Abstract
Background:
No meta-analysis has assessed the pooled frequencies of adverse events (AEs) induced by concomitant nivolumab plus ipilimumab regimen for anticancer-medications-naïve malignancies. Furthermore, no meta-analysis has compared detailed safety profiles between four doses of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks (N3I1) and four doses of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (N1I3). Objectives of this study was estimating AE frequencies, and comparison of AE frequencies between N3I1 and N1I3 regimens.
Methods:
Four major electronic databases were searched; both interventional and observational studies were included. All primary cancer types were permitted. Patients should not have been previously treated with any anti-cancer medications. The frequency of AEs was pooled using a random-model meta-analysis using the generic inverse variance method. Protocol registration: UMIN000044090.
Results:
Forty articles representing 48 populations with 4,677 patients were included in the study. The pooled frequencies for key indicators were as follows: any AE, 81.3% (95% confidence interval (CI) 77.5-85.1); grade 3 or higher AE, 40.6% (95% CI: 35.7–45.5); serious AE, 32.7% (95% CI: 22.4–43.1); AE leading to discontinuation, 28.3% (95% CI: 23.7–32.8); and treatment-related death, 0.7% (95% CI: 0.4–1.1). AEs with the highest incidence were fatigue (27.9%, 95% CI: 22.6–33.3), followed by diarrhea (26.0%, 95% CI: 21.5–30.5), pruritus (24.6%, 95% CI: 20.3–28.8), rash (24.0% 95% CI: 19.3–28.7), and elevated aspartate aminotransferase (21.2%, 95% CI: 14.9–27.5). Subgroup analyses demonstrated that N3I1, compared to N1I3, less frequently induced any AE (N1I3 95.7%, N3I1 84.5%, p = 0.003), grade 3 or higher AE (N1I3 64.3%, N3I1 35.7%, p < 0.001), and serious AE (N1I3 61.4%, N3I1 47.8%, p = 0.004).
Conclusions:
Approximately 40% of patients had grade 3 or higher AE. The N3I1 regimen was substantiated to trigger fewer any AEs, high grade AEs, and serious AE than the N1I3 regimen.
Keywords: antineoplastic agents, clinical trial, drug-related side effects and adverse reactions, monoclonal antibodies, neoplasms
Backgrounds
Immune checkpoint inhibitors (ICIs) block the inhibitory signals suppressing T cell activation generated by tumor. Recent use of ICIs has drastically improved the survival of cancer patients. Ipilimumab, a monoclonal antibody targeting the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) protein receptor that inactivates T-cell recognition and proliferation against cancer cells, was approved for the treatment of melanoma in 2011 by the U.S. Food and Drug Administration.1,2 Nivolumab, which is another type of ICI that enhances T cells to attack tumor cells by inhibiting programmed death-ligand 1 (PD-L1) from binding to programmed cell death protein-1 (PD-1), was subsequently developed in adjunct to ipilimumab and has been approved for many types of major malignancies. 1 ICIs were first used in the form of a single-agent regimen; however, trials suggested possible advantages of combined ICI regimens over single-agent regimens. 1 Among these, the nivolumab-ipilimumab combination is one of the most commonly used regimens. Frontline setting phase III trials revealed prolonged survival of patients with treatment-naïve malignant pleural mesothelioma, 3 renal cell carcinoma, 4 non-small cell lung cancer, 5 and melanoma 6 who were treated with this particular combination regimen. In addition, this combination regimen has also been explored for recurrent cancers7,8 and in perioperative settings.9,10
The combination of nivolumab and ipilimumab is known to be highly effective in treating some cancers, but there are significant concerns about its safety profile. 11 A few systematic reviews and meta-analyses have evaluated the safety profile of the nivolumab plus ipilimumab regimen.12 –15 However, these reviews did not focus on the first-line setting and included sequential regimens. Thus, no meta-analysis has assessed the pooled frequencies of AEs induced by concomitant nivolumab plus ipilimumab regimens for chemo-naïve ICI-naïve cancers. Furthermore, no meta-analysis has compared the safety profiles among various nivolumab plus ipilimumab regimens. Currently, the most common dual ICI regimen is four courses of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (N1I3 q3w x4). However, another regimen, four courses of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks (N3I1 q3w x4), has been reported to cause fewer grade 3 or higher AEs in a phase III trial. 16 Another randomized phase II melanoma study, OpACIN-neo, suggested that N3I1 q3w x2 might induce less grade 3 or higher AE than N1I3 q3w x2. 9 Since the nivolumab plus ipilimumab combination regimen is becoming the standard of care for various malignancies, clinicians need to know the AE profile in detail. However, no systematic review has evaluated organ-specific AEs triggered by the combination of nivolumab and ipilimumab combination. The current systematic review and meta-analysis aimed to clarify per-person frequencies of AEs due to nivolumab plus ipilimumab used in patients without previous anticancer-medication treatment.
Methods
Protocol registration
The protocol of this systematic review, in compliance with Meta-analyses Of Observational Studies in Epidemiology guidelines (Supplemental material Table 1), was registered in the University Hospital Medical Information Network (UMIN) Center website (ID: UMIN000044090).17,18
Study search
The electronical database search formula for PubMed, Web of Science Core Collection, Cochrane Advanced Search, and EMBASE are presented elsewhere (Supplemental material Text 1). We searched these databases on April 27, 2021. Additional manual searches were independently conducted by two review authors (K.S. and N.H.).
