Abstract
Case series summary
This case series describes three shelter-housed cats concurrently diagnosed with feline infectious peritonitis (FIP). The cats were from a cohort of seven surrendered from the site of a house fire. The three cats presented with mild upper respiratory signs. Within 10 days they clinically declined: progressive signs included pyrexia, icterus, lethargy, anorexia and cavitary effusions. Necropsy followed by histopathology and immunohistochemistry confirmed a diagnosis of FIP in all three. Molecular analysis of the causative feline coronavirus (FCoV) revealed varied amino acid alterations in the spike gene both between cats and between sample types in individual cats. A fourth cat from the cohort remained healthy in the shelter but succumbed to FIP 6 weeks post-adoption.
Relevance and novel information
This case series places FCoV genetic sequences in the context of clinical signs in a small shelter outbreak. Each of the three cats concurrently developed a slightly different clinical presentation. PCR amplification and genetic sequencing revealed that two cats shared an S1/S2 cleavage site mutation (R790S) previously described to be associated with the development of FIP; one of the cats had an additional S1/S2 cleavage site mutation (R793S). The third cat had a single, identical S1/S2 point mutation (R790G) unique from the other two cats; the R790G mutation has not been previously reported. This case series provides interesting data on point mutations associated with the development of FIP and provides support for a ‘circulating virulent–avirulent theory’ of FIP pathogenesis in a small shelter outbreak.
Keywords: Feline infectious peritonitis, feline coronavirus, spike protein, FIP
Introduction
Feline infectious peritonitis (FIP) is a deadly disease of cats caused by the ubiquitous feline coronavirus (FCoV). Seroprevalence for FCoV is estimated at 25% in single-cat households, and as high as 75–90% in group-housed cats.1 The vast majority of FCoV infections are asymptomatic or cause mild enteritis as a consequence of enterocyte infection. The fraction of FCoV-infected cats that go on to develop FIP is estimated to be 5–12%.2,3 In these cases the virus mutates, sustainably infects monocytes and disseminates systemically. Complex interactions between these activated monocytes and the host’s immune system then result in granulomatous lesions and vasculitis, the hallmark findings in FIP.3–5 Risk factors for individuals to develop FIP include those that influence viral transmission and replication rates, including young age, compromised immune status, physiological stress, viral load in the environment, group housing and genetic predisposition.4
Coronaviruses are highly subject to genomic mutation based on RNA replication and their large genome. Point mutations within the 3, 7b, M and S genes have been investigated to elucidate the transformation of FCoV from a biotype causing mild enteric disease, commonly referred to as feline enteric coronavirus (FECV), to the highly pathogenic biotype causing FIP, often referred to as FIPV.4,6–12 Although mutations in the 3, M and 7b genes were originally considered predictive of FIP, more recent work has shifted investigative focus to the S gene.11–17
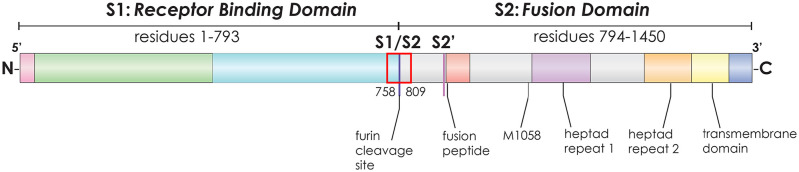
The spike (S) protein is a key mediator of host cell binding and membrane fusion. Additionally, S gene mutations are prone to an unusually high rate of selection based on functional and antigenic pressure. Several S gene mutations have been proposed to drive viral pathogenesis, notably M1058L and mutations within the S1/S2 furin cleavage site (Figure 1).10,18 The M1058L mutation has been widely studied and sequenced in cats both with and without FIP; the current view is that M1058L is not predictive of the development of FIP, but is associated with systemic spread of FCoV.9,12,14,15,19
Figure 1.
