Abstract
Objectives:
Retaining on antiretroviral therapy is essential for reducing HIV-related morbidity and mortality. However, attrition in HIV-positive children remains a critical challenge in resource-limited settings, including Ethiopia. This study aims to determine the incidence and predictors of attrition among children on antiretroviral therapy at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Methods:
An institution-based retrospective follow-up study was conducted among 357 HIV-positive children at the University of Gondar Comprehensive Specialized Hospital from 1 January 2005 to 30 December 2018 (G.C.). Data were collected by chart review using a structured and pre-tested data abstraction checklist. SPSS 22 and STATA 14.0 were used for data entry and analysis, respectively. In the Cox proportional hazard model, bivariables had a 0.25 computed to multivariable, and variables with a p-value of 0.05 at a 95% confidence interval were considered statistically significant predictors of attrition incidence.
Results:
A total of 344 child records with a completeness rate of 96.4% were reviewed and included in the analysis. The median follow-up period was 4.3 (interquartile range = 4.3 ± 4.7) years, and the median survival time was 132 months. The incidence rate of attrition was 6.6 per 100 person year observation (PYO) (95% confidence interval = 5.5, 8.0). In all, 105 (30.5%) children were recorded as attrition in the follow-up period. Baseline WHO clinical staging 3 and 4 (adjusted hazard ratio = 2.3 (95% confidence interval = 1.3, 4.0)), baseline weight-for-age −2 Z-score (adjusted hazard ratio = 3.1 (95% confidence interval = 1.7, 5.3)), cotrimoxazole non-users (adjusted hazard ratio = 2.5 (95% confidence interval = 1.4, 4.3)), and baseline hemoglobin levels 10 mg/dL (adjusted hazard ratio = 2.7 (95% confidence interval = 1.5, 47)) were found to be a predictor of attrition.
Conclusion:
The overall incidence of the rate of attrition was high. Baseline WHO clinical staging 3/4, baseline hemoglobin 10 mg/dL, cotrimoxazole (cotrimoxazole preventive therapy) non-user, and underweight at baseline (weight-for-age 2 Z-score) were found to be the main predictors of attrition.
Keywords: Attrition, incidence, predictors, antiretroviral therapy, Ethiopia
Introduction
Early detection of Human Immunodeficiency Virus (HIV) infection, linking and retaining them on antiretroviral therapy (ART), is essential for reducing morbidity and improving survival. 1 Ensuring adequate retention in ART care is one of the critical steps needed to maximize the benefits of ART as a strategy to prevent death and disease transmission. 2 However, the incidence of attrition in children on ART is higher in resource-limited settings when compared to developed countries, and it remains a critical challenge for HIV-positive children in ART programs.3,4
A study in five Asian countries found the incidence of attrition was 6.5 per 100 child-years. 5 In the pediatric West African Database, the incidence of attrition included Benin, Cote d’Ivoire, Gambia, Ghana, Senegal, and Mali was 26.2 per 100 child-years. 6
In 2014, the United Nations Program on HIV/AIDS (UNAIDS) launched the ambitious “90–90–90” targets to help end the AIDS epidemic by 2030.7,8 The programmatic feature of their targets is not only expanding access to diagnosis and treatment, but also focusing on the quality of care in terms of retention and suppression, which are key to optimal HIV outcomes. 7
Scale-up of ART has led to significant declines in HIV-related morbidity and mortality in Ethiopia. However, attrition from ART care remains a major public health concern, 9 and it leads to early morbidity and mortality, which possibly increases the risk of HIV transmission, the progression of the disease, and resistance to different drug regimens.10,11
In Ethiopia, the 2015–2020 HIV/AIDS prevention care and treatment strategic plan aims to pave the path to ending AIDS by 2030. The targets set in this investment case are in line with the three 90’s (90–90–90) treatment targets set by UNAIDS to help end the AIDS epidemic. However, attrition, mainly in children, has been a challenge in resource-limited settings, including Ethiopia, because attrition is interlinked with different predictor variables. Furthermore, children are more vulnerable to attrition in ART services than adults because they rely on their parents or caregivers to gain access to health care services. 12
A limited study conducted in Ethiopia provided important insights about the incidence and predictors of attrition among HIV-positive children on ART. Many of the studies conducted in the previous era were cross-sectional in nature and had a short-term follow-up period. Furthermore, determining the rate of attrition and its predictors with a longer-term retrospective follow-up study is critical in Ethiopia in general and in the study area in particular in order to meet the ambitious plan by 2030. Hence, this study aims to determine the incidence and predictors of attrition among children on ART at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia.
Method and material
Study design and period
A retrospective follow-up study was conducted from 1 January 2005 to 30 December 2018.
Study setting
The study was conducted at the University of Gondar Compressive Specialized Hospital ART clinic, and the hospital is located in Gondar, which is located in the North Gondar zone of northwestern Ethiopia, far from Addis Ababa, Ethiopia’s capital city. The institution is found in Northwest Ethiopia and serves more than five million people in North Gondar and neighboring zones. The services provided by the University of Gondar Compressive Specialized Hospital include pediatrics, surgery, gynecology and obstetrics, internal medicine, psychiatry, ophthalmology, ART, and so on in its inpatient and outpatient clinics. 13
According to the Ethiopian Demographic and Health Survey 2016, the national HIV prevalence was 0.9% and the urban prevalence was 2.9%. 14 According to the hospital’s ART case team report, ART service started in 2005, and a total of 8581 adults and 1138 children (i.e. less than 15 years of age) were enrolled in HIV care until March 2017. 15 Moreover, the patients are seen in the clinic every 3 months for regular follow-up. The healthcare providers in the ART clinic have used national guidelines for comprehensive HIV prevention, care, and treatment. The guidelines should include a full range of services that should be provided in conjunction with HIV testing, such as counseling (pre-test information and post-test counseling), referral to appropriate HIV prevention, treatment, and care services, and other clinical services.
Study population
All children under 15 years old living with HIV from 1 January 2005 to 30 December 2018 at the University of Gondar Compressive Specialized Hospital.
Inclusion criteria
Children whose age was less than 15 years and who followed their treatment during the study period were eligible for this study.
