Abstract
Introduction
We aimed to describe shear wave elastography parameters of non-mass lesions of the breast and to assess the measures of diagnostic accuracy of shear wave elastography in the differentiation of non-mass lesions compared with conventional ultrasound, using histopathologic results as the reference standard.
Methods
This retrospective study included breast ultrasound-detected non-mass lesions with a confirmed pathologic diagnosis during a two-year study period. B-mode ultrasound and shear wave elastography were performed for all lesions before biopsy. Ultrasound features, shear wave elastography parameters (mean elasticity and maximum stiffness color), as well as Breast Imaging-Reporting and Data System categories were recorded for each lesion. Measures of diagnostic accuracy of ultrasound and ultrasound + shear wave elastography were also assessed.
Results
From a total of 567 breast lesions requiring core-needle biopsy, 49 (8.6%) were considered as non-mass lesions. Based on histopathologic reports, 32 patients (65.3%) had non-high-risk benign lesions, five (10.2%) had high-risk benign lesions, five (10.2%) had ductal carcinoma in situ, and seven (14.3%) had invasive carcinoma. There was no significant difference in patients’ age and palpability between benign and malignant lesions (p = 0.16 and p = 0.12, respectively). Mean elasticity values and Breast Imaging-Reporting and Data System categories were significantly higher among malignant lesions compared with benign non-mass lesions (both p < 0.001). Furthermore, the addition of shear wave elastography to grayscale ultrasound increased the specificity, positive predictive value, and diagnostic accuracy.
Conclusion
The complementary use of shear wave elastography with conventional ultrasound might help in the differentiation of non-mass breast lesions and has the potential to decrease the frequency of unnecessary biopsies performed for benign non-mass lesions.
Keywords: Breast, non-mass lesion, shear wave elastography, ultrasound, BI-RADS
Introduction
Breast cancer is the most common malignancy and the first cause of cancer-related mortality among women. Therefore, early diagnosis of breast cancer as well as the development of novel therapeutic strategies is of utmost importance for improving the prognosis of breast cancer patients.1–3 Ultrasound (US) has been conventionally used for the evaluation of breast lesions that are initially detected on mammography. Evidence suggests that breast cancer can occasionally manifest as a non-mass lesion (NML) on breast US. With the availability and the widespread use of high-resolution breast US, the detection rate of breast NMLs has increased in recent years.4–6 According to studies performed in Korea, the incidence of such lesions is estimated to be approximately 1–5.3% on screening breast US.5,7 While masses are defined as space occupying lesions that are distinguished from adjacent tissue by their anatomic border, NMLs are characterized by ill-defined areas with hypoechogenicity or tissue distortion. 8
NMLs have not yet been defined by the Breast Imaging-Reporting and Data System (BI-RADS) lexicon of the American College of Radiology, but studies have shown that these lesions have a more than 2% risk of malignancy.5,6,9 The differential diagnosis of NMLs comprises a broad range of benign and malignant lesions, including fibrocystic changes, fibrosis, adenosis, mastitis, ductal carcinoma in situ (DCIS), invasive lobular carcinoma, and sometimes invasive ductal carcinoma. These lesions are difficult to detect and recognize, especially within a heterogeneous and dense breast parenchyma.8,10 Attempts have been made in categorizing NMLs into four subcategories, including ductal hypoechoic structures as type I, hypoechoic area without ductal appearance as type II, areas of tissue distortion as type III, and hypoechoic areas with posterior acoustic shadowing as type IV. Types I and II are further subdivided into two groups, a and b, which are dependent on the presence of internal calcification.11,12
Shear wave elastography (SWE) has recently gained popularity as a supplementary method to advance the diagnostic accuracy of conventional US in the diagnosis of superficial lesions, specifically those of the breast. 13 Compared to other methods of elastography, SWE has been shown to be less operator-dependent and more reproducible.14,15 In comparison to strain elastography, which produces qualitative and semi-quantitative breast tissue elasticity properties based on strain ratio and width ratio, SWE is able to generate quantitative values. 13 In addition, some believe that for isotropic tissues, such as breast, liver, and thyroid, the diagnostic value of SWE is superior to that of acoustic radiation force impulse. 16 SWE improves the specificity of conventional US in differentiating malignant from benign breast masses.17,18 Few studies have described SWE characteristics of NMLs; therefore, we conducted this study to define SWE characteristics of NMLs and to assess the measures of diagnostic accuracy of grayscale US and US + SWE for differentiation of benign and malignant NMLs, using histopathologic results as a gold standard.