Schematic of the type I feline coronavirus (FCoV-1) spike protein gene demonstrating the binding and membrane fusion portions, as well as the S1/S2 cleavage site and the location of the 156 base-pair region sequenced (corresponding to amino acids 758–809; red box) and the M1058 amino acid residue, along with other key features. Convention is to describe amino acid substitutions by initials surrounding the numbered amino acid residue location (eg, M1058L = methionine is replaced by leucine at position 1058)
Other S mutations of particular interest include those within a structural loop of the spike protein controlling activation during cell entry, referred to as the S1/S2 furin cleavage site.10,18,19 Several investigations have focused on S1/S2 mutations.16–19 These mutations are predicted to eliminate or reduce furin cleavage, likely reducing transmissibility of the virus but increasing its tropism for monocytes.18
Ante-mortem diagnosis of FIP can be difficult. The gold standard for diagnosing FIP is labeling FCoV antigen in macrophages using immunohistochemistry (IHC) in tissues with granulomatous lesions typical of FIP.20 Tissue samples for IHC are best collected via laparotomy, which is invasive and carries risk for the ill patient. Unfortunately, the use of IHC or immunocytochemistry on samples attained via less invasive methods such as needle biopsy or fine-needle aspiration are not as reliable as tissue biopsies in confirming or excluding a diagnosis of FIP.20–22
Diagnostic test development is currently focused on molecular methods. In the past, FECV replication was thought to be restricted to the intestinal epithelium.5,23 However, using molecular methods, FCoV has been detected in monocytes and macrophages in blood samples and extra-intestinal tissues of cats without FIP-associated pathological changes.5,14,23 Commercially available molecular diagnostic tests that focus on mutation analysis, which can be performed ante-mortem on effusions or fine-needle aspirate samples, are reserved to two specific S mutations and cannot always distinguish the biotype FIPV from FECV.14,20,24 No testing modality can predict which cats with FECV will develop FIP given similar exposures and risk factors.
Outbreaks of FIP are rare. Two theories account for why, given the high rate of FCoV transmission, there is low risk of an individual developing FIP. The first, known as the ‘internal mutation theory’, suggests each case of FIP arises independently when an FECV mutates to a FIPV within the infected host.4,5 This theory has long predominated the discussion of FIP pathogenesis and implies that FIPV is not directly transmitted between cats. The second theory of transmission, the ‘horizontal transmission theory’, proposes that cats may at times shed and transmit FIPV directly.4,25 This route of transmission is thought to be extremely rare; however, a horizontal transmission event has been described in a shelter outbreak of a type II FCoV with an identical recombination site in S and an identical nonsense mutation in 3c.25 Therefore, the potential for horizontal transmission remains a concern in outbreak scenarios.
A current variation on the ‘internal mutation theory’, referred to as the ‘circulating virulent–avirulent theory’ of FIP pathogenesis, suggests that distinctive strains of FECV circulate in a population, and that FIP will develop only in cats more susceptible to the selection of virulent strains following internal mutation.5,26 This alternate theory would account for clusters of cases in populations of cats in catteries and shelters.
This case series reports the clinical presentations, pathological features and molecular sequences of the S1/S2 cleavage site from tissue and effusion samples of three cats from a single location that all developed concurrent clinical features of FIP following entry to a shelter. Our investigation provides support for a ‘circulating virulent–avirulent theory’ of FIP pathogenesis.
Case series description
In March 2019, a residence caught fire. In the following days, neighbors spotted a number of cats around the remains of the home. Seven of the cats were trapped and surrendered to a private, limited admission animal shelter (annual intake 1500 animals). All seven were sexually intact domestic shorthair cats with age estimates ranging from 6 to 18 months. Their genetic relationship to one another was unknown. Upon presentation to the shelter, each was subject to standard intake protocols for adult cats: vaccination with a killed rabies vaccine and modified-live feline viral rhinotracheitis calicivirus panleukopenia vaccine; screening for Microsporum canis with a Wood’s lamp; a combination ELISA for feline leukemia virus antigen and feline immunodeficiency virus antibody; and medications for internal and external parasites, including pyrantel pamoate (10 mg/kg PO), praziquantel (22.7 mg PO) and imidacloprid topically.
