Abstract
Introduction
Bedside lung sonography is recognized as a reliable diagnostic modality in trauma settings due to its ability to detect alterations both in lung parenchyma and in pleural cavities. In severe blunt chest trauma, lung ultrasound can identify promptly life-threatening conditions which may need direct intervention, whereas in minor trauma, lung ultrasound contributes to detection of acute pathologies which are often initially radio-occult and helps in the selection of those patients that might need further investigation.
Topic Description
We did a literature search on databases EMBASE, PubMed, SCOPUS and Google Scholar using the terms ‘trauma’, ‘lung contusion’, ‘pneumothorax’, ‘hemothorax’ and ‘lung ultrasound’. The latest articles were reviewed and this article was written using the most current and validated information.
Discussion
Lung ultrasound is quite accurate in diagnosing pneumothorax by using a combination of four sonographic signs; absence of lung sliding, B-lines, lung pulse and presence of lung point. It provides a rapid diagnosis in hemodynamically unstable patients. Lung contusions and hemothorax can be diagnosed and assessed with lung ultrasound. Ultrasound is also very useful for evaluating rib and sternal fractures and for imaging the pericardium for effusion and tamponade.
Conclusion
Bedside lung ultrasound can lead to rapid and accurate diagnosis of major life-threatening pathologies in blunt chest trauma patients.
Keywords: Lung ultrasound, pneumothorax, hemothorax, lung contusion
Introduction
Over the past few decades, ultrasonography (US) has become an attractive tool in a growing number of clinical specialties which allows to address specific clinical problems at the bedside with rapid examinations that can be repeated without additional radiation risks to patients. 1 US has been recognized as a reliable method for the examination of the adult trauma patient, providing accurate information and quickly identifying disorders of the chest wall. 2
Generally, US-based examination of the lungs relies on the correct interpretation of direct or indirect image features. On the one hand, there can be immediate visualization of a pathologic condition, such as a pleural effusion or consolidation, while on the other hand, the diagnosis of pneumothorax (PTX) and interstitial lung disorders relies on the presence of artifacts generated by the interface between the parietal pleura and the underlying lung parenchyma. In the setting of trauma, lung ultrasound (LUS) can recognize pathologies that may need prompt life-saving treatment, such as hemothorax, PTX and contusions. 3 , 4 Although computed tomography (CT) is the gold standard for evaluating lung parenchyma, it may not be feasible in trauma patients due to their hemodynamic instability or the lack of scanner availability. 5 Under these circumstances, LUS is of great value for diagnosis and rapid treatment in the trauma bay. 6
Lung ultrasound technique in blunt chest trauma
Transducer selection
Ideally, a convex array transducer of 3 to 7 MHz is desirable for optimal lung visualization of the lung. In trauma, the convex transducer enables a complete assessment including lung, abdomen, heart and inferior vena cava. 6 A linear high-frequency transducer (5 to 10 MHz) can visualize the most superficial pleural layers and define details of small sub-pleural consolidations.
Scanning technique