Material and methods
Patients and breast lesions
Our institutional review board approved this retrospective study and informed consent was waived for reviewing patients’ medical records and radiological images. In this study, we retrospectively identified patients in whom a core-needle biopsy (CNB) was performed following the detection of a breast lesion on breast US by searching our institutional databases between April 2018 and April 2020. The inclusion criteria for this study were women with breast US-detected NMLs who had a confirmed pathologic diagnosis. All of the patients with a recorded NML had undergone SWE imaging before biopsy. In total, 49 patients with a mean age of 47.5 years (range, 28–75 years) who had NML lesions were included in this study. The patients’ medical records were reviewed for recorded demographic data, clinical information and histopathologic reports.
US examination and image interpretation
Conventional B-mode US and SWE were performed by two board-certified radiologists with more than 10 years of experience each in breast imaging before US-guided CNB. B-mode and SWE were done using a supersonic US device (SuperSonic, Aix-en-Provence,France) with a 12 MHz superficial linear array transducer.
Data pertaining to B-mode US imaging were reviewed by radiologists on the picture archiving and communication system and information regarding breast composition (homogenous fat, homogenous fibroglandular, and heterogeneous) and types of NMLs, as defined in a previous study, 12 were recorded.
For each lesion, quantitative and qualitative SWE parameters (mean elasticity (based on Young’s modulus (kPa)) and maximum stiffness color) were collected. The maximum stiffness color scale was displayed according to the color of the stiffest area of each lesion on SWE as follows: dark blue, 0–36 kPa; light blue, 36–72 kPa; green to yellow, 72–108 kPa; orange, 108–144 kPa; and red, 144–180 kPa. Quantitative stiffness values were calculated by drawing a 2 mm circular region of interest (ROI) over the area with maximum stiffness (including the lesion’s margin), and another ROI over adjacent normal breast tissue.
BI-RADS categories were determined by consensus for each lesion with grayscale US (after obtaining at least two orthogonal grayscale images for each NML) and US + SWE. Biopsy was performed for all lesions with BI-RADS 4a (n = 22), 4b (n = 8), 4c (n = 5), 5 (n = 5), and BI-RADS 3 (n = 9) at the patient’s or physician’s preference.
All imaging data were retrospectively reviewed and reported by experienced radiologists other than those who performed the studies, and had no knowledge of the clinical findings, histological results, and clinical outcomes.
Biopsy results
US-guided CNB was performed by expert radiologists using a 14 G 16 cm automated biopsy needle (BARD Medical Division, Covington, GA, USA) with at least four passes per lesion.
Histopathologic results were divided into benign and malignant lesions. Benign lesions were then subdivided into high-risk and non-high-risk lesions, and malignant lesions were subdivided into DCIS and invasive carcinoma categories. Papillary lesions, atypical ductal hyperplasia and flat epithelial hyperplasia were classified as high-risk benign lesions. All other benign histopathologic entities, including fibrocystic change, fibroadenoma, sclerosing adenosis, and fat necrosis, were assumed as non-high-risk benign lesions.
Statistical analysis
All numeric data were evaluated for normal distribution using Kolmogorov–Smirnov test. Mean elasticity values were compared between histopathologic entities using the nonparametric Mann–Whitney and Kruskal–Wallis tests. For parametric data, independent samples t-test and chi-square test were used for comparison between groups. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of B-mode US and US + SWE were calculated for differentiating malignant from benign NMLs. Sensitivity and specificity of B-mode US were compared with those of US + SWE by using the McNemar test. In this study, BI-RADS category 4a or higher was considered as positive for malignancy and CNB histopathologic results were considered as the reference standard. Statistical analysis was performed using SPSS statistical software (SPSS Inc., Chicago, IL, version 23). p-Values < 0.05 were considered as statistically significant.