Each of the cats was placed in individual housing and examined by a staff veterinarian. None showed clinical signs attributed to actually having been in the fire. Two of the seven were healthy on intake and remained healthy throughout their shelter stay; these two cats were neutered and made available for adoption. Another two presented with diarrhea but were otherwise systemically healthy; these two were positive on fecal flotation for roundworm eggs and treated with pyrantel pamoate (10 mg/kg PO). Once the diarrhea resolved, these two cats were neutered and adopted. The three remaining cats – hereby known as cats 1024, 1025 and 1026 – presented with varied signs of upper respiratory infection (URI). All three cats were housed in isolation and received standard shelter treatments for URI, including doxycycline (10 mg/kg PO q24h), subcutaneous fluids as needed, mirtazapine (1.8 mg PO) and warmed wet food to encourage appetite and daily monitoring by medical staff.
Over the next 10 days, all three cats became progressively lethargic and inappetent, and developed additional clinical signs suggestive of FIP (Table 1).
Table 1.
Clinical presentation and relevant diagnostic test results of three cats from a single intake location that developed feline infectious peritonitis concurrently in a shelter
| Cat 1024 | Cat 1025 | Cat 1026 | |
|---|---|---|---|
| Signalment* | 6 months old, 2.7 kg, MI DSH | 1 year old, 3 kg, MI DSH | 1.5 years old, 4 kg, MI DSH |
| Progressive clinical signs | URI (nasal discharge, conjunctivitis, sneezing), gingivitis, anorexia, diarrhea, febrile (⩽103.9°F), tachypnea, dyspnea | URI (ocular discharge, third eyelid elevation, nasal discharge), gingivitis, BCS 3/9, febrile (⩽104.5°F), mild icterus, tachypnea | URI (nasal discharge), febrile (⩽104.5°F), icterus |
| Relevant bloodwork results (RIs) | Not performed | PCV/TP 23/7 (29–48%/5.9–7.5 g/dl), A/G ratio
0.8 (0.8–1.5), total bilirubin 0.4 (0.0–0.1 mg/dl) |
PCV/TP 22/8 (29–48%/ 5.9–7.5 g/dl), A/G ratio 0.7 (0.8–1.5), total bilirubin 3.1 (0.0–0.1 mg/dl) |
| Ultrasound evidence of effusion | Peritoneal, pleural, pericardial | Peritoneal, pleural | Peritoneal |
Ages were estimated by veterinarians using dentition and signs of sexual maturity
MI = male intact; DSH = domestic shorthair; URI = upper respiratory infection; BCS = body condition score; RI = reference interval; PCV = packed cell volume; TP = total protein; A/G = albumin/globulin
All three cats developed waxing–waning fevers with temperatures reaching as high as 104.5°F. Cat 1026 developed icterus first, followed by cat 1025. Bloodwork performed on cats 1026 and 1025 revealed anemia, a low or low–normal albumin-to-globulin ratio and hyperbilirubinemia. Cat 1024 had relatively mild signs initially and no icterus, so no blood chemistry was performed. Within 10 days, cats 1024 and 1025 developed tachypnea, and 1024 developed profound dyspnea. Ultrasound examinations revealed moderate peritoneal fluid in cat 1026; moderate fluid in the pleural and peritoneal cavities of cat 1025; and significant fluid accumulation in the pleural, peritoneal and pericardial cavities of cat 1024. Abdominocentesis was performed on cat 1026: fluid was straw-colored, proteinaceous (5.9 g/dl) and of low cellularity (few macrophages and non-degenerate neutrophils). Owing to rapid decline and strong suspicion for FIP, all three cats were euthanized and submitted as clinical research cases for necropsy, tissue sampling and molecular analysis.
