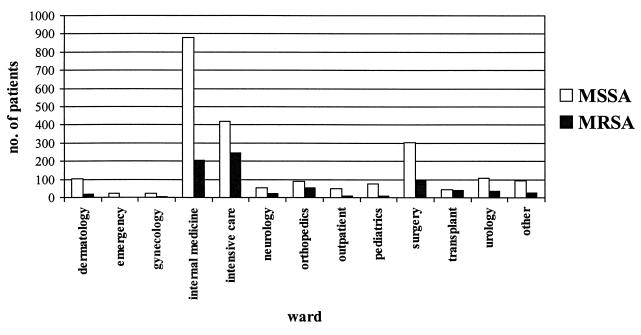
Considerable differences were observed when the distributions of MRSA isolates in different wards were compared (Fig. 1). Almost 38% of the S. aureus isolates from intensive care units (ICUs) and 22.6% of the isolates from internal medicine wards were MRSA, whereas 0% of the isolates from emergency rooms and 1% of the isolates from outpatient departments were MRSA. This partly reflects the relative sizes of some specialties, but it also reflects the fact that some patients, e.g., critically ill patients in ICUs, have a greater chance of becoming colonized or infected. Our results concerning the prevalence of MRSA in different wards are largely in accordance with recent data from the United States. However, we were not able to confirm the extremely high prevalence of MRSA in ICUs described in the European Prevalence of Infection in Intensive Care study (17). The low prevalence of MRSA in emergency rooms and outpatient departments suggests that the level of MRSA in the community is still lower than that in hospitals (5, 9).
FIG. 1.
Distributions of MSSA and MRSA isolates for different wards within the hospital.
The distributions of both MSSA and MRSA among different age groups were similar. However, with the exception of newborns, S. aureus infections were more often found with increasing age, but their prevalence declined after 75 years of age. Compared to the age distribution for all infections with other organisms, no significant differences in the age distributions of individuals with MRSA infections were observed.
The distributions of the MICs for the isolates were as follows: ≤0.06 μg/ml, 18.5% (n = 565); 0.12 μg/ml, 21.1% (n = 645); 0.25 μg/ml, 12.7% (n = 388); 0.5 μg/ml, 16.4% (n = 501); 1 μg/ml, 4.5% (n = 137); 2 μg/ml, 1.7% (n = 51); 4 μg/ml, 1.1% (n = 35); 8 μg/ml, 1.7% (n = 51); and >8 μg/ml, 22.2% (n = 678). In 2.25% of the MRSA isolates, for all of which the oxacillin MIC was 4 μg/ml, the mecA gene could not be detected by PCR (data not shown). Oxacillin resistance in these isolates may be explained by undetected penicillin-binding protein alterations or the production of large amounts of β-lactamase (1, 8, 16).
The comparative in vitro activities of 21 antimicrobial agents against MSSA and MRSA isolates are listed in Tables 2 and 3, respectively. Of the MSSA isolates tested, 84.7% were resistant to penicillin, while MRSA isolates are, by definition, resistant to all β-lactam antibiotics. There is an obvious relationship between oxacillin resistance and resistance to other antibiotics (Table 2). The percentage of MSSA isolates which were susceptible to erythromycin (77.5%) was more than eightfold higher than the percentage of MRSA isolates which were susceptible to erythromycin. While 94% of the MSSA isolates were susceptible to clindamycin, only 23% of the MRSA isolates exhibited susceptibility. Eighty-eight percent of the erythromycin-resistant MRSA isolates and 37% of the erythromycin-resistant MSSA isolates displayed a constitutive macrolide-lincosamide-streptogramin B (MLS) resistance phenotype on the basis of the MICs. The other erythromycin-resistant S. aureus isolates had an inducible MLS resistance phenotype. The percentage of MRSA isolates showing susceptibility to gentamicin (22.8%) was more than fourfold lower than that of MSSA isolates. While susceptibility to tetracyclines fell from 88.5% among MSSA isolates to 40.5% among MRSA isolates, this decrease was far less pronounced for the structurally related compounds minocycline and doxycycline, to which some 90% of the MRSA showed in vitro susceptibility. More then 90% of all MSSA isolates were susceptible to ciprofloxacin, whereas less than 10% of all MRSA isolates tested were susceptible to ciprofloxacin (Table 2).
TABLE 2.