Trauma patients are often immobilized in a supine or semi-recumbent position. Therefore, anterior and lateral chest walls are mainly accessible to assessment by US. Initial scanning should be performed in the longitudinal plane, perpendicular to the ribs, allowing correct identification of the pleural line between two adjacent ribs with acoustic shadows (‘bat-sign’). 1 The transducer may then be slightly rotated anti-clockwise (with the transducer marker facing backwards towards the vertebral body) to obtain an oblique scan along one intercostal space which permits visualization of a larger portion of the pleura and lung parenchyma away from rib shadows. Therefore, priority should be given to scanning specific chest regions where the chance to detect even the smallest PTX is highest. These areas, also known as ‘hot zones’, are located on both sides of the upper and lower anterior chest wall. 7
Normal patterns in lung ultrasound
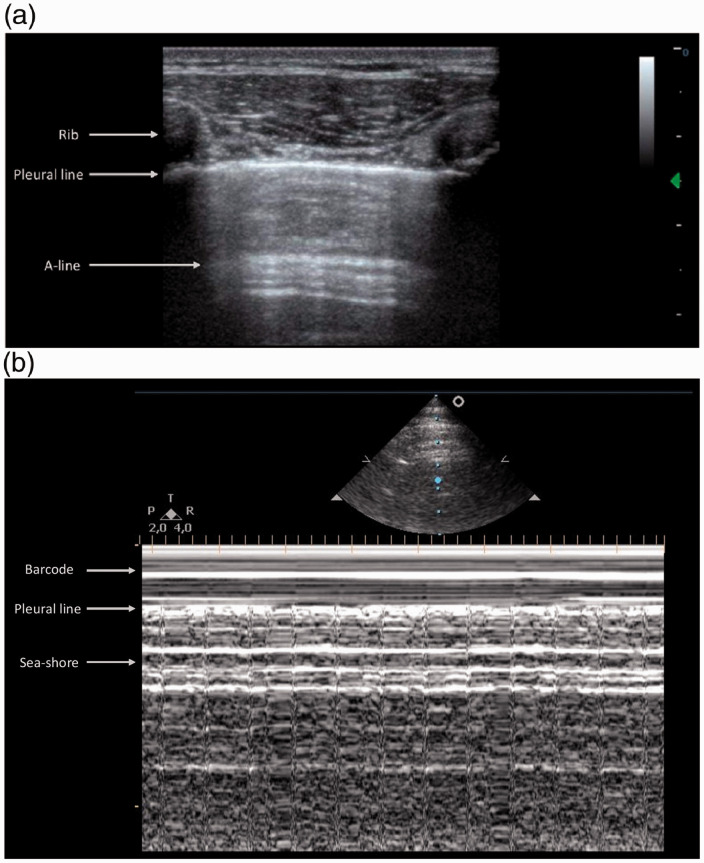
Ultrasound images consist of both chest structures that are directly visualized and imaging artifacts that require careful interpretation. An image of structures situated between skin and the hyperechoic line of the parietal pleura can be obtained by directly applying the transducer to the chest wall. Below the parietal pleura, alveolar air content of the lung creates an acoustic interface with the pleura and soft tissue of the chest wall preventing a direct view of the lung parenchyma. Lung tissue is replaced by artifacts that are the result of US waves reflecting off the chest wall above the pleural line. This reverberation phenomenon is known as the ‘mirror effect’ of the lung. 4 As a result, the horizontal lines that can be visualized at constant intervals in the artifact zone below the pleura correspond to multiple reflections of the pleural line and are called A lines 8 (Figure 1(a)).
Figure 1.
Normally aerated lung: B-mode and M-mode view. (a) Longitudinal scan of a normal lung surface. Upper rib (on the left), shimmering pleural line, and lower rib (on the right) showing the ‘bat sign’. From the pleural line (arrow) to the A line below (arrowhead) the image represents a reflection of the chest wall (mirror effect). (b) The motion of the lung changes the brightness of the echoes creating a speckled appearance like grains of sand beneath the bright pleural line whereas the extra-pleural structures are represented by calm waters washing on the sandy beach. As a result, the M-mode pattern generated by normal lung is called the ‘seashore sign’.
Two specific dynamic movements of the lung against the chest wall represent the hallmark of chest ultrasound and they are the lung sliding and the lung pulse. The former is a horizontal rhythmic movement that occurs between the parietal and the visceral pleura in synchronization with respiration, with the two pleural surfaces either being directly opposed or separated by a thin layer of fluid. The presence of lung sliding indicates that the lung is ventilating in the inspected area (Additional file 1). The latter is due to the transmission of the cardiac beat to the lung, which results in movement directed towards the chest wall. The lung pulse can be visualized only when the patient’s lung rests between respirations or the lung is not ventilating. 9 The pulsation is horizontal or vertical depending on the angle by which the transducer is applied to the chest wall (Additional file 2).
Use of M-mode function might be of interest for assessment of some patients in blunt chest trauma. In normal aerated lung, when the M-mode cursor is placed correctly on the pleural line, different patterns are displayed on the screen. The most superficial portion of the screen corresponds to the motionless portion of the chest wall structures above the pleura which is described as a ‘wavy pattern’, while the physiological sliding below the pleural line creates a ‘sandy pattern’. 1 As a result, the M-mode pattern generated by normal lung is called the ‘seashore sign’ 8 (Figure 1(b)).