Results
In total, 567 US-guided CNBs that corresponded to 567 patients were performed between April 2018 and April 2020 at our institution, of which 49 biopsies (8.6%) evaluated NMLs. Histopathologic reports were available for all of the 49 patients; 32 patients (65.3%) had non-high-risk benign lesions, five patients (10.2%) had high-risk benign lesions, five patients (10.2%) had DCIS, and seven patients (14.3%) had invasive carcinoma. Patients with benign and malignant NMLs had a mean age of 46.2 (±11.0) and 51.5 (±11.1) years, respectively (p = 0.16). There were 26 non-palpable lesions (53.1%), including 22 benign and 4 malignant lesions (Figures 1 and 2). No significant association was seen between palpability of lesions and their histopathology (benign or malignant) (p = 0.12). Most lesions (83.7%) had a heterogeneous echotexture on B-mode US, and the overall breast composition was not significantly different between benign and malignant NMLs (p = 0.35) (Table 1).
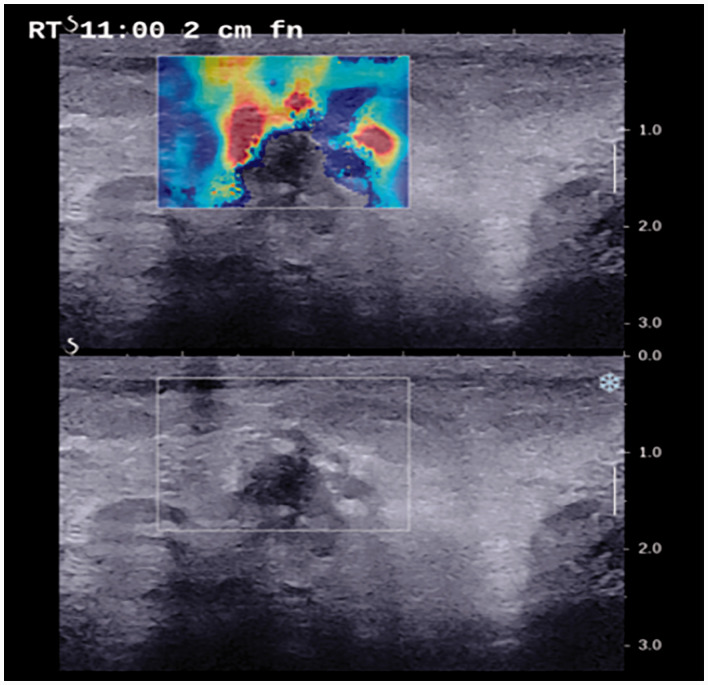
Figure 1.
A 45-year-old woman who presented with a palpable abnormality in the upper inner quadrant of the right breast. Target B-mode ultrasound and shear wave elastography showed a type IIa non-mass lesion with a stiff rim. Pathology confirmed a diagnosis of ductal carcinoma in situ.
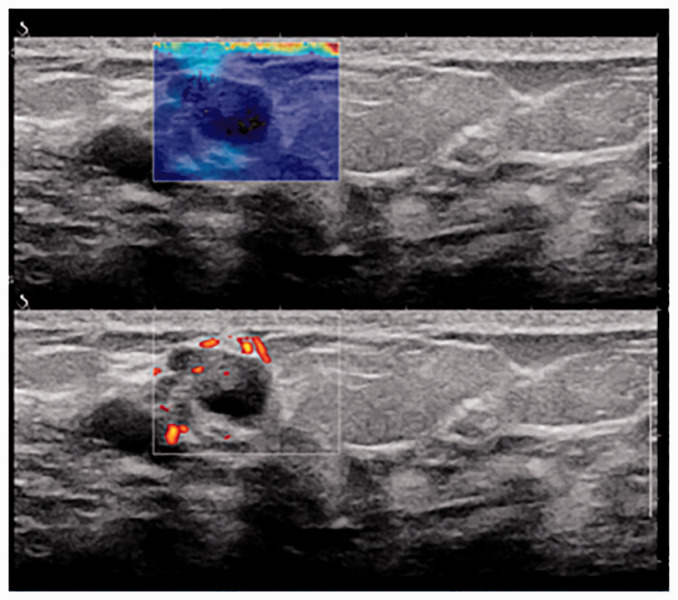
Figure 2.
A 54-year-old woman with a non-palpable abnormality detected on breast ultrasound. Shear wave elastography showed a dark blue color and histopathologic examination revealed ductal hyperplasia.
Table 1.