Exclusion criteria
The medical charts and ART registration logbooks of the study participants with unrecorded values of the outcome variable (i.e. the status of attrition) were excluded.
Sample size determination and sampling procedure
The sample size was calculated using Epi info package software of the two-population proportion formula by considering the following assumptions: 95% confidence level (CI), 80% optimum statistical power, and taking type one error of 5%. By considering the previous study conducted in the University of Gondar Compressive Specialized Hospital, Ethiopia, 15 and taking fair or poor adherence as the exposed group (i.e. adjusted hazard ratio (AHR) = 3.5; 95% CI = 1.7, 7.5) denoted by p1 (0.22), and good adherence as the non-exposed group denoted by q2 (0.10), the total sample size after adding 10% incomplete medical records was 357. A total of 1196 children who started ART during the study period were identified in the pediatric ART clinics using the ART registration logbook as a sampling frame. The investigator assigned the registration numbers from January 2005 to December 2018, in chronological order. Of these, the investigator drew 357 samples that fulfilled the inclusion criteria after reviewing the medical charts and ART registration logbook, and then 13 medical records that did not fulfill the inclusion criteria were excluded.
Operational definition
Attrition (i.e. event) defined as a child either dead or lost to follow-up (LTFU) reported in the child’s medical record in the follow-up period. 16
The follow-up time or period is measured from time zero (the start of the study or from the point at which the participant is considered to be at risk) until the event occurs or the study ends. Mortality is defined as a child’s medical record being recorded as “dead” on the patient’s exit form, and loss to follow-up is defined as a child who missed 3 months or more after the last scheduled visit.
Data collection tools and procedures
The data extraction checklist is adapted from national HIV follow-up forms, the data extraction checklist. 17 Data were extracted from children’s charts in terms of socio-demographic, clinical, and treatment-related variables. The data were collected by three BSc nurses, with one supervisor having an MSc in pediatrics. Both the data collector and the supervisor took comprehensive HIV care training. Once extracted, data from children’s charts were coded in order to avoid duplication. A pre-test was conducted among 18 (5%) medical records to check the consistency of the abstraction tool; one-day training and briefings were given about how to review the documents and extract data from them. The filled formats were checked for completeness by the supervisor each day, and double data entry was performed to reduce errors, and data cleaning was performed before data analysis.
Statistical analysis
The data were entered in SPSS version 22 and transferred to STATA version 14 for analysis. Descriptive and summary statistics were carried out through the table and figures. The incidence rate of attrition was calculated by dividing the number of a combination of children either children died or LTFU during the follow-up period by the child-years of follow-up. A Kaplan–Meier curve was used to estimate median survival time and a log-rank test was entered into the multivariable to see the difference between categorical variables. The life table was used to determine the cumulative probability of survival at 12, 24, and 36 months. Based on the Akaike Information Criteria (AIC) comparison, the Cox proportional hazard model was identified to be an efficient model. The Cox proportional hazards model assumption was checked for variables by the Schoenfeld residual and for goodness-of-fitness by the Cox Snell residual test. Both bi-variable and multivariable analyses were used to identify predictors of attrition. In bi-variable having a p-value less than 0.25 computed into the multivariable analysis, p-value 0.05 and 95% CI of hazard ratio (HR) in the multivariable analysis were considered to declare significance.
Results
Socio-demographic characteristics of children on ART
A total of 357 HIV-positive children’s medical records with a completeness rate of 96.4% were reviewed and 13 (3.6%) were excluded from the analysis due to missing data on baseline WHO clinical stage, baseline cluster of differentiation 4 (CD4) count, hemoglobin level, height/weight for age, or level of adherence. The remaining 344 HIV-positive children’s medical records were included in the analysis. The mean age of the study participants was 7.3 (standard deviation (SD) = 3.3) years, and 133 (38.7%) of them were in the age group of 9–14 years. Males made up nearly half of the 178 (51.7%). Two hundred seventy-five (79.9%) of the respondents lived in urban areas.
About 236 (68.6%) of the children had both parents alive. Furthermore, 321 (93.3%) of the caregivers of the children were biological families. Approximately two-thirds (77.3%) of the children’s caregivers were HIV positive (Table 1).
Table 1.
Socio-demographic characteristics of HIV-positive children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018 (n = 344).
| Characteristics | Frequency (n = 344) | Percent (%) |
|---|---|---|
| Age (years) | ||
| <3 | 84 | 24.4 |
| 4–8 | 133 | 36.9 |
| 9–14 | 127 | 38.7 |
| Sex | ||
| Male | 178 | 51.7 |
| Female | 166 | 48.3 |
| Residence | ||
| Urban | 275 | 79.9 |
| Rural | 69 | 20.1 |
| Parent status | ||
| Both alive | 236 | 68.6 |
| Mother alive, but father dead | 52 | 15.1 |
| Mother dead, but father alive | 18 | 5.2 |
| Both dead | 38 | 11 |
| Relation of caregiver | ||
| Biological family | 321 | 93.3 |
| Non-biological caregivers | 23 | 6.7 |
| Caregiver/parent | ||
| HIV status | ||
| Positive | 266 | 77.3 |
| Negative | 38 | 11 |
| Not tested | 40 | 11.6 |
Clinical and treatment-related characteristics of children on ART
A total of 255 (74.1%) of children were in WHO clinical stages 1 and 2, and 55 (16%) of those children had CD4 counts below the threshold level at baseline.
Seventy-eight (22.4%) of children had experienced an initial regiment change during the follow-up period. Of these, 32 (9.3%) were due to toxicity or side effects. A total of 212 (61.6%) HIV-positive children started on ART within 6 months, and 267 (77.6%) children had a good level of adherence to ART. Thirty-four (9.9%) of the children had experienced treatment failure. Of these, 12 (3.5%) children were due to clinical failure. Children who had a baseline hemoglobin level of 10 mg/dL were 43 (12.5%). A majority of 226 (91.1%) and 85 (88.6%) were working in functional status and appropriate in motor development status, respectively, and 176 (51.2%) and 141 (41.2%) were underweight (−2 Z-score) and stunted (−2 Z-score) at baseline, respectively.