Lung ultrasound abnormalities in blunt chest trauma
Pneumothorax
Bedside US can detect pneumothoraces early. Ultrasound has a sensitivity of 82.9% 10 for diagnosing PTX, which far exceeds the sensitivity of chest radiography (CXR) at just 20.9%. 11 LUS at the bedside may expedite the diagnosis, treatment and resuscitation of a patient who may have otherwise decompensated. 11 The high sensitivity of LUS identifies small, ‘occult’ PTX, which otherwise may go undetected. 12
Definition
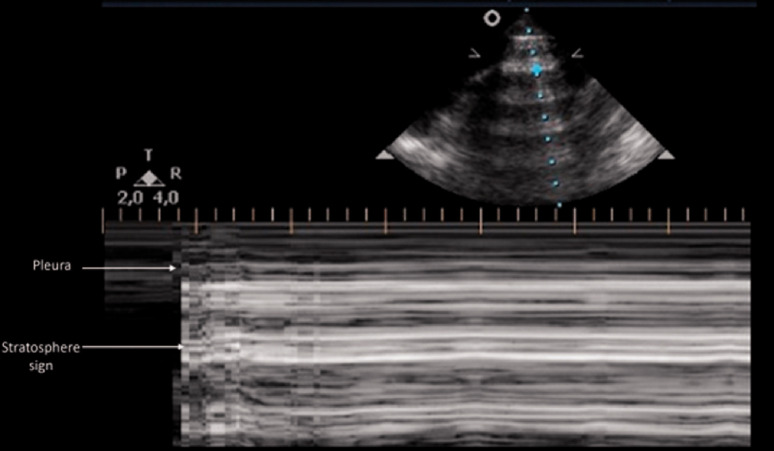
Air in the interpleural space cannot be directly visualized and prevents the penetration of the US beam beyond the pleural layers. In PTX, the static ultrasound image of the lung is similar to the normally aerated lung view (Figure 1(a)), but air between the two pleural layers limits visualization of any movement of the lung, and both lung sliding and lung pulse can no longer be seen (Additional file 3). The resultant M-mode tracing in a PTX will replace the normal ‘seashore’ pattern with the ‘stratosphere’ or ‘barcode’ sign, described as a distinctive pattern of parallel horizontal lines above and below the pleural line due to the lack of pleural movement 8 (Figure 2). Other sonographic signs of PTX are discussed below.
Figure 2.
Pneumothorax: M-mode view. In pneumothorax, the granular sand appearance representing the lung pattern is absent. Instead, M-mode shows a linear pattern above and below the pleural line. This pattern is described as the ‘barcode’ or ‘stratosphere’ sign.
Lung sliding
Visualization of lung sliding rules out PTX with a very high negative predictive value, 99.2–100% 11 but its absence is not pathognomonic for PTX. 13 Some lung conditions such as atelectasis, adult respiratory distress syndrome, selective lung intubation, pneumonectomy, apnea and pleurodesis abolish the physiological lung sliding. Therefore, with absent lung sliding in a stable patient, it is necessary to look for other basic sonographic signs to confirm diagnosis. 6 , 14
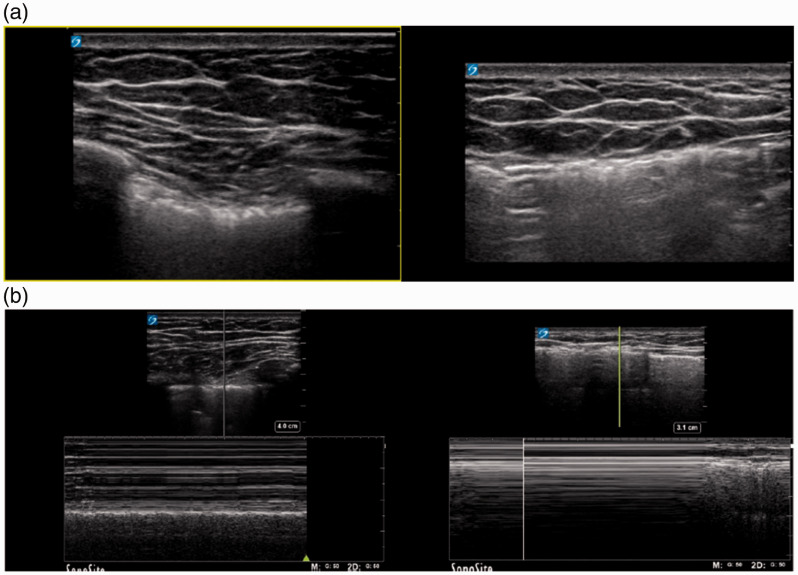
Where sliding is subtle, M-mode function might contribute to detecting pleural sliding over time. In patients with subcutaneous emphysema, visibility of pleura is limited due to air interposition in the shallow chest wall structures (Figure 3(a)). M-mode is more sensitive than B-mode sonography in the detection of lung sliding. The advantage of M-mode in a patient with subcutaneous emphysema is twofold: it focuses on a smaller suitable acoustic window compared to B-mode and its high resolution facilitates the recognition of even subtle motion artifacts caused by lung sliding 13 , 15 (Figure 3(b)).