Characteristics of non-mass breast lesions.
|
Type of breast lesion (n = 49) |
|||||
|---|---|---|---|---|---|
|
Benign (n = 37) |
Malignant (n = 12) |
||||
| Variables | High-risk | Non high-risk | DCIS | Invasive carcinoma | p-Valuesa |
| Frequency | 5 (10.2) | 32 (65.3) | 5 (10.2) | 7 (14.3) | – |
| Patients’ age, mean ± SD, years | 50.0 ± 2.5 | 45.7 ± 11.7 | 48.0 ± 11.6 | 54.0 ± 10.9 | 0.16 |
| Palpability | |||||
| Palpable | 2 (40.0) | 13 (40.6) | 3 (60.0) | 5 (71.4) | 0.12 |
| Non-palpable | 3 (60.0) | 19 (59.4) | 2 (40.0) | 2 (28.6) | |
| Overall breast composition | |||||
| Homogenous fat | 3 (50.0) | 3 (50.0) | 0 (0) | 0 (0) | |
| Homogenous fibroglandular | 0 (0) | 2 (100.0) | 0 (0) | 0 (0) | 0.35 |
| Heterogeneous | 2 (4.9) | 27 (65.9) | 5 (10.2) | 7 (14.3) | |
Note: Data are presented as n (%), unless stated otherwise.
aAcross benign and malignant lesions.
DCIS: ductal carcinoma in-situ; SD: standard deviation.
The majority (77.8%) of type Ia NMLs were non-high-risk benign lesions, while about one-third of type Ib lesions were reported as DCIS. Among type IIa and IIb lesions, the most common histopathology was non-high-risk benign lesions. Type III NMLs were more likely to have a benign pathology; however, this was not statistically significant. Most of type IV lesions, which are associated with posterior acoustic shadowing, were invasive carcinoma (75%). Overall, there was no statistically significant association between types of NMLs and benign and malignant histopathology (p = 0.12). Similar to breast mass lesions, higher BI-RADS categories were associated with increased rate of malignancy in NMLs (p < 0.001) (Table 2).
Table 2.
Radiologic features of non-mass breast lesions (n = 49).
|
Type of breast lesion |
||||||
|---|---|---|---|---|---|---|
|
Benign |
Malignant |
|||||
| Variables | High-risk | Non high-risk | DCIS | Invasive carcinoma | p-Valuesa | p-Valuesb |
| Type of lesion | ||||||
| Type Ia | 2 (12.5) | 12 (75.0) | 1 (6.3) | 1 (6.3) | ||
| Type Ib | 0 (0) | 2 (66.7) | 1 (33.3) | 0 (0) | ||
| Type IIa | 1 (5.9) | 13 (76.5) | 2 (11.8) | 1 (5.9) | 0.07 | 0.12 |
| Type IIb | 0 (0) | 3 (75.0) | 0 (0) | 1 (25.0) | ||
| Type III | 2 (40.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | ||
| Type IV | 0 (0) | 1 (25.0) | 0 (0) | 3 (75.0) | ||
| BI-RADSc | ||||||
| BI-RADS 3 | 1 (11.1) | 7 (77.8) | 1 (11.1) | 0 (0) | ||
| BI-RADS 4a | 2 (9.1) | 19 (86.4) | 0 (0) | 1 (4.5) | <0.001 | <0.001 |
| BI-RADS 4b | 0 (0) | 6 (75.0) | 0 (0) | 2 (25.0) | ||
| BI-RADS 4c | 2 (40.0) | 0 (0) | 2 (40.0) | 1 (20.0) | ||
| BI-RADS 5 | 0 (0) | 0 (0) | 2 (40.0) | 3 (60.0) | ||
Note: Data are presented as n (%).
aAcross all breast lesion types.
bAcross benign and malignant lesions.
cBased on SWE and gray-scale ultrasound.
DCIS: ductal carcinoma in-situ; BI-RADS: Breast Imaging-Reporting and Data System.
Table 3 demonstrates the quantitative and qualitative parameters of SWE for different histopathologic entities. According to the results of this study, the average mean elasticity values were significantly higher among malignant NMLs compared with benign lesions (93.8 ± 83.5 kPa vs 23.9 ± 23.9 kPa; p < 0.001). The highest stiffness was observed in lesions histopathologically identified as DCIS (mean ± SD = 132.9 ± 97.4 kPa). Maximum stiffness color was also significantly different among histopathologic subtypes of NMLs (p = 0.002). Most NMLs showing a dark blue color on SWE were non-high-risk benign lesions (81%), whereas NMLs showing a red or orange color on SWE imaging had higher rates of malignancy (66.7%).