A total of 215 (62.5%) children’s initiations of ART were before the year 2014. Thirty-seven (10.8%) of the children had tuberculosis (TB) status. Furthermore, 301 (87.5%) children were cotrimoxazole preventive therapy (CPT) prophylaxis users, and 161 (46.8%) children were on ART for a duration of 12–59 months. About 28 (8.1%) of the initial ART regimens were protease inhibitor (PI)-based, and 167 (48.5%) of the children disclosed their HIV status (Table 2).
Table 2.
Clinical and treatment related of HIV-positive children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018 (n = 344).
| Variables | Frequency (n = 344) | Percent (%) |
|---|---|---|
| Baseline WHO clinical stage | ||
| Stages 1 and 2 | 255 | 74.1 |
| Stages 3 and 4 | 89 | 25.9 |
| Baseline CD4 count | ||
| Below threshold | 55 | 16.0 |
| Above threshold | 289 | 84.0 |
| Disclosure status | ||
| Disclosed | 167 | 48.5 |
| Non-disclosed | 177 | 51.5 |
| ART adherence | ||
| Good | 267 | 77.6 |
| Fair and poor | 77 | 22.4 |
| Initial regiment change | ||
| Yes | 78 | 22.7 |
| No | 266 | 77.3 |
| Reason for regiment change | ||
| Side effect/toxicities | 32 | 9.3 |
| Stock out | 12 | 3.5 |
| Treatment failure | 34 | 9.9 |
| Treatment failure | ||
| Yes | 34 | 9.9 |
| No | 310 | 90.1 |
| Immunologic failure | ||
| Yes | 9 | 2.6 |
| No | 335 | 97.4 |
| Clinical failure | ||
| Yes | 12 | 3.5 |
| No | 332 | 96.5 |
| Both immunologic failure and clinical failure | ||
| Yes | 6 | 1.7 |
| No | 338 | 98.3 |
| Virological failure | ||
| Yes | 7 | 2.0 |
| No | 337 | 98.0 |
| Baseline hemoglobin level | ||
| ⩽10 mg/dL | 43 | 12.5 |
| >10 mg/dL | 301 | 87.5 |
| Baseline height for age | ||
| Stunting | 141 | 41.0 |
| Normal | 203 | 59.0 |
| Baseline weight for age | ||
| Underweight | 176 | 51.2 |
| Normal | 168 | 48.8 |
| Baseline functional status >5 years (n = 248) | ||
| Working | 226 | 91.1 |
| Ambulatory | 19 | 7.7 |
| Bedridden | 3 | 1.2 |
| Baseline development status ⩽5 years (n = 96) | ||
| Appropriate | 85 | 88.6 |
| Delayed | 8 | 8.3 |
| Regressed | 3 | 3.1 |
| Timing of initiation | ||
| Early (⩽6 months) | 212 | 61.6 |
| Late (>6 months) | 132 | 38.4 |
| Year of initiations | ||
| ⩽2013 | 215 | 62.5 |
| ⩾2014 | 129 | 37.5 |
| Presence of TB | ||
| Yes | 37 | 10.8 |
| No | 307 | 89.2 |
| OI other than TB | ||
| Yes | 113 | 32.8 |
| No | 231 | 67.2 |
| CPT | ||
| Yes | 301 | 87.5 |
| No | 43 | 12.5 |
| IPT | ||
| Yes | 105 | 30.5 |
| No | 339 | 69.5 |
| Initial ART regiment-based | ||
| PI (protease inhibitor)-based | 28 | 8.1 |
| NVP/EVZ-based | 316 | 91.6 |
| Duration on ART | ||
| <12 months | 35 | 10.2 |
| 12 months to 60 months | 161 | 46.8 |
| >60 months | 148 | 43.0 |
NVP: nevirapine; EVZ: efavirenz; ART: antiretroviral therapy; OI: opportunistic infection; TB: tuberculosis; WHO: World Health Organization; CPT: cotrimoxazole prophylactic therapy; IPT: isoniazid prophylactic therapy; CD4: cluster of differentiation 4.
Incidence of attrition during follow-up period
Three hundred forty-four children were followed for different periods (4 months to 167 months) that gave a total of 19,081 child-months or 1590.1 child-years of observation. The median follow-up period was 4.3 (interquartile range (IQR) = 4.3 ± 4.7 years). At the end of follow-up, 239 (69.5%) of the children were under active follow-up, while 105 (31.5%) were not. Of these, 29 (8.4%) died and 76 (22.1%) lost follow-up. The cumulative probability of survival rate of retention at the end of 12, 24, and 36 months of follow-up years was 92.2%, 80.5%, and 77.5%, respectively. The incidence rate of attrition was 6.6 per 100 PYO (95% CI = (5.5, 8.0)). Of these, lost follow-up and mortality were 4.8 per 100 PYO (95% CI = 3.8, 6.0) and 1.8 per 100 PYO (95% CI = 1.3, 2.6) respectively. The incidence of attrition among HIV-positive children on ART in different categories of predictor variable was estimated (Table 3).
Table 3.
Incidence of attrition per 100 PYO stratified by different categories among children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018 (n = 344).