Figure 3.
Subcutaneous emphysema. (a) Longitudinal scan of normal aerated lung (left) and presence of trapped air in the soft tissue of the chest wall beneath the skin (right). (b) Longitudinal M-mode scan in the same patient showing the ‘seashore’ sign in the normal aerated lung (left) and pathological findings in the presence of subcutaneous emphysema (right).
B lines
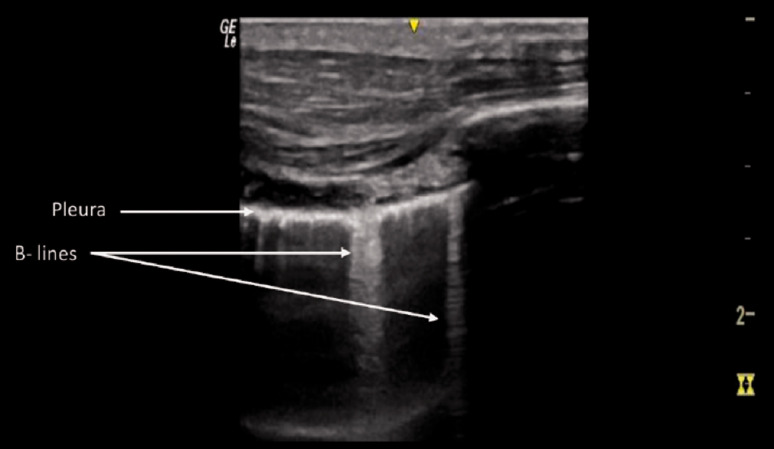
Conditions under which the normal air/fluids balance in the lung changes, e.g. when air is replaced by fluid, generate vertical laser-like imaging artifacts that arise from the pleura and extend to the edge of the screen (Figure 4). These are known as B lines. 14 B lines can blur or completely erase the physiological A lines. 4 The presence of multiple and diffuse B lines indicates loss of lung aeration and increase in interstitial fluid. 1 , 5 Air in the pleural space prevents visualization of any B lines. 14 Thus, in absence of lung sliding, direct visualization of one single isolated B line is sufficient to rule out PTX with a negative predictive value of 98–100%. 11 In contrast, the absence of both dynamic lung sliding and B lines does not rule in PTX.
Figure 4.
B lines pattern. Longitudinal scan of pathological lung with increased fluid: the reflection of the ultrasound beam creates reverberation artifacts called B lines.
Lung pulse
The lung pulse is a normal dynamic movement of the lung during apnea (in between two normal respiratory cycles). Thus, it can only be visualized when the two pleural layers adhere (Additional file 2). 9 Even with absence of both lung sliding and B lines, visualization of the slightest lung pulse excludes PTX. 16 The beat of an intercostal artery or a thoracic internal artery in the parasternal region may mimic a lung pulse and thus mislead the US operator to ruling out PTX. 17 , 18
Specific sonographic signs of PTX
Lung point
In the case of a partially collapsed lung, signs such as visible dynamic lung sliding and/or B lines and/or lung pulse in the unaffected lung region and PTX in the affected area (just parietal pleura seen with no sliding) will be visible. The interface between two distinct sonographic patterns is known as the lung point and identification of this is 100% specific for PTX. At this point, the visceral pleura (and the lung below it) returns close to the parietal layer (attached to the chest wall) creating a transition from a pathological to a normal pattern. This manifests as a respiratory synchronous movement of the sliding lung that replaces the non-sliding zone 19 (Additional file 4). Although highly specific, a lung point is not found in all PTX cases (sensitivity ∼65%), but mainly in the most severe situations when the lung is totally collapsed. In patients with chronic pathologies, such as restrictive pattern disorders or in case of a bleb point in bullous lung disease, the lung point may be falsely positive. 10
Heart point sign
As the heart fills with blood in diastole, it enlarges and displaces the air from the pre-cardiac space, allowing the heart to transiently contact the left-sided anterior chest wall and be therefore visualized during scanning. During systole, the heart contracts and in the presence of PTX, air will fill the space between the heart and the anterior chest wall and it disappears from the view. US operators should be aware of this phenomenon as it may be the first clue to the presence of an unsuspected PTX. 20
Speckle tracking
Recently, speckle tracking techniques, i.e. the detection and tracking of the deformation of the anatomical structure over time by analyzing acoustic markers, have been applied in determining the presence of PTX. 21
Estimation of size of pneumothorax
LUS can estimate the PTX size reliably. Taking the sternum as a reference point and moving laterally onto the chest wall with the patient in supine position, when the lung point is found before the anterior axillary line (AAL), lung collapse is less than 10%, defined as a small PTX. Medium-sized PTX is associated with a lung point that is located somewhere in the area between the AAL and mid-axillary line (MAL) with a lung collapse of 10 to 15%. When the lung point is found at or posterior to the MAL, the PTX is greater than 15%. 22
Pitfalls
Despite the accuracy of LUS in detecting PTX, there are several complex situations of which the ultrasound user should be aware to avoid misdiagnosis.