Table 3.
Shear wave elastography features of non-mass breast lesions.
|
Type of breast lesion |
||||||
|---|---|---|---|---|---|---|
|
Benign |
Malignant |
|||||
| Variable | High-risk | Non high-risk | DCIS | Invasive carcinoma | p-Valuesa | p-Valuesb |
| Mean elasticity, mean ± SD, kPa | 40.6 ± 46.2 | 21.3 ± 18.3 | 132.9 ± 97.4 | 65.9 ± 65.4 | 0.002 | <0.001 |
| Maximum color, n (%) | ||||||
| Dark blue | 2 (9.5) | 17 (81.0) | 1 (4.8) | 1 (4.8) | ||
| Light blue | 1 (5.9) | 13 (76.5) | 1 (5.9) | 2 (11.8) | 0.002 | 0.01 |
| Green to yellow | 0 (0) | 2 (40.0) | 1 (20.0) | 2 (40.0) | ||
| Orange | 1 (33.3) | 0 (0) | 0 (0) | 2 (66.7) | ||
| Red | 1 (33.3) | 0 (0) | 2 (66.7) | 0 (0) | ||
DCIS: ductal carcinoma in-situ; SD: standard deviation.
a Across all breast lesion types.
b Across benign and malignant lesions.
When comparing the mean elasticity of NMLs on SWE across BI-RADS categories, we found that lesions classified as BI-RADS 4 and 5 had significantly higher values than lesions with BI-RADS category 3 (p = 0.001) (Table 4).
Table 4.
Mean elasticity of non-mass breast lesions on shear wave elastography, by BI-RADS category.
| BI-RADS category | Mean elasticitya | p-Values |
|---|---|---|
| BI-RADS 3 (n = 9) | 15.1 ± 10.0 | |
| BI-RADS 4a (n = 22) | 23.1 ± 17.9 | |
| BI-RADS 4b (n = 8) | 31.4 ± 28.2 | 0.001 |
| BI-RADS 4c (n = 5) | 69.1 ± 47.7 | |
| BI-RADS 5 (n = 5) | 154.0 ± 97.0 |
aMean ± SD, kPa.
BI-RADS: Breast Imaging-Reporting and Data System.
Measures of diagnostic accuracy of B-mode US and US + SWE for differentiation of malignant and benign NMLs are presented in Table 5. The addition of SWE to conventional gray scale US increased the specificity, PPV, and diagnostic accuracy, but reduced the sensitivity and NPV. While the increase in specificity was borderline significant (p = 0.06), the decrease in sensitivity was not statistically significant (p = 1.00).
Table 5.
Measures of diagnostic accuracy with grayscale ultrasound and shear wave elastography.
| Measure | Grayscale ultrasound | Grayscale ultrasound+ SWE |
|---|---|---|
| Sensitivity | 100 | 91.7 |
| Specificity | 8.1 | 21.6 |
| Positive predictive value | 26.1 | 27.5 |
| Negative predictive value | 100 | 88.9 |
| Diagnostic accuracy | 30.6 | 38.7 |
Note: Data are presented as percentages (%).
SWE: shear wave elastography.
Discussion
Currently, there is lack of an established classification system for breast NMLs detected by conventional breast US. The interpretation of such lesions is of high clinical importance as both benign and malignant breast lesions can appear as NMLs on US. In routine clinical practice, benign NMLs are frequently biopsied by radiologists and thus, identification of a highly reliable non-invasive imaging modality can aid in reducing the number of unnecessary biopsies. Previous studies have shown that certain ultrasonographic features, including microcalcification, posterior shadowing, architectural distortion, and linear-segmental distribution are associated with malignancy in NMLs of the breast.12,17,19 Nevertheless, information regarding the characteristics of NMLs on SWE imaging is scarce. Therefore, we investigated the SWE parameters of breast NMLs detected by conventional US and assessed the measures of diagnostic accuracy of SWE in the differentiation of breast NMLs compared with grayscale US, using histopathologic results as the reference standard.