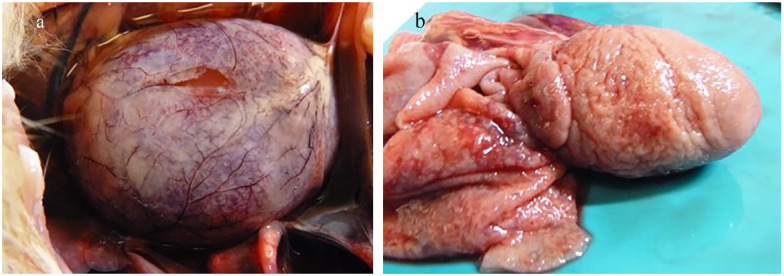
Necropsy revealed numerous and common lesions consistent with FIP, including cavitary effusions; fibrinous serositis; and multifocal granulomas in the liver, kidneys, spleen and mesentery. Although not seen initially on ultrasound examination, cat 1026 also had pleural effusion. Additionally, cat 1024 had pericardial effusion and fibrinous and granulomatous pericarditis and epicarditis (Figure 2).
Figure 2.
Necropsy images from cat 1024. (a) Heart in situ. Puncture through the pericardium reveals severe pericardial effusion with multifocal white plaques (granulomas) noted throughout the pericardium. (b) Heart removed with pericardium reflected. The entire pericardium and epicardial surface are irregular and thickened by granulomatous inflammation
Representative samples of all organs were fixed in 10% neutral buffered formalin and routinely paraffin wax embedded; sections were cut, stained with hematoxylin and eosin, and analyzed via light microscopy. Histology of select gross lesions revealed classic FIP lesions, including vasculitis, serositis and perivascular pyogranulomatous inflammation. IHC for FCoV was performed using monoclonal antibody FIPV3-70 (1:1000), Affinity Purified Anti-Mouse IgG and Bond Polymer Refine Red Detection (Leica Microsystems). IHC confirmed immunolabeling within the cytoplasm of macrophages in the granulomatous inflammation in all three cats.
PCR of frozen tissue samples collected at necropsy also confirmed the presence of FCoV in numerous tissue samples for all three cats. RNA was extracted from tissues using MagMAX Express (Life Technologies). RNA from feces was extracted using methods previously reported.27 Quantitative PCR analysis was performed and cycle threshold (Ct) values of positive samples are summarized in Table 2.
Table 2.
Real-time reverse transcriptase (RT)-PCR cycle threshold (Ct) values by cat and by sample type
| Cat 1024 sample type | Real-time RT-PCR Ct value |
Cat 1025 sample type |
Real-time RT-PCR Ct value |
Cat 1026 sample type |
Real-time RT-PCR Ct value |
|---|---|---|---|---|---|
| Brain | 31.72 | Brain | 36.33 | Brain | 35.04 |
| Kidney | 28.15 | Kidney | 34.93 | Kidney | 35.81 |
| Liver | 30.03 | Liver | 28.92 | Liver | 36.12 |
| Lung | 34.78 | Lung | 30.56 | Lung | 34.16 |
| Lymph node | 31.41 | Lymph node | 31.30 | Lymph node | 31.87 |
| Small intestine | 37.38 | Small intestine | 29.53 | Small intestine | 30.09 |
| Feces | 36.08 | Feces | 37.95 | Feces | 33.28 |
| Urine | ND | Urine | 33.40 | Urine | NT |
| Whole blood | 36.71 | Whole blood | ND | Whole blood | ND |
| Conjunctiva swab | ND | Conjunctiva swab | ND | Conjunctiva swab | ND |
| Nasal swab | ND | Nasal swab | ND | Nasal swab | ND |