| Variables | Total (n = 344) | Censored (n = 239) | Attrition (n = 105) | IR of attrition (95% CI) | PPY |
|---|---|---|---|---|---|
| Age (years) | |||||
| <3 | 84 | 59 | 25 | 7.6 (5.1, 11.2) | 329.9 |
| 4–8 | 127 | 93 | 34 | 5.2 (3.7, 7.3) | 654.8 |
| 9–14 | 133 | 87 | 46 | 7.6 (5.7, 10.1) | 605.4 |
| Sex | |||||
| Male | 178 | 126 | 52 | 6.5 (5.0, 8.5) | 800 |
| Female | 166 | 113 | 53 | 6.7 (5.1, 8.8) | 790.1 |
| Residence | |||||
| Rural | 69 | 46 | 23 | 7.7 (5.1, 11.6) | 298.3 |
| Urban | 275 | 193 | 82 | 6.3 (5.1, 7.9) | 1291.8 |
| Parent status | |||||
| Both alive | 236 | 168 | 68 | 6.4 (5.1, 8.2) | 1056.3 |
| Both or either dead | 108 | 71 | 37 | 6.9 (5.0, 9.6) | 533.6 |
| Caregiver of the child | |||||
| Biological family | 321 | 226 | 95 | 6.4 (5.2, 7.8) | 1493.3 |
| Other | 23 | 13 | 10 | 10.3 (5.6, 19.2) | 96.8 |
| Baseline CD4 count | |||||
| Below threshold | 55 | 33 | 22 | 9.2 (6.1, 14.0) | 238.5 |
| above threshold | 289 | 206 | 83 | 6.1 (5.0, 7.6) | 1351.6 |
| Baseline hemoglobin | |||||
| ⩽10 mg/dL | 43 | 10 | 33 | 22.6 (16.1, 31.8) | 146 |
| ⩾−10 mg/dL | 301 | 229 | 72 | 5.0 (4.0, 6.3) | 1444.1 |
| Baseline weight for age | |||||
| <Under weight | 176 | 94 | 82 | 10.9 (8.8, 13.6) | 751.3 |
| Normal | 168 | 145 | 23 | 2.7 (1.8, 4.1) | 828.8 |
| Baseline height for age | |||||
| Stunting | 141 | 93 | 48 | 7.4 (5.6, 9.8) | 649.1 |
| Normal | 203 | 146 | 57 | 6.1 (5.1, 7.9) | 941 |
| Initial regimen given | |||||
| PI-based | 28 | 14 | 14 | 12.1 (7.2, 20.4) | 115.7 |
| NVP/EVZ-based | 316 | 225 | 91 | 6.2 (5.0, 7.6) | 1474.4 |
| Disclosure status | |||||
| Non-disclosed | 176 | 130 | 46 | 5.7 (4.3, 7.6) | 807.3 |
| Disclosed | 168 | 109 | 59 | 7.5 (5.8, 9.7) | 782.8 |
| Duration on ART | |||||
| <12 months | 35 | 9 | 26 | 107 (73, 150) | 24.2 |
| 12 months to 59 months | 161 | 102 | 59 | 13.2 (10.2, 17) | 447.8 |
| 59 months | 148 | 128 | 20 | 1.8 (1.1, 2.7) | 1118.2 |
| Baseline WHO stage | |||||
| Stages 1 and 2 | 255 | 203 | 52 | 4.3 (3.3, 5.6) | 1209.3 |
| Stages 3 and 4 | 89 | 36 | 53 | 13.9 (10.6, 18.2) | 380.8 |
| Presence of TB | |||||
| Yes | 37 | 17 | 20 | 12.1 (7.8, 18.7) | 165.8 |
| No | 307 | 222 | 85 | 6.0 (4.8, 7.4) | 1424.3 |
| OI other than TB | |||||
| Yes | 113 | 76 | 37 | 7.2 (5.2, 9.9) | 516.8 |
| No | 231 | 163 | 68 | 6.3 (4.9, 8.0) | 1073.3 |
| CPT prophylaxis | |||||
| Yes | 301 | 225 | 76 | 5.2 (4.2, 6.5) | 1454 |
| No | 43 | 14 | 29 | 21 (14.9, 30.7) | 136 |
| IPT prophylaxis | |||||
| Yes | 105 | 73 | 32 | 6.9 (4.9, 9.7) | 465.8 |
| No | 239 | 166 | 73 | 6.5 (5.2, 8.2) | 1124.3 |
| Timing of initiation | |||||
| Early (⩽6 months) | 212 | 141 | 71 | 7.3 (5.8, 9.1) | 977.5 |
| Late (>6 months) | 132 | 98 | 34 | 5.6 (3.9, 7.8) | 612.5 |
| Year of initiations | |||||
| ⩽2013 | 215 | 131 | 84 | 7.2 (5.8, 8.9) | 1165.3 |
| ⩾2014 | 129 | 108 | 21 | 4.9 (3.2, 7.8) | 424.8 |
| Baseline functional status <5 years (n = 248) | |||||
| Working | 226 | 160 | 66 | 6.0 (4.7, 7.7) | 1092.3 |
| Bedridden/ambulatory | 22 | 7 | 15 | 16.6 (10.0, 27.5) | 90.3 |
| Baseline developmental status ⩽5 years (n = 96) | |||||
| Appropriate | 85 | 63 | 22 | 6.0 (4.0, 9.1) | 367.8 |
| Delayed/regressed | 11 | 9 | 2 | 5.0 (1.3, 20.0) | 39.7 |
| Regimen change | |||||
| Yes | 78 | 49 | 29 | 8.6 (6.0, 12.4) | 336.3 |
| No | 267 | 190 | 76 | 6.1 (4.8, 7.6) | 1253.8 |
| Treatment failure | |||||
| Yes | 34 | 23 | 11 | 7.3 (4.1, 13.2) | 151.1 |
| No | 309 | 215 | 94 | 6.5 (5.3, 8.0) | 1439 |
| ART adherence | |||||
| Good | 267 | 187 | 80 | 6.4 (5.1, 7.9) | 1266.8 |
| Fair and poor | 77 | 52 | 25 | 7.7 (5.3, 12.0) | 323.3 |
PI: protease inhibitor; NVP: nevirapine; EVZ: efavirenz; ART: antiretroviral therapy; OI: opportunistic infection; TB: tuberculosis; WHO: World Health Organization; CPT: cotrimoxazole prophylactic therapy; IPT: isoniazid prophylactic therapy; CD4: cluster of differentiation 4; PPY: person per year.
