Abstract
Introduction
Testicular adrenal rest cell tumours (TART) are rare benign adrenocorticotropic hormone-dependent testicular tumours, which can develop in patients with congenital adrenal hyperplasia. If left untreated, they can cause testicular tissue damage and infertility. Ultrasound is the imaging modality of choice allowing for non-invasive diagnosis provided that characteristic features are seen. In recent times, magnetic resonance imaging characteristics have also been described to aid diagnosis.
Case Report
This case describes the imaging features of multiple bilateral TART in a young patient with congenital adrenal hyperplasia. Traditional greyscale and colour Doppler ultrasound demonstrated intratesticular, predominantly hypoechoic areas, with increased surrounding colour Doppler flow. In addition, contrast-enhanced ultrasound and strain elastography were performed, showing increased TART vascularisation and increased stiffness in the hypoechoic areas. Subsequent magnetic resonance imaging confirmed bilateral lobulated, well demarcated, intratesticular lesions, which appeared predominantly isointense on T1-weighted imaging, hypointense on T2-weighted imaging, with heterogeneous enhancement following gadolinium administration.
Discussion
This case illustrates the sonographic features including greyscale, colour Doppler, contrast and elastography ultrasound of TART in a patient with congenital adrenal hyperplasia. Both contrast-enhanced ultrasound and elastography can provide information about tissue properties not normally derived from conventional ultrasound and aid accurate diagnosis. Additional magnetic resonance imaging is not normally required with typical ultrasound appearances. The unusual findings seen on the ultrasound examination were likely secondary to poor compliance with treatment.
Conclusion
Familiarity and recognition of characteristic and uncommon imaging features of these tumours are important to avoid misdiagnosis and surgical intervention.
Keywords: Testicular adrenal rest tumour, ultrasonography, elastography, 21-hydroxylase deficiency, congenital adrenal hyperplasia, multi-parametric ultrasound
Introduction
Congenital adrenal hyperplasia (CAH) is an autosomal recessive inherited disorder affecting steroid synthesis in the adrenal gland. More than 90% of cases of CAH are caused by 21-α-hydroxylase deficiency which results in impaired cortisol and aldosterone production, leading to subsequent overproduction of adrenocorticotropic hormone (ACTH) from the pituitary gland and adrenal gland hyperplasia.1,2 One of the most significant complications in male CAH patients is that of testicular adrenal rest tumours (TART), which are thought to arise from aberrant adrenal cells in the testes secondary to chronically elevated ACTH concentrations, a consequence of inadequate hormone therapy or non-compliance. 3 Histologically, TART resemble adrenocortical tissue, but the underlying aetiology is incompletely understood. These tumours are bilateral in 80% of cases, in distinction to Leydig cell tumours (the main differential diagnosis), which are bilateral in only 3% of cases. Whilst TART are benign, they have serious implications for male fertility through obstruction of the seminiferous tubules, leading to irreversible damage of testicular tissue. 4 TART may be easily missed on clinical examination if small in size but also due to their most common location within the rete testis. Ultrasound (US) is therefore invaluable in the detection and assessment of these tumours. We present a case of TART with US characteristics, including unusual greyscale appearances, colour Doppler, contrast-enhanced US and strain elastography and associated magnetic resonance (MR) imaging features.
Case report
A 21-year-old male presented to the endocrinology clinic as part of a routine follow-up for known history of CAH secondary to 21-hydroxylase deficiency. He was physically well on presentation and completely asymptomatic, taking regular oral steroids (hydrocortisone 10 mg a.m. and midday, 7.5 mg p.m. and 100 mcg fludrocortisone a.m.). Testicular examination revealed bilateral nodular testicles with no associated pain. The examining endocrinologist arranged for imaging, where the patient underwent testicular US (including greyscale, colour Doppler, contrast-enhanced US and strain elastography). The patient also underwent a scrotal and pelvic MR examination (T1, T2-weighted imaging and T1-weighted fat saturation imaging following gadolinium injection).
