Abstract
Objective
Socioeconomic and other demographic factors are associated with outcomes in head and neck cancer. This study uses a national cancer database to explore how patient race, ethnicity, and socioeconomic status (SES) are associated with esthesioneuroblastoma outcomes, including 5-year disease-specific survival (DSS), conditional DSS, stage at diagnosis, and treatment.
Study Design
Retrospective cohort analysis.
Setting
Patients with esthesioneuroblastomas between 1973 and 2015 from the SEER registry (Surveillance, Epidemiology, and End Results).
Methods
The National Cancer Institute Yost Index, a census tract–level composite score composed of 7 parameters, was used to categorize the SES of patients. Kaplan-Meier analysis and Cox regression were conducted to assess DSS. Conditional DSS was calculated per estimates from simplified Cox models. Logistic regression was conducted to identify risk factors for advanced cancer stage at diagnosis and the likelihood of receiving multimodal therapy.
Results
Complete data were included for 561 patients. DSS was significantly associated with SES (log-rank, P < .01) but not race. According to Cox regression, DSS was worse for the lowest SES tertile vs the highest (hazard ratio, 1.70 [95% CI, 1.05-2.75]; P = .03). Patients of the lowest SES tertile exhibited an increased risk of advanced cancer stage at diagnosis as compared with the highest SES tertile (odds ratio, 1.84 [95% CI, 1.06-3.30]; P = .035). Black patients (odds ratio, 0.44 [95% CI, 0.24-0.84]; P = .011) were less likely than other patients to receive multimodal therapy. SES alone was not associated with receiving multimodal therapy.
Conclusion
SES is significantly associated with DSS and conditional DSS for patients with esthesioneuroblastomas. Inequalities in access to care and treatment likely contribute to these disparities.
Keywords: esthesioneuroblastoma, health disparities, conditional survival, SEER
Racial, ethnic, and socioeconomic disparities influence outcomes for many cancers.1-10 Studies have consistently demonstrated decreased survival for solid tumors as well as hematologic malignancies among patients of certain racial and socioeconomic backgrounds.11,12 Mechanisms underlying these disparities include differences in access to care and follow-up, screening utilization, and health behaviors.13,14 For specific malignancies, studies have also illustrated that the likelihood of receiving standard-of-care treatment may vary by patient race. 15 Therefore, disparities in oncologic outcomes are likely influenced by key sociodemographic variables. This variance presents an important avenue of study and intervention to improve cancer treatment and outcomes for patients in high-risk cohorts.
Esthesioneuroblastomas, or olfactory neuroblastomas, are malignant tumors that arise from the olfactory neuroepithelium. These tumors are uncommon, accounting for only 2% to 3% of nasal cavity neoplasms. 16 There is a bimodal distribution in the age of diagnosis, with most presenting in the second decade or fourth to sixth decade of life. 17 Severe disease can include intracranial and orbital invasion, in addition to regional nodal and distant metastasis. 18 The most common form of treatment combines surgery and adjuvant radiotherapy, which shows superior outcomes to using either modality alone.18-23 The role of chemotherapy remains unclear, but it is commonly used for disseminated and locally advanced disease. 24 Overall 5-year disease-specific survival (DSS) is approximately 69%. 25
The effect of race, ethnicity, and SES on survival patterns for rare sinonasal tumors such as esthesioneuroblastomas has not been well established. 26 This study aims to explore the association of sociodemographics with several outcome metrics for patients with esthesioneuroblastoma, such as DSS, 5-year conditional DSS (CDSS), stage at diagnosis, and treatment received. Specifically, we use data from the SEER registry (Surveillance, Epidemiology, and End Results) and the National Cancer Institute’s Yost Index—a validated census tract–level composite score of socioeconomic status (SES)—to explore the effects of race, ethnicity, and SES on these outcomes.10,27,28 CDSS characterizes the likelihood of continued survival based on the duration of time already survived since diagnosis. This measure of survival can effectively inform treatment plans and surveillance patterns and provide a more accurate assessment of prognosis for patients.29-34 Findings from this study may not only help to characterize disparities in outcomes for esthesioneuroblastomas but also inform future studies and intervention strategies to improve survival for patients in high-risk cohorts.
Materials and Methods
Data Source and Study Population
This study is an analysis of the National Cancer Institute’s SEER registry. This database acquires data from 18 population-based registries that represent approximately 30% of the US population. With a dedicated team of trained data curators, SEER accurately captures information on patient demographics, tumor characteristics, treatment factors, and survival statistics. Race and ethnicity were categorized separately at the case level by using 2 variables in the SEER database. Both variables were included in multivariable analyses.