Potentially included research articles were screened and fully checked (K.S. and N.H.). A third author was involved in the discussion when the two authors could not resolve the disagreement.
Publication type and trial design
We allowed both interventional and observational studies. However, case reports were excluded. Only articles written in English were considered eligible. Both full articles and conference abstracts were permitted.
Patients
All primary cancer types were permitted because it does not largely affect the safety profile, as long as the same regimen was selected. Patients should not have been previously treated with anti-cancer medications such as cytotoxic drugs, molecular targeted therapy, or ICIs. No restrictions were set for performance status or age. Patients who underwent transplantation were excluded from the study.
Treatment
Concurrent nivolumab plus ipilimumab combination therapy, regardless of dosing and scheduling, was the main treatment concern. Concomitant administration of cytotoxic agents, molecular-targeted medications, and other ICIs was prohibited in our analysis. Consequent administration of nivolumab and ipilimumab, such as three cycles of nivolumab followed by three cycles of ipilimumab, was not accepted. The dose, scheduling, and total number of administrations of the nivolumab plus ipilimumab regimen were not questioned. Treatment with radiotherapy was not allowed in our analysis. Adjuvant and neoadjuvant perioperative therapies were accepted.
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale for cohort studies was used for quality assessment. 19
Outcomes
Pooled binomial frequencies of key AE indicators (any AE, grade 3 or higher AE, serious AE, AE leading to discontinuation, and treatment-related death) were pooled. In addition, 22 specific AEs, such as alanine aminotransferase elevation and diarrhea were reported.
Data extraction
Key study characteristics such as author name, publication year, country of origin, study name, and number of patients were extracted by two review authors (K.S. and N.H.). If one study assessed two or more of nivolumab plus ipilimumab regimens provided AE profile for each regimen, then these regimens were counted in different populations. If necessary, author groups were contacted via e-mail for detailed data.
Subgroup analysis
Subgroup analyses focusing on N1I3 q3w x4 and N3I1 q3w x4 were performed. Maintenance treatment after the four doses of these treatments were ignored for the subgroup analysis.
Statistics
The frequency of each AE was pooled using a random-model meta-analysis utilizing the generic inverse variance method (RevMan ver 5.4. Cochrane Collaboration, London, UK). Standard error was calculated using Agrestia’s method. 20 Subgroup differences were expressed using the p value for heterogeneity based on the RevMan random-model analysis, with a significance threshold of p < 0.05. Heterogeneity was assessed using I 2 statistics.
Results
Study selection process
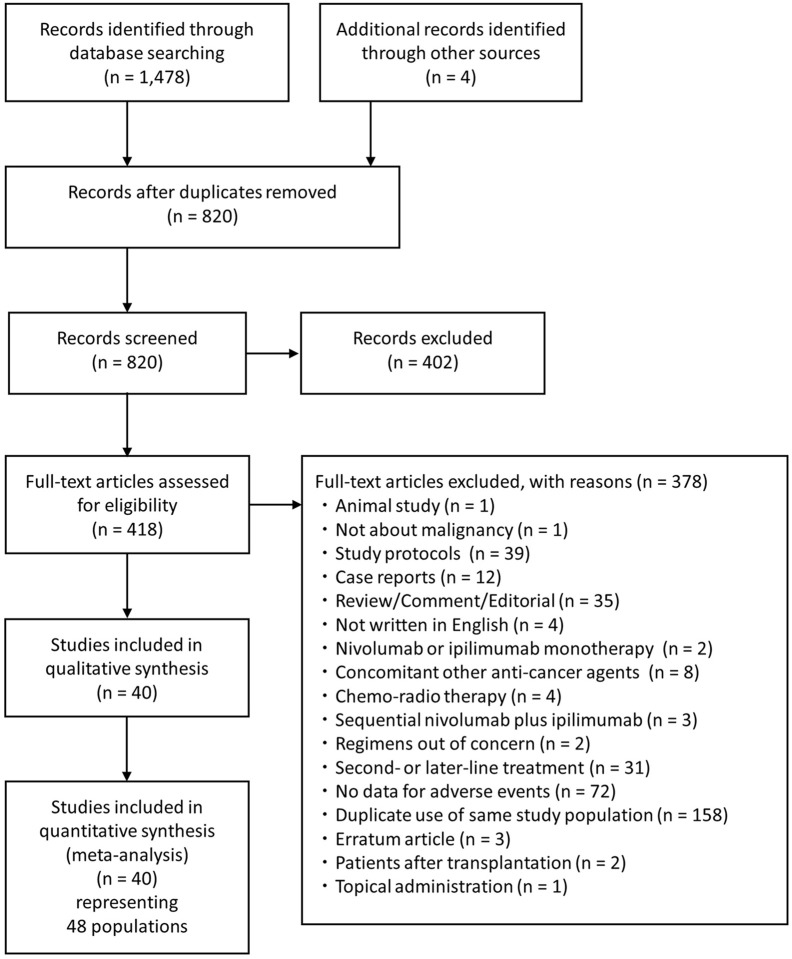
An electronic search using four major databases detected 1,478 articles, and manual searching identified four additional articles (Figure 1). After duplication removal (n = 662), screening of title and abstracts (n = 402) and full text (n = 378), 40 eligible articles representing 48 populations were finally regarded as eligible for quantitative analysis (Figure 1, Supplemental material Table 2).