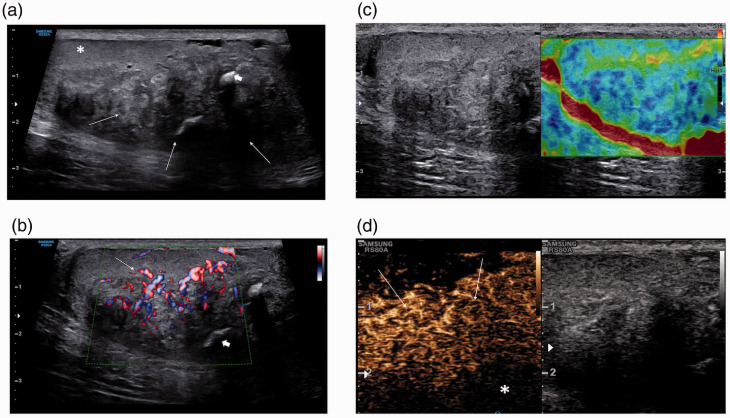
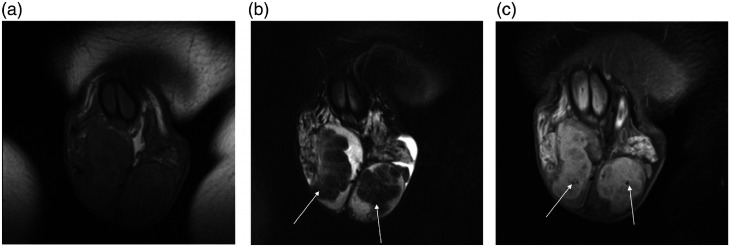
On US examination, using a Samsung RS85 (Samsung Medison, Seoul, Korea) and a L3-12A linear transducer, both testes were abnormal; there were multiple coalescing areas of hypo- and hyperechoic changes, predominantly in the lower aspect of the testis, occupying most of the testis. Curvilinear areas of focal calcification were noted, with some remaining normal testicular parenchyma present (Figure 1(a)). An increase in vascularity surrounding the areas of abnormality was depicted by increased colour Doppler flow, with a paucity of colour Doppler flow present within the lesions (Figure 1(b)). The strain elastography images demonstrated increased stiffness (Figure 1(c)). Following the administration of SonoVue™ (Bracco SpA, Milan, Italy) 4.8 mL, the mixed echo lesion demonstrated increased enhancement compared to the surrounding normal testicular parenchyma and subsequent late wash-out (Figure 1(d)). The US features were considered unusual for TART but most likely to represent chronic changes in relation to the underlying CAH and possible inadequate treatment. Subsequent MR imaging demonstrated bilateral focal lobulated intratesticular abnormalities surrounding the testicular mediastinum, seen as isointense on T1-weighted images (Figure 2(a)), and hypointense on T2-weighted images compared to normal testicular parenchyma (Figure 2(b)). There was heterogeneous enhancement following administration of gadolinium. In addition, hypointense foci were seen in keeping with calcification seen on US examination (Figure 2(c)).
Figure 1.
(a) Greyscale ultrasound of the right testis shows multiple, coalescing areas of hypo- and hyperechoic changes (arrows), predominantly at the lower aspect of the testis, and occupying most of the testis. Curvilinear areas of focal calcification were noted (arrowheads), with some remaining normal testicular parenchyma present (asterisk). (b) An increase in vascularity surrounding the areas of abnormality was depicted by colour Doppler flow (arrows), with a paucity of colour Doppler flow present within the lesion (short arrows). (c) Strain elastography of the right testis shows increased stiffness in the abnormal mixed echogenic areas, as demonstrated by an increase in ‘blue’ depicting tissue hardness. (d) Following the administration of SonoVue™ 4.8 mL, the mixed echogenic lesion (arrows) demonstrates increased enhancement in comparison to the surrounding normal testicular parenchyma (asterisk) and subsequent late wash-out.
Figure 2.
(a) Axial T1-weighted MR image demonstrates that the abnormal intratesticular areas are isointense to the normal surrounding testicular parenchyma. (b) Axial T2-weighted image MR image shows lobulated and heterogenous but predominantly hypointense masses within both testes (arrows). (c) Axial T1-weighted MR image with fat saturation following gadolinium administration demonstrates heterogeneous enhancement of both testicular masses. In addition, there are hypointense foci within the masses in keeping with calcification (arrows).
In line with the imaging findings of bilateral TART, biochemical investigation confirmed the suspected inadequate hormonal control; the patient was found to have an elevated serum 17-medroxyprogesterone 501 (0.0–5.0 nmol/L), ACTH 651 (0–46 ng/L), androstenedione >35.0 (1.4–9.1 nmol/L) and FSH 15.2 (1.8–10 IU/L), but normal serum aldosterone 379 (100–400 pmol/L), LH 7.6 (1.5–9.3 IU/L) and testosterone 10.5 (10–30 nmol/L). Following review of the blood biochemistry and imaging findings, the patient failed to attend scheduled endocrinology follow-up but had been advised to adjust steroidal treatment and start prednisolone 2.5 mg twice daily. He was also referred for outpatient follow-up in a fertility clinic. Following the initial US in 2019, the patient underwent follow-up US imaging in September 2020; this demonstrated unchanged intratesticular appearances with no clear evidence of tumour regression. This may be indicative of ongoing poor treatment compliance.
