Summary
Background
Non-protein antigen classes can be presented to T cells by near-monomorphic antigen-presenting molecules such as CD1, MR1, and butyrophilin 3A1. Such T cells, referred to as donor unrestricted T (DURT) cells, typically express stereotypic T cell receptors. The near-unrestricted nature of DURT cell antigen recognition is of particular interest for vaccine development, and we sought to define the roles of DURT cells, including MR1-restricted MAIT cells, CD1b-restricted glucose monomycolate (GMM)-specific T cells, CD1d-restricted NKT cells, and γδ T cells, in vaccination against Mycobacterium tuberculosis.
Methods
We compared and characterized DURT cells following primary bacille Calmette-Guerin (BCG) vaccination in a cohort of vaccinated and unvaccinated infants, as well as before and after BCG-revaccination in adults.
Findings
BCG (re)vaccination did not modulate peripheral blood frequencies, T cell activation or memory profiles of MAIT cells, CD1b-restricted GMM-specific and germline-encoded mycolyl-reactive (GEM) cells or CD1d-restricted NKT cells. By contrast, primary BCG vaccination was associated with increased frequencies of γδ T cells as well as a novel subset of CD26+CD161+TRAV1-2− IFN-γ-expressing CD4+ T cells in infants.
Interpretation
Our findings, that most DURT cell populations were not modulated by BCG, do not preclude a role of BCG in modulating other qualitative aspects of DURT cells. More studies are required to understand the full potential of DURT cells in new TB vaccine strategies.
Funding
Aeras, the National Institutes of Health, and the Bill and Melinda Gates Foundation.
Keywords: BCG, Tuberculosis, Vaccination, Unconventional T cells, Memory
Research in Context.
Evidence before this study
Bacillus Calmette-Guerin (BCG), the only licensed tuberculosis vaccine, provides variable protection against tuberculosis, the deadliest disease caused by a single infectious agent. While many efforts have been made to investigate the role of Th1 cytokine-producing, MHC-restricted T cells in protection against TB, other arms of immunity, including donor-unrestricted T (DURT) cells, have not been substantially explored. Evidence from non-human primates and human studies suggest that DURT cells are activated during natural infection with Mycobacterium tuberculosis, providing a rationale to investigate if BCG vaccination can modulate these T cell populations in humans.
Added value of this study
We aimed to determine if BCG vaccination modulates peripheral blood frequencies, activation and expression of memory markers of MR1-restricted MAIT cells, CD1b-restricted glucose monomycolate (GMM)-specific T cells, CD1d-restricted NKT cells, and γδ T cells. We performed flow cytometric analysis in unvaccinated and BCG vaccinated infants and BCG re-vaccinated adults to profile DURT cells. Our findings show that neonatal BCG vaccination was associated with an increase in γδ T cells as well as a novel subset of BCG-reactive IFN-γ-producing CD26+CD161+TRAV1–2 − T cells that express CD4. However, BCG (re)vaccination did not modulate the other three DURT cell subsets.
Implications of all the available evidence
Our results lend further support to prior evidence that γδ T cell responses play a role in immunity induced by whole cell vaccination against TB to BCG, and highlight a new BCG-reactive IFN-γ-producing CD4 T cell subset that deserves investigation.
Alt-text: Unlabelled box
Introduction
Current approaches to develop vaccines against tuberculosis (TB), one of the top 10 causes of death globally, are based primarily on the induction of conventional, major-histocompatibility complex (MHC)-restricted, Th1 cytokine-producing T cells to protein antigens. While proteins constitute the majority of known antigens recognized by αβ T cell receptor (TCR)-bearing cells, other chemical classes including lipids, small-molecule metabolites and specially modified peptides are also antigenic and can be presented to T cells by the non-polymorphic molecules, CD1, MR1, and butyrophilin 3A1, respectively.1, 2, 3 Unlike MHC-restricted T cells, these T cell subsets generally bear T cell receptors that show limited diversity, and are therefore referred to as donor-unrestricted T (DURT) cells.3 DURT cells are activated through their TCR in a manner that is unrestricted by donor origin: response is mediated by antigen presentation molecules of limited polymorphism, such that DURT responses can be shared among genetically diverse individuals.1 This nature of antigen recognition by DURT cells is of particular interest for vaccine development, since immunogens could be designed to elicit universal, population-wide T cell responses, irrespective of host MHC-encoded genetic factors.
Studies in mouse and non-human primate models have demonstrated the importance of T cells in conferring protection against challenge with Mycobacterium tuberculosis (M.tb).4, 5, 6 However, it remains unclear which and how many mycobacteria-derived antigens should be targeted by protective T cell responses, and which T cell functional, homing and memory attributes are required for protection.7 Given the complexity of host-pathogen interactions, TB vaccination strategies that exploit immunological diversity and target additional arms of the immune system, including DURT cells, such as the CD1d-restricted NKT cells, CD1b-restricted glucose monomycolate (GMM)-specific T cells and germline-encoded mycolyl-reactive (GEM) cells, γδ T cells, and MR1-restricted MAIT cells, should be explored.1
Current studies provide extensive evidence that DURT subtypes are activated during natural M.tb infection in humans and non-human primates. Analysis of the T cell response during human M.tb infection indicates that infected individuals have increased CD1-restricted T cell responses to lipid antigens compared to M.tb naïve controls.8, 9, 10 A recent study in non-human primates also reported that glucose monomycolate (GMM)-specific CD1c-restricted T cells expanded after intravenous bacille Calmette-Guerin (BCG) administration.11 Vaccination of guinea pigs with mycobacterial lipids formulated in liposomes was also associated with a reduction in the number of lesions, severity of pathology, and reduction of bacterial load upon challenge with M.tb.12
Activation of MR1-restricted MAIT cells, which recognize vitamin B metabolites presented by the monomorphic MHC class 1-related molecule (MR1), has been shown in response to a variety of bacteria including BCG, Francisella tularensis, Klebsiella pneumonia, and M.tb.13 Despite previous evidence that MAIT cells can respond to M.tb infection, there is conflicting data on the importance of MAIT cells in controlling infection. In the murine model, endogenous MAIT cell responses have been implicated in early innate responses to M.tb infection,14 but more recently additional studies suggest they are less important for adaptive host defenses against M.tb infection.15, 16, 17 Moreover, early MAIT cell responses impeded the priming and induction of conventional peptide-specific CD4 T cell responses.15
Butyrophilin 3A1 facilitates recognition of phosphoantigens, small molecules like isopentyl pyrophosphate, by γδ T cells, which rapidly respond to pathogen infection in mucosal tissues, and are induced by both M.tb infection and BCG vaccination in humans and non-human primates.18, 19, 20, 21, 22 Importantly, induction of Vγ2Vδ2 T cells specific for the phosphoantigen, (E)−4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), in non-human primates was associated with increased Th1-like Vγ2Vδ2 T cells in the airways, facilitated earlier recruitment of conventional Th1 cytokine-expressing CD4 and CD8 T cell to the lungs, and was associated with containment of M.tb after pulmonary challenge.20
Another feature of DURT cells that makes them attractive as vaccine targets is their inherent immediate effector function, such as secretion of inflammatory cytokines and cytotoxic molecules upon recognition of microbial antigens.23 Immediate cytokine secretion allows DURT cells to modulate the antimicrobial function of other cells before recruitment or induction of conventional MHC-restricted T cells has occurred. For example, MAIT cell secretion of CCL4 mediates recruitment of NK cells, monocytes, and other inflammatory cells to infected tissues.24 Similarly, immediate or early secretion of pro-inflammatory cytokines that can facilitate the development of adaptive immunity has been described for NK cells,25 γδ T cells,26 and NKT cells.27 The relatively high baseline precursor frequency in blood and tissues of DURT cells allows them to recognize antigens and respond in large numbers without requiring extensive clonal expansion, implying that they can act simultaneously with innate cells as sensors of infection or immune dysregulation. Despite these attributes, it remains unknown whether DURT cells possess immunological memory such that they can be selectively expanded by vaccination to provide long-term, antigen-specific protective immunity.
In this study, we sought to determine if a live attenuated vaccine, BCG, modulates frequencies, phenotypes or functions of DURT cells in humans. The key premise of this study is that live BCG contains peptide and non-peptide antigens that can be recognized by DURT cells. We employed a unique study design in which BCG was given at birth, as is routine, or in which BCG was delayed until sampling, to allow comparison of a vaccinated and unvaccinated group of infants. We also evaluated BCG effects on DURT cells in M.tb-infected adults who received investigational BCG revaccination. This allows ascertainment of the modulatory effect of BCG vaccination on DURT cells to inform whether changes are durable such that they constitute immunological memory. Ours is the first study that aimed to systemically profile DURT cells in a case/control design.