Kaplan–Meier curve of attrition-free survival probability
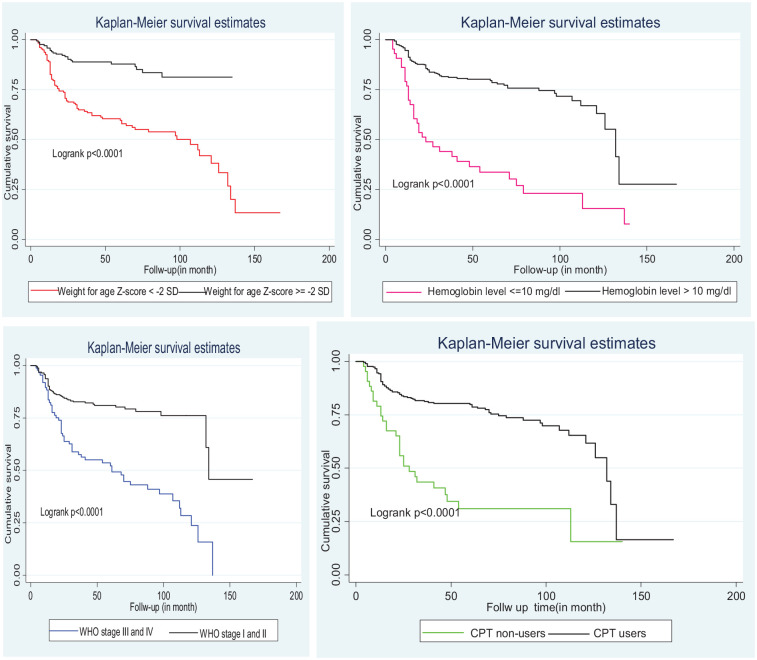
The cumulative probability of survival rate of retention at the end of 12, 24, and 36 months of follow-up years was 92.2%, 80.5%, and 77.5%, respectively. The median survival time was 132 months (Figure 1). Moreover, the Kaplan–Meier attrition-free survival probability of the main predictor variable was estimated (Figure 2).
Figure 1.
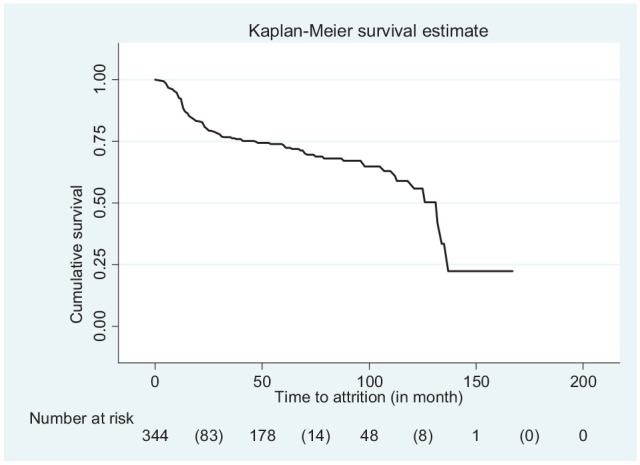
Kaplan–Meier curve of attrition-free survival probability among children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018.
Figure 2.
Kaplan–Meier of attrition-free survival of main predictor variable among children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018.
In the bi-variate Cox proportional hazard model, age, baseline CD4 count, baseline WHO clinical staging, baseline weight for age, baseline hemoglobin level, TB status, regimen change, baseline functional status, disclosure status, cotrimoxazole (CPT) non-users, the relationship of the caregiver, year of initiation, and initial regimen were statistically significant. However, in the multivariable Cox proportional hazard model, baseline WHO clinical staging, baseline weight for age, baseline hemoglobin level, and non-users of cotrimoxazole (CPT) were predictors of attrition at 5% significance level remained statistically significant (Table 4). The fitness of the Cox’s proportional hazard model was checked through the proportional-hazards assumption based on the Schoenfeld residuals test.
Table 4.
Multivariable analysis using Cox regression model for predictors of attrition among children on antiretroviral therapy at University of Gondar Compressive Specialized Hospital in Northwest Ethiopia, from January 2005 to December 2018.
| Variables | Survival status | CHR (95% CI) | p-value | AHR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| Attrition (n = 105) | Censored (n = 239) | |||||
| Age (years) | ||||||
| <3 | 25 | 59 | 0.9 (0.7, 1.5) | 0.753 | – | |
| 4–8 | 34 | 93 | 0.7 (0.4, 1.4) | 0.116 | – | |
| 9–14 | 46 | 87 | 1 | |||
| Sex | ||||||
| Male | 52 | 126 | 1 | |||
| Female | 53 | 113 | 1.1 (0.7, 1.6) | 0.35 | – | |
| Residence | ||||||
| Rural | 23 | 46 | 1 | |||
| Urban | 82 | 193 | 0.8 (0.5, 1.3) | 0.467 | – | |
| Parent status | ||||||
| Both alive | 68 | 168 | 1 | |||
| Both or either dead | 37 | 71 | 1.2 (0.8, 1.8) | 0.405 | – | |
| Caregiver of child | ||||||
| Biological family | 96 | 225 | 0.6 (0.3, 1.2) | 0.125 | 0.9 (0.4, 1.8) | 0.768 |
| Other | 10 | 13 | 1 | 1 | ||
| Baseline CD4 count | ||||||
| Below threshold | 22 | 33 | 1.4 (0.9, 2.3) | 0.151 | 1.4 (0.8, 2.5) | 0.257 |
| Above threshold | 83 | 206 | 1 | 1 | ||
| Baseline hemoglobin | ||||||
| ⩽10 mg/dL | 33 | 10 | 3.9 (2.6, 6.0) | 0.000 | 2.7 (1.5, 4.7) | 0.001 |
| >10 mg/dL | 72 | 229 | 1 | 1 | ||
| Baseline weight for age | ||||||
| Underweight | 82 | 114 | 3.8 (2.4, 6.0) | 0.000 | 3.1 (1.7, 5.3) | 0.000 |
| Normal | 23 | 125 | 1 | 1 | ||
| Baseline height for age | ||||||
| Stunting | 48 | 93 | 1.2 (0.8, 1.8) | 0.300 | – | |
| Normal | 57 | 146 | 1 | |||
| Initial regimen | ||||||
| PI-based | 14 | 14 | 1.7 (1.0, 3.1) | 0.064 | 1.6 (0.8, 3.2) | 0.227 |
| NVP/EVZ-based | 91 | 225 | 1 | 1 | ||
| Disclosure status | ||||||
| Non-disclosed | 46 | 130 | 1 | 1 | ||
| Disclosed | 59 | 109 | 1.4 (0.9, 2.0) | 0.098 | – | |
| Baseline WHO stage | ||||||
| Stages 1 and 2 | 52 | 197 | 1 | 1 | ||
| Stages 3 and 4 | 53 | 42 | 3.2 (2.2, 4.7) | 0.000 | 2.3 (1.3, 4.0) | 0.002 |
| Presence of TB | ||||||
| Yes | 20 | 17 | 2.1 (1.3, 3.4) | 0.003 | 1.0 (0.5, 1.9) | 0.979 |
| No | 85 | 222 | 1 | 1 | ||
| OI other than TB | ||||||
| Yes | 37 | 76 | 1.1 (0.7, 1.6) | 0.659 | – | |
| No | 68 | 163 | 1 | |||
| CPT prophylaxis | ||||||
| Yes | 76 | 225 | 1 | 0.000 | 1 | 0.001 |
| No | 29 | 14 | 3.5 (2.3, 5.4) | 2.5 (1.4, 4.3) | ||
| IPT prophylaxis | ||||||
| Yes | 32 | 73 | 1 | |||
| No | 73 | 166 | 1.0 (0.7, 1.6) | 0.824 | – | |
| Timing of initiation | ||||||
| Early (⩽6 months) | 71 | 141 | 1.2 (0.8, 1.9) | 0.331 | – | |
| Late (>6 months) | 34 | 98 | 1 | |||
| Year of initiations | ||||||
| ⩽2013 | 84 | 131 | 1.9 (1.1, 3.1) | 0.013 | – | |
| ⩾2014 | 21 | 108 | 1 | |||
| Baseline functional status (n = 196) | ||||||
| Working | 66 | 149 | 1 | 1 | ||
| Bedridden/ambulatory | 15 | 18 | 2.7 (1.5, 4.7) | 0.001 | 0.8 (0.4, 1.6) | 0.491 |
| Regimen change | ||||||
| Yes | 29 | 49 | 1.4 (0.9, 2.2) | – | ||
| No | 76 | 190 | 1 | |||
| Treatment failure | ||||||
| Yes | 23 | 11 | 1.1 (0.6, 2.1) | 0.802 | – | |
| No | 94 | 216 | 1 | |||
| ART adherence | ||||||
| Good | 80 | 187 | 1 | |||
| Fair and poor | 25 | 52 | 1.2 (0.8, 1.9) | 0.462 | – | |