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta- analyses flow chart.
Study characteristics
Of the 40 reports, 22 were full articles, 17 were conference abstracts, and one was a letter article. More than half of the articles were from the United States (n = 22); the rest were from the Netherlands (n = 5), Japan (n = 4), France (n = 2), Spain (n = 2), and other countries (n = 5) (Table 1). The eligible articles included five phase I studies, one phase Ib/II study, thirteen phase II studies, four phase III studies, two phase IIIb/IV studies, and one phase IV study, and eleven retrospective reports, whereas some did not describe study phase (Table 1). Melanoma was the most frequently studied (n = 19), followed by renal cell carcinoma (n = 9), non-small cell lung cancer (n = 8), colorectal cancer (n = 1), hepatocellular carcinoma (n = 1), malignant pleural mesothelioma (n = 1), oral cavity carcinoma, and soft tissue sarcomas (n = 1). The majority of studies (n = 28) evaluated first-line nivolumab plus ipilimumab, whereas five studies were for adjuvant therapy, six were for neoadjuvant treatment, and another reported AE from neoadjuvant and adjuvant therapies collectively. The article-level median score of the Newcastle-Ottawa quality assessment scale was 4 ranging from 3 to 6. The total number of patients analyzed patients was 4,677 (Table 1).
Table 1.
Characteristics of included populations.
| Population | Country | Report | Design | Cancer | Stage | Regimen | Setting | n | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Allison (2021) | UK | CA | Retrospective | RCC | metastatic | NS | 1L | 69 | 3 |
| Antonia (2014) | USA | CA | P1 | NSCLC | advanced | (N1I3 q3w or N3I1) x4 | 1L | 46 | 4 |
| Baas et al. 3 | Netherlands | FA | P3 | MPM | unresectable | N3 q2w + I1 q6w | 1L | 300 | 5 |
| Barlesi (2019 A) | France | CA | P3b/4 | NSCLC | advanced | N240 q2w + I1 q6w | 1L | 391 | 5 |
| Barlesi (2019 A1) | France | CA | P3b/4 | NSCLC | advanced | N240 q2w + I1 q6w | 1L | 198 | 5 |
| Blank (2018) | Netherlands | Letter | P1b, RCT | Melanoma | stage III | N1I3 q3w x4 | Adj | 10 | 5 |
| Cascone (2021) | USA | FA | P2, RCT | NSCLC | operable | N3 q2w x3 + I1 once | NeoAdj | 21 | 5 |
| Chen (2021) | China | FA | Retrospective | Soft tissue sarcomas | metastatic | N1I3 q3w x4 > N3 q2w | 1L | 74 | 3 |
| Cocorocchio (2020) | Italy | CA | P2, 1-arm | Melanoma | locally advanced or oligometastatic | N3I1 q3w neoadj x4 | NeoAdj | 21 | 4 |
| Constantinou (2021) | USA | FA | Interventional | Melanoma | high risk resected, IIc-IV | N3 q2w x12 + I1 q6w x4 | Adj | 21 | 4 |
| Desai (2020) | USA | CA | Retrospective | RCC | metastatic | NS | 1L | 46 | 3 |
| Haanen (2017) | USA | CA | P1 | Melanoma | brain metastasis | N1I3 q3w x4 > N3 q2w | 1L | 10 | 4 |
| Hellmann (2015 arm1) | USA | CA | P1 | NSCLC | advanced | N1I1 q3w x4 > N3 q2w | 1L | 31 | 4 |
| Hellmann (2015 arm2) | USA | CA | P1 | NSCLC | advanced | N1 q2w + I1 q6w | 1L | 40 | 4 |
| Hellmann (2015 arm3) | USA | CA | P1 | NSCLC | advanced | N3 q2w + I1 q12w | 1L | 38 | 4 |
| Hellmann (2015 arm4) | USA | CA | P1 | NSCLC | advanced | N3 q2w + I1 q6w | 1L | 39 | 4 |
| Hellmann (2017 q12w) | USA | FA | P1 | NSCLC | advanced | N3 q2w I1 q12w | 1L | 38 | 4 |
| Hellmann (2017 q6w) | USA | FA | P1 | NSCLC | advanced | N3 q2w I1 q6w | 1L | 39 | 4 |
| Hellmann et al. 5 | USA | FA | P3 | NSCLC | advanced | N3 q2w I1 q6w | 1L | 576 | 5 |
| Kaseb (2020) | USA | CA | P2, RCT | HCC | resectable | N240I1 q2w x3 NeoAdj > N240I1 q6w x4 Adj |
NeoAdj Adj | 14 | 5 |
| Khushalani et al. 21 | USA | CA | interventional | Melanoma | stage IIIb/IV | N1I3 q3w x4 > N3 q2w | Adj | 20 | 4 |
| Khushalani et al. 21 | USA | CA | interventional | Melanoma | stage IIIb/IV | N3I1 q3w x4 > N3 q2w | Adj | 20 | 4 |
| Kido (2021) | Japan | FA | Retrospective | RCC | metastatic | N3I1 q3w x4 > N3 q2w | 1L | 52 | 3 |