Methods
Study participants
All participants were enrolled at the South African Tuberculosis Vaccine Initiative Field site in Worcester near Cape Town, South Africa. We collected blood from two cohorts of participants who were vaccinated with BCG.
The first cohort comprised two groups of healthy, 9-week-old infants. In one group (BCG vaccinated), infants received BCG at birth as is routine according to the World Health Organization Expanded Program on Immunization (EPI) in South Africa. In the second group (BCG naïve), BCG vaccination was delayed until after blood collection at 9 weeks, for whole blood stimulation and PBMC cryopreservation. In the BCG naïve (with delayed BCG vaccination) cohort, mothers and infants were closely monitored by our clinical team to maximize the likelihood of identifying those with TB exposure, in order to provide clinical care. Mothers were also sensitized about TB signs and symptoms and had access to the study team for any medical concern. Infants who were born preterm (<37 weeks of gestation), with a low birth weight (<2.5 kg), had congenital malformations or perinatal complications, were in close contact with someone with TB disease or had suspected TB, received isoniazid preventive therapy or immunosuppressant therapy, or had any acute or chronic disease were excluded.
The second cohort, the TBRU adult BCG revaccination trial,28, 29 comprised healthy tuberculin skin test (TST)-positive, HIV-negative adults who received at least 6 months of isoniazid preventive therapy before, or 7 months after, BCG revaccination (NCT01119521). Peripheral blood was collected before BCG revaccination and on days 21, 35, and 365 post-vaccination and peripheral blood mononuclear cells (PBMCs) cryopreserved.
Sample size simulations indicate that the study was well powered to detect a standardized effect size of 2 or more (see Data Analysis for details) using a Mann-Whitney test under a variety of distributions with varying degrees of skewness. Power was 67% under the statistical distribution with the least power (gamma distribution) when the family-wise type I error rate was controlled at 0.05 across five tests (Supplementary Figure 1); however, typically the data were more normally distributed. Depending on the distribution and the number of tests across which the type I error rate was controlled, power varied around 50% for a standardized effect size of 1, and was negligible when the standardized effect size was 0.1.
Ethical approvals
The studies were conducted in accordance with Good Clinical Practice (GCP) and guidelines set out by the World Medical Association's Declaration of Helsinki. The studies, cohort protocols and all procedures were reviewed and approved by the Human Research Ethics Committee (HREC) of the University of Cape Town as follows: BCG revaccination trial (Ref. 387/2008), infants vaccinated at birth (Ref. 126/2006) and infants with delayed BCG vaccination (Ref. 177/2011). The BCG revaccination study was also reviewed and approved by the University Hospital Cleveland Medical Center Institutional Review Board. Written informed consent was obtained from all adult participants and from a parent or legal guardian of infants before enrollment.
Flow cytometry assays
PBMC staining
Cryopreserved PBMCs were thawed in a 37 °C water bath, washed in PBS, and then stained with LIVE/DEAD dye according to the manufacturer's instructions. Thereafter, PBMCs were stained with the fluorochrome-conjugated tetramers (CD1b-GMM,30 CD1d-PBS57, MR1-5-OP-RU, and their respective controls) for 45–60 min at room temperature, followed by CCR7 staining at 37 °C for 30 min, and thereafter stained for other phenotypic markers (antibody panels described in Supplemental Table 1) at 4 °C for another 30 min.
Whole blood intracellular cytokine staining (WB-ICS) assay and staining
Heparinized blood was processed, within 75 min of blood collection, using a standardized 12 h WB-ICS assay protocol.31 Briefly, blood was stimulated with BCG Vaccine SSI (Biovac, Cape Town, South Africa) reconstituted with RPMI (final concentration 1.2×106 CFU/ml), PHA (Sigma-Aldrich; positive control at 5g/ml) or RPMI (negative control). For all stimulation conditions, co-stimulants anti-CD28 and anti-CD49d (BD Biosciences; San Diego, USA) were added at 0.25 μg/ml. Blood was stimulated for 7 h at 37 C, after which Brefeldin-A (Sigma-Aldrich) was added at a concentration of 10 μg/ml for the remaining 5 h of stimulation. At the end of the stimulation, 2 mM of EDTA (Sigma-Aldrich) was added, red blood cells were lysed using 1:10 FACS Lysing solution (BD Biosciences) and fixed white blood cells were cryopreserved in liquid nitrogen. Cryopreserved fixed white blood cells from infant participants were thawed, washed in PBS, permeabilized in Perm/Wash buffer (BD Biosciences) and stained with antibody panels (Supplemental Table 2, 3) for 30 min at 4 C.
Flow cytometry
Stained samples were acquired on a BD-LSR-II flow cytometer configured with 4 lasers: Solid state Blue (488 nm; 100 mW; 3 detectors), Solid state Violet (405 nm; 25nW; 8 detectors), HeNe gas Red (635 nm; 70 mW; 3 detectors), and Diode-pumped Coherent Compass (532 nm; 150 mW; 8 detectors). We used mouse κ chain BD CompBeads stained with each individual antibody conjugate (for tetramer reagents, fluorochrome-matched antibody conjugates were used) to compensate all parameters. Samples were acquired with optimal photomultiplier tube voltages calibrated daily using targets for SPHERO Rainbow Fluorescent Particles (Spherotech, Inc.).
Data analysis
Flow cytometric data were analyzed using FlowJo (version 10.5.3). Statistical analyses were performed in R (version 3.6.3). Within a particular age and vaccination status, as well as the effect of BCG revaccination after a certain number of days since revaccination, estimates of the median and 95% confidence interval thereof were performed by quantile regression using the quantreg package.32, 33 The effect of BCG within infants and the effect of age (adults versus infants) within BCG-vaccinated individuals was calculated using quantile regression and tested against the null hypothesis using a Wald test,34 controlling for sex and ethnicity. Other measured potential variables were deemed to have little effect on the response based on graphical assessment and prior experience. When comparing a single time point since vaccination to pre-vaccination, the effect of BCG within adults was assessed using the Wilcoxon signed-rank test. The test for an effect of BCG revaccination at any time-point was a likelihood ratio test for linear mixed-effects models,35 using race and sex as confounders. The type I family-wise error rate was controlled throughout using the Bonferroni procedure, with an adjusted p-value of less than 0.05 considered statistically significant.
The standardized effect size (and associated standardized confidence interval) was estimated by dividing the estimated effect size (and associated confidence interval bounds) by the standard deviation of the raw residuals, after accounting for vaccination status (as well as ethnicity and sex, where appropriate).
Role of funding source
The study was funded by Aeras and the Bill and Melinda Gates Foundation. The delayed BCG infant study was funded by NIH R01 grant AI087915 and the adult BCG revaccination study was funded by NIH grant NO1-AI70022. CD1b tetramers, ligands and their validation were supported by R01 AI049313. Anele Gela was supported by postdoctoral fellowships from the Claude Leon Foundation and the Harry Crossley Foundation. Melissa Murphy was supported by a Masters and Doctoral Innovation Scholarship from the National Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Participant enrollment and demographic data
We enrolled two cohorts from communities residing in a TB endemic setting, in South Africa. The infant cohort comprised two groups of healthy 9-week-old infants, born to HIV negative mothers, who either received routine BCG at birth as part of the expanded program on immunization (EPI) by the World Health Organization, or in whom BCG vaccination was delayed until after blood collection at 9 weeks. There were no significant differences in sex or ethnicity distribution between the vaccinated and the delayed BCG arm. The adult cohort included tuberculin skin test (TST)-positive, HIV-negative adults who received BCG revaccination after pre-treatment with isoniazid as part of the TBRU adult BCG revaccination trial.28, 29 Peripheral blood samples were analyzed before revaccination and on days 21, 35, and 365 post-vaccination. The demographics of the study populations are summarized in Table 1.
Table 1.