Patients were identified with the SEER histologic code for esthesioneuroblastoma (9522-3) with a primary site in the nasal cavity and sinuses (SEER site code C30.0-31.9) or intracranial location (C70.0-71.9). Patients were included if they had (1) a diagnosis of esthesioneuroblastoma between 1973 and 2015 and (2) complete data available on age at diagnosis, sex, stage at diagnosis, race, ethnicity, SES, treatment regimen (surgery, radiation, and/or chemotherapy), and follow-up visits to calculate DSS. After application of exclusion criteria, only patients after 2000 were included.
Although esthesioneuroblastomas are generally staged with the Kadish and other systems, these data are not available in the SEER registry. 35 Instead, the standard SEER staging system was used, which stratifies disease into 3 main categories: localized disease, defined as a malignancy isolated to the primary site; regional disease, characterized as infiltration into surrounding tissues and/or spread to regional lymph nodes; and distant disease, such as malignances with metastatic spread to remote tissues or lymphatic systems.
Yost Index
The National Cancer Institute utilizes a composite score known as the Yost Index to quantify SES.10,27 Based on geocoded patient-level data, the index is a validated census tract–level composite score integrating 7 SES parameters: median household income, median home value, median rent, percentage of individuals <150% of the federal poverty line, percentage unemployment, percentage working-class occupation, and educational achievement index. The use of census tract–level data is superior to other county-level measurements of SES, as census tracts are smaller and more targeted and provide greater resolution and specificity of SES characterization. The Yost Index is divided into tertiles, of which the first is the lowest SES and the third is the highest. Several studies have illustrated the validity of the Yost Index in cancer outcomes.27,36
Primary and Secondary Outcomes
DSS is the primary outcome analyzed in this study, which was based on SEER data on the cause of death (cancer vs noncancer related). Patients who died from causes other than esthesioneuroblastoma or had an unknown cause of death were censored in survival analysis. Five-year CDSS was calculated according to simplified DSS estimates controlling for stage and SES.
Secondary outcomes included (1) stage of disease at diagnosis and (2) use of multimodal therapy (defined as receiving any ≥2 of surgery, radiation, and/or chemotherapy).
Statistical Analysis
Kaplan-Meier with log-rank statistic was used to examine the association between DSS and SES tertile, race, and ethnicity. A Cox proportional hazard regression model was generated while adjusting for age, sex, race, ethnicity, stage, Yost Index, and treatment (surgery, radiation, and/or chemotherapy). This model was utilized to understand the independent influence of SES, race, and ethnicity on survival and other outcomes. Multivariable logistic regression models were then generated to determine the association between secondary outcomes (stage at time of diagnosis and use of multimodal therapy) and SES, race, and ethnicity. Notably, logistic regression predicting the use of multimodal therapy was adjusted for stage of disease among other covariates.
A simplified Cox proportional hazard, S(x), regression model controlling for cancer stage and Yost Index was used to estimate CDSS. The 5-year CDSS is calculated through conditional survival: CS(5 +t | t) = S(5 +t) / S(t). S is the function for DSS based on the Cox proportional hazard regression model controlling for stage and Yost Index. This equation establishes the probability that a patient will live an additional 5 years, given that the patient has already survived t years since diagnosis. For example, the CDSS after 4 years since diagnosis is calculated as S(9)/S(4).
Statistical analysis was conducted in R version 4.03.1 (RStudio). Statistical significance was set at P < .05. This study was exempted by the Columbia University Institutional Review Board due to the deidentified nature of the data.
Results
In this study, 561 patients with complete data were included for analysis. Forty-four percent (n = 247) were in the third tertile, representing the highest SES, 32% (n = 182) in the second tertile, and 24% (n = 132) in the first tertile. Table 1 illustrates various demographic characteristics stratified by Yost Index tertiles. Most patients were White (79%), followed by Asian/Pacific Islander (10.7%) and Black (9.3%). Seventy-three patients (13.0%) were Hispanic. Patients in the highest SES tertile had the lowest mortality rate (16%), while patients in the lowest SES tertile exhibited the highest mortality (29%). The total incidence of deaths across the 3 SES tertiles was significantly different (P = .009).
Table 1.
