Abstract
Background
The glycosylation alterations of serum and IgG are involved in a variety of autoimmune and inflammatory diseases and have shown great potential in biomarker field. The diagnosis of immune thrombocytopenia (ITP) is exclusive. Our study aimed to discover the potential glyco‐biomarkers for auxiliary diagnosis of ITP.
Methods
The serum samples were obtained from 61 ITP patients and 35 healthy controls, and IgG samples were purified from 34 out of 61 ITP patients and 35 healthy controls. DNA sequencer‐assisted fluorophore‐assisted carbohydrate electrophoresis (DSA‐FACE) was used to analyze serum and IgG N‐glycan profiling.
Results
6 of 12 serum N‐glycan peaks, 6 of 7 IgG N‐glycan peaks, serum fucosylation, and IgG galactosylation were significantly different between ITP patients and healthy controls (p < 0.05). IgG peak 7 showed good diagnostic efficacy for discriminating ITP patients from healthy individuals (AUC 0.967). ITP patients with severe thrombocytopenia had a significantly lower serum fucosylation than ITP patients with mild and moderate thrombocytopenia (p < 0.05). Serum fucosylation and serum peak 5 were correlated with platelet counts in ITP patients with severe thrombocytopenia, and the absolute values of correlation coefficient were both over 0.5.
Conclusions
The specific N‐glycan patterns of serum and IgG were observed in ITP patients. IgG peak 7 was a potential biomarker for auxiliary diagnosis of ITP.
Keywords: biomarker, diagnosis, glycosylation, immune thrombocytopenia, N‐glycan profiling
We analyzed serum and IgG N‐glycan profiling in patients with immune thrombocytopenia (ITP) and healthy controls, and identified N‐glycans as potential biomarkers for auxiliary diagnosis of ITP. Our research enriched the studies on the glyco‐biomarkers for autoimmune diseases.

1. INTRODUCTION
Primary immune thrombocytopenia (ITP) is a common autoimmune bleeding disease, in which immune‐mediated excessive platelet clearance and insufficient platelet production result in a decrease in platelet count (<100 × 109/L). The clinical manifestation varies from asymptomatic to mucocutaneous bleeding, visceral bleeding, and even life‐threatening intracranial hemorrhage.
Among mechanisms of platelet clearance in ITP, the clearance mediated by anti‐platelet autoantibodies is considered the classical pathway, and the type of autoantibodies is mainly immunoglobulin G (IgG). IgG antibodies are glycoproteins and undergo post‐translational modification. The Fc portion of IgG has a uniquely conserved glycosylation site at asparagine 297 (Asn297). The exact composition of the attached N‐glycan affects IgG‐mediated effector functions such as antibody‐dependent cell‐mediated cytotoxicity (ADCC), complement‐dependent cytotoxicity (CDC), and antibody‐dependent cellular phagocytosis (ADCP) through modification of the IgG Fc binding affinity for Fcγ receptors (FcγRs) and complement protein C1q complex. 1 , 2 , 3 , 4 , 5 Glycosylation of IgG Fc is critical for modulating the pro‐inflammatory and anti‐inflammatory activities of IgG and may contribute to autoimmune and inflammatory diseases. It has been reported that a variety of autoimmune and inflammatory diseases such as autoimmune hemolytic anemia, systemic lupus erythematosus, inflammatory bowel disease, and rheumatoid arthritis have skewed IgG glycosylation pattern, 6 , 7 , 8 , 9 and in some diseases, the skewed IgG glycosylation is associated with disease severity and treatment response. 10 , 11 In addition to IgG, the altered glycosylation pattern of total serum proteins, which is considered reflecting the physiological and pathological state of the body, is also associated with autoimmune and inflammatory diseases. 12 Nowadays, the disease‐related glycosylation alterations of serum and IgG have shown great potential in the biomarker field. 12 , 13 , 14 , 15 , 16
The researches on glycosylation alteration in ITP are scarce and inconsistent. One study showed IgG galactosylation of ITP patients was higher than that of healthy controls, 17 while another did not detect any difference in IgG glycosylation between ITP patients and healthy controls. 18 Whether the glycosylation patterns of serum and IgG are altered in ITP, similar to other autoimmune disorders, needs further investigations. In this study, we compared the N‐glycan profiling of serum proteins and purified IgG fraction (hereinafter referred to as serum and IgG respectively) between ITP patients and healthy controls and investigated the correlations of N‐glycans with platelet counts, and the specific N‐glycan patterns of serum and IgG were observed in ITP patients.