Demographic characteristics of participants.
| Variable | Infants |
Adults (N=25) | |
|---|---|---|---|
| No BCG (N=25) | BCG (N=50) | ||
| BCG (re)vaccination | No | Yes | Yes |
| Median age | 9 weeks | 9 weeks | 24 years |
| Range | (8–11) | (8–11) | (19–39) |
| Sex, n (%) | |||
| Male | 13 (52) | 25 (50) | 7 (28) |
| Female | 12 (48) | 25 (50) | 18 (72) |
| Ethnicity, n (%) | |||
| African | 4 (16) | 13 (26) | 5 (20) |
| Caucasian | 0 (0) | 0 (0) | 1 (4) |
| Mixed ancestry | 21 (84) | 37 (74) | 19 (76) |
DURT cell subset characterization by flow cytometry
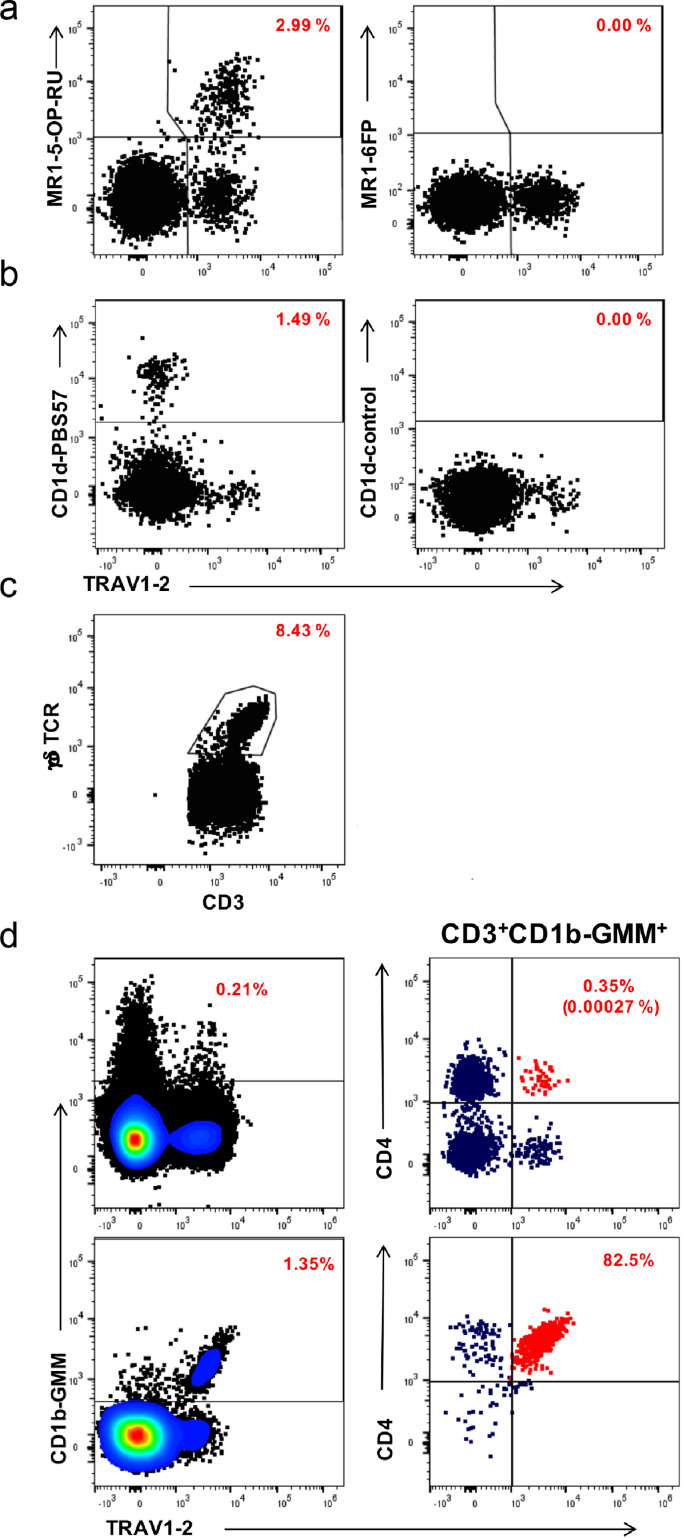
We quantified CD1b-, CD1d-, MR1-restricted, and γδ T cells in peripheral blood using a combination of tetramers and monoclonal antibody staining against phenotypic markers. DURT cell subsets were defined by flow cytometry as MR1-5-OP-RU+TRAV1-2+(or CD26+CD161+) MAIT cells, CD1d-PBS57+NKT cells, γδ-T cells, and CD1b-GMM+CD4+TRAV1–2+GEM cells (Figure 1).
Figure 1.
Representative flow cytometry plots demonstrating detection of DURT cell populations in a typical infant. Plots represent live, CD3+ T cells co-stained with the indicated antibody-conjugate on the X-axis and the indicated tetramer reagent or antibody-conjugate on the Y-axis. (a) MR1-restricted MAIT cells (MR1-5-OP-RU tetramer on the left and MR1-6FP tetramer as negative control on the right). (b) CD1d-restricted NKT cells (CD1d-PBS57 tetramer on the left and unloaded [empty] CD1d tetramer as negative control on the right). (c) γδ T cells, defined as CD3 T cells that stained positive with anti-pan γδ TCR antibody. (d) Definition of CD1b-restricted glucose monomycolate (GMM)-specific T cells and germline-encoded mycolyl-reactive (GEM) cells in an infant sample (top plots) or a PBMC sample that was spiked with GEM T cell clone cells (bottom plots). GMM-specific T cells (left plots) were defined as CD1b-GMM tetramer+ T cells and GEM cells were defined as CD1b-GMM tetramer+ T cells that co-expressed CD4 and the TCR variable chain TRAV1–2 (red dots in right plots). Red numbers in all plots denote the percentages of T cells in that plot that stain positive for each population-defining marker out of CD3+ T cells. The red numbers in parentheses in the top, right plot in panel d denote the percentage of GEM cells out of all CD3+ T cells.
Frequencies of γδ T cell, but not other DURT subsets, increase after BCG vaccination in infants
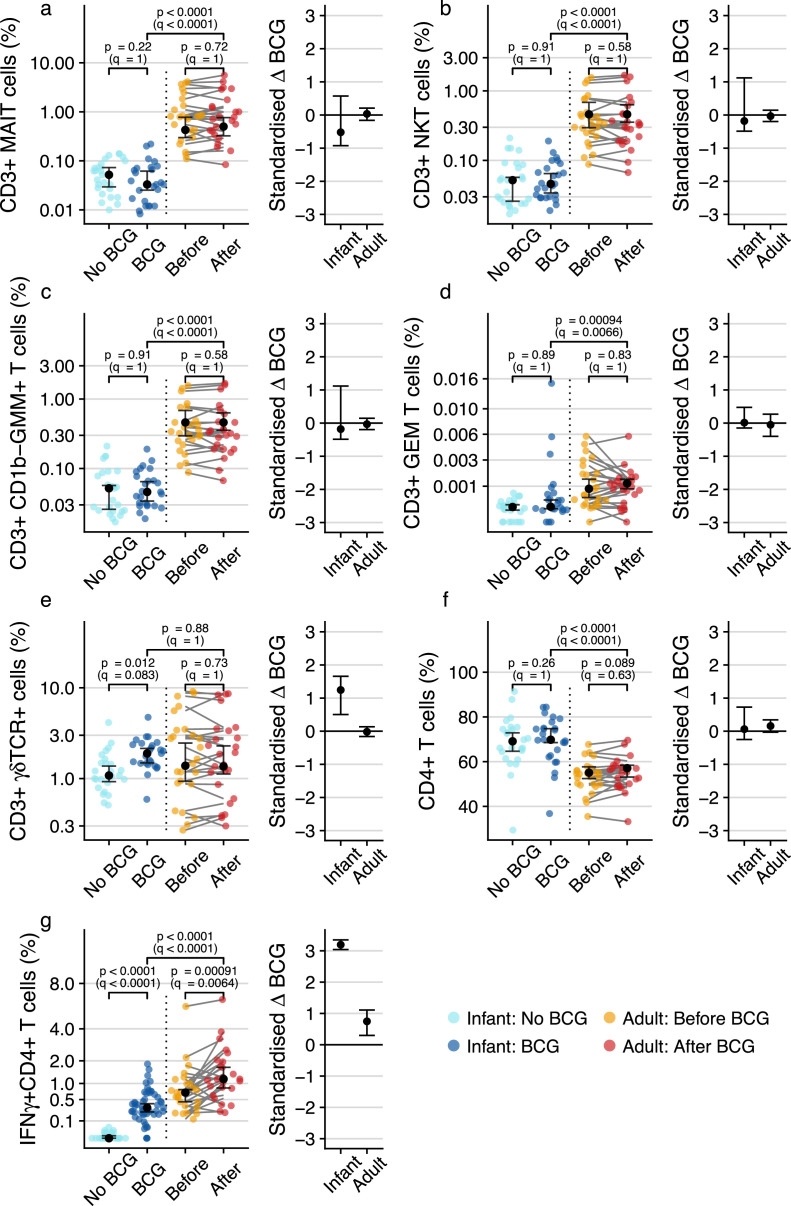
Previous studies showed that neonatal BCG vaccination boosts antigen-specific T cell responses that peak 6–10 weeks of age,36, 37 while BCG revaccination in adults transiently boosts frequencies of antigen-specific T cells, including γδ T cells, that peak 3–5 weeks post-vaccination.38 An unresolved question is whether BCG revaccination induces durable changes in frequencies of DURT cells in peripheral blood. To determine if BCG vaccination alters DURT cell abundance, we measured frequencies of DURT cell subsets in infants and adults at 35 days post revaccination. Frequencies of the different DURT subsets were generally lower in infants compared to adults, but there was high heterogeneity among the adults, especially for frequencies of MAIT and γδ T cell populations (Figure 2a-e). Frequencies of MAIT cells, CD1d- restricted NKT cells, CD1b-restricted GMM-specific and germline-encoded mycolyl-reactive (GEM) cells were not significantly modulated by primary BCG vaccination, nor by BCG re-vaccination at all time-points (Fig 2a-d, data not shown for day 21 and 365). However, frequencies of γδ T cells were significantly higher in BCG-vaccinated infants compared to their unvaccinated counterparts (fold change 1.47, 95% CI 1.27–2.24; p=0.012 by Wald test, q=0.083) (Fig 2e). No difference in γδ T cell frequencies was observed before and after BCG revaccination in adults at any of the post-vaccination time points (Fig 2e, data not shown for day 21 and 365).