| Oropharyngeal swab | ND | Oropharyngeal swab | ND | Oropharyngeal swab | ND |
| Pleural fluid | 32.93 | Pleural fluid | 29.34 | Pleural fluid | 29.84 |
| Peritoneal fluid | 29.39 | Peritoneal fluid | 31.27 | Peritoneal fluid | 32.11 |
| Pericardial fluid | 31.45 |
The lower the Ct value, the higher the amount of feline coronavirus (FCoV) RNA in the sample
ND = not detected; NT = not tested
For PCR-positive tissue and effusion samples with a Ct value <36, a central 156 base-pair (bp) region of the spike gene, including the S1/S2 activation site, was PCR amplified and sequenced as described by Licitra et al,18 and shown in Figure 1, with the following modifications: 25 μl reverse transcription PCRs were performed with qScript XLT 1-Step RT PCR kit (Quantbio); PCR conditions were 20 mins at 50°C, 3 mins at 95°C and 40 cycles of 10 s at 95°C, 20 s at 55°C, 40 s at 72°C, then 10 mins at 72°C. PCR products were purified using Diffinity RapidTips (Diffinity Genomics). All FCoV sequences were consistent with type I FCoV based on sequence alignment with reference genomes over the S1/S2 area sequenced. Based on the limited utility of other FCoV mutations in determining FIP outcome, including M1058L, genomic data outside of the central 156 bp region of the spike gene was not collected.
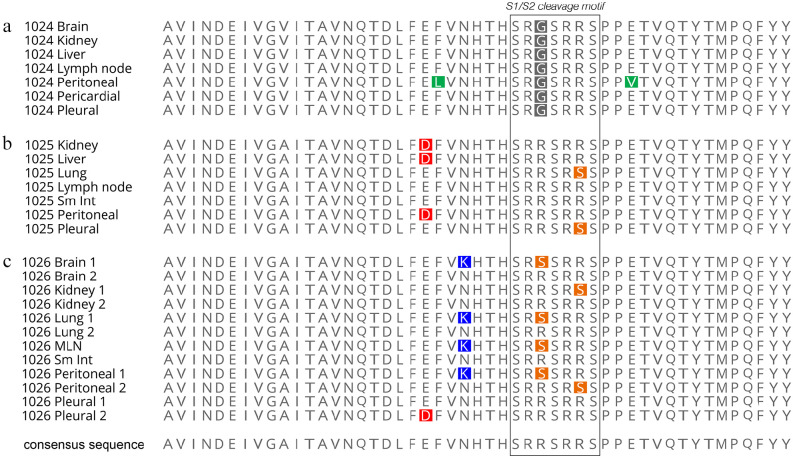
Samples from the three cats had different sequencing results. Within the S1/S2 cleavage site, FCoV recovered from cats 1025 and 1026 shared identical amino acid point mutations at the 793 position (R793S); FCoV recovered from cat 1026 had an additional, alternate mutation (R790S) in samples from brain, lung, lymph node and peritoneal effusion. Virus from cat 1024 had a point substitution at the 790 position (R790G) distinct from that of cats 1025 and 1026 and identical in all tissues. The partial spike gene sequences generated are shown in Figure 3. These substitutions are all consistent with individual point mutations affecting furin cleavage, as outlined in Licitra et al.18
Figure 3.
Schematic of the amino acid sequences from (a) cat 1024, (b) cat 1025 and (c) cat 1026 and the tissues analyzed. Amino acid changes from the consensus (non-mutated) sequences are shown in colored highlights, with colors based on RasMol convention (http://www.openrasmol.org/)
Non-mutated (consensus) S1/S2 sequences were recovered alongside mutated sequences in samples from the kidney, brain and lung of cat 1026. Additionally, only non-mutated S1/S2 sequences were recovered from the small intestine and pleural effusion from cat 1026, and from the kidney, liver, lymph node, small intestine and peritoneal fluid of cat 1025. Outside of the S1/S2 cleavage site, additional mutations were present in many samples, but the functional outcome of these mutations is not clear.