| Larkin (2015) | USA | FA | P3 | Melanoma | advanced | N1I3 q3w x4 | 1L | 313 | 6 |
| Lebbé et al. 16 N1I3 | France | FA | P3b/4 | Melanoma | metastatic | N1I3 q3w x4 > N480 q4w | 1L | 178 | 5 |
| Lebbé et al. 16 N3I1 | France | FA | P3b/4 | Melanoma | metastatic | N3I1 q3w x4 > N480 q4w | 1L | 180 | 5 |
| Lenz (2018) | USA | CA | P2 non-RCT | Colorectal | metastatic | N3q2w + I1 q6w | 1L | 45 | 5 |
| Ma (2021) | USA | FA | Retrospective | Melanoma | advanced | N1I3 q3w x4 > N (3 regimens) | 1L | 110 | 3 |
| Meerveld-Eggink (2020) | Netherlands | CA | Retrospective | RCC | metastatic | NS | 1L | 52 | 3 |
| Motzer (2018) | USA | FA | P3 | RCC | advanced | N3I1 q3w x4 > N3 q2w | 1L | 547 | 5 |
| Namikawa (2020) | Japan | FA | 1-arm P2 | Melanoma | stage III, IV, Rec | N1I3 q3w x4 > N3 q2w | 1L | 30 | 4 |
| Oberoi (2019) | Spain | CA | Retrospective | Melanoma | metastatic | NS | 1L | 42 | 3 |
| Piulats (2021) | Spain | FA | Single-arm P2 | Melanoma, uveal | metastatic | N1I3 q3w x4 > N3 q2w | 1L | 52 | 4 |
| Postow (2015) | USA | FA | P2 RCT | Melanoma | metastatic | N1I3 q3w x4 > N3 q2w | 1L | 94 | 6 |
| Rauwerdink (2020) | USA | CA | Retrospective | Melanoma | metastatic | NS | 1L | 57 | 3 |
| Ready (2019) | USA | FA | P2, 1-arm | NSCLC | stage IIIb, IV | N3 q2w I1 q6w | 1L | 288 | 4 |
| Reddy (2017) | USA | CA | RCT | Melanoma | advanced | N1I3 q3w x3 | NeoAdj | 23 | 5 |
| Reuss (2020) | USA | FA | 1-arm phase 1b/2 | NSCLC | stage Ib-IIIa | N3I1 x1 + N3 q2w x2 | NeoAdj | 9 | 4 |
| Rozeman et al. 9 | Netherlands | FA | P2 RCT | Melanoma | stage III | N1I3 q3w x2 | NeoAdj | 30 | 6 |
| Rozeman et al. 9 | Netherlands | FA | P2 RCT | Melanoma | stage III | N3I1 q3w x2 | NeoAdj | 30 | 5 |
| Schoenfeld (2020) | USA | FA | P2 RCT | Oral Cavity | stage II | N3 q2w x2 + I1 once | NeoAdj | 15 | 5 |
| Schwarze (2019) | Belgium | CA | P2, 2-arm | melanoma | resectable | N10 q2w x4 + I50 x1 | Adj | 34 | 5 |
| Tachibana (2021) | Japan | FA | Retrospective | RCC | metastatic | NS | 1L | 30 | 3 |
| Tanaka (2020) | Japan | FA | Retrospective | RCC | metastatic | NI q3w x4 > N q2w | 1L | 52 | 3 |
| Tawbi (2018) | USA | FA | P2 single-arm | melanoma | metastatic | N1I3 q3w x4 > N3q2w | 1L | 94 | 4 |
| Tykodi (2021) | USA | CA | P4 | RCC | advanced/metastatic | N3I1 q3w x4 > N480 q4w | 1L | 52 | 4 |
| Zeijl (2019) | Natherlands | CA | Retrospective | melanoma | advanced | NS | 1L | 151 | 3 |
| Zimmer (2020) | Germany | FA | P2 RCT | melanoma | resectable | N1I3 q3w x4 > N3q2w | Adj | 55 | 6 |
Barlesi 2019 A cohort recruited patients with Eastern Cooperative Oncology Group performance status of 0-1. Barlesi 2019 A1 cohort recruited those with performance status 0-1 who had brain metastasis, hepatic impairment, renal impairment, or HIV infection and those with performance status of 2. Adj, adjuvant therapy; CA, conference abstract; FA, full article; HCC, hepatocellular carcinoma; I1, ipilimumab 1 mg/kg; I3, ipilimumab 3 mg/kg; MPM, malignant pleural mesothelioma; n, number of patients; NeoAdj, neo-adjuvant therapy; NOS, score of The Newcastle-Ottawa Quality Assessment Scale for Cohort Studies wherein higher score means better quality; NS, not specified; NSCLC, non-small cell lung cancer; N1, nivolumab 1 mg/kg; N3, nivolumab 3 mg/kg; N240, nivolumab 240 mg/body; P1-4, phase 1-4, we judged trial phase of each study as it was stated by the authors of original studies; q2-6w, every 2–6 weeks; RCC, renal cell carcinoma; RCT, randomized controlled trial; x2-4, administrated total 2–4 times; 1 L, first line; >, then.
Detailed information of referred articles are provided in the Supplementary File.
Since data of two nivolumab plus ipilimumab regimens were obtained from five articles and another article independently presenting data of four arms, we eventually analyzed 48 independent populations (Table 1, Figure 1). The population sized ranged from 9 to 576, with a median of 46.