Figure 2.
T cell subset frequencies measured by flow cytometry in BCG-vaccinated or unvaccinated infants or before and after BCG revaccination in adults. Peripheral blood frequencies and standardized BCG effect sizes for (a) MR1-5-OP-RU tetramer+ MAIT cells, (b) CD1d-PBS57 tetramer+ NKT cells, (c) CD1b-GMM tetramer+T cells, (d) CD1b-GMM tetramer+ CD4+TRAV1–2+ GEM cells, (e) γδ T cells and (f) “conventional” CD4+ T cells in individual infants (blue colors) or adults before (yellow) or 35 days after (red) BCG re-vaccination. (g) Peripheral blood frequencies of BCG-reactive “conventional” CD4 T cells expressing IFN-γ in individual infants (blue colors) or adults (yellow and red). For frequency plots, the black dots represent the estimated median while error bars represent 95% confidence intervals. For standardized effect size plots, black dots represent the standardized estimated difference in medians between infants who were vaccinated with BCG and those who were not, or in adults between the pre- and post- BCG revaccination time-points. Error bars represent 95% confidence intervals. Q-values are Bonferroni-adjusted p-values, with q < 0.05 considered statistically significant.
Infants also had higher frequencies of bulk CD4 T cells than adults, but these were not modulated by BCG in either age group (Figure 2f). In contrast to these DURT cell subsets, antigen-specific CD4 T cells were significantly modulated by BCG vaccination, as expected for peptide-responsive, MHC-restricted T cells; primary BCG vaccination induced significant increases in BCG-reactive IFN-γ+ CD4 T cells in infants (fold change 5.34, 95% CI 5.01 – 6.05; p < 0.0001 by Wald test, q < 0.0001), while BCG re-vaccination boosted pre-existing BCG-reactive CD4 T cells in adults (fold change 1.43, 95% CI 1.15 – 1.7; p=0.0009 by Wilcoxon signed-rank test, q=0.0064) (Figure 2g). Taken together, these results show that with the exception of a significant increase in total γδ T cells in BCG-vaccinated infants, DURT cell frequencies in peripheral blood were not modulated by BCG vaccination.
BCG vaccination does not modulate the activation status of DURT cells in peripheral blood
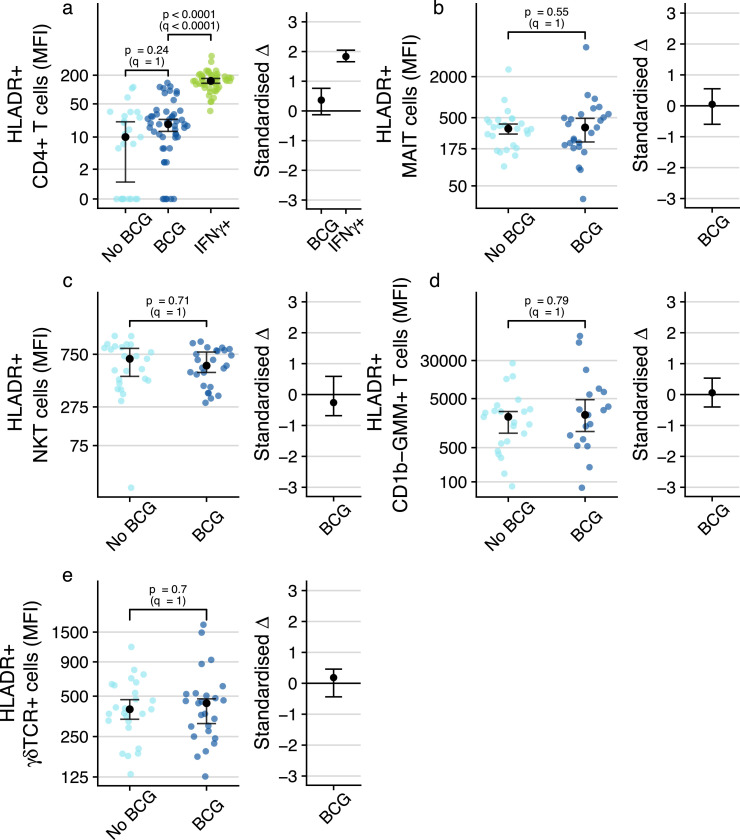
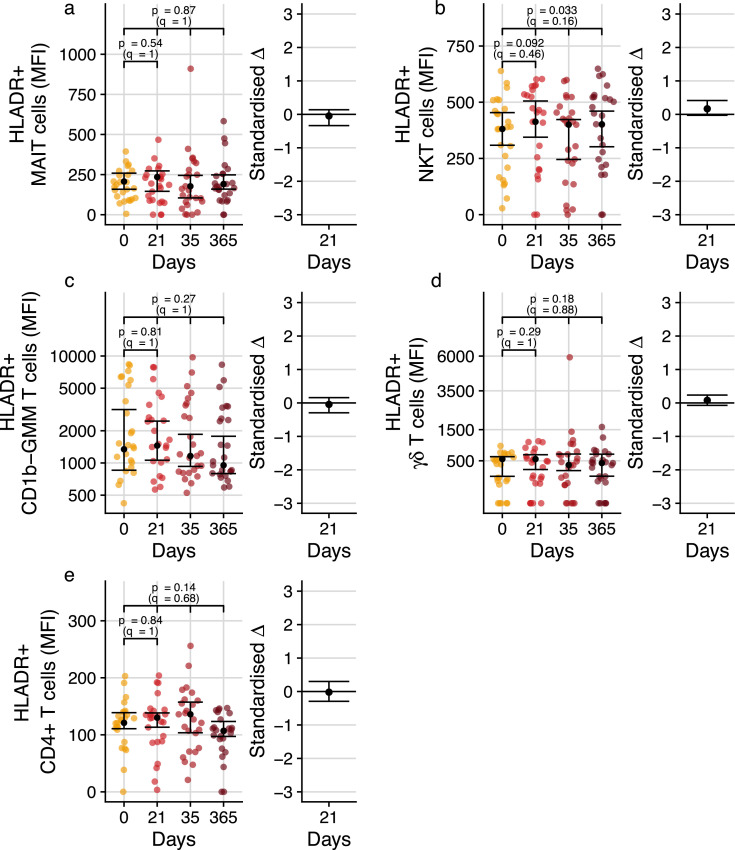
We next sought to determine if BCG vaccination results in activation of DURT subsets in peripheral blood, since evidence of T cell activation may indicate that DURT cells sense and respond to the vaccine, or its downstream effects. Our previous study of T cell response kinetics after neonatal BCG vaccination indicated that antigen-specific CD4 T cell activation also peaked around 6 weeks after vaccination.39 Consistent with this, BCG-specific IFN-γ-expressing CD4 T cells detected at 9 weeks of age in the BCG vaccinated infants expressed significantly higher levels of the in-vivo activation marker, HLA-DR, than bulk CD4 T cells (Figure 3a). BCG-specific CD4 T cell responses were not detected in unvaccinated infants, precluding analysis of activation status of these CD4 T cells in this group (Figure 3a). By contrast, at the corresponding post-BCG time point, measured at 9 weeks of age, HLA-DR expression levels on all DURT cell subsets were not different between BCG-vaccinated and unvaccinated infants (Figure 3b-e). Similarly, levels of HLA-DR expression by DURT cell subsets or bulk CD4 T cells were not different between pre- and post- BCG-revaccination time points in BCG-revaccinated adults (Figure 4a-e). Taken together, these data suggest that DURT cells in the peripheral blood were not activated by BCG vaccination at the time points that we assessed.