Discussion
This case illustrates the sonographic and MR imaging features of TART in a patient with poorly controlled CAH. TART are very common in patients with CAH with a reported prevalence up to 94% and usually present between the ages of 20–40 years. 5 US is the first line imaging modality with the advantages of being low cost and readily available. Whilst US and MR imaging characteristics of TART have been described previously,3,5 this is the first description of contrast-enhanced US and strain elastography appearances. TART are usually described as sonographically round or lobular elongated masses with sharp margins, but we found somewhat unusual greyscale characteristics with patchy hypo- and hyperechogenicity associated with intratesticular calcification. In our patient, there was increased surrounding vascularity on colour Doppler, with increased perilesional and intralesional flow which has been reported previously. 5
The use of contrast-enhanced US is advantageous in that it provides real time imaging of vascular flow, even at a microvascular level, and thus tissue perfusion. 6 We found that the lesions were predominantly hypervascular, indicating increased tissue perfusion compared to that of the surrounding testicular tissue. Disorganised or increased vascularity on contrast-enhanced US is usually considered a feature of malignancy and ‘wash-out’ a further marker of potential malignancy. 7
Strain elastography can provide complementary information to conventional US by adding stiffness as an additional qualitative and quantitative measure of tissue properties; malignant lesions are usually considered ‘harder’ (less compressible and stiffer) than benign lesions. 8 This case demonstrates, however, that benign lesions, such as TART, can also demonstrate both increased vascularity and stiffness compared to that of normal testicular tissue. It is therefore important to recognise that both contrast-enhanced US and elastography on its own cannot differentiate between benign and malignant lesions but rather provide information about tissue properties not normally derived from conventional US. MR imaging characteristics were similar to those seen previously, with TART being iso- or slightly hyperintense on T1-weighted imaging, hypointense on T2-weighted imaging, with variable but usually homogeneous enhancement, and discrete margins. MR imaging is not normally required with diagnostic US appearances but was required in this case due to atypical findings and size of the bilateral lesions. The atypical findings seen on the US examination were likely a consequence of progressive growth of the TART in a non-treatment compliant patient.
It is known that TART have no malignant features, and there is no need to remove the tumours at an early stage. 9 However, because of the localisation of the tumours in the rete testis, the tumours may compress the seminiferous tubules leading to obstructive azoospermia. Therefore, it is important to detect and treat the tumours before permanent damage of the testis has occurred. Currently, CAH patients do not routinely undergo US screening for TART, but prepubertal, pubertal and even annual US have been proposed previously in order to avoid complications such as infertility. In addition, US can be used to assess treatment response in some patients, where improved hormonal control leads to tumour regression. 10 The main differential to an intratesticular lesion is that of germ cell tumours, which can demonstrate similar imaging features to TART, but these are only bilateral in 5% of cases; 9 thus the clinical history of CAH and bilateral nature of TART will be the most useful diagnostic aid. Concurrent TART and Leydig tumours are considered extremely rare. The patient did not undergo biopsy or histological confirmation due to the re-assuring imaging findings of benign testicular lesions on a known background of CAH.
Conclusion
US characterisation and evaluation are important due to the high prevalence of TART in CAH patients, which often goes undetected on clinical examination. Increased awareness and accurate characterisation of benign TART are pertinent to avoid unnecessary surgery for presumed underlying testicular malignancy. The addition of elastography and contrast-enhanced US aids confidence in establishing a diagnosis, but full multiparametric imaging, a clinical history of CAH and the bilateral nature of TART are important.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: PSS received lecture fees from Bracco SpA.
Ethics Approval: Ethical approval was not required for this case report, however, written consent to publish the details was obtained from the patient.
Guarantor: PSS.
Contributorship: NMM and PSS researched the literature and designed the case study. NMM and PSS obtained written consent from patient for publication of case report. PSS and DYH acquired the images used for the case report. NMM wrote the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Acknowledgments: N/A.
ORCID iDs: Nina M Mansoor https://orcid.org/0000-0003-3146-082X
Dean Y Huang https://orcid.org/0000-0002-5637-6723
Paul S Sidhu https://orcid.org/0000-0003-1928-4077
References
- 1.Speiser PW, Azziz R, Baskin LS, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95: 4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab 2010; 95: 5110–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avila NA, Shawker TS, Jones JV, et al. Testicular adrenal rest tissue in congenital adrenal hyperplasia: serial sonographic and clinical findings nib. AJR Am J Roentgenol 1999; 172: 1235–1238. [DOI] [PubMed] [Google Scholar]
- 4.Claahsen-van der Grinten HL, Hermus ARMM, Otten BJ. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol 2009; 2009: 624823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delfino M, Elia J, Imbrogno N, et al. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia. J Ultrasound Med 2012; 31: 383–388 [DOI] [PubMed] [Google Scholar]
- 6.Huang DY, Sidhu PS. Focal testicular lesions: colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography as adjuvants to the diagnosis. Br J Radiol 2012; 85: S41–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf GT, Rafailidis V, Moore S, et al. The role of contrast-enhanced ultrasound (CEUS) in the evaluation of scrotal trauma: a review. Insights Imaging 2020; 11: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigrist RMS, Liau J, El Kaffas A, et al. Ultrasound elastography: review of techniques and clinical applications. Theranostics 2017; 7: 1303–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieckmann KP, Boeckmann W, Brosig W, et al. Bilateral testicular germ cell tumors. Report of nine cases and review of the literature. Cancer 1986; 57: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 10.Claahsen-van der Grinten HL, Sweep FCGJ, Blickman JG, et al. Prevalence of testicular adrenal rest tumors in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol 2007; 157: 339–344. [DOI] [PubMed] [Google Scholar]