CHR: crud hazard ratio; AHR: adjusted hazard ratio; PI: protease inhibitor; NVP: nevirapine; EVZ: efavirenz; ART: antiretroviral therapy; OI: opportunistic infection; TB: tuberculosis; WHO: World Health Organization; CPT: cotrimoxazole prophylactic therapy; IPT: isoniazid prophylactic therapy; CD4: cluster of differentiation 4.
Children with underweight were 3.1 times at higher risk of attrition than a children with normal weight (AHR = 3.1 (95% CI = 1.7, 5.3)). Similarly, children with hemoglobin level ⩽10 mg/dL were 2.7 times at higher risk of attrition than children with hemoglobin >10 mg/dL (AHR = 2.7 (95% CI = 1.5, 4.7)). Children with WHO clinical stages 3 and 4 were 2.3 times at higher risk of attrition than children with WHO clinical stages 1 and 2 (AHR = 2.3 (95% CI= 1.3, 4.0)). Likewise, children with CPT non-users were 2.5 times at higher risk of attrition than children with CPT users (AHR = 2.5 (95% CI = 1.4, 4.3)) (Table 4).
Discussion
The main goal of this study was to determine the incidence of attrition and its predictors in HIV-infected children with a 13-year long-term follow-up period. The focus of this article may be important for policymakers and subsequent researchers to reduce HIV-related morbidity and mortality associated with attrition and to achieve the ambitious plan by the year 2030 of ending the HIV pandemic in Ethiopia in general and in the study area in particular. In fact, reducing the incidence of attrition for HIV-positive children is critical to retaining and promoting quality of life.
From 1 January 2005 to 30 December 2018, the incidence rate of attrition at the University of Gondar Comprehensive Specialized Hospital was found to be 6.6 per 100 PYO (95% CI = 5.5, 8.0). The rates of lost follow-up and mortality were 4.8 per 100 PYO (95% CI = 3.8, 6.0) and 1.8 per 100 PYO (95% CI = 1.3, 2.6). The baseline WHO clinical staging 3/4, baseline hemoglobin 10 mg/dL, cotrimoxazole (CPT) non-user, and underweight at baseline (weight-for-age 2 Z-score) were found to be the main predictors of attrition.
The finding was consistent with a study conducted in Addis Ababa, Ethiopia, which found 8.3 per 100 PYO (95% CI = 5.4–12.1). 16 This consistency could be because LTFU was defined as a child whose treatment was interrupted for at least the first 3 months or more after the last visit date. In addition, this may be explained by the fact that the study participants had similar socio-demographic characteristics and monitoring and recording of data formats in the follow-up period in the ART service prepared from the Federal Ministry of Health Ethiopian Guidelines for Pediatric HIV/AIDS Care and Treatment in Ethiopia, which is a similar scenario in this study. 17 The findings are also consistent with a study conducted in the Asia Pediatric HIV Observational Database in 11 sites, including Cambodia, India, Indonesia, Malaysia, and Thailand, where the incidence of attrition rate was 6.3 per 100 person-years 18 and in five other Asia-Pacific region countries, the incidence of attrition was 6.5 per 100 child-years. 5 This finding resemblance could be attributed to the time frame/follow-up period. Although the heterogeneity of the data is inherent in a multinational cohort and the types of clinical centers, all health centers were in middle-income countries. However, our finding was lower than that which has been reported in Tigray (8.8 per 100 PYO). 19 This difference could be because the operational definition of attrition was defined as a combination of LTFU, having discontinued ART, death, or having transferred out. In addition to this, the majority of study participants were adults, who might be more fearful of social isolation and stigma than children, which might increase the risk of attrition. The study design was from the January 2013 and December 2014 prospective cohorts. 19 The finding was also lower than in a study conducted. In Uganda, the incidence of attrition was 14.4 per 100 person-years. 20 The pediatric West African Database to evaluate attrition from 2000 to 2008 included Benin, Côte d’Ivoire, Gambia, Ghana, Senegal, and Mali was 26.2 per 100 child-years. 6 In Kenya, Lesotho, Mozambique, Rwanda, and Tanzania, the incidence of attrition rate at primary health facilities was 15 per 100 person-years and at secondary and tertiary health facilities was 26.2 per 100 person-years. 21 The possible reason for this difference might be due to the operational definition of LTFU. The interval between the last clinic visit registered in the database was >6 months in the pediatric West African Database. 6 The study participants in Uganda were adolescents and children, 20 and the time frame/follow-up period, the heterogeneity of the study participants, and the types of clinical centers were also considered. This study had a long time frame/follow-up period. This has allowed us to measure incidence for various time intervals and also assess the hazard function experienced in the short-term and long-term.