MIC distributions, antimicrobial susceptibilities, and spectra of activity of the different antimicrobial agents tested for the MRSA isolates testeda
| Antimicrobial agent | No. of isolates for which MIC (mg/liter) is:
|
MIC50/MIC90b | % Susceptible | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.03 | 0.06 | ≤0.12 | 0.12 | 0.25 | ≤0.5 | 0.5 | 1 | 2 | >2 | ≤4 | 4 | >4 | 8 | >8 | |||
| Erythromycin | 7 | 28 | 40 | 13 | 17 | 7 | 8 | 644 | >8/>8 | 4.8 | |||||||
| Clindamycin | 103 | 60 | 5 | 4 | 1 | 2 | 7 | 572 | >8/>8 | 23.3 | |||||||
| Gentamicin | 21 | 58 | 75 | 6 | 5 | 9 | 16 | 574 | >16/>16 | 22.8 | |||||||
| Tetracycline | 309 | 11 | 444 | >8/>8 | 42.9 | ||||||||||||
| Doxycycline | 300 | 5 | 22 | 150 | 287 | 4/8 | 85.2 | ||||||||||
| Minocycline | 132 | 16 | 103 | 74 | 27 | 2/4 | 92.3 | ||||||||||
| Ciprofloxacin | 1 | 9 | 16 | 23 | 9 | 12 | 9 | 605 | >2/>2 | 9.2 | |||||||
| Gatifloxacinc | 18 | 23 | 19 | 5 | 11 | 79 | 376 | 233 | 2/4 | ||||||||
| Trovafloxacinc | 43 | 14 | 7 | 9 | 97 | 255 | 195 | 144 | 1/>4 | ||||||||
| Rifampin | 33 | 240 | 9 | 20 | 78 | 334 | 2/>2 | 46.1 | |||||||||
| Chloramphenicol | 1 | 1 | 2 | 2 | 10 | 180 | 438 | 130 | 8/16 | 83.0 | |||||||
| Quinupristin-dalfopristin | 24 | 167 | 427 | 111 | 14 | 3 | 12 | 6 | 0.5/1 | 99.5 | |||||||
| Linezolidcd | 0 | 6 | 7 | 166 | 220 | 13 | 0 | 0 | 2/2 | ||||||||
| Teicoplanin | 5 | 35 | 158 | 260 | 236 | 63 | 5 | 2 | 1/2 | 99.7 | |||||||
| Vancomycin | 1 | 1 | 65 | 493 | 200 | 4 | 0 | 0 | 1/2 | 100 | |||||||
A total of 764 MRSA isolates were tested.
MIC50/MIC90, MICs at which 50%/90% of isolates are inhibited.
Investigational drug. No susceptibility breakpoints are available (10).
Only 412 MRSA isolates were tested, unless indicated otherwise.
TABLE 3.
MIC distributions, antimicrobial susceptibilities, and spectra of activity of the different antimicrobial agents tested for the MSSA isolates testeda
| Antimicrobial agent | No. of isolates for which (mg/liter) is:
|
MIC50/MIC90 | % Susceptible | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | ≤0.12 | 0.12 | 0.25 | ≤0.5 | 0.5 | 1 | 2 | >2 | ≤4 | 4 | >4 | 8 | >8 | 16 | >16 | |||
| Penicillin | 351 | 28 | 33 | 61 | 93 | 130 | 182 | 297 | 1,463 | 16/>32 | 15.4 | ||||||||
| Ampicillin | 323 | 44 | 47 | 87 | 137 | 150 | 177 | 289 | 1,033 | 16/>16 | 16.1 | ||||||||
| Amoxicillin-clavulanate | 315 | 181 | 462 | 805 | 326 | 80 | 30 | 31 | 57 | 1/2 | 94.8 | ||||||||
| Ceftriaxone | 0 | 4 | 13 | 146 | 1,604 | 372 | 47 | 22 | 79c | 2/4 | 95.6 | ||||||||