Demographic Factors and Mortality Rate Stratified by SES Tertiles. a
| SES tertile, No. (%) | |||||
|---|---|---|---|---|---|
| Third (n = 247) | Second (n = 182) | First (n = 132) | Total | P value b | |
| Age, y | .489 | ||||
| <45 | 56 (23) | 43 (24) | 42 (32) | 141 (25.1) | |
| 45-59 | 98 (40) | 71 (39) | 43 (33) | 212 (37.8) | |
| 60-79 | 81 (33) | 57 (31) | 38 (29) | 176 (31.4) | |
| ≥80 | 12 (4.9) | 11 (6.0) | 9 (6.8) | 32 (5.7) | |
| Race | |||||
| White | 194 (79) | 147 (81) | 102 (77) | 443 (79.0) | <.001 |
| Black | 12 (4.9) | 17 (9.3) | 23 (17) | 52 (9.3) | |
| Asian/Pacific Islander | 36 (15) | 17 (9.3) | 7 (5.3) | 60 (10.7) | |
| Alaskan Native/Native American | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Other | 5 (2.0) | 1 (0.5) | 0 (0) | 6 (1.1) | |
| Hispanic | <.001 | ||||
| Non-Hispanic | 226 (91) | 162 (89) | 100 (76) | 488 (87.0) | |
| Hispanic | 21 (8.5) | 20 (11) | 32 (24) | 73 (13.0) | |
| Sex | .312 | ||||
| Female | 110 (45) | 68 (37) | 57 (43) | 235 (41.9) | |
| Male | 137 (55) | 114 (63) | 75 (57) | 326 (58.1) | |
| Mortality | .009 | ||||
| Censored | 208 (84) | 139 (76) | 94 (71) | 441 (78.6) | |
| Dead due to cancer | 39 (16) | 43 (24) | 38 (29) | 120 (21.4) | |
Abbreviation: SES, socioeconomic status.
The third tertile indicates the highest SES, and the first tertile indicates the lowest SES.
Chi-square test of independence. Bold indicates P < .05.
Table 2 illustrates the tumor characteristics of the patients. The largest number of patients presented with regional disease (42%). Thirty percent of patients received chemotherapy, 86% surgery, and 71% radiation. The likelihood of being treated with chemotherapy (P = .020) and surgery (P = .006) was also significantly different across Yost Index tertiles. Patients in the highest SES tertile exhibited the lowest rates of chemotherapy treatment (25%), and patients in the lowest SES tertile had the lowest rates of surgery (79%).
Table 2.
Tumor and Treatment Characteristics Stratified by SES Tertiles.
| SES tertile, No. (%) | ||||
|---|---|---|---|---|
| Third (n = 247) | Second (n = 182) | First (n = 132) | P value a | |
| Stage of disease | .082 | |||
| Localized | 69 (28) | 43 (24) | 21 (16) | |
| Regional | 105 (43) | 74 (41) | 59 (45) | |
| Distant | 73 (30) | 65 (36) | 52 (39) | |
| Chemotherapy: yes | 61 (25) | 60 (33) | 50 (38) | .020 |
| Surgery: yes | 224 (91) | 155 (85) | 104 (79) | .006 |
| Radiation: yes | 178 (72) | 129 (71) | 90 (68) | .730 |
Abbreviation: SES, socioeconomic status.
Chi-square test of independence. Bold indicates P < .05.
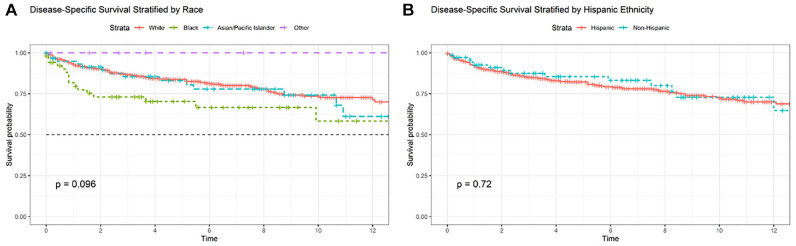
Kaplan-Meier analysis ( Figures 1 and 2 ) illustrated a significant difference in DSS when stratified by Yost Index (log-rank P < .001) but not race (P = .096) or Hispanic ethnicity (P = .720). According to Cox proportional hazard models ( Table 3 ), only SES measured by the Yost Index was associated with DSS after controlling for tumor stage, treatment, and other demographic factors. The lowest SES tertile exhibited worse DSS vs the highest (hazard ratio, 1.70 [95% CI, 1.05-2.75]; P = .03).
Figure 1.