After confirming the clinical diagnosis of FIP in all three cats, the remaining four cats from the cohort were carefully re-examined by a veterinarian for any signs of systemic illness. All four cats appeared to be in excellent health. A memorandum for potential adopters was added to each cat’s file explaining FIP and its clinical appearance, and emphasizing that these cats could be at elevated risk for developing FIP. All four cats were adopted. Six weeks post-adoption one became acutely ill, reportedly showing signs consistent with FIP, and was euthanized by a primary care veterinarian. No necropsy or diagnostic testing was performed. Of the remaining three cats, two were in good health in their adoptive homes 2 years later and one was lost to follow-up.
Discussion
Definitive diagnosis of FIP by molecular diagnosis is challenging using currently available testing methods, primarily due to FCoV’s large viral genome and limited knowledge of the significance of particular mutations.20 In this outbreak investigation, IHC was performed on tissues of affected cats to confirm the diagnosis of FIP; viral RNA was then PCR amplified and sequenced to characterize a fragment of the spike protein in all cats and in all major sampled tissues. A key question was whether the three cats shared an identical mutation given their common origin and concurrent development of disease.
PCR and sequencing revealed each cat was infected with FCoVs containing different S1/S2 sequences. The sequences in cats 1025 and 1026 display a range of molecularly connected changes centered on the key residues needed for cleavage by the protease furin.18 These findings suggest a similar sequence of mutations occurred in cats 1025 and 1026. The R790S mutation in cat 1026 has previously been reported as being associated with the development of FIP.16,18 The R793S mutation seen in cats 1025 and 1026 is also consistent with a loss of function for furin cleavage and FIP development.16,18 The tissues and effusion from cat 1024 contained a distinct but consistent mutation (R790G), notably also in a key amino acid position for furin cleavage, which has not been previously reported.
This cluster of FIP cases is most consistent with a ‘circulating virulent–avirulent’ variation of the ‘internal mutation theory’, presumably with the three cats sharing a distinct but common FCoV that acquired additional key mutations in the S1/S2 cleavage site to drive FIP development. The simultaneous development of signs in these three cats, while unusual, was likely fueled by physiological and immune stressors, including the house fire and admission to a shelter, acting on a parent FCoV that was primed for the transition to virulence.
This case series is also an interesting illustration of an outbreak in a closely associated cohort of cats with slightly different manifestations of disease and with varied diagnostic results. Often effusion is the most readily collected sample for ante-mortem diagnosis; however, recovery of FCoV RNA and sequences in effusion samples of these cats varied. Cat 1026 had two different S1/S2 furin cleavage-associated point mutations in its peritoneal effusion sample, but a non-mutated FCoV sequence in its pleural effusion sample; conversely, cat 1025 had an S1/S2 mutation in its pleural effusion sample but a non-mutated FCoV sequence in its peritoneal effusion sample.
Our investigation also demonstrated that both mutated and non-mutated sequences can be found concurrently in tissue samples. This is not surprising given the relatively recent discovery that FECVs can disseminate in monocytes and be present in tissues of cats both with and without FIP.5,14,20,23
This investigation had several limitations. Without full genomic sequencing of the FCoV RNA recovered from each cat it was not possible to compare the remainder of their sequences. Furthermore, the study did not gather contemporaneous molecular sequencing data from the unaffected cats from the cohort of seven, nor prior to the stressful event, which could otherwise provide a helpful baseline for comparison to further analyze these results.
Examination of the known risk factors for developing FIP provides ample rationale for each of these cats to have developed the disease. Each was less than 2 years old and concurrently admitted to a shelter from a single location with numerous cats. These cats may have shared food bowls, litter boxes or other living areas where FECV would be readily transmitted. Age estimates suggest they were not littermates, but they could have been genetically related in other ways. Each was subject to major stressors: surviving a fire, experiencing homelessness and being trapped and placed in an unfamiliar shelter environment. In population settings, an outbreak of FIP can raise concern for a horizontal transmission of FIPV based on the rare reports of such events in the literature; however, more common risk factors in the population and environmental management practices should always be investigated.