The study findings were slightly higher than a study conducted in Myanmar in which the ART attrition rate was 4 per 100 person-years of follow-up (95% CI = 3, 4) and the pre-ART attrition rate was 19 per 100 person-years of follow-up (95% CI = 17, 21) since early ART initiation significantly decreased morbidity and mortality in HIV-infected children. 2 This slight difference might be due to socio-demographic, sample size, and follow-up period. In addition, this may be explained by the improvements in health care services in the later periods of follow-up as compared with earlier periods, and may promote the use of fewer toxic regimens and more strategic laboratory monitoring than in this study. In fact, a long time frame/follow-up period reduced the rate of attrition with a specific year of observation because most deaths and LTFU occurred early in the initiation of ART, which could be due to age, drug side effects, or fear of social isolation, as supported by different literatures.2,16
In this study, children with baseline hemoglobin levels of 10 mg/dL had a nearly threefold higher risk of attrition (AHR = 2.7 (95% CI = 1.5, 4.7)) than those with hemoglobin levels of >10 mg/dL. This finding is in line with a study conducted in Ethiopia, Myanmar, Tanzania, and Mozambique.2,16,22,23 This could be because anemia has been shown to influence the natural history of HIV disease by accelerating the rate of disease progression and increasing mortality in developed and developing countries, which is supported by a study conducted in Rwanda. 24
Similarly, children who had a lower baseline weight-for-age −2 Z-score had an almost threefold higher risk of attrition (AHR = 3.1 (95% CI = 1.7, 5.3)) as compared with children who had a weight-for-age >−2 Z-score. This finding is in line with previous research from Ethiopia, Tanzania, Myanmar, Sub-Saharan Africa, and Asia-Pacific region countries.2,5,22,25 Underweight children may be exposed to and experience an exacerbation of opportunistic infection (OI), as well as a decrease in CD4 count, which can lead to social isolation and stigma before dying or losing ART follow-up. Malnutrition causes immune system dysfunction and increases the host’s vulnerability to infections and immunological deficiency, which increases the severity of the disease and also delays recovery time. 26
We have also found that children who had a baseline advanced WHO clinical staging 3/4 had a higher risk of attrition nearly by twofold (AHR = 2.3 (95% CI = 1.3, 4.0)) as compared with those children who had a WHO clinical stage 1/2. Similar findings have been reported in Sub-Saharan Africa, A Systematic Review in Resource Limited Settings, and Myanmar.2,27,28 This might be due to advanced WHO clinical staging, which can weaken immunity and lead to severe sickness as a result of viral replication, CD4 count depletion, and the increased burden of these diseases, further complicating treatment outcomes. 29
Children who were not taking cotrimoxazole (CPT) had a nearly threefold increased risk of developing attrition (AHR = 2.5 (95% CI = 1.4, 4.3)) compared to children who were. Similar results have been reported from Ethiopia, West Africa, Tanzania, Mozambique, Rwanda, and Kenya.6,30–32 In fact, cotrimoxazole (CPT) can prevent or reduce the occurrence of OIs and further complications. Therefore, it is important to increase the immune status of children to decrease viral replication, which increases their survival rate by preventing and treating OI infections, which is supported by a study conducted in Ethiopia. 15 Cotrimoxazole (CPT) prophylaxis has been recommended for the benefit of HIV/AIDS-infected individuals to prevent OI since it is a simple and effective intervention to reduce morbidity, improve quality of life, and increase rates of retention in ART services. 33
This study has some limitations. To begin, data were collected from routine medical care records, and there was limited information on potential predictors of attrition, such as socio-demographics such as distance from the hospital and clinical characteristics such as baseline viral loads. Second, baseline clinical characteristics for the unrecorded data were excluded in the analysis, and most children had attrition but did not start follow-up. That might have made the result an underestimation.
Conclusion
The overall incidence of the rate of attrition was high. Baseline WHO clinical staging 3/4, baseline hemoglobin ⩽10 mg/dL, cotrimoxazole (CPT) non-user, and underweight at baseline (weight-for-age <−2 Z-score) were found to be the main predictors of attrition.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221077843 for Incidence and predictors of attrition among children on antiretroviral therapy at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2019: Retrospective follow-up study by Ermias Sisay Chanie, Debrework Tesgera Beshah and Amare Demsie Ayele in SAGE Open Medicine
Acknowledgments
The authors thank Debre Tabor University for financial support that made this study possible and the University of Gondar, College of Medicine and Health Sciences, for support with all necessary services. In addition, the authors appreciate the support from hospital administrations and data collectors.
Footnotes
Author contributions: All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval for the version to be published, and have agreed to be accountable for all aspects of the work.
Availability of data and materials: Data will be had upon request from the corresponding author.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval was obtained from the Institutional Review Board (IRB) of the University of Gondar on behalf of the Ethical Review Committee of the School of Nursing (reference number SN/HP/1600/2018).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: A permission letter was obtained from the hospital administration and the ART focal person in the hospital. Besides, the verbal informed consent approved by IRB was obtained from legally authorized representatives before data collection.
ORCID iD: Ermias Sisay Chanie  https://orcid.org/0000-0002-3124-5380
https://orcid.org/0000-0002-3124-5380
Supplemental material: Supplemental material for this article is available online.
References
- 1. Global HIV and AIDS statistics—2018 fact sheet, UNAIDS, http://www.unaids.org/en/resources/fact-sheet (accessed 13 December 2018).