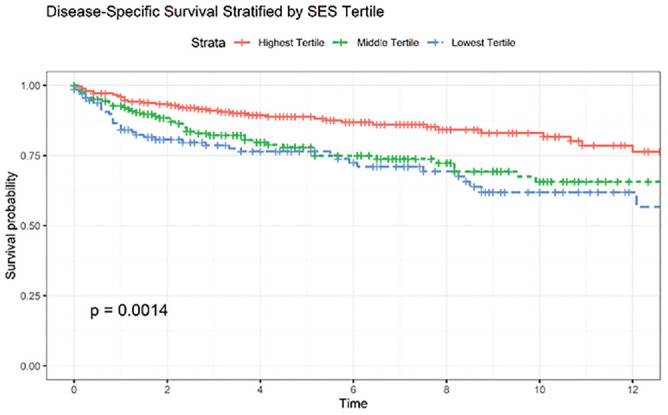
Kaplan-Meier curve stratified by socioeconomic status tertiles. Significance values are results of log-rank statistics.
Figure 2.
Kaplan-Meier survival curve stratified by (a) race and (b) Hispanic ethnicity with log-rank statistics.
Table 3.
Cox Proportional Hazard Model for Disease-Specific Survival. a
| HR | 95% CI | P value b | |
|---|---|---|---|
| Race | |||
| White | — | — | |
| Black | 1.32 | 0.75-2.32 | .30 |
| Asian/Pacific Islander | 1.30 | 0.73-2.30 | .40 |
| Other | 0.00 | 0.00-Inf | >.9 |
| Sex | |||
| Female | — | — | |
| Male | 1.47 | 1.00-2.17 | .05 |
| Hispanic ethnicity | |||
| Non-Hispanic | — | — | |
| Hispanic | 0.91 | 0.50-1.65 | .80 |
| Yost Index, SES tertile | |||
| Highest | — | — | |
| Middle | 1.48 | 0.95-2.32 | .08 |
| Lowest | 1.70 | 1.05-2.75 | .03 |
Abbreviations: HR, hazard ratio; SES, socioeconomic status.
Controlling for age, stage, treatment, and age in addition to variables in table.
Bold indicates P < .05.
Table 4 depicts logistic regression models predicting advanced-stage cancer, defined as regional or distant spread, at presentation. Patients of the lowest SES tertile exhibited an increased likelihood of a having an advanced-stage lesion at the time of diagnosis as compared with the highest SES tertile (odds ratio, 1.84 [95% CI, 1.06-3.30]; P = .035). Additionally, we examined predictors of multimodal therapy ( Table 5 ). Black patients were significantly less likely than their White counterparts to receive multimodal therapy (odds ratio, 0.44 [95% CI, 0.24-0.84]; P = .011). SES was not associated with the likelihood of receiving multimodal therapy.
Table 4.
Logistic Regression Model Predicting Late-Stage Disease at Diagnosis. a
| OR | 95% CI | P value b | |
|---|---|---|---|
| Sex | |||
| Female | — | — | |
| Male | 1.34 | 0.89-1.99 | .157 |
| Race | |||
| White | — | — | |
| Black | 2.4 | 1.05-6.48 | .055 |
| Asian/Pacific Islander | 1.74 | 0.89-3.68 | .123 |
| Other | 0.39 | 0.07-2.18 | .261 |
| Hispanic ethnicity | |||
| Non-Hispanic | — | — | |
| Hispanic | 1.26 | 0.68-2.44 | .479 |
| Yost Index, SES tertile | |||
| Highest | — | — | |
| Middle | 1.19 | 0.76-1.88 | .444 |
| Lowest | 1.84 | 1.06-3.30 | .035 |
Abbreviations: OR, odds ratio; SES, socioeconomic status.
Controls for age in addition to variables in table.
Bold indicates P < .05.
Table 5.
Logistic Regression Model Predicting Use of Multimodal Therapy. a
| OR | 95% CI | P value b | |
|---|---|---|---|
| Sex | |||
| Female | — | — | |
| Male | 0.9 | 0.61-1.32 | .584 |
| Race | |||
| White | — | — | |
| Black | 0.44 | 0.24-0.84 | .011 |
| Asian/Pacific Islander | 1.12 | 0.58-2.27 | .744 |
| Other | 0.07 | 0.00-0.48 | .019 |
| Hispanic ethnicity | |||
| Non-Hispanic | — | — | |
| Hispanic | 0.96 | 0.53-1.78 | .894 |
| Yost Index, SES tertile | |||
| Highest | — | — | |
| Middle | 0.9 | 0.58-1.41 | .644 |
| Lowest | 0.78 | 0.47-1.29 | .327 |
Abbreviations: OR, odds ratio; SES, socioeconomic status.