Figure 3.
Assessment of BCG-associated T cell activation of the various cell subsets in BCG-vaccinated or unvaccinated infants. (a) T cell activation, measured by expression levels of HLA-DR, by total CD4+ T cells in BCG-vaccinated or unvaccinated infants or by IFN-γ-expressing, BCG-reactive CD4+ T cells in BCG-vaccinated infants (IFN- γ-expressing CD4+ T cells in unvaccinated infants were too infrequent to quantify HLA-DR expression). T cell activation of (b) MR1-5-OP-RU tetramer+ MAIT cells, (c) CD1d-PBS57 tetramer+ NKT cells, (d) CD1b-GMM tetramer+T cells or (e) γδ T cells. CD1b-GMM tetramer+ CD4+TRAV1–2+ GEM cells were too infrequent to reliably quantify HLA-DR expression. MFI, median fluorescence intensity. For frequency plots, the black dots represent the estimated median; for standardized effect size plots, the black dots represent the standardized estimated difference in medians. Error bars represent 95% confidence intervals. Q-values are Bonferroni-adjusted p-values, with q < 0.05 considered statistically significant.
Figure 4.
Longitudinal analysis of DURT cell activation profiles in adults before and after BCG revaccination. T cell activation, measured by expression levels of HLA-DR, on MR1-5-OP-RU tetramer+ MAIT cells (a), CD1d-PBS57 tetramer+ NKT cells (b), CD1b-GMM tetramer+T cells (c), γδ T cells (d) or total CD4+ T cells (e). CD1b-GMM tetramer+ CD4+TRAV1–2+ GEM cells were too infrequent to reliably quantify HLA-DR expression. MFI, median fluorescence intensity. For frequency plots, the black dots represent the estimated median; for standardized effect size plots, the black dots represent the standardized estimated difference in medians between the pre- and post- BCG revaccination time-points. Error bars represent 95% confidence intervals. Q-values are Bonferroni-adjusted p-values, with q < 0.05 considered statistically significant.
BCG vaccination modulates a novel CD26+CD161+ CD4 T cell subset in infants
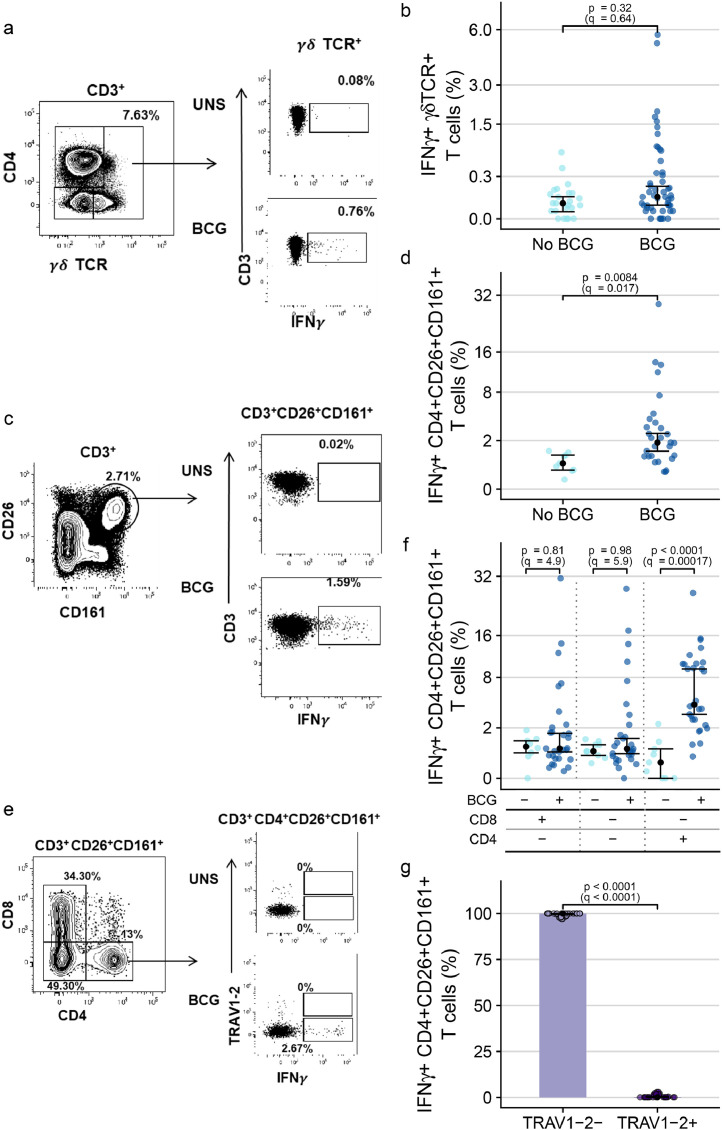
We also sought to evaluate functional characteristics of DURT subsets. In a subgroup of the infant cohort, we quantified IFN-γ-expressing BCG-reactive γδ and phenotypically defined MAIT cells using a 12-h whole blood intracellular cytokine-staining (WB-ICS) assay. Although a number of BCG-vaccinated infants appeared to have markedly higher frequencies of BCG-reactive IFN-γ-expressing γδ T cells than the range of IFN-γ-expressing γδ T cells in unvaccinated infants, no significant difference between the two groups was observed (Figure 5a-b). On the other hand, frequencies of BCG-reactive IFN-γ-expressing CD26+CD161+ CD3+ T cells, which phenotypically resemble the definition for MAIT cells,40 were significantly elevated in BCG vaccinated compared to BCG naïve infants (fold change 3.46, 95% CI 2.28 – 4.72; p=0.0084 by Wald test, q=0.017) (Figure 5c-d). Further phenotypic characterization revealed that this result was driven by an IFN-γ-expressing, BCG-reactive CD4-positive CD26+CD161+ T cell subset (fold change 9.69, 95% CI 6.19 – 13.4; p < 0.0001 by Wald test, q=0.00017), and not the typical CD8+ subset that characterizes MAIT cells (Figure 5e-f). These BCG-reactive CD4+CD26+CD161+ T cells also did not express TRAV1–2, the canonical TCRα variable gene associated with MAIT cells (fold change 0.001, 95% CI 0.001 - 0.001; p < 0.0001 by Wald test, q < 0.0001) (Figure 5g). Unfortunately, the MR1 tetramer was not included in the flow cytometry panel used to analyze stimulated whole blood, and therefore we could not determine whether these cells were MR1-restricted. In summary, these data indicate that neonatal BCG vaccination modulates a functional subset of CD4+ T cells with a CD26+CD161+TRAV1–2−phenotype.
Figure 5.
IFN-γ-expressing unconventional T cells elicited by infant BCG vaccination. (a) Representative flow cytometry plots depicting γδ TCR expressing CD3+lymphocytes (left) and IFN- γ expression in unstimulated and BCG-stimulated γδ T cells (right). (b) Frequencies of BCG-reactive γδ T cells expressing IFN-γ in BCG-vaccinated (dark blue) and unvaccinated (light blue) infants. (c) Representative flow cytometry plots of (left) CD26 and CD161 expression by CD3+lymphocytes to identify CD26+CD161+ T cells producing IFN-γ in unstimulated and BCG-stimulated infant blood samples (right). (d) Frequencies of BCG-reactive IFN-γ-expressing CD3+CD26+CD161+ T cells in BCG-vaccinated (dark blue) and unvaccinated (light blue) infants. (e) Representative flow cytometry plot depicting CD8 and CD4 staining and gating in CD3+CD26+CD161+ T cells (left). Plot depicting TRAV1-2 and IFN-γ staining among CD4+CD26+CD161+ T cells (right). (f) Proportions of BCG-reactive IFN-γ+CD3+CD26+CD161+ T cells that are CD8+, double negative for CD8 and CD4 or that are CD4+ in BCG-vaccinated (dark blue) and unvaccinated (light) infants. (g) Proportions of BCG-reactive IFN-γ+CD4+CD26+CD161+ T cells that express TRAV1–2 in BCG-vaccinated infants. The black dots represent the estimated median; the error bars represent 95% confidence intervals. Q-values are Bonferroni-adjusted p-values, with q < 0.05 considered statistically significant.
T cell memory profiles of DURT subsets differ from conventional CD4+ T cells
DURT cells are known to display immediate effector functions, such as secretion of inflammatory cytokines and cytotoxic molecules.41 By comparison, conventional MHC-restricted T cells fully develop effector functions only after antigen-induced priming and differentiation into memory and effector cells. To investigate possible effects of BCG on differentiation and memory marker expression, we assessed proportions of DURT cells expressing CCR7 and/or CD45RA.