2. MATERIALS AND METHODS
2.1. Patients and controls
61 patients with ITP (23 males and 38 females; 22–84 years old; median age 54 years) were enrolled between January 2020 and November 2021 in the Department of Hematology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, China (hereinafter referred to as Yueyang Hospital), after an informed consent was obtained from each patient. All the patients were diagnosed according to Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). 19 35 age‐ and sex‐matched healthy controls (16 males and 19 females; 39–63 years old; median age 54 years) were randomly selected from the database established by Shanghai Eastern Hepatobiliary Surgery Hospital as a control group (http://206.189.76.64/NGFP/login.php). The study was approved by the Institutional Review Boards at the leading study center (EHBHKY2020‐02‐012). Serum samples were stored until analysis (−80°C).
2.2. Serum N‐glycan profiling
Fluorescent glucose electrophoresis based on DNA sequencer (DSA‐FACE) was used to analyze N‐glycan profiling of desialylated sera as previously described. 20 In brief, the N‐glycans were released from the glycoproteins in 2 μL serum with peptide‐N‐glycosidase‐F (PNGase F) (New England Biolabs, MA) and labeled with fluorescein APTS (8‐amino‐naphthalene‐1,3,6‐ trisulfonic acid) (Invitrogen, CA); then, sialic acid at the end of the oligosaccharide was removed with arthrobacter ureafaciens sialidase (Roche Bioscience, Palo Alto, CA). The processed samples were analyzed by capillary electrophoresis‐based ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA) and the GeneMapper software (Applied Biosystems). 12 obvious N‐glycan peaks were detected, and the relative structure abundance of each N‐glycan peak was described numerically by normalizing its peak height to the sum of the heights of all peaks. Subsequently, the levels of fucosylation and galactosylation were calculated. The level of fucosylation was described as the sum of relative abundances of all the peaks containing core fucose (peak 1 + peak 2 + peak 3 + peak 4 + peak 6 + peak 7 + peak 9p + peak 10), and the level of galactosylation was described as the sum of relative abundances of all the peaks containing galactose (peak 3 + peak 4 + peak 5 + peak 6 + peak 7 + peak 8 + peak 9 + peak 9p + peak 10 + peak 11).
2.3. IgG N‐glycan profiling
IgG was isolated from the whole serum using protein A/G Spin kit according to the manufacture's instruction (Thermo Scientific, USA). IgG was eluted with formic acid and dried in a vacuum concentrator; then, N‐glycans were released from IgG by PNGase F. The N‐glycan profiling was performed by DSA‐FACE as described above. 7 IgG N‐glycan peaks corresponding to peak 1 to peak 7 of serum N‐glycan peaks were detected, and the relative abundance of each N‐glycan was described as above. The level of fucosylation was described as the sum of relative abundances of all the peaks containing core fucose (peak 1 + peak 2 + peak 3 + peak 4 + peak 6 + peak 7), and the level of galactosylation was described as the sum of relative abundances of all the peaks containing galactose (peak 3 + peak 4 + peak 5 + peak 6 + peak 7).