| Cefepime | 3 | 5 | 20 | 431 | 1,268 | 886 | 34 | 12 | 68 | 2/4 | 96.5 | ||||||||
| Imipenem | 1,974 | 196 | 27 | 9 | 6 | 11 | 14 | 50d | 0.12/0.25 | 97.2 | |||||||||
| Erythromycin | 21 | 716 | 1,011 | 149 | 6 | 13 | 10 | 361 | 0.5/>8 | 77.5 | |||||||||
| Clindamycin | 1,661 | 460 | 20 | 6 | 2 | 0 | 0 | 138 | 0.12/0.25 | 93.7 | |||||||||
| Gentamicin | 222 | 56 | 1,034 | 293 | 47 | 7 | 7 | 117 | 0.5/1 | 94.6 | |||||||||
| Tetracycline | 2,023 | 20 | 244 | ≤4/8 | 89.7 | ||||||||||||||
| Doxycycline | 2,019 | 41 | 94 | 62 | 71 | ≤0.5/1 | 97.7 | ||||||||||||
| Minocycline | 1,145 | 9 | 11 | 23 | 21 | ≤0.25/≤0.25 | 92.3 | ||||||||||||
| Ciprofloxacin | 19 | 169 | 868 | 793 | 185 | 37 | 13 | 203 | 0.25/1 | 90.6 | |||||||||
| Gatifloxacine | 471 | 1,110 | 468 | 34 | 25 | 33 | 83 | 63 | 0.06/0.25 | ||||||||||
| Trovafloxacine | 1,470 | 504 | 90 | 28 | 37 | 82 | 46 | 30 | ≤0.03/0.12 | ||||||||||
| Rifampin | 1,840 | 366 | 13 | 6 | 10 | 52 | 0.03/0.25 | 97.4 | |||||||||||
| Chloramphenicol | 0 | 3 | 5 | 12 | 41 | 637 | 1,474 | 115 | 8/8 | 96.3 | |||||||||
| Quinupristin-dalfopristin | 185 | 1,372 | 656 | 61 | 0 | 6 | 0 | 0 | 0.25/0.5 | 95.3 | |||||||||
| Linezolidef | 2 | 1 | 11 | 139 | 838 | 86 | 0 | 0 | 2/2 | ||||||||||
| Teicoplanin | 10 | 327 | 1,574 | 313 | 55 | 7 | 1 | 0 | 0.5/1 | 100 | |||||||||
| Vancomycin | 0 | 3 | 260 | 1,954 | 67 | 3 | 0 | 0 | 0.5/1 | 100 | |||||||||
A total of 2,287 MSSA isolates were tested, unless indicated otherwise.
See footnote b of Table 2.
For 9 isolates the MIC was 32 mg/liter, and for 70 isolates the MIC was >32 mg/liter.
MIC, >8 mg/liter.
Investigational drug. No susceptibility breakpoints are available for this drug (10).
Only 1,075 MSSA isolates were tested.
While 99.5% of the MSSA isolates were susceptible to quinupristin-dalfopristin, this rate was slightly decreased to 95.3% for the MRSA isolates. Vancomycin and linezolid were the only compounds tested to which reduced susceptibility was not recognized for any of the S. aureus isolates tested. One MRSA isolate was resistant to teicoplanin, whereas a second one was intermediate resistant.
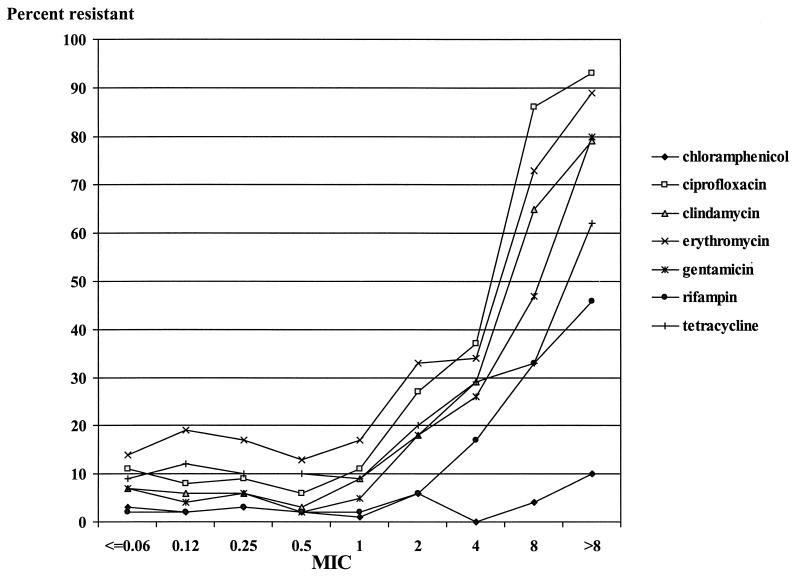
The percentage of isolates resistant to all of the antibiotics listed in Fig. 2 with the exception of chloramphenicol was quite stable among the population of S. aureus isolates for which oxacillin MICs were ≤0.06 to 1 μg/ml, but the percentage increased significantly with an increase in the oxacillin MIC to >2 μg/ml.