Key adverse event indicators
Random-model meta-analysis using data of 4,224 patients from 35 populations suggested that the pooled frequency of any AE was 81.3% (95% confidence interval (CI): 77.5–85.1) (Table 2, Supplemental material Figure 1). The pooled frequency of grade 3 or higher AE in 38 populations (n = 4,044) was 40.6% (95% CI: 35.7–45.5) (Table 2, Supplemental material Figure 2). Analysis of 11 populations with 1,740 cancer patients showed an estimated frequency of serious AEs of 32.7% (95% CI: 22.4–43.1) (Table 2, Supplemental material Figure 3). Discontinuation due to AE was deemed to occur in 28.3% (95% CI: 23.7–32.8) of patients who were treated with nivolumab plus ipilimumab (33 populations, 4146 patients) (Table 2, Supplemental material Figure 4). Our meta-analysis of 4,272 patients from 38 populations suggested that the nivolumab plus ipilimumab regimen caused treatment-related death in 0.7% (95% CI: 0.4–1.1) of patients in the nivolumab plus ipilimumab arm (Table 2, Supplemental material Figure 5).
Table 2.
Estimated incidence of adverse events.
| N | n | Incidence (95% CI) | |
|---|---|---|---|
| Key adverse event indicators | |||
| Any AE | 35 | 4,224 | 81.3 (77.5–85.1) |
| Grade 3 or higher AE | 38 | 4,044 | 40.6 (35.7–45.5) |
| Serious AE | 11 | 1,740 | 32.7 (22.4–43.1) |
| AE leading to discontinuation | 33 | 4,146 | 28.3 (23.7–32.8) |
| Treatment-related death | 38 | 4,272 | 0.7 (0.4–1.1) |
| Gastrointestinal | |||
| Aspartate aminotransferase | 14 | 1,659 | 21.2 (14.9–27.5) |
| Alanine aminotransferase | 14 | 1,659 | 18.1 (13.1–23.2) |
| Amylase | 11 | 1,033 | 9.4 (6.2–12.7) |
| Lipase | 15 | 2,457 | 11.9 (8.7–15.2) |
| Diarrhea | 23 | 3,594 | 26.0 (21.5–30.5) |
| Colitis | 16 | 2,053 | 8.2 (5.5–10.8) |
| Decreased appetite | 15 | 2,792 | 12.1 (10.3–14.0) |
| Nausea | 22 | 3,547 | 15.1 (12.1–18.1) |
| Vomiting | 15 | 2,527 | 8.6 (5.9–11.4) |
| Dermatological | |||
| Rash | 21 | 3,242 | 24.0 (19.3–28.7) |
| Maculopapular rash | 12 | 1,877 | 12.4 (8.8–16.0) |
| Vitiligo | 7 | 944 | 7.1 (5.3–8.8) |
| Pruritus | 18 | 2,832 | 24.6 (20.3–28.8) |
| Hormonal | |||
| Hypothyroidism | 20 | 3,190 | 13.1 (11.2–15.1) |
| Hyperthyroidism | 15 | 1,701 | 11.0 (7.7–14.4) |
| Adrenal insufficiency | 12 | 1,091 | 4.8 (2.8–6.7) |
| Hypopituitarism | 6 | 335 | 9.5 (5.7–13.2) |
| Other adverse events | |||
| Fatigue | 23 | 3,555 | 27.9 (22.6–33.3) |
| Pyrexia | 17 | 1,282 | 14.8 (10.7–18.9) |
| Headache | 11 | 1,035 | 13.5 (9.9–17.1) |
| Arthralgia | 12 | 830 | 9.6 (6.5–12.7) |
| Pneumonitis | 13 | 1,386 | 6.5 (5.1–7.9) |
Incidence (95% CI), pooled incidence using random-model meta-analysis and its 95% CI. AE, adverse event; CI, confidence interval; N, number of populations; n, number of patients.
Sensitivity analyses focusing RCT were presented in Supplemental material Table 3. According to this sensitivity analysis, frequency of any AE (91.4%) was higher than estimated by the main analysis above. For the other key indicators, 95% CI from both analyses overlapped.
Specific adverse events
Fatigue had the AE with the highest pooled incidence of 27.9% (95% CI: 22.6–33.3) (Table 2). Gastrointestinal AEs, including diarrhea (26.0%, 95% CI: 21.5–30.5) and aspartate aminotransferase elevation (21.2%, 95% CI: 14.9–27.5) were also observed in more than 20% of patients. Nearly a quarter of the evaluated patients experienced pruritus (24.6%, 95% CI: 20.3–28.8) and rash (24.0% 95% CI: 19.3–28.7). Among hormonal AEs, hypothyroidism (13.1%, 95% CI: 11.2–15.1) was the most frequently observed, followed by hyperthyroidism (11.0%, 95% CI: 7.7–14.4), and hypopituitarism (9.5%, 95% CI: 5.7–13.2) (Table 2).