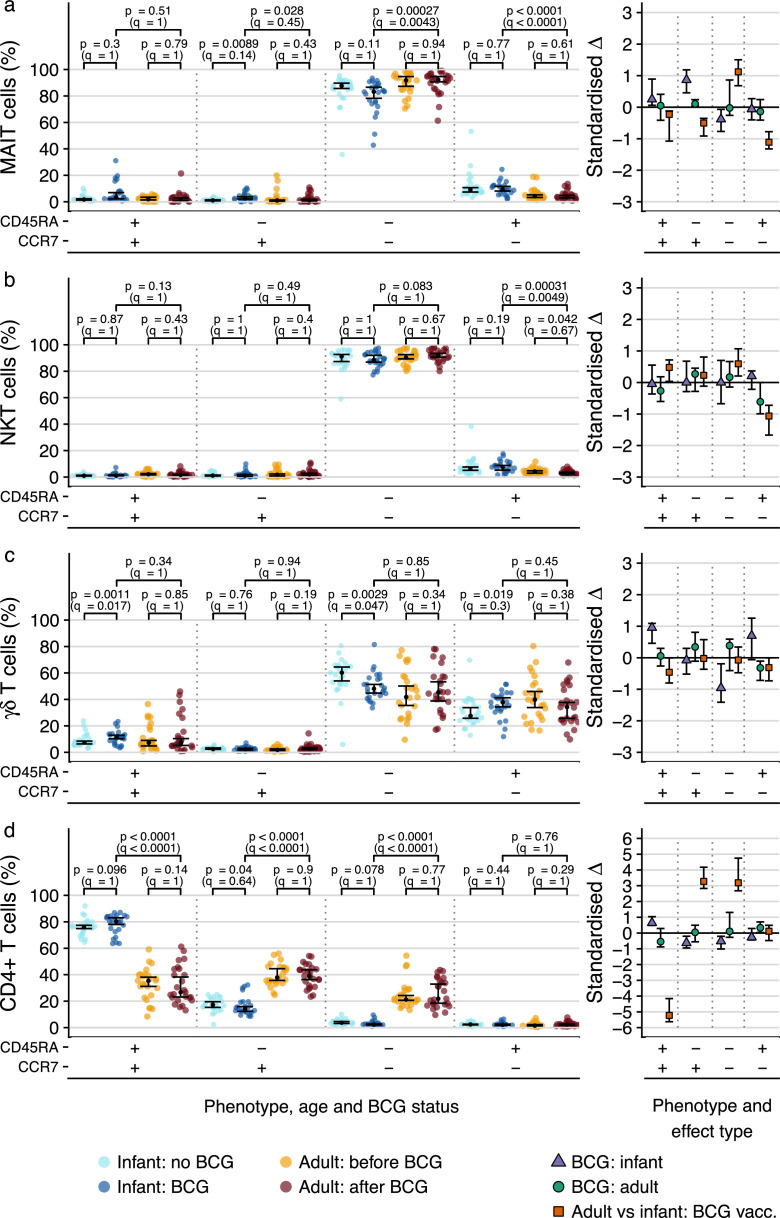
In both infants and adults, MAIT and NKT cells predominantly expressed a CCR7−CD45RA− phenotype at all time points, consistent with effector memory T cells (Figure 6a-b). In infants, the proportion of CD45RA−CCR7+ MAIT cells was marginally higher in the BCG vaccinated group (fold change 2.41, 95% CI 1.81 – 3.02; p=0.009 by Wald test, q=0.14) with a concomitant decrease in CD45RA−CCR7− MAIT cells (fold change 0.95, 95% CI 0.89 – 0.99; p=0.11 by Wald test, q=1). However, the standardized effect size confidence intervals were at most consistent with minor to moderate effect sizes, indicating minor effects that do not change the overall phenotypic profile. Regarding adults, our data did not reveal any BCG-associated effects. The “effector-like” CD45RA−CCR7− phenotype of these cell subsets was as prominent in infants as in the adult population, suggesting early development of this phenotype of MAIT and NKT cells in infants with little change to its predominance thereafter. Longitudinal analysis of memory profiles of MAIT and NKT cells in the adult cohort before and after BCG-revaccination suggested that BCG does not modulate CCR7 or CD45RA expression up to one year post-vaccination (Suppl. Figure 2).
Figure 6.
Memory phenotypes of DURT cell subsets or CD4 T cells in infants or adults. T cell memory profiles, measured by CCR7 and CD45RA co-expression patterns, on (a) MR1-5-OP-RU tetramer+ MAIT cells, (a) CD1d-PBS57 tetramer+ NKT cells, (c) γδ T cells or (d) total CD4 T cells in BCG-vaccinated and unvaccinated infants or in adults before and 365 days after BCG re-vaccination. Relative proportions of cells that fall into each of the possible combinations of CCR7 and CD45RA are represented as percentages. For percentage plots, the black dots represent the estimated median; for standardized effect size plots, the black dots represent the standardized estimated difference in medians. Error bars represent 95% confidence intervals. Q-values are Bonferroni-adjusted p-values, with q < 0.05 considered statistically significant.
γδ T cells, on the other hand, exhibited a more mixed phenotype comprising CCR7−CD45RA− (consistent with effector memory) and CCR7−CD45RA+ (terminally differentiated effector) T cells (Figure 6c). Contrasting with NKT and MAIT cells, vaccination in infants was associated with a reduction in the proportions of CD45RA−CCR7− γδ T cells (fold change 0.79, 95% CI – 0.7 – 0.96; p=0.0029 by Wald test, q=0.047) and increased the proportions of CD45RA+CCR7+ cells (fold change 1.66, 95% CI 1.3 – 1.76; p = 0.0011 by Wald test, q=0.017) and possibly CD45RA+CCR7− cells (fold change 1.25, 95% CI 0.98 – 1.44; p=0.019 by Wald test; q=0.3). Again, vaccination in adults was associated with little to no effect on the phenotypic profile.
As expected from previous work, bulk CD4+ T cells in infants were comprised predominantly of naïve (CCR7+CD45RA+) and some central memory (CCR7+CD45RA−) T cells, whereas adults had comparatively lower proportions of naïve and higher proportions of central memory and effector memory CD4+ T cells (q < 0.0001 for all phenotypes except terminally differentiated) (Figure 6d).
Discussion
DURT cells and their roles in protective and pathogenic immune responses have received much attention in recent years.42 Because these cell populations are not restricted by donor genotype, are relatively abundant and are intrinsically poised for rapid effector function, there is growing interest in whether DURT cells can be harnessed to improve efficacy of vaccination against organisms that express ligands for DURT TCRs.1 We characterized frequencies, phenotypic and functional characteristics of DURT cell populations in the peripheral blood following BCG vaccination to explore their potential as possible immunological targets for TB vaccination. Evidence that γδ T cells, MAIT, NKT and GEM T cells can respond to mycobacteria15,43, 44, 45, 46, 47 provides a strong rationale to investigate if BCG vaccination can modulate these T cell populations.
A key finding was that frequencies of γδ T cells, and perhaps frequencies of IFN-γ-expressing γδ T cells detected by intracellular cytokine staining after in vitro BCG stimulation, were increased in BCG-vaccinated compared with unvaccinated infants. We also previously reported that BCG revaccination induced an increase of BCG-reactive IFN-γ-expressing γδ T cells in adults,38 however, here we show that γδ T cell frequencies were not significantly modulated. Our results are also consistent with data from non-human primates, in which a rapid and robust expansion of γδ T cells has been observed after primary BCG vaccination or M.tb-infection.18 In cattle, γδ T cells have been shown to accumulate in the early phases of M. bovis infection, but quickly dissipate in peripheral blood upon the arrival of other cells. Moreover, circulating γδ T cells from these animals produced increased amounts of IFN-γ and CCL2, and expressed high amounts of cytolytic molecules and lysed BCG-infected target cells with greater efficiency compared to cells from uninfected animals.48 Our findings are thus consistent with the previously reported role of γδ T cells as early responders to BCG vaccination, and may contribute to or enhance the ensuing immune response by recruiting or activating other key immune and effector response players. However, these results are at odds with findings of another recent study of γδ T cell development in early life. Frequencies and differentiation profiles of γδ T cells, assessed in cord blood and at 10 weeks of age, revealed a rapid expansion of Vγ9Vδ2 T cells with cytotoxic phenotypes after birth.49 Notably, this early and robust change in T cell responses was not attributable to newborn BCG vaccination, since Vγ9Vδ2 T cell responses between BCG-vaccinated and unvaccinated infants were not different. The data suggest a possible role of environmental phosphoantigen exposure in the priming of these cells in early life.49 The discrepancy between the BCG-attributable effects on γδ T cells between this study and our present one most likely relate to the different subsets of γδ T cells detected in each study. Although Vγ9Vδ2 are the main γδ T cell subset, in this study we measured the total γδ T cell population, and we cannot exclude that other minor subsets may have contributed to the differences observed here.