FIG. 2.
Percent resistance to selected antibiotics for S. aureus isolates for which oxacillin MICs varied.
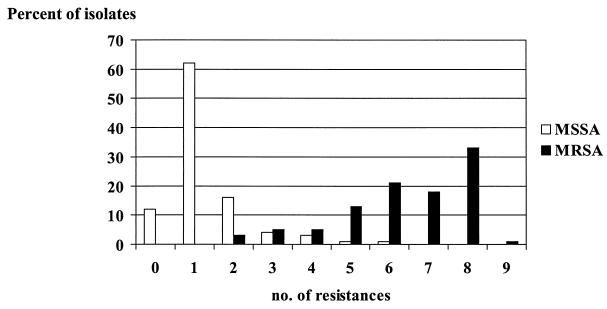
Isolates were considered to be multidrug resistant when they displayed resistance to five (or more) of the following antibiotics, which represented different antibiotic classes: oxacillin, penicillin, erythromycin, clindamycin, gentamicin, ciprofloxacin, tetracycline, rifampin, and chloramphenicol. MRSA is, by definition, also resistant to penicillin (10). Thus, all MRSA isolates were resistant to at least two classes of antibiotics. The results are shown in Fig. 3. Only 2% of the MSSA isolates were multidrug resistant. However, 87% of the MRSA isolates were multidrug resistant and only 3% of the MRSA isolates were resistant to β-lactam antibiotics only.
FIG. 3.
Number of drugs to which S. aureus isolates were resistant for selected antibiotics (oxacillin, penicillin, gentamicin, erythromycin, clindamycin, ciprofloxacin, tetracycline, rifampin, and chloramphenicol) (number of resistances). Note that MRSA isolates were always resistant to two of these antimicrobial agents.
The rates of susceptibility of the European S. aureus population were comparable to those determined from the data of Voss et al. (18). The results from the SENTRY Antimicrobial Surveillance Program for blood isolates from the United States, Canada, and Latin America generally showed higher percentages of susceptibility for MSSA isolates to most antimicrobial agents with the exception of erythromycin, chloramphenicol, and rifampin (13). This pattern was also observed for MRSA isolates from the United States and Latin America. A similar result was obtained when the European data were compared to the data from the SCOPE program (7), which investigated the susceptibilities of S. aureus isolates implicated in nosocomial bloodstream infections in the United States.
The glycopeptide agent vancomycin is still the drug of choice for the treatment of life-threatening infections caused by multidrug-resistant MRSA strains. Recent studies have suggested that treatment of infections with staphylococci currently considered susceptible according to the standards of the National Committee for Clinical Laboratory Standards but for which vancomycin MICs are 4 μg/ml might lead to therapeutic failures and that such isolates might be precursors of vancomycin-resistant S. aureus strains (14). Although we did not find MRSA isolates with reduced susceptibility to vancomycin in the European S. aureus population, emerging vancomycin resistance is a constant threat since the first glycopeptide-intermediate-resistant S. aureus (GISA) isolates and hetero-GISA isolates have also been detected in Europe (3). For seven strains (0.23%) in the present European collection, vancomycin MICs were 4 μg/ml. Recently, we investigated the seven strains for which the vancomycin MIC was 4 μg/ml for their hetero-glycopeptide-intermediate resistance status. However, neither GISA nor hetero-GISA was detected (15). Nevertheless, it is important to carefully monitor the prevalence of (hetero-)GISA, especially in MRSA populations because of the almost invariable multidrug-resistant nature of MRSA.
Acknowledgments
We thank Miriam Klootwijk, Karlijn Kusters, Alice Florijn, and Stefan de Vaal for expert technical assistance.
The SENTRY Antimicrobial Surveillance Program is funded by an educational grant from Bristol-Myers Squibb Pharmaceutical Company and by the European Network for Antimicrobial Resistance and Epidemiology (ENARE) through a grant (grant ERBCHRCT940554) from the European Union.