Safety comparison of N1I3 q3w x4 and N3I1 q3w x4
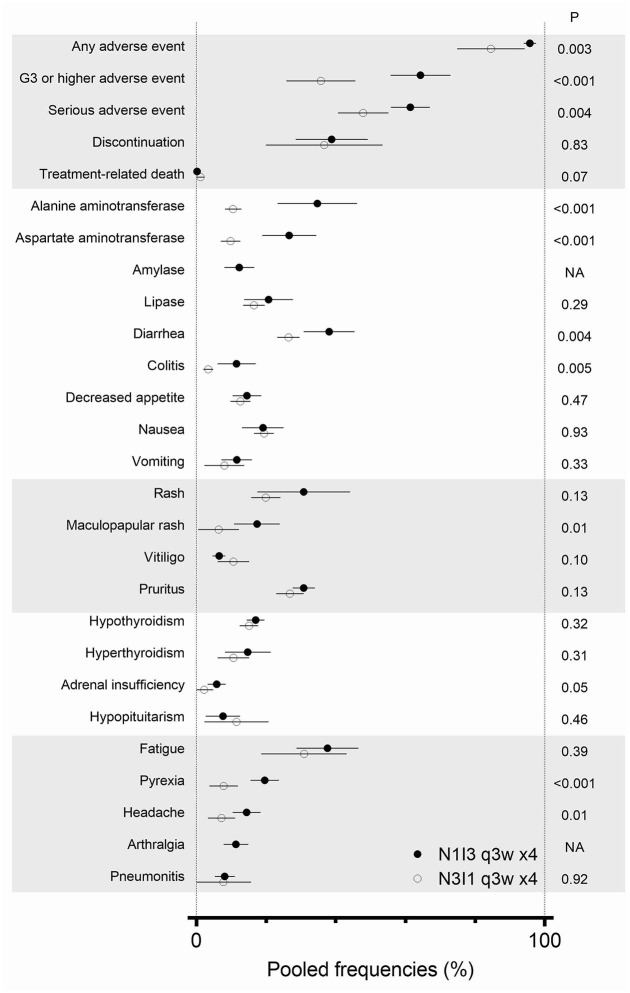
Subgroup analyses demonstrated that N3I1 q3w x4 regimen, compared to N1I3 q3w x4 regimen, less frequently induced any AE (N1I3, 8 populations, 826 cases, estimated 95.7%, 95% CI: 93.9–97.5; N3I1, 3 populations, 779 cases, estimated 84.5% 95% CI: 74.8–94.2; p = 0.003) (Figures 2 and 3). Furthermore, grade 3 or higher AEs (N1I3, 9 populations, 836 cases, estimated 64.3%, 95% CI: 55.7–72.9; N3I1, 4 populations, 800 cases, estimated 35.7%, 95% CI: 25.8–45.6; p < 0.001) and serious AEs (N1I3, 3 populations, 285 cases, estimated 61.4% 95% CI: 55.8–67.0; N3I1, 1 population, 180 cases, estimated 47.8% 95% CI: 40.6–55.1; p = 0.004) were less common among patients who were treated with the N3I1 q3w x4 regimen (Figures 2 and 3). No difference was observed in AE leading to discontinuation (N1I3, 12 populations, 1,040 cases; 4 populations, 799 cases; p = 0.96) or treatment-related death (N1I3, 8 populations, 870 cases; N3I1, 5 populations, 852 cases; p = 0.07) (Figures 2 and 3).
Figure 2.
Forest plots to compare N1I3 and N3I1 regimens for key adverse event indicators. (a) Any adverse event, (b) grade 3 or higher adverse event, (c) serious adverse event, (d) adverse event leading to discontinuation, and (e) treatment-related death.
AE, adverse event; IV, generic inverse variance; N1I3 q3w x4, four doses of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks; N3I1 q3w x4, four doses of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks; 95% CI, 95% confidence interval.
Figure 3.
Comparison of adverse event between N1I3 and N3I1 regimens.
An error bar indicates 95% confidence interval. N1I3 q3w x4, four doses of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks; N3I1 q3w x4, four doses of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks; NA, not available.
According to the two RCTs that directly compared N3I1 and N1I3, any AE, grade 3 or higher AE, serious AE were less frequently observed in N3I1 arm (Supplemental material Figure 6). Among the 22 specific AEs, no AE was frequent in N3I1 populations, whereas seven AEs were more frequent in N1I3 populations: alanine aminotransferase (p < 0.001), aspirate aminotransferase (p < 0.001), diarrhea (p = 0.004), colitis (p = 0.005), maculopapular rash (p = 0.01), pyrexia (p < 0.001), and headache (p = 0.01) (Figure 3, Supplemental material Table 4).
Discussion
This is the first comprehensive systematic review focused on chemo-naïve ICI-naïve patients with malignancies to clarify the safety profile of the combined nivolumab plus ipilimumab regimen. More than 80% of patients experienced any AE and approximately 40% of patients experienced grade 3 or higher AEs (Table 2). Nearly 30% of patients were forced to quit the regimen due to AEs. However, treatment-related death occurred in 0.7% of the cases (Table 2). Because AEs are inevitable for medical cancer treatment and the combination therapy enhances toxicity,12,14,15 we believe that our data provide useful information for physicians, pharmacists, and patients who should be aware of the risk-benefit balance when considering the combined nivolumab plus ipilimumab treatment.