During a two-year study period, a total of 567 US-detected breast lesions requiring CNB were recorded at our institution, of which 49 lesions (8.6%) were identified as NMLs. Among these lesions, the majority (37/49, 75.5%) were benign. In a study by Zhang et al., among a total of 928 patients with US-detected breast lesions, 79 individuals had lesions recognized as NMLs, 43.7% of which were benign. 20 More recently, a study including 715 NMLs found that 53.8% of the lesions were benign. 19 In our study, palpability was not significantly associated with greater risk of malignancy among NMLs. Also, there was no statistically significant difference in age and breast composition between benign and malignant NMLs. This is in accordance with several other studies, which found that age and the percentage of palpable lesions were not statistically different between benign and malignant NMLs.19–21
Based on the classification for NMLs provided by Ko et al. in 2015, 12 most lesions were either type Ia or IIa (33/49, 67.3%). In other words, architectural distortion and posterior acoustic shadowing were not common findings in NMLs. No significant association was found between histopathologic entities and types of NMLs, which means that the type of NML cannot be representative of its pathology. However, the percentage of malignancy was higher among type I and II NMLs with accompanying calcification compared with those without calcification. This suggests that the presence of calcification within type I and II NMLs might be a characteristic feature of malignancy. Although Ko and colleagues also found that type Ib and IIb lesions had a significantly higher likelihood of malignancy, 12 further confirmatory studies are required.
Qualitative color assessment of maximum elasticity, which has been shown to be the best-performing feature of SWE, 22 demonstrated that lesions showing a dark blue or light blue color on SWE were mainly benign, but those showing a green to yellow, orange or red color mostly had a malignant histopathologic report. Furthermore, quantitative measurement of mean elasticity of NMLs revealed that malignant lesions had significantly higher mean elasticity values compared with benign lesions. Earlier studies also indicated that mean and maximum elasticity values were markedly higher in malignant lesions in comparison to benign lesions.21,23,24 Just like breast masses, high stiffness of NMLs is thought to be a risk factor for malignancy.20,25 An unexpected finding of the present study was that lesions reported as DCIS had markedly higher mean elasticity values than NMLs diagnosed as invasive carcinoma.
Among BI-RADS categories, category 5 and 3 NMLs had the highest and lowest mean elasticity values, respectively. In addition, BI-RADS categories based on US + SWE were significantly higher in malignant lesions than in benign lesions, a finding that is supported by Park et al. and Choi et al.21,26
According to the results of our study, B-mode US had sensitivity and specificity of 100% and 8% for differentiating NMLs. Meanwhile, the addition of SWE to B-mode US significantly increased the specificity to 22% while decreasing, although not significantly, the sensitivity to 92%. In a study evaluating NMLs by different ultrasonic methods, the sensitivity and specificity for US and US + strain elastography were 100% and 29%, and 97.5% and 58.1%, respectively. 20 However, the sensitivity, specificity, PPV, and NPV of breast US and US + SWE for differentiation of malignant and benign NMLs vary widely among several other published studies.12,21,24,27
One limitation of this study is its small sample size and single-institution design; larger multicenter studies would allow for the better evaluation of SWE parameters in breast NMLs. Another limitation of our study is its retrospective design. More robust prospective studies are needed to determine if accurately assessing and formulating new criteria for the assessment of breast NMLs may help to limit the amount of unnecessary biopsies. In addition, the development of a validated practical guideline for reporting features of NMLs on breast US seems necessary.
Overall, our results showed that the majority of patients with breast NMLs had non-high-risk benign lesions based on histopathologic reports. As expected, mean elasticity values were significantly higher among malignant lesions compared with benign NMLs. Furthermore, the addition of SWE to B-mode ultrasonography increased the diagnostic accuracy, specificity, and PPV for differentiating breast NMLs.
Conclusion
Our findings indicate that the complementary use of SWE with conventional US might help in the differentiation of breast NMLs and has the potential to decrease the frequency of unnecessary biopsies performed for benign NMLs. However, further large-scale studies are needed to draw definite conclusions on the utility of SWE in the assessment of breast NMLs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: This study was approved by the institutional review board of Shiraz University of Medical Sciences and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Guarantor: Sara Haseli.
Contributors: All authors contributed to the study conception and design. Material preparation and data collection were performed by S.S., S.H., V.B., P.K., and B.ZR. N.K. wrote the draft of the manuscript and performed data analysis. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments: None.