Controls for stage and age in addition to variables in table.
Bold indicates P < .05.
Figure 3 illustrates conditional survival curves stratified by stage of disease and SES tertiles. For localized disease, 5-year conditional survival estimates remain consistently favorable, with limited differences among SES tertiles. With increasing stage, there were stronger improvements in CDSS in the first 2 years after diagnosis, but these improvements diminished with increased survivorship, as shown by the flattening slope. This finding suggests that prognosis does not dramatically improve with increased survival. Qualitatively, the differences among SES tertiles appear to become more pronounced as stage at diagnosis becomes more advanced, with the lowest SES tertile consistently exhibiting the worst conditional survival predictions.
Figure 3.
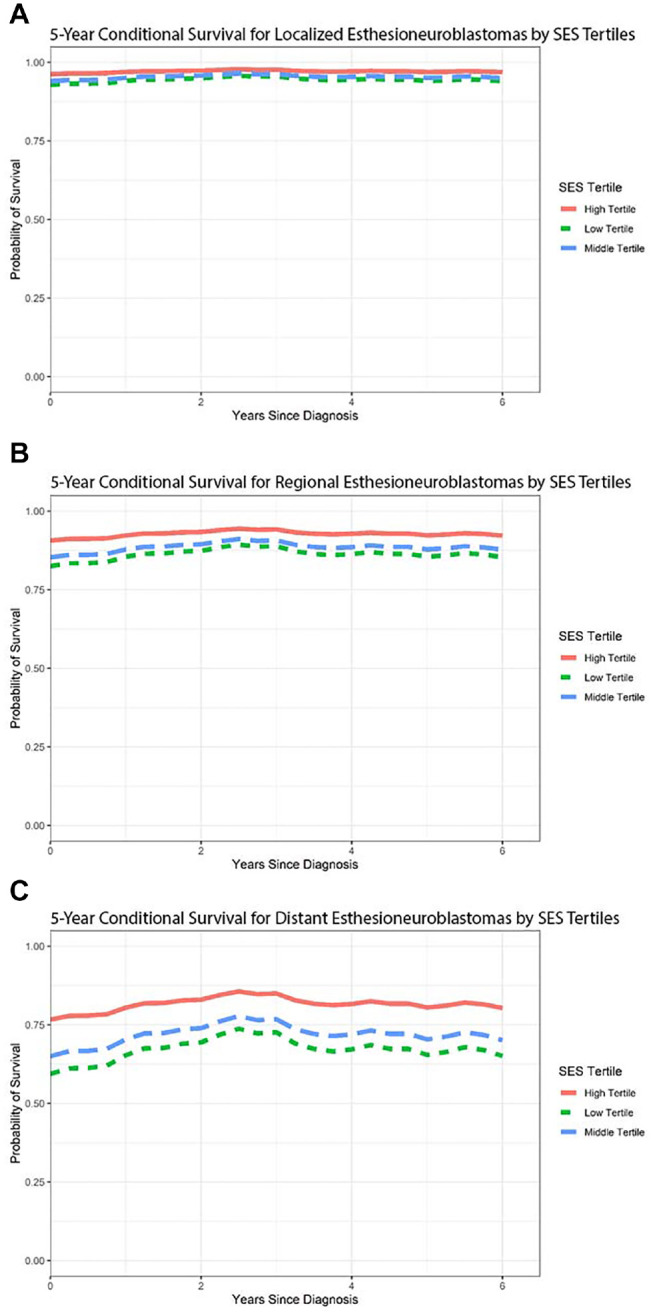
Conditional survival graphs predicting disease-specific survival of (a) localized, (b) regional, and (c) distant esthesioneuroblastoma, stratified by socioeconomic status (SES) tertiles.
Discussion
In this study, we analyzed >500 patients with esthesioneuroblastoma in the National Cancer Institute’s SEER registry to understand the association of race, ethnicity, and SES with several outcome metrics, such as DSS, use of multimodal therapy, and cancer stage at presentation. Additionally, we examined the effect of SES on 5-year conditional survival, a powerful metric in cancer outcomes that more accurately characterizes prognosis and can effectively inform surveillance strategies. While the effect of race and SES on cancer outcomes has been well described for various malignancies, few studies have examined their role in esthesioneuroblastoma outcomes, and none have examined conditional survival.