Some published systematic reviews assessed the AE caused by the combined nivolumab plus ipilimumab regimen.12 –15 The first meta-analysis for this topic was written by Zhou et al. 14 in 2019. They analyzed the data of 2,946 patients from four studies and showed that the dual combination treatment increased immune-related AEs and higher-grade immune-related AEs. 14 Another systematic review by Almutairi et al. 12 compared immune-related AsE caused by ICI monotherapies (nivolumab or ipilimumab) and ICI combination therapies. They also alerted clinicians about increased risks of AEs due to the combination therapies. 12 Furthermore, Liu et al. 15 conducted a network meta-analysis comparing numerous kinds of treatments joining chemotherapies, ICIs, molecular-targeted medicines, and radiotherapy. Results revealed that, compared to ICI monotherapy, ICI combination therapy triggered more grade 3 or higher AEs. 15 Therefore, these three systematic reviews have consistently mentioned that combined ICI regimen is generally more toxic than single ICI therapy; thus, identifying dual ICI regimen with less AEs was needed. Chen et al. 13 reported a systematic review and meta-analysis incorporating two studies for treatment-naïve cases and six studies for previously treated cases to compare N1I3 and N3I1 regimens. Their study revealed that the N3I1 regimen caused less grade 3 or higher AEs than the N1I3 regimen; however, their analysis concluded that both regimens led to similar risks for all-grade AEs. A comprehensive meta-analysis with a larger number of studies is still needed to clarify this discrepancy.
Since the standard dosing of nivolumab plus ipilimumab has not yet been established, one of our primary interests was to compare two popular regimens: N1I3 q3w x4 and N3I1 q3w x4. This systematic review clarified that the N3I1 q3w x4 regimen induced fewer AEs than the N1I3 q3w x4 regimen. Along with safety profile, efficacy is another main concern when comparing the two regimens. CheckMate 511 revealed almost compatible progression-free survival (hazard ratio = 1.06), overall survival (hazard ratio = 1.09), and objective response rate among the two first-line regimens for advanced melanoma. 16 The OpACIN-neo study also showed compatible radiological and pathological response rates between two doses of the regimens as a neo-adjuvant treatment for melanoma. 9 Khushalani et al. 21 performed a trial concerning adjuvant therapy for resected melanoma Results revealed that the relapse rate and activity were similar for the two regimens. So far, the majority of researchers have selected the N1I3 regimen (Table 1); however, the N3I1 regimen seems to be the safer option (Figures 2 and 3).9,16,21 In 2013, Wolchok et al. reported a phase I study determining the best dose for nivolumab and ipilimumab combination. In this study, N1I3 q3w x4 (any AE, 100%; grades 3–4 AE, 65%; objective response rate, 53%; aggregate clinical activity rate, 65%) resulted in similar efficacy and trend toward poorer treatment-related safety profile than N3I1 q3w x4 (any AE, 81%, grades 3–4 AE 44%; objective response rate, 40%; aggregate clinical activity rate, 73%). 22 For melanoma patients, N1I3 regimen and N3I1 regimen indicated similar efficacy. Therefore, future phase III trial were warranted to address the relative efficacy for numerous cancers. In any case, a less toxic N3I1 regimen could be a reasonable option for patients with poor performance status and as well as for the elderly.
In addition to melanoma, the nivolumab plus ipilimumab combination is considered as the first-choice regimen for advanced non-small-cell lung cancer, renal cell carcinoma, and malignant pleural mesothelioma based on the CheckMate trials.3 –5 The Open-label CheckMate 227 phase III trial assigned patients with non-small-cell lung cancer to the nivolumab plus ipilimumab combination and standard chemotherapies. Patients in the ICI combination arm had longer overall survival (hazard ratio, 0.79; 95% CI: 0.65–0.96). 5 Furthermore, the CheckMate 214 trial compared the first-line N3I1 q3w x4 regimen and sunitinib for advanced renal cell carcinoma. In this phase III study, the dual ICI therapy led to improved response rate and overall survival. 4 According to the CheckMate 743 phase III trial with 605 randomized malignant pleural mesothelioma cases, combined nivolumab-ipilimumab therapy resulted in longer survival than the standard chemotherapy. 3 Therefore, nivolumab plus ipilimumab combination will be the first choice for a wider variation of carcinomas, where detailed AE data from this treatment becomes more useful.
One serious limitation of our study is that our regimen comparison was not based on a meta-analysis of direct comparison based on randomized controlled trials. Nonetheless, we believe that the N3I1 regimen is safer because of our straightforward analytical method and sufficient sample size, 4,677 (Table 1). Furthermore, our data did not conflict with those of the previous RCTs. CheckMate 511 was a phase III/IV melanoma trial assessing whether the N3I1 regimen was superior to the N1I3 regimen regarding grades 3–5 AE in the front-line setting. 16 Both the CheckMate 511 trial, which randomized 360 patients with advanced melanoma, and our analysis with nearly 5,000 cases similarly demonstrated that the N3I1 regimen was less toxic regarding grade 3 or higher AE (Figures 2 and 3). 16 In addition, OpACIN-neo was a phase II RCT that treated 20 patients with each of the N1I3 and N3I1 regimens and compared the incidence of grades 3–4 AEs as the primary endpoint. 9 Although the OpACIN-neo trial failed to detect significantly different AE incidences, it suggested a potential difference in grades 3–4 AE incidence: 40% in the N1I3 arm and 20% in the N3I1 arm. 9 Meta-analyses of direct comparison data from two RCTs supported our main finding (Supplemental material Figure 6). Another limitation of our study included the heterogeneous cancer types and the study designs of the original studies. However, this may extend the external validity of our data.