2.4. Statistical analysis
Statistical analyses were performed in SPSS 26.0, and SPSS 26.0 and GraphPad Prism 5.0 were used for graphing. Categorical variables were expressed as numbers and percentages (n, %), and the comparison of categorical variables between groups was performed using chi‐square test. Continuous variables were expressed as mean ±standard deviation (SD) and median with range according to data distribution, and comparisons of continuous variables between groups were performed by Mann‐Whitney U test, unpaired t test and paired t test. The diagnostic efficacies of the biomarkers were evaluated by receiver operating characteristic curve (ROC). A two‐tailed p values < 0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
The main demographic features and platelet counts of healthy controls and ITP patients were summarized in Table 1. There were no significant differences in age and sex between control group and ITP group.
TABLE 1.
Demographic and clinical characteristics of healthy controls and ITP patients
| Characteristics |
Control (n = 35) |
ITP (n = 61) |
P Value |
|---|---|---|---|
| Age in years | |||
| Median | 54 | 54 | 0.855 |
| Range | 39–63 | 22–84 | |
| Gender, n (%) | |||
| Male | 16(45.71) | 23(37.70) | 0.442 |
| Female | 19(54.29) | 38(62.30) | |
| PLT (×109/L) | |||
| Mean ±SD | 235.63 ± 50.95 | 39.59 ± 22.30 | 0.000* |
| Range | 140–359 | 2–94 |
PLT, platelet count. * p < 0.05, the difference is statistically significant.
3.2. Alterations of N‐glycan profiling in ITP
We analyzed serum N‐glycan profiling in 35 healthy controls and 61 ITP patients, and IgG N‐glycan profiling in 35 healthy controls and 34 out of 61 ITP patients, and the flow chart was shown on Supplementary data, Figure S1. 12 serum N‐glycan structures (peaks) and 7 IgG N‐glycan peaks were identified. The 12 serum N‐glycan peaks comprised 7 biantennary glycans, 4 tri‐antennary glycans, and 1 tetra‐antennary glycan, and the detailed structure of each N‐glycan peak was described previously by Liu et al. 21 The representative serum and IgG profiling patterns were shown in Figure 1. 6 out of 12 directly measured serum N‐glycan peaks showed statistically significant differences between healthy controls and ITP patients. In ITP patients, the relative abundances of peak 5 (NA2), peak 9p (NA3F), and peak 11 (NA4) were increased, while the relative abundances of peak 3 (NG1A2F), peak 4 (NG1A2F), and peak 6 (NA2F) were decreased. We also analyzed the derived N‐glycan traits, namely fucosylation and galactosylation, which were commonly differentially expressed in autoimmune diseases. The level of fucosylation in ITP patients was significantly decreased compared with healthy controls, whereas no statistically significant difference for galactosylation was observed between two groups (Table 2).
FIGURE 1.
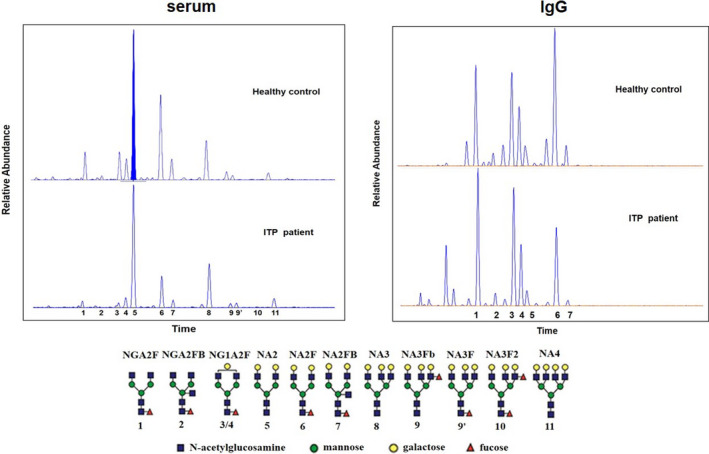
Representative desialylated N‐glycan profiling for serum and IgG of healthy controls and ITP patients. 12 peaks were identified in all serum samples, and 7 peaks were identified in all IgG samples, and the structures of the N‐glycan peaks are shown below the pane. Blue square indicates β‐linked N‐acetylglucosamine (GlcNAc); green circle indicates α/β‐linked mannose; yellow circle indicates β‐linked galactose; and red triangle indicates α/β‐1,3/6 linked fucose
TABLE 2.