Pleural adhesions can be due to previous surgical pleurodesis or lung contusion in a trauma setting. Adherence of pleural layers in some specific areas prevents air from moving freely inside the pleural cavity 23 , 24 and results in the loss of respiratory movement visualization (absence of lung sliding). In contrast to normal physiology, air may also collect in the lower chest area. In a stable patient with known pleural adhesions, the absence of lung sliding alone should not be considered diagnostic for PTX and the entire chest surface should be properly scanned. A more detailed CT investigation may be required.
A very small (loculated) PTX may show the ‘double lung point’ sign. This phenomenon consists of two lung points moving with respiration intermittently appearing at the two opposite sides of the scan field. It does not have clinical relevance unless in ventilated patients and in those requiring aeromedical transport. In these two specific situations, even the smallest PTX needs to be monitored or may warrant prophylactic drainage. 24
Subcutaneous emphysema may mimic PTX. Air collections between the skin and the chest wall prevent visualization of the pleural line. When the pleural layer cannot be seen, LUS cannot be used to draw any diagnostic conclusions concerning PTX. However, sonographic or physical signs of subcutaneous emphysema in the setting of chest trauma in an otherwise healthy patient are indicative of PTX and/or rupture of the upper airways. 23
Pulmonary contusion
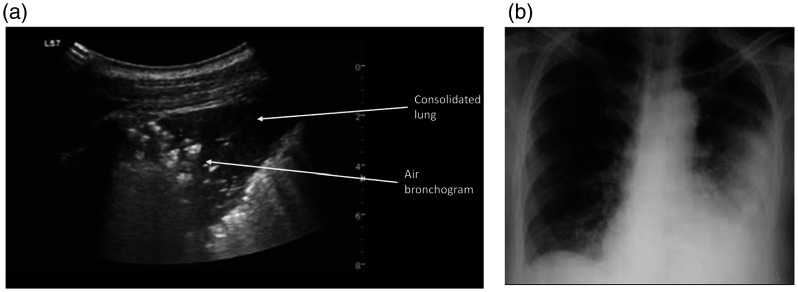
Pulmonary contusion typically results from blunt trauma to the chest wall. In the emergency setting, LUS is a more accurate method for early detection of lung contusion (sensitivity 73%) 3 as compared to conventional radiography and clinical examination combined (sensitivity 66%). 3 , 25 The lower sensitivity of CXR initially is because classic radiologic signs may take several hours before appearing on a plain film. Contused lung parenchyma is characterized by a hemorrhagic area formation as a result of direct effect of the trauma. Progression towards an edematous phase, due to infiltrates and air replaced by fluid, occurs within one or two hours after injury. It is possible to monitor strictly those pattern variations with LUS. 25 The first level of aeration loss is the ‘interstitial pattern’, represented by multiple B lines often associated with irregularity of the pleural line. The ‘consolidation pattern’ represents the final step and varies from an anechoic blurred dendrite-like air bronchogram to a ‘tissue-like’ pattern. In real time, air can be seen moving through bronchi, which is known as a dynamic air bronchogram, whereas the tissue-like pattern is often referred to as ‘hepatization of the lung parenchyma’ due to its gray scale density. Both interstitial and consolidation patterns can occur in sequence or coexist in the same scan view (Figure 5(a) and (b)). Boundaries of consolidated lung segment are defined by a pleural line, adjacent aerated lung and any effusion that may be present. Although highly sensitive in detecting lung contusion, similar sonographic findings may be found in other lung parenchymal injuries such as pneumonia, ARDS and pulmonary edema, thus limiting the specificity of lung US. However, such sonographic signs in the setting of blunt chest trauma are highly suspicious for lung contusion but should be interpreted within the clinical context.