Conclusions
This report provides a review of the clinical features, pathological findings and molecular sequences of the S1/S2 spike gene in samples from three adult domestic shorthiair, shelter cats affected concurrently with rapidly progressive effusive FIP. The cats were admitted to the shelter from a single location but were of unknown genetic relation. Molecular analysis of tissues collected at necropsy and positive on IHC revealed each cat was infected with a type 1 FCoV, each with distinct patterns of mutations in the S1/S2 spike protein gene sequence.
The variation in clinical signs, sequences and dissemination of sequences in samples in this case series illustrates the complexity of definitively diagnosing FIP in a live patient, particularly as ante-mortem testing modalities are typically reliant on effusion or small tissue samples.20 In this case series, molecular sequencing of tissue samples obtained at necropsy clearly identified that this outbreak was not due to a horizontal transmission. Additionally, a previously unreported S1/S2 cleavage site mutation (R790G) was sequenced. Ongoing investigation and sequencing of mutations recovered from FIP patients may provide important information for future diagnostic test development.
Acknowledgments
The authors are grateful for Beth Licitra for her feedback prior to publishing this manuscript.
Footnotes
Correction (January 2023): Article updated to correct a minor spelling error in fourth co-author’s surname.
Accepted: 28 December 2021
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Work in the Whittaker Lab is funded by the Michael Zemsky Fund for Feline Diseases at the Cornell Feline Health Center. Work of Maddie’s Shelter Medicine Program at Cornell is supported by Maddie’s Fund, as well as many private donors. Salary for Eleni Healey’s position at Penn Vet during writing and editing was supported by the Arnall Family Foundation.
Ethical approval: This work involved the use of non-experimental animals only (including owned or unowned animals and data from prospective or retrospective studies). Established internationally recognized high standards (‘best practice’) of individual veterinary clinical patient care were followed. Ethical approval from a committee was therefore not specifically required for publication in JFMS Open Reports.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). For any animals or humans individually identifiable within this publication, informed consent (either verbal or written) for their use in the publication was obtained from the people involved.
ORCID iDs: Nicole M Andre  https://orcid.org/0000-0002-3703-5026
https://orcid.org/0000-0002-3703-5026
Gary R Whittaker  https://orcid.org/0000-0001-8037-3816
https://orcid.org/0000-0001-8037-3816
Elizabeth A Berliner  https://orcid.org/0000-0001-5513-379X
https://orcid.org/0000-0001-5513-379X
References
- 1. Rohrbach BW, Legendere AM, Baldwin CA, et al. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J Am Vet Med Assoc 2001; 218: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 2. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartmann K. Feline infectious peritonitis: new developments in pathogenesis, diagnosis, and management. Fred Scott Feline Health Symposium; 2016; Ithaca, NY, USA. [Google Scholar]
- 4. Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet J 2014; 201: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kipar A, Meli ML. Feline infectious peritonitis: still an enigma? Vet Pathol 2014; 51: 505–526. [DOI] [PubMed] [Google Scholar]
- 6. Oguma K, Ohno M, Yoshida M, et al. Mutation of the S and 3c genes in genomes of feline coronaviruses. J Vet Med Sci 2018; 80: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vennema H, Poland A, Foley J, et al. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology 1998; 243: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang HW, de Groot RJ, Egberink HF, et al. Feline infectious peritonitis: insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J Gen Virol 2010; 91: 415–420. [DOI] [PubMed] [Google Scholar]