- 2. Minn AC, Kyaw NTT, Aung TK, et al. Attrition among HIV positive children enrolled under integrated HIV care programme in Myanmar: 12 years cohort analysis. Glob Health Action 2018; 11(1): 1510593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Post-HAART outcomes in pediatric populations: comparison of resource-limited developed countries, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3025421/ (accessed 13 December 2018). [DOI] [PMC free article] [PubMed]
- 4. Vermund SH, Belvins M, Moon TD, et al. Poor clinical outcomes for HIV infected children on antiretroviral therapy in rural Mozambique: need for program quality improvement and community. PLoS ONE 2014; 9: e110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumbiganon P, Kariminia A, Aurpibul L, et al. Survival of HIV-infected children: a cohort study from the Asia-Pacific region. J Acquir Immune Defic Syndr 1999; 56(4): 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health 2011; 11: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies MA, Pinto J. Targeting 90–90–90—don’t leave children and adolescents behind. J Int AIDS Soc 2015; 18(Suppl. 6): 20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desmonde S, Tanser F, Vreeman R, et al. Access to antiretroviral therapy in HIV-infected children aged 0–19 years in the international epidemiology databases to evaluate AIDS (IeDEA) global cohort consortium, 2004–2015: a prospective cohort study. PLoS Med 2018; 15(5): e1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassan AS, Mwaringa SM, Ndirangu KK, et al. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health 2015; 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu H, Napravnik S, Eron J, et al. Attrition among Human Immunodeficiency Virus (HIV)-infected patients initiating antiretroviral therapy in China, 2003–2010. PLoS ONE 2012; 7(6): e0039414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massavon W, Mosha LB, Mugenyi L, et al. Factors determining survival and retention among HIV-infected children and adolescents in a community home-based care and a facility-based family-centred approach in Kampala, Uganda: A Cohort Study. ISRN AIDS 2014; 2014: 852489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Global AIDS Monitoring. 2018: Indicators for monitoring the 2016 United Nations Political Declaration on Ending AIDS—World. ReliefWeb, https://reliefweb.int/report/world/global-aids-monitoring-2018-indicators-monitoring-2016-united-nations-political (accessed 3 December 2018).
- 13. Taddese AA, Gashaye KT, Dagne H, et al. Maternal and partner’s level of satisfaction on the delivery room service in University of Gondar Referral Hospital, northwest, Ethiopia: a comparative cross-sectional study. BMC Health Serv Res 2020; 20(1): 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Central Sattistical Agency/CSA/Ethiopia and ICF. Ethiopia Demographic and Health Survey 2016, 2017, https://dhsprogram.com/publications/publication-fr328-dhs-final-reports.cfm (accessed 2 December 2021).
- 15. Atalell KA, Birhan Tebeje N, Ekubagewargies DT. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 2018; 13(5): e0197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biru M, Hallström I, Lundqvist P, et al. Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: A prospective cohort study. PLoS ONE 2018; 13(2): e0189777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guidelines for Paediatric HIV/AIDS Care Treatment in Ethiopia, 2007, http://apps.who.int/medicinedocs/en/m/abstract/Js19145en/ (accessed 22 May 2019).
- 18. Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr 1999; 62(2): 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bucciardini R, Fragola V, Abegaz T, et al. Predictors of attrition from care at 2 years in a prospective cohort of HIV-infected adults in Tigray, Ethiopia. BMJ Glob Health 2017; 2(3): e000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. William M, Rebecca L, Paola C, et al. Attrition and loss to follow-up among children and adolescents in a community home-based care HIV programme in Uganda. Pediatr Ther 2013; 3(5): 1–7. [Google Scholar]
- 21. Fayorsey RN, Saito S, Carter RJ, et al. Decentralization of pediatric HIV care and treatment in five Sub-Saharan African countries. J Acquir Immune Defic Syndr 1999; 62(5): e124–e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mwiru RS, Spiegelman D, Duggan C, et al. Nutritional status and other baseline predictors of mortality among HIV–infected children initiating antiretroviral therapy in Tanzania. J Int Assoc Provid AIDS Care 2015; 14(2): 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vermund SH, Blevins M, Moon TD, et al. Poor clinical outcomes for HIV infected children on antiretroviral therapy in Rural Mozambique: need for program quality improvement and community engagement. PLoS ONE 2014; 9(10): e0110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masaisa F, Gahutu JB, Mukiibi J, et al. Anemia in human immunodeficiency virus–infected and uninfected women in Rwanda. Am J Trop Med Hyg 2011; 84(3): 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda and Tanzania. J Acquir Immune Defic Syndr 1999; 62(3): e70–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on immune system. Clin Dev Immunol 2012; 2012: 784704, https://www.hindawi.com/journals/jir/2012/784740/ (accessed 14 May 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics 2012; 130(3): e591–e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abuogi LL, Smith C, McFarland EJ. Retention of HIV-infected children in the first 12 months of anti-retroviral therapy and predictors of attrition in resource limited settings: a systematic review. PLoS ONE 2016; 11(6): e0156506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dejen D, Jara D, Yeshanew F, et al. Attrition and its predictors among adults receiving first-line antiretroviral therapy in woldia town public health facilities, Northeast Ethiopia: a retrospective cohort study. HIVAIDS Auckl NZ 2012; 13: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koye DN, Ayele TA, Zeleke BM. Predictors of mortality among children on Antiretroviral Therapy at a referral hospital, Northwest Ethiopia: a retrospective follow up study. BMC Pediatr 2012; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teshome W, Belayneh M, Moges M, et al. Do loss to follow-up and death rates from ART care vary across primary health care facilities and hospitals in south Ethiopia? A retrospective follow-up study. HIV AIDS 2015; 7: 167–174, https://www.dovepress.com/do-loss-to-follow-up-and-death-rates-from-art-care-vary-across-primary-peer-reviewed-fulltext-article-HIV (accessed 28 November 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med 2011; 8(6): e1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Date AA, Vitoria M, Granich R, et al. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ 2010; 88(4): 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221077843 for Incidence and predictors of attrition among children on antiretroviral therapy at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2019: Retrospective follow-up study by Ermias Sisay Chanie, Debrework Tesgera Beshah and Amare Demsie Ayele in SAGE Open Medicine