Our analysis illustrates that patients of lower SES status experienced higher mortality and worse DSS after controlling for a number of potential confounders, including stage at diagnosis and treatment modality. Notably, patients of the lowest SES tertile experienced a dramatic 70% worse DSS when compared with the highest SES tertile. However, race and ethnicity alone were not statistically associated with differences in DSS in this cohort.
Disease presentation and treatment modalities are significantly associated with sociodemographic factors. We illustrated that patients of the lowest SES tertile were almost 85% more likely to present with advanced-stage cancer than patients in the highest SES tertile, a dramatic disparity. Disease stage at presentation is often considered a proxy for access to specialized tertiary care, as a delay in access to care represents an opportunity for disease progression. While there was no association between SES tertiles and use of multimodal therapy, race has a considerable association with treatment course. Black patients were >60% less likely to receive multimodal therapy than White patients. These findings suggest that there is treatment equality across SES but not across different races, which is interesting as SES and race are traditionally closely related. Of note, the nuances and intricacies of treatment frequency and duration are not effectively captured within our database, which may explain some of these discrepancies.
Socioeconomic factors, ranging from marital status to neighborhood characteristics, have been associated with disease stage at presentation as well as quality of care. However, the interplay of SES and cancer outcomes is complex and multifaceted.37,38 Proper management of oncologic disease involves numerous components, such as access to and quality of health care and material resources, all of which are intertwined with economic and social policies. Patients of lower SES may present at later stages for numerous reasons—for example, a lower frequency of primary and specialist care utilization or disproportionate exposure to carcinogenic environments. However, as few risk factors for esthesioneuroblastoma have been identified at this time, delayed acknowledgment of symptoms by the patient and a low index of clinical suspicion by providers may also contribute to late-stage diagnosis. Other possible factors could include scrutinizing the health literacy of patients, distribution of specialty referral networks, adherence to therapy protocols, and surveillance strategies.
Finally, our analysis of CDSS helps to elucidate the possible mechanisms for reduced survival by SES tertiles. Notably, SES does not primarily affect conditional survival estimates for localized tumors but does for regional and distant staged tumors. There was no convergence among the tertiles even several years after diagnosis. Factors affecting long-term survival metrics in regional and distant tumors commonly include adherence to surveillance and treatment modalities, both of which may be affected by SES and access to care. Therefore, the mechanisms of SES and survival may be due to long-term over short-term factors, although further studies scrutinizing recurrence and related outcomes are necessary. It is also noteworthy that our data demonstrate a relatively constant 5-year CDSS for esthesioneuroblastoma. In other words, a patient who has survived 5 years since the time of diagnosis still has about the same 5-year survival as when the diagnosis was made. This survival outcome is unusual for head and neck cancers. In general, head and neck cancer recurrence and mortality are thought to occur most often within the first 5 years after diagnosis and treatment, and continued survivorship is associated with an improved DSS and prognosis; this relationship would be demonstrated with an upward-sloping, increasing CDSS over time, rather than the relatively constant CDSS that we have identified for esthesioneuroblastoma.
Esthesioneuroblastoma is a rare lesion; therefore, large statistical analyses require the use of a large database. However, such studies have several inherent limitations. Large national databases such as the SEER registry help study rare diseases such as esthesioneuroblastoma, but they carry limitations regarding the specificity and nuances of data. While robust and generalizable, the SEER registry lacks comorbidity data, detailed treatment information, and recurrence data, all of which affect survival in these cohorts. Additionally, the Yost Index uses census tract–level data. Although this is one of the most effective geospatial measurements of SES available, it does not replace prospectively collected SES data at the individual patient level. Additionally, there are inherent limitations to the characterization of race and ethnicity in the SEER database. SEER uses a fairly narrow representation of race and ethnicity, which does not capture the rich diversity of ethnic-racial backgrounds in the United States, as well as those who are from multiple racial and ethnic backgrounds. Additionally, SEER captures only a portion of the US population, which carries limitations when generalizing to more racially/ethnically diverse regions of the county or internationally. Finally, this cohort comprises patients who were diagnosed over a time span of nearly 40 years. Changing treatment paradigms and management strategies during this time may influence survival estimates as well.
Overall, we believe that this study provides insight on the association of race, ethnicity, and SES with clinical outcomes for patients with esthesioneuroblastoma. These data not only provide a foundation for further study to determine the mechanisms of the associations between sociodemographic factors and cancer outcomes but also represent an opportunity for otolaryngologists to explore interventions to mitigate these considerable disparities for esthesioneuroblastoma.