ORCID iDs: Sara Haseli https://orcid.org/0000-0003-0300-5491
Banafsheh Zeinali-Rafsanjani https://orcid.org/0000-0002-4815-0345
References
- 1.Munoz D, Near AM, Van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst 2014; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machida Y, Shimauchi A, Okuma H, et al. Shear wave speed of the lesion in preoperative breast ultrasonography: association with disease-free survival of patients with primary operable invasive breast cancer. Acad Radiol 2018; 25: 1003–1009. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4.Burgess MD, O’Neal EL. Breast ultrasound for the evaluation of benign breast disease. Curr Radiol Rep 2019; 7: 9. [Google Scholar]
- 5.Lee J, Lee JH, Baik S, et al. Non-mass lesions on screening breast ultrasound. Med Ultrason 2016; 18: 446–451. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZL, Li N, Li M, et al. Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med 2015; 120: 905–910. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Park YM, Jung HK. Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. Ultrasound Med 2014; 33: 421–430. [DOI] [PubMed] [Google Scholar]
- 8.Choe J, Chikarmane SA, Giess CS. Nonmass findings at Breast US: definition, classifications, and differential diagnosis. RadioGraphics 2020; 40: 326–335. [DOI] [PubMed] [Google Scholar]
- 9.Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer 2012; 19: 295–301. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Zhou X, Zhao X, et al. B-Mode ultrasound combined with color Doppler and strain elastography in the diagnosis of non-mass breast lesions: a prospective study. Ultrasound Med Biol 2017; 43: 2582–2590. [DOI] [PubMed] [Google Scholar]
- 11.Ko KH, Jung HK, Kim SJ, et al. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol 2014; 24: 305–311. [DOI] [PubMed] [Google Scholar]
- 12.Ko K-H, Hsu H-H, Yu J-C, et al. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol 2015; 84: 77–85. [DOI] [PubMed] [Google Scholar]
- 13.Ng WL, Rahmat K, Fadzli F, et al. Shearwave elastography increases diagnostic accuracy in characterization of breast lesions. Medicine 2016; 95: e3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y-L, Gao Y, Chang C, et al. Ultrasound shear wave elastography of breast lesions: correlation of anisotropy with clinical and histopathological findings. Cancer Imaging 2018; 18: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosgrove DO, Berg WA, Doré CJ, et al. Shear wave elastography for breast masses is highly reproducible. Eur Radiol 2012; 22: 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian M, Jiang D, Su C, et al. Value of real-time shear wave elastography versus acoustic radiation force impulse imaging in the diagnosis of female bladder neck obstruction. J Ultrasound Med 2019; 38: 2427–2435. [DOI] [PubMed] [Google Scholar]
- 17.Park JW, Ko KH, Kim E-K, et al. Non-mass breast lesions on ultrasound: final outcomes and predictors of malignancy. Acta Radiol 2017; 58: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 18.Youk JH, Gweon HM, Son EJ. Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography 2017; 36: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park KW, Park S, Shon I, et al. Non-mass lesions detected by breast US: stratification of cancer risk for clinical management. Eur Radiol 2021; 31: 1693–1706. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Xiao X, Xu X, et al. Non-mass breast lesions on ultrasound: feature exploration and multimode ultrasonic diagnosis. Ultrasound Med Biol 2018; 44: 1703–1711. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Choi JS, Han B-K, et al. Shear wave elastography in the diagnosis of breast non-mass lesions: factors associated with false negative and false positive results. Eur Radiol 2017; 27: 3788–3798. [DOI] [PubMed] [Google Scholar]
- 22.Berg WA, Cosgrove DO, Doré CJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012; 262: 435–449. [DOI] [PubMed] [Google Scholar]
- 23.Xu P, Wu M, Yang M, et al. Evaluation of internal and shell stiffness in the differential diagnosis of breast non-mass lesions by shear wave elastography. World J Clin Cases 2020; 8: 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZL, Li Y, Wan WB, et al. Shear-wave elastography: could it be helpful for the diagnosis of non-mass-like breast lesions? Ultrasound Med Biol 2017; 43: 83–90. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Chang JM, Kim WH, et al. Added value of shear-wave elastography for evaluation of breast masses detected with screening US imaging. Radiology 2014; 273: 61–69. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Han B-K, Ko EY, et al. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol 2016; 26: 3542–3549. [DOI] [PubMed] [Google Scholar]
- 27.Aslan H, Pourbagher A, Ozen M. The role of Shear-Wave elastography in the differentiation of benign and malign non-mass lesions of the breast. Ann Ital Chir 2018; 89: 385–391. [PubMed] [Google Scholar]