- 9. Lewis CS, Porter E, Matthews D, et al. Genotyping coronaviruses associated with feline infectious peritonitis. J Gen Virol 2015; 96: 1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang HW, Egberink HF, Halpin R, et al. Spike protein fusion peptide and feline coronavirus virulence. Emerg Infect Dis 2012; 18: 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yin Y, Li T, Wang C, et al. A retrospective study of clinical and laboratory features and treatment on cats highly suspected of feline infectious peritonitis in Wuhan, China. Sci Rep 2021; 11: 5208. DOI: 10.1038/s41598-021-84754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Decaro N, Mari V, Lanave G, et al. Mutation analysis of the spike protein in Italian feline infectious peritonitis virus and feline enteric coronavirus sequences. Res Vet Sci 2021; 135: 15–19. [DOI] [PubMed] [Google Scholar]
- 13. Emmler L, Felten S, Matiasek K, et al. Feline coronavirus with and without spike gene mutations detected by real-time RT-PCRs in cats with feline infectious peritonitis. J Feline Med Surg 2020; 22: 791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barker E, Stranieri A, Helps C, et al. Limitations of using feline coronavirus spike protein gene mutations to diagnose feline infectious peritonitis. Vet Res 2017; 48: 60. DOI: 10.1186/s13567-017-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Felten S, Matiasek K, Leutenegger CM, et al. Diagnostic value of detecting feline coronavirus RNA and spike gene mutations in cerebrospinal fluid to confirm feline infectious peritonitis. Viruses 2021; 13: 186. DOI: 10.3390/v13020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. André NM, Miller AD, Whittaker GR. Feline infectious peritonitis virus-associated rhinitis in a cat. JFMS Open Rep 2020; 6. DOI: 10.1177/2055116920930582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andre NM, Cossic B, Davies E, et al. Distinct mutation in the feline coronavirus spike protein cleavage activation site in a cat with feline infectious peritonitis-associated meningoencephalomyelitis. JFMS Open Rep 2019; 5. DOI: 10.1177/2055116919856103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Licitra BN, Millet JK, Regan AD, et al. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerg Infect Dis 2013; 19: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Porter E, Tasker S, Day MJ, et al. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet Res 2014; 45: DOI: 10.1186/1297-9716-45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felten S, Hartmann K. Diagnosis of feline infectious peritonitis: a review of the current literature. Viruses 2019; 11: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giordano A, Paltrinieri S, Bertazzolo W, et al. Sensitivity of tru-cut and fine needle aspiration biopsies of liver and kidney for diagnosis of feline infectious peritonitis. Vet Clin Pathol 2005; 34: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Felten S, Hartmann K, Doerfelt S, et al. Immunocytochemistry of mesenteric lymph node fine-needle aspirates in the diagnosis of feline infectious peritonitis. J Vet Diagn Invest 2019; 31: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kipar A, Meli ML, Baptiste KE, et al. Sites of feline coronavirus persistence in healthy cats. J Gen Virol 2010; 91: 1698–1707. [DOI] [PubMed] [Google Scholar]
- 24. IDEXX Reference Laboratories. IDEXX Reference Laboratories now offers the Feline Infectious Peritonitis (FIP) Virus RealPCR Test to aid in the diagnosis of this devastating feline disease. https://ca.idexx.com/files/rfip-virus-pct-dxu-update.pdf (2014, accessed June 29, 2021).
- 25. Wang YT, Su BL, Hsieh LE, et al. An outbreak of feline infectious peritonitis in a Taiwanese shelter: epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Vet Res 2013; 44. DOI: 10.1186/1297-9716-44-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown MA, Troyer JL, Pecon-Slattery J, et al. Genetics and pathogenesis of feline infectious peritonitis virus. Emerg Infect Dis 2009; 15: 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stout AE, Hofmar-Glennon HG, André NM, et al. Infectious disease surveillance of apparently healthy horses at a multi-day show using a novel nanoscale real-time PCR panel. J Vet Diagn Invest 2021; 33: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]