We found that infants generally had lower peripheral blood frequencies of MAIT, NKT, GMM-specific and GEM T cells compared to adults, a finding consistent with our previous study.50 By contrast, frequencies of γδ T cells were not different between infants and adults. As expected, BCG vaccination markedly induced conventional, IFN-γ-expressing CD4 T cell responses. However, we show that frequencies of MAIT, NKT, GMM-specific and GEM T cell populations were not modulated by intradermal BCG administration in either infants or adults. BCG vaccination also did not affect DURT cell activation and only induced minor alterations in the expression of the T cell memory markers CCR7 and CD45RA on DURT populations in infants. DURT cell activation was assessed by quantifying HLA-DR expression by the different DURT subsets, with the rationale that TCR-mediated recognition of BCG antigens in vivo would lead to increased HLA-DR expression. Despite the use of HLA-DR as a marker to measure vaccine-induced activation in studies of MHC-restricted T cells,37,51,52 it is not known if this marker is ideal for measuring DURT cell activation. Inclusion of additional activation markers, such as Ki67, would have allowed a more comprehensive ascertainment of the activation status on these cells. The memory phenotypes observed for the DURT subsets is not surprising given that the DURT subsets exhibited very dominant CCR7− CD45RA− effector-like phenotypes in infants and adults, consistent with the well-described intrinsic effector function of these innate-like T cell populations.1
The finding that BCG vaccination did not modulate DURT subsets can be explained by the well-described intrinsic innate-like and poor proliferative capacity generally attributed to DURT cells, which prescribe that these cells do not adapt to antigenic stimulation in the same manner as conventional, adaptive CD4 and CD8 T cells do. A recent study illustrated this elegantly by employing transcriptomic analyses of conventional T cells, MAIT cells, iNKT cells, γδ T cells and NK cells to investigate the apparent trade-off between potential for cellular proliferation and rapid effector function.53 The analyses revealed that these cell subsets can be ranked according to their “innateness” with conventional, adaptive CD4 T cells the least innate and NK cells the most, and that this innateness was characterized by pre-formed mRNA encoding effector molecules, while impaired proliferation was marked by decreased baseline expression of ribosomal genes.
We acknowledge the possibility that the timing of our analyses, performed at 9 weeks after neonatal BCG vaccination and 3, 5 weeks and 1 year after adult BCG revaccination, may have missed transient DURT cell responses to BCG vaccination. In a controlled human Salmonella challenge model, MAIT cells were shown to be activated at the peak of infection (day 10) and this activation state was maintained even after antibiotic treatment.54 By contrast, there was an early decrease in MAIT frequencies after bacterial challenge (day 8), which recovered after antibiotic treatment (day 28).54 Greene et al.55 also reported significant, but transient activation of MAIT cells in peripheral blood of rhesus macaques in response to BCG, which peaked around 3 weeks after vaccination. Notably however, no significant modulation of MAIT cell frequencies in the peripheral blood was observed, highlighting that clonal expansion may be subtle and easy to miss. We also note that the adult BCG revaccinated cohort enrolled individuals with tuberculin skin test (TST) indurations exceeding 15 mm, consistent with prior M.tb infection. These individuals therefore had high levels of baseline anti-mycobacterial immune responses, which may have led to a masking effect such that subtle changes in BCG-induced immune responses were not detected. Future studies should also include TST or IGRA-negative individuals which are difficult to enroll in a high TB incidence setting like South Africa, to allow investigation of the effects of prior sensitization on vaccine-induced DURT responses. Our findings do not preclude that BCG vaccination may shape other qualitative aspects of DURT cells, such as their homing capacity, redistribution of the clonal TCR repertoire and/or proliferative potential.
Neonatal BCG vaccination was associated with a marked increase of an interesting and novel subset of BCG-reactive, IFN-γ-expressing CD26+CD161+ CD4 T cells. Further phenotypic characterization of this subset revealed that these cells did not express the canonical MAIT TCR alpha variable gene segment, TRAV1–2. MAIT cells have traditionally been phenotypically identified as TRAV1–2+CD161+ or CD26+CD161+ CD8+ cells, and most studies have focused exclusively on adults. This peculiar cell subset was not detectable before and after BCG revaccination in adults. We recently showed that MR1–5-OP-RU tetramer-positive MAIT cells in neonates and infants do also include CD4+ and TRAV1–2-negative T cells.50 In addition, in a TCR transgenic MAIT cell murine model, CD4+ MAIT cells were associated with pulmonary protective immunity, recruited into the lung in response to M.tb aerosol challenge.56 However, these BCG-reactive, IFN-γ-expressing CD26+CD161+ CD4 T cells could also be a subset of conventional, activated CD4 T cells or a novel DURT subset that are induced by BCG vaccination. Interestingly, these cells share characteristics with a recently described CD4+CD26+CD161+CCR6+cell subset that expresses IL-17 and IL-22 and was preferentially enriched in a Peruvian cohort of TB non-progressors.57 These findings warrant further clinical and mechanistic investigation of these cells in clinical studies and animal models.
In conclusion, our study suggests that intradermal BCG vaccination does not modulate MAIT, NKT, GMM-specific and GEM T cell frequencies. Newborn BCG vaccination was associated with increased frequencies of γδ T cells; however, there was no significant increase in IFN-γ expressing γδ T cells in response to BCG. More studies are required to understand the full potential of DURT cells for TB vaccination and whether the modulation of γδ T cells is durable. Given their immediate effector properties, the monoclonal or oligoclonal use of a TCR, and their role in regulating other key immune cell subsets and functions, future research should also explore their potential adjuvant effects.
Contributors
AG, DFH, WAH, WHB, JLJ, DFH, SAJ, THMO, SS, DBM, DL, MH, CS, EN and TJS designed the experiments or clinical studies. WAH, HB, and MH performed clinical investigations. AG, MM, MR, KH and SS processed samples, performed assays, and/or analyzed data. AG, MM, MR, KH, SS, EN and TJS verified and interpreted the data. AG, MM, MR, CS, EN and TJS wrote the manuscript. All authors reviewed and approved the manuscript.
Declaration of interests
No competing interests.
Acknowledgments
Acknowledgments
We would like to thank the participants who gave their time and dedication to this study. This study was initiated and designed by members of the Collaboration for TB Vaccine Discovery (CVTD), DURT Research Community, with support from the Bill and Melinda Gates Foundation. The MR1 tetramer technology was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne.
Data sharing statement
All datasets and metadata have been deposited in Zivahub (https://figshare.com/s/91b7b121ea2dcf7b5b36), an open access data repository hosted by the University of Cape Town's institutional data repository powered by Figshare for Institutions.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103839.