Conclusion
Esthesioneuroblastoma presentation, treatment, and survival outcomes are significantly associated with demographic factors such as race, ethnicity, and SES. This study is the first to examine CDSS for this specific pathology, which illustrates an unusual lack of improved DSS with increasing survivorship. Further studies should be devoted to the nuances of these observations to mitigate these risks for vulnerable patient populations.
Author Contributions
Rahul K. Sharma, data analysis, data interpretation, manuscript drafting, editing, design; Alexandria L. Irace, data interpretation, manuscript drafting, editing; Jonathan B. Overdevest, data interpretation, manuscript drafting, editing; Justin H. Turner, data interpretation, manuscript drafting, editing; Zara M. Patel, data interpretation, manuscript drafting, editing; David A. Gudis, data analysis, data interpretation, manuscript drafting, editing, design.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
Footnotes
The abstract of this article was presented at the 67th Annual Meeting of the American Rhinologic Society; October 1-2, 2021; Los Angeles, California.
References
- 1. Saini AT, Genden EM, Megwalu UC. Sociodemographic disparities in choice of therapy and survival in advanced laryngeal cancer. Am J Otolaryngol. 2016;37(2):65-69. doi: 10.1016/j.amjoto.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 2. Adams SA, Smith ER, Hardin J, et al. Racial differences in follow-up of abnormal mammography findings among economically disadvantaged women. Cancer. 2009;115(24):5788-5797. doi: 10.1002/cncr.24633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhambhvani HP, Rodrigues AJ, Medress ZA, et al. Racial and socioeconomic correlates of treatment and survival among patients with meningioma: a population-based study. J Neurooncol. 2020;147(2):495-501. doi: 10.1007/s11060-020-03455-2 [DOI] [PubMed] [Google Scholar]
- 4. Carey RM, Parasher AK, Workman AD, et al. Disparities in sinonasal squamous cell carcinoma short- and long-term outcomes: analysis from the national cancer database. Laryngoscope. 2018;128(3):560-567. doi: 10.1002/lary.26804 [DOI] [PubMed] [Google Scholar]
- 5. Daly MC, Jung AD, Hanseman DJ, et al. Surviving rectal cancer: examination of racial disparities surrounding access to care. J Surg Res. 2017;211:100-106. doi: 10.1016/j.jss.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 6. De La Cruz LM, Shulman LN. “Under” surveillance: impact of race and socioeconomic status on post-treatment breast cancer imaging. Ann Surg Oncol. 2018;25(6):1456-1457. doi: 10.1245/s10434-018-6418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanson HA, Martin C, O’Neil B, et al. The relative importance of race compared to health care and social factors in predicting prostate cancer mortality: a random forest approach. J Urol. 2019;202(6):1209-1216. doi: 10.1097/JU.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozdemir BC, Dotto GP. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3(3):181-197. doi: 10.1016/j.trecan.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith ER, Adams SA, Das IP, et al. Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2882-2890. doi: 10.1158/1055-9965.EPI-08-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yost K, Perkins C, Cohen R, et al. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 11. Division of Cancer Control and Population Sciences, National Cancer Institute. Cancer stat facts: cancer disparities. Accessed December 4, 2020. https://seer.cancer.gov/statfacts/html/disparities.html
- 12. National Cancer Institute. Cancer disparities. Published 2020. https://www.cancer.gov/about-cancer/understanding/disparities#ui-id-22021
- 13. Owoyemi O, Aakhus E. Underrepresentation in oncology: identifying and addressing structural barriers. Oncologist. Published online April 3, 2021. doi: 10.1002/onco.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minas TZ, Kiely M, Ajao A, et al. An overview of cancer health disparities: new approaches and insights and why they matter. Carcinogenesis. 2021;42(1):2-13. doi: 10.1093/carcin/bgaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel ZM, Li J, Chen AY, et al. Determinants of racial differences in survival for sinonasal cancer. Laryngoscope. 2016;126(9):2022-2028. doi: 10.1002/lary.25897 [DOI] [PubMed] [Google Scholar]
- 16. Liermann J, Syed M, Held T, et al. Advanced radiation techniques in the treatment of esthesioneuroblastoma: a 7-year single-institution’s clinical experience. Cancers (Basel). 2018;10(11):457. doi: 10.3390/cancers10110457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svane-Knudsen V, Jorgensen KE, Hansen O, et al. Cancer of the nasal cavity and paranasal sinuses: a series of 115 patients. Rhinology. 1998;36(1):12-14. [PubMed] [Google Scholar]