REFERENCES
- 1.Barg N, Chambers H, Kernodle D. Borderline susceptibility to antistaphylococcal penicillins is not conferred exclusively by the hyperproduction of β-lactamase. Antimicrob Agents Chemother. 1991;35:1975–1979. doi: 10.1128/aac.35.10.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmond M B, Wallace S E, McClish D K, Pfaller M A, Jones R N, Wenzel R P. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 3.Geisel R, Schmitz F J, Thomas L, Berns G, Zetsche O, Ulrich B, Fluit A C, Labischinsky H, Witte W. Emergence of heterogeneous intermediate vancomycin resistance in Staphylococcus aureus isolates in the Dusseldorf area. J Antimicrob Chemother. 1999;43:846–848. doi: 10.1093/jac/43.6.846. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto H, Inoue M, Hayashi I. A survey of Staphylococcus aureus for typing and drug-resistance in various areas of Japan during 1992 and 1993. Jpn J Antibiot. 1994;47:618–626. [PubMed] [Google Scholar]
- 5.Herold B C, Immergluck L C, Maranan M C, Lauderdale D S, Gaskin R E, Boyle-Vavra S, Leitch C D, Daum R S. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 6.Jones M E, Schmitz F J, Fluit A C, Acar J, Verhoef J the SENTRY Antimicrobial Surveillance Program Participants Group. The frequency of occurrence and antimicrobial susceptibility of bacterial pathogens associated with skin and soft tissue infections during 1997, for the SENTRY Antimicrobial Surveillance Programme. Eur J Clin Microbiol Infect Dis. 1998;18:403–408. doi: 10.1007/s100960050308. [DOI] [PubMed] [Google Scholar]
- 7.Marshall S A, Wilke W W, Pfaller M A, Jones R N. Staphylococcus aureus and coagulase-negative staphylococci from blood stream infections: frequency and occurrence, antimicrobial susceptibility, and molecular (mecA) characterization of oxacillin resistance in the SCOPE program. Diagn Microbial Infect Dis. 1998;30:205–214. doi: 10.1016/s0732-8893(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 8.McDougal L K, Thornsberry C. The role of β-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986;23:832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno F, Crisp C, Jorgensen J H. Methicillin-resistant Staphylococcus aureus as a community organism. Clin Infect Dis. 1995;21:1308–1312. doi: 10.1093/clinids/21.5.1308. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial tests for bacteria that grow aerobically. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 11.Panlilio A L, Culver D H, Gaynes R P, Banerjee S, Henderson T S, Tolson J S, Martone W J. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect Control Hosp Epidemiol. 1992;3:582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller M A, Jones R N, Doern G V, Kugler K the SENTRY Participants Group. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997) Antimicrob Agents Chemother. 1998;42:1762–1770. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller M A, Jones R N, Doern G V, Sader H S, Kugler K C, Beach M L the SENTRY Participants Group. Survey of blood stream infections attributable to gram-positive cocci: frequency of occurrence and antimicrobial susceptibility of isolates collected in 1997 in the United States, Canada, and Latin America from the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis. 1999;33:283–297. doi: 10.1016/s0732-8893(98)00149-7. [DOI] [PubMed] [Google Scholar]
- 14.Sahm D F, Marsilio M K, Piazza G. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin Infect Dis. 1999;29:259–263. doi: 10.1086/520195. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz F-J, Krey A, Geisel R, Verhoef J, Heinz H-P, Fluit A C. Susceptibility of 302 methicillin-resistant Staphylococcus aureus isolates from 20 European university hospitals to vancomycin and alternative antistaphylococcal compounds. SENTRY Participants Group. Eur J Clin Microbiol Infect Dis. 1999;18:528–530. doi: 10.1007/s100960050340. [DOI] [PubMed] [Google Scholar]
- 16.Tomasz A, Drugeon H B, DeLencastre H, Jabes D, McDougall L, Bille J. New mechanism for methicillin-resistance in Staphylococcus aureus: clinical isolates that lack the PBP2a gene and contain normal penicillin-binding proteins with modified penicillin-binding capacity. Antimicrob Agents Chemother. 1989;33:1869–1874. doi: 10.1128/aac.33.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent J L, Bihari D J, Suter P M, Bruining H A, White J, Nicolas-Chanoin M H, Wolff M, Spencer R C, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 18.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl V T, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe. Eur J Clin Microbiol Infect Dis. 1994;13:50–55. doi: 10.1007/BF02026127. [DOI] [PubMed] [Google Scholar]