Appendix. Supplementary materials
References
- 1.Joosten S.A., Ottenhoff T.H.M., Lewinsohn D.M., et al. Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine. 2019;37(23):3022–3030. doi: 10.1016/j.vaccine.2019.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zajonc D.M., Flajnik M.F. CD1, MR1, NKT, and MAIT: evolution and origins of non-peptidic antigen recognition by T lymphocytes. Immunogenetics. 2016;68(8):489–490. doi: 10.1007/s00251-016-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rhijn I., Moody D.B. Donor unrestricted T cells: a shared human T cell response. J Immunol. 2015;195(5):1927–1932. doi: 10.4049/jimmunol.1500943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behar S.M., Dascher C.C., Grusby M.J., Wang C.R., Brenner M.B. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189(12):1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin P.L., Rutledge T., Green A.M., et al. CD4 T cell depletion exacerbates acute Mycobacterium tuberculosis while reactivation of latent infection is dependent on severity of tissue depletion in cynomolgus macaques. AIDS Res Hum Retroviruses. 2012;28(12):1693–1702. doi: 10.1089/aid.2012.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mogues T., Goodrich M.E., Ryan L., LaCourse R., North R.J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193(3):271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen P., Scriba T.J. Moving tuberculosis vaccines from theory to practice. Nat Rev Immunol. 2019;19(9):550–562. doi: 10.1038/s41577-019-0174-z. [DOI] [PubMed] [Google Scholar]
- 8.Layre E., Collmann A., Bastian M., et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. 2009;16(1):82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Gilleron M., Stenger S., Mazorra Z., et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199(5):649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moody D.B., Ulrichs T., Muhlecker W., et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404(6780):884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 11.Layton E.D., Barman S., Wilburn D.B., et al. T Cells Specific for a Mycobacterial Glycolipid Expand after Intravenous Bacillus Calmette-Guérin Vaccination. J Immunol. 2021;206(6):1240–1250. doi: 10.4049/jimmunol.2001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiromatsu K., Dascher C.C., LeClair K.P., et al. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J Immunol. 2002;169(1):330–339. doi: 10.4049/jimmunol.169.1.330. [DOI] [PubMed] [Google Scholar]
- 13.Gold M.C., Napier R.J., Lewinsohn D.M. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev. 2015;264(1):154–166. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua W.J., Truscott S.M., Eickhoff C.S., Blazevic A., Hoft D.F., Hansen T.H. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80(9):3256–3267. doi: 10.1128/IAI.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai S., Kauffman K.D., Oh S., Nelson C.E., Barry C.E., 3rd, Barber D.L. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021;14(1):199–208. doi: 10.1038/s41385-020-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorkas C.K., Levy O., Skular M., Li K., Aube J., Glickman M.S. Efficient 5-OP-RU-induced enrichment of mucosa-associated invariant T cells in the murine lung does not enhance control of aerosol mycobacterium tuberculosis infection. Infect Immun. 2020;89(1) doi: 10.1128/IAI.00524-20. e00524-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H., Yang A., Derrick S., et al. Artificially induced MAIT cells inhibit M. bovis BCG but not M. tuberculosis during in vivo pulmonary infection. Sci Rep. 2020;10(1):13579. doi: 10.1038/s41598-020-70615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y., Zhou D., Qiu L., et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295(5563):2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai X., Shen Y., Zhou D., et al. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin Exp Immunol. 2003;133(2):182–192. doi: 10.1046/j.1365-2249.2003.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen L., Frencher J., Huang D., et al. Immunization of Vgamma2Vdelta2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc Natl Acad Sci USA. 2019;116(13):6371–6378. doi: 10.1073/pnas.1811380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoft D.F., Brown R.M., Roodman S.T. Bacille Calmette-Guérin vaccination enhanceshuman gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161(2):1045–1054. [PubMed] [Google Scholar]
- 22.Hoft D.F., Worku S., Kampmann B., et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis. 2002;186(10):1448–1457. doi: 10.1086/344359. [DOI] [PubMed] [Google Scholar]
- 23.Salio M., Cerundolo V. Regulation of lipid specific and vitamin specific non-MHC restricted T cells by antigen presenting cells and their therapeutic potentials. Front Immunol. 2015;6:388. doi: 10.3389/fimmu.2015.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepore M., Kalinichenko A., Colone A., et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Fontecha A., Thomsen L.L., Brett S., et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 26.Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerundolo V., Silk J.D., Masri S.H., Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 28.Hatherill M., Geldenhuys H., Pienaar B., et al. Safety and reactogenicity of BCG revaccination with isoniazid pretreatment in TST positive adults. Vaccine. 2014;32(31):3982–3988. doi: 10.1016/j.vaccine.2014.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suliman S., Geldenhuys H., Johnson J.L., et al. Bacillus Calmette-Guerin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol. 2016;197(4):1100–1110. doi: 10.4049/jimmunol.1501996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton E.D., Yu K.K.Q., Smith M.T., Scriba T.J., De Rosa S.C., Seshadri C. Validation of a CD1b tetramer assay for studies of human mycobacterial infection or vaccination. J Immunol Methods. 2018;458:44–52. doi: 10.1016/j.jim.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagina B.M., Mansoor N., Kpamegan E.P., et al. Qualification of a whole blood intracellular cytokine staining assay to measure mycobacteria-specific CD4 and CD8 T cell immunity by flow cytometry. J Immunol Methods. 2015;417:22–33. doi: 10.1016/j.jim.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenker R. Confidence intervals for regression quantiles. Heidelberg: physica-Verlag HD; 1994.
- 33.Koenker R.W., D'Orey V. Algorithm AS 229: computing regression quantiles. J R Stat Soc. 1987;36(3):383–393. [Google Scholar]
- 34.Koenker R.W., Bassett G. Robust tests for heteroscedasticity based on regression quantiles. Econometrica. 1982;50(1):43–61. [Google Scholar]
- 35.Brooks M.E., Magnusson A., Berg C.W., et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9(2):378–400. [Google Scholar]
- 36.Soares A.P., Scriba T.J., Joseph S., et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180(5):3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares A.P., Kwong Chung C.K., Choice T., et al. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis. 2013;207(7):1084–1094. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suliman S., Geldenhuys H., Johnson J.L., et al. Bacillus Calmette-Guerin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol. 2016;197(4):1100–1110. doi: 10.4049/jimmunol.1501996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares A.P., Chung C.K.C.K., Choice T., et al. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis. 2013;207(7):1084–1094. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma P.K., Wong E.B., Napier R.J., et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145(3):443–453. doi: 10.1111/imm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Godfrey D.I., Uldrich A.P., McCluskey J., Rossjohn J., Moody D.B. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 42.Ruibal P., Voogd L., Joosten S.A., Ottenhoff T.H.M. The role of donor-unrestricted T-cells, innate lymphoid cells, and NK cells in anti-mycobacterial immunity. Immunol Rev. 2021 doi: 10.1111/imr.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layton E.D., Barman S., Wilburn D.B., et al. T cells specific for a mycobacterial glycolipid expand after intravenous bacillus calmette-guerin vaccination. J Immunol. 2021 doi: 10.4049/jimmunol.2001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boom W.H. Gammadelta T cells and Mycobacterium tuberculosis. Microbes Infect. 1999;1(3):187–195. doi: 10.1016/s1286-4579(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 45.Ordway D.J., Pinto L., Costa L., et al. Gamma delta T cell responses associated with the development of tuberculosis in health care workers. FEMS Immunol Med Microbiol. 2005;43(3):339–350. doi: 10.1016/j.femsim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Li Z., Yang B., Zhang Y., et al. Mycobacterium tuberculosis-specific memory NKT cells in patients with tuberculous pleurisy. J Clin Immunol. 2014;34(8):979–990. doi: 10.1007/s10875-014-0090-8. [DOI] [PubMed] [Google Scholar]
- 47.Chancellor A., Tocheva A.S., Cave-Ayland C., et al. CD1b-restricted GEM T cell responses are modulated by Mycobacterium tuberculosis mycolic acid meromycolate chains. Proc Natl Acad Sci USA. 2017;114(51):E10956–E10E64. doi: 10.1073/pnas.1708252114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGill J.L., Sacco R.E., Baldwin C.L., Telfer J.C., Palmer M.V., Waters W.R. The role of gamma delta T cells in immunity to Mycobacterium bovis infection in cattle. Vet Immunol Immunopathol. 2014;159(3–4):133–143. doi: 10.1016/j.vetimm.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Papadopoulou M., Dimova T., Shey M., et al. Fetal public Vgamma9Vdelta2 T cells expand and gain potent cytotoxic functions early after birth. Proc Natl Acad Sci USA. 2020;117(31):18638–18648. doi: 10.1073/pnas.1922595117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swarbrick G.M., Gela A., Cansler M.E., et al. Postnatal expansion, maturation, and functionality of MR1T cells in humans. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.556695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adekambi T., Ibegbu C.C., Cagle S., et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest. 2015;125(9):3723. doi: 10.1172/JCI83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller J.D., van der Most R.G., Akondy R.S., et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez-Arcelus M., Teslovich N., Mola A.R., et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun. 2019;10(1):687. doi: 10.1038/s41467-019-08604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howson L.J., Napolitani G., Shepherd D., et al. MAIT cell clonal expansion and TCR repertoire shaping in human volunteers challenged with Salmonella Paratyphi A. Nat Commun. 2018;9(1):253. doi: 10.1038/s41467-017-02540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greene J.M., Dash P., Roy S., et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 2017;10(3):802–813. doi: 10.1038/mi.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakala I.G., Kjer-Nielsen L., Eickhoff C.S., et al. Functional heterogeneity and antimycobacterial effects of mouse mucosal-associated invariant T cells specific for riboflavin metabolites. J Immunol. 2015;195(2):587–601. doi: 10.4049/jimmunol.1402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nathan A., Beynor J.I., Baglaenko Y., et al. Multimodally profiling memory T cells from a tuberculosis cohort identifies cell state associations with demographics, environment and disease. Nat Immunol. 2021;22(6):781–793. doi: 10.1038/s41590-021-00933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.