In conclusion, this exhaustive systematic review summarized the AEs caused by the combined nivolumab plus ipilimumab regimen for antineoplastic-drug naïve patients, incorporating 4,677 patients from 48 populations. Our data showed the incidence of any AE (81.3%), grade 3 or higher AEs (40.6%), serious AEs (32.7%), AEs leading to discontinuation (28.3%), treatment-related death (0.7%), and 22 specific adverse events. In addition, the N3I1 q3w x4 regimen was substantiated to trigger fewer AEs, high-grade AEs, and serious AEs than the N1I3 q3w x4 regimen.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211058393 for Adverse events induced by nivolumab and ipilimumab combination regimens by Kohei Somekawa, Nobuyuki Horita, Ayami Kaneko, Yoichi Tagami, Nobuhiko Fukuda, Hiromi Matsumoto, Ho Namkoong, Yu Fujiwara, Kaoru Minegishi, Takeshi Fukumoto, Keisuke Watanabe, Yu Hara, Nobuaki Kobayashi and Takeshi Kaneko in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: KS worked for data acquisition, analysis of data, the interpretation, and drafting. NH contributed to the conception of the work, data acquisition, analysis of data, the interpretation, and drafting. AK, YT, NF, HM, YF, KM, TF, KW, YH, NK, TK performed the interpretation, and critical revision. HN conducted data acquisition and critical revision. All the authors provided final approval and took the accountability.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.W. received lecture fee outside of this work from Ono Pharmaceutical. N.K. received lecture fee outside of this work from Ono Pharmaceutical and Bristol Myers Squibb. T.K. received lecture fee outside of this work from Bristol Myers Squibb. The other authors nothing to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Nobuyuki Horita  https://orcid.org/0000-0002-8200-0340
https://orcid.org/0000-0002-8200-0340
Nobuhiko Fukuda  https://orcid.org/0000-0002-8498-2915
https://orcid.org/0000-0002-8498-2915
Nobuaki Kobayashi  https://orcid.org/0000-0002-7064-320X
https://orcid.org/0000-0002-7064-320X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kohei Somekawa, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuyuki Horita, Department of Pulmonology, Yokohama City University Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama 236-0004, Japan.
Ayami Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Yoichi Tagami, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuhiko Fukuda, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Hiromi Matsumoto, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Ho Namkoong, Department of Infectious Diseases, Keio University School of Medicine, Tokyo, Japan.
Yu Fujiwara, Department of Medicine, Icahn School of Medicine at Mount Sinai and Mount Sinai Beth Israel, New York, NY, USA.
Kaoru Minegishi, Department of Stem Cell and Immune Regulation, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Takeshi Fukumoto, Division of Dermatology, Department of Internal Related, Kobe University Graduate School of Medicine, Kobe, Japan.
Keisuke Watanabe, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Yu Hara, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Nobuaki Kobayashi, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
Takeshi Kaneko, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
References
- 1. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018; 62: 29–39. DOI: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 2. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. DOI: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397: 375–386. DOI: 10.1016/s0140-6736(20)32714. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 2020; 8:e000891. DOI: 10.1136/jitc-2020-000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019; 381: 2020–2031. DOI: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 6. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19: 1480–1492. DOI: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 7. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17: 883–895. DOI: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 8. Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med 2019; 7: 260–270. DOI: 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 9. Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol 2019; 20: 948–960. DOI: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- 10. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–1835. DOI: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 1721–1728. DOI: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almutairi AR, McBride A, Slack M, et al. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol 2020; 10: 91. DOI: 10.3389/fonc.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Li S, Yao Q, et al. The efficacy and safety of combined immune checkpoint inhibitors (nivolumab plus ipilimumab): a systematic review and meta-analysis. World J Surg Oncol 2020; 18: 150. DOI: 10.1186/s12957-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou S, Khanal S, Zhang H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manag 2019; 15: 211–221. DOI: 10.2147/TCRM.S193338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu T, Jin B, Chen J, et al. Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: a network meta-analysis. Ther Adv Med Oncol. Epub ahead of print July 2020. DOI: 10.1177/1758835920940927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lebbé C, Meyer N, Mortier L, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol 2019; 37: 867–875. DOI: 10.1200/jco.18.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. DOI: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18. UMIN Center. UMIN Clinical Trials Registry (UMIN-CTR), https://www.umin.ac.jp/ctr/ctr_regist.htm (accessed 29 June 2021).
- 19. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 29 June 2021).
- 20. Agresti A, Coull BA. Approximate is better than ‘exact’ for interval estimation of binomial proportions. Am Stat 1998; 52: 119–126. DOI: 10.2307/2685469. [DOI] [Google Scholar]
- 21. Khushalani NI, Kim Y, Gibney GT, et al. Adjuvant nivolumab (NIVO) plus ipilimumab (IPI) for resected high-risk stages IIIC/IV melanoma (MEL). J Clin Oncol 2016; 34: 9586. [Google Scholar]
- 22. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–133. DOI: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211058393 for Adverse events induced by nivolumab and ipilimumab combination regimens by Kohei Somekawa, Nobuyuki Horita, Ayami Kaneko, Yoichi Tagami, Nobuhiko Fukuda, Hiromi Matsumoto, Ho Namkoong, Yu Fujiwara, Kaoru Minegishi, Takeshi Fukumoto, Keisuke Watanabe, Yu Hara, Nobuaki Kobayashi and Takeshi Kaneko in Therapeutic Advances in Medical Oncology