- 18. Ow TJ, Bell D, Kupferman ME, et al. Esthesioneuroblastoma. Neurosurg Clin N Am. 2013;24(1):51-65. doi: 10.1016/j.nec.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 19. Chao KS, Kaplan C, Simpson JR, et al. Esthesioneuroblastoma: the impact of treatment modality. Head Neck. 2001;23(9):749-757. doi: 10.1002/hed.1107 [DOI] [PubMed] [Google Scholar]
- 20. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501). Head Neck. 2005;27(10):843-850. doi: 10.1002/hed.20279 [DOI] [PubMed] [Google Scholar]
- 21. Foote RL, Morita A, Ebersold MJ, et al. Esthesioneuroblastoma: the role of adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1993;27(4):835-842. doi: 10.1016/0360-3016(93)90457-7 [DOI] [PubMed] [Google Scholar]
- 22. Platek ME, Merzianu M, Mashtare TL, et al. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2011;6:41. doi: 10.1186/1748-717X-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozsahin M, Gruber G, Olszyk O, et al. Outcome and prognostic factors in olfactory neuroblastoma: a rare cancer network study. Int J Radiat Oncol Biol Phys. 2010;78(4):992-997. doi: 10.1016/j.ijrobp.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 24. Nishimura H, Ogino T, Kawashima M, et al. Proton-beam therapy for olfactory neuroblastoma. Int J Radiat Oncol Biol Phys. 2007;68(3):758-762. doi: 10.1016/j.ijrobp.2006.12.071 [DOI] [PubMed] [Google Scholar]
- 25. Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32(5):706-714. doi: 10.1227/00006123-199305000-00002 [DOI] [PubMed] [Google Scholar]
- 26. Berger MH, Lehrich BM, Yasaka TM, et al. Characteristics and overall survival in pediatric versus adult esthesioneuroblastoma: a population-based study. Int J Pediatr Otorhinolaryngol. 2021;144:110696. doi: 10.1016/j.ijporl.2021.110696 [DOI] [PubMed] [Google Scholar]
- 27. Moss JL, Stinchcomb DG, Yu M. Providing higher resolution indicators of rurality in the Surveillance, Epidemiology, and End Results (SEER) database: implications for patient privacy and research. Cancer Epidemiol Biomarkers Prev. 2019;28(9):1409-1416. doi: 10.1158/1055-9965.EPI-19-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu M, Tatalovich Z, Gibson JT, et al. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81-92. doi: 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 29. Bouvier AM, Remontet L, Hedelin G, et al. Conditional relative survival of cancer patients and conditional probability of death: a French national database analysis. Cancer. 2009;115(19):4616-4624. doi: 10.1002/cncr.24489 [DOI] [PubMed] [Google Scholar]
- 30. Janssen-Heijnen ML, Gondos A, Bray F, et al. Clinical relevance of conditional survival of cancer patients in europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010;28(15):2520-2528. doi: 10.1200/JCO.2009.25.9697 [DOI] [PubMed] [Google Scholar]
- 31. Merrill RM, Henson DE, Barnes M. Conditional survival among patients with carcinoma of the lung. Chest. 1999;116(3):697-703. doi: 10.1378/chest.116.3.697 [DOI] [PubMed] [Google Scholar]
- 32. Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15(8):873-882. doi: 10.1634/theoncologist.2009-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah MM, Meyer BI, Rhee K, et al. Conditional survival analysis of hepatocellular carcinoma. J Surg Oncol. Published online June 12, 2020. doi: 10.1002/jso.26049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zabor EC, Radivoyevitch T, Singh AD, et al. Conditional survival in uveal melanoma. Ophthalmol Retina. Published online September 27, 2020. doi: 10.1016/j.oret.2020.09.015 [DOI] [PubMed] [Google Scholar]
- 35. Elkon D, Hightower SI, Lim ML, et al. Esthesioneuroblastoma. Cancer. 1979;44(3):1087-1094. doi: [DOI] [PubMed] [Google Scholar]
- 36. Rajeshuni N, Zubair T, Ludwig CA, et al. Evaluation of racial, ethnic, and socioeconomic associations with treatment and survival in uveal melanoma, 2004-2014. JAMA Ophthalmol. 2020;138(8):876-884. doi: 10.1001/jamaophthalmol.2020.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhanvadia SK, Psutka SP, Burg ML, et al. Financial toxicity among patients with prostate, bladder, and kidney cancer: a systematic review and call to action. Eur Urol Oncol. Published online April 7, 2021. doi: 10.1016/j.euo.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 38. Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315-332. doi: 10.1038/s41416-020-01038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]