Figure 5.
Lung contusion: (a) Oblique scan of a lobar consolidation with hypoechoic heterogeneous echotexture. The finding is isoechoic with the liver and is described as lung ‘hepatization’. Air bronchograms can be appreciated as multiple hyperechoic 1 mm-long air inlets or as hyperechoic branching tubular structures within the consolidation. Focal interstitial syndrome finding is also present in the same scan. (b) Chest radiograph after several days showing findings which correspond to the sonographic appearance.
Hemothorax
Fluid in the pleural space creates an acoustic window allowing complete penetration of the ultrasound beam. Effusion is visualized as an anechoic space within the two pleural layers, with a cyclic variation of the inter-pleural distance during respiration. Fluid collects in the most dependent lateral–basal area of the chest wall in the supine patient. When an effusion is absent, the sonographic image of a lung pattern will intermittently overlap and hide the image of the diaphragm and the underlying abdominal organs. This phenomenon is known as the ‘curtain’ sign. Specifically, in a trauma setting with an otherwise healthy patient, visualization of anechoic fluid between the two pleural layers is highly indicative of hemorrhagic effusion. 26 LUS outperforms CT in determining the nature of the effusion, due to the ability to distinguish internal echoes and mobile particles; findings highly suggestive of hemothorax 25 (Additional file 5).
Other uses of bedside ultrasound in chest trauma
Along with LUS, other anatomical structures of the thoracic cavity and wall can be examined.
Airway assessment
LUS has proved to be a useful aid for confirmation of endotracheal tube placement. Absence of lung sliding bilaterally and a ‘double trachea’ sign suggest esophageal intubation, whereas the presence of sliding just in one lung indicates selective intubation. 27
Pneumonia and pleural empyema
The persistence of interstitial syndrome and anechoic loculated fluid can suggest pneumonia and empyema in the right clinical setting. Both conditions can be easily diagnosed with the help of LUS. 28
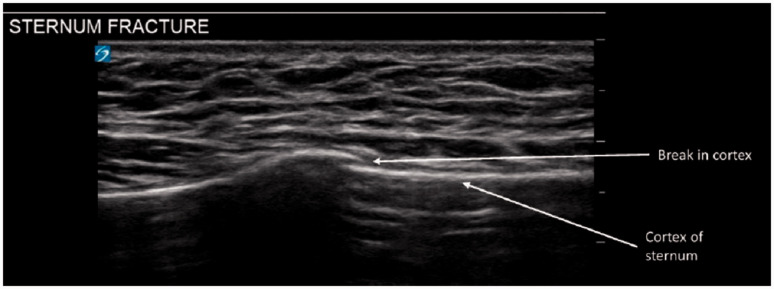
Sternal and rib fractures
Compared to plain radiography, ultrasound has higher diagnostic sensitivity even in cases demonstrating minimal discontinuity of bone cortex (Figure 6). Diagnostic criteria for fractures include direct evidence of a cortical step or indirect evidence of a local hematoma 29 (Additional file 6).
Figure 6.
Sternum fracture. Sternal fractures on thoracic ultrasound highlighted by the discontinuity of the cortical bone (white line).
Hemopericardium
Free fluid in the pericardial sac should be ruled out in the context of blunt chest trauma, as it can potentially lead to hemodynamic shock. 4 According to the ATLS algorithm, US is the diagnostic tool for the assessment of traumatic pericardial effusion as per the e-FAST examination. 4
Conclusion
Trauma patients may benefit from rapid US-guided evaluation at the bedside due to its ease of use, low cost, no risk of ionizing radiation and reproducibility. In blunt chest trauma, LUS has demonstrated accuracy and safety superior to conventional radiography for conditions such as PTX, lung contusion and early stage hemothorax.
Supplementary Material
Acknowledgments
We would like to thank Professor Volker H Haase for reviewing the manuscript and Christy Moore for her valuable support on image acquisition for this project.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Not required.
Guarantor: SR.
Contributorship: SR, SN and DO interpreted the current literature on blunt chest trauma; GV and LV reviewed image acquisition technique and the main lung ultrasound findings in trauma settings. All authors read and approved the final manuscript.
ORCID iDs: Serena Rovida https://orcid.org/0000-0003-4017-5078
Luigi Vetrugno https://orcid.org/0000-0003-3745-8368
Supplemental material: Supplemental material for this article is available online.
References
- 1.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015; 147: 1659–1670. [DOI] [PubMed] [Google Scholar]
- 2.Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand 2008; 52: 776–784. [DOI] [PubMed] [Google Scholar]
- 3.Hyacinthe AC, Broux C, Francony G, et al. Diagnostic accuracy of ultrasonography in the acute assessment of common thoracic lesions after trauma. Chest 2012; 141: 1177–1183. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick AW, Sirois M, Laupland KB, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma Inj Infect Crit Care 2004; 57: 288–295. [DOI] [PubMed] [Google Scholar]
- 5.Phung NTN, Vo TTT, Hon KLE. The role of lung ultrasonography in etiologic diagnosis of acute dyspnea in a resource limited setting. Bull Emerg Trauma 2020; 8: 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 7.Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein DA, Mezière G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med 2005; 33: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein DA, Lascols N, Prin S, et al. The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 2003; 29: 2187–2192. [DOI] [PubMed] [Google Scholar]
- 10.Dahmarde H, Parooie F, Salarzaei M. Accuracy of ultrasound in diagnosis of pneumothorax: a comparison between neonates and adults – a systematic review and meta-analysis. Can Resp J 2019; 2019: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husain LF, Hagopian L, Wayman D, et al. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock 2012; 5: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soldati G, Testa A, Sher S, et al. Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008; 133: 204–211. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest 1995; 108: 1345–1348. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein D, Mezière G, Biderman P, et al. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999; 25: 383–388. [DOI] [PubMed] [Google Scholar]
- 15.Berlet T, Etter R. Favourable experience with M-mode sonography in the diagnosis of pneumothorax in two patients with thoracic subcutaneous emphysema. Case Rep Radiol 2014; 2014: 90617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steenvoorden TS, Hilderink B, Elbers PWG, et al. Lung point in the absence of pneumothorax. Intensive Care Med 2018; 44: 1329–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco P and, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J 2016; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copetti R. Lung pulse with pneumothorax: examine the thoracic artery and veins. Anesthesiology 2019; 131: 666. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein D, Mezière G, Biderman P, et al. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 2000; 26: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 20.Stone MB, Chilstrom M, Chase K, et al. The heart point sign: description of a new ultrasound finding suggesting pneumothorax. Acad Emerg Med 2010; 17: e149–e150. [DOI] [PubMed] [Google Scholar]
- 21.Duclos G, Bobbia X, Markarian T, et al. Speckle tracking quantification of lung sliding for the diagnosis of pneumothorax: a multicentric observational study. Intensive Care Med 2019; 45: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 22.Volpicelli G, Boero E, Sverzellati N, et al. Semi-quantification of pneumothorax volume by lung ultrasound. Intensive Care Med 2014; 40: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 23.Soldati G, Sher S and, Copetti R. If you see the contusion, there is no pneumothorax. Am J Emerg Med. 2010; 28: 106–107. [DOI] [PubMed] [Google Scholar]
- 24.Volpicelli G and, Audino B. The double lung point: an unusual sonographic sign of juvenile spontaneous pneumothorax. Am J Emerg Med 2011; 29: 355.e1–2. [DOI] [PubMed] [Google Scholar]
- 25.Brogi E, Bignami E, Sidoti A, et al. Could the use of bedside lung ultrasound reduce the number of chest X-rays in the intensive care unit? Cardiovasc Ultrasound 2017; 15: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. J Trauma Nurs 1998. [DOI] [PubMed] [Google Scholar]
- 27.Senussi MH, Kantamneni PC, et al. Protocolized tracheal and thoracic ultrasound for confirmation of endotracheal intubation and positioning: a multicenter observational study. Crit Care Explorations 2020; 2: e0225. [Google Scholar]
- 28.Orso D, Guglielmo N and, Copetti R. Lung ultrasound in diagnosing pneumonia in the emergency department: a systematic review and meta-analysis. Eur J Emerg Med 2018; 25: 312–321. [DOI] [PubMed] [Google Scholar]
- 29.Battle C, Hayward S, Eggert S, et al. Comparison of the use of lung ultrasound and chest radiography in the diagnosis of rib fractures: a systematic review. Emerg Med J 2019; 36: 185–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.