Serum and IgG N‐glycans in healthy controls and ITP patients
| Peak or glycosylation | Serum | IgG | ||||
|---|---|---|---|---|---|---|
|
Control (n = 35) |
ITP (n = 61) |
P1 |
Control (n = 35) |
ITP (n = 34) |
P2 | |
| Peak 1 | 7.01 ± 1.77 | 7.37 ± 2.55 | 0.468 | 22.32 ± 4.99 | 28.41 ± 7.51 | 0.000* |
| Peak 2 | 1.05 ± 0.35 | 1.10 ± 0.39 | 0.480 | 3.61 ± 1.07 | 4.42 ± 1.64 | 0.018* |
| Peak 3 | 6.56 ± 1.03 | 5.89 ± 1.38 | 0.014* | 19.81 ± 1.78 | 22.81 ± 3.14 | 0.002* |
| Peak 4 | 5.82 ± 0.96 | 5.28 ± 0.86 | 0.006* | 12.82 ± 1.37 | 14.54 ± 2.33 | 0.000* |
| Peak5 | 39.15 ± 3.14 | 42.27 ± 4.36 | 0.000* | 5.52 ± 1.22 | 5.22 ± 1.24 | 0.315 |
| Peak 6 | 21.39 ± 3.62 | 18.93 ± 2.93 | 0.000* | 29.47 ± 5.93 | 22.65 ± 6.85 | 0.000* |
| Peak 7 | 5.66 ± 1.21 | 5.34 ± 1.19 | 0.212 | 6.44 ± 1.11 | 2.95 ± 1.36 | 0.000* |
| Peak 8 | 7.95 ± 1.81 | 8.20 ± 2.39 | 0.599 | ‐ | ‐ | ‐ |
| Peak 9 | 2.35 ± 1.13 | 1.90 ± 1.15 | 0.067 | ‐ | ‐ | ‐ |
| Peak 9p | 0.87 ± 0.30 | 1.05 ± 0.44 | 0.031* | ‐ | ‐ | ‐ |
| Peak 10 | 0.32 ± 0.29 | 0.29 ± 0.11 | 0.491 | ‐ | ‐ | ‐ |
| Peak 11 | 1.86 ± 0.53 | 2.18 ± 0.71 | 0.025* | ‐ | ‐ | ‐ |
| Fucosylation | 48.68 ± 4.68 | 45.25 ± 5.93 | 0.004* | 94.48 ± 1.22 | 94.78 ± 1.24 | 0.315 |
| Galactosylation | 91.94 ± 2.03 | 91.32 ± 2.81 | 0.259 | 74.07 ± 5.83 | 67.17 ± 8.48 | 0.000* |
Data were expressed as means ±standard deviation (SD); PLT: platelet count; P1: comparison of serum N‐glycans between control and ITP; P2: comparison of IgG N‐glycans between control and ITP. * p<0.05, the difference is statistically significant.
In the aspect of IgG, 6 out of 7 IgG N‐glycan peaks showed statistically significant differences between healthy controls and ITP patients. In ITP patients, the relative abundances of agalactosyl peak 1 and peak 2, and monogalactosyl peak 3 and peak 4 were increased, while the relative abundances of bigalactosyl peak 6 and peak 7 were decreased. With regard to glycosylation, IgG galactosylation in ITP patients was significantly decreased compared with healthy controls, whereas IgG fucosylation was not significantly different between two groups (Table 2).
ITP patients with platelet counts (PLT) below 30 × 109/L were usually in high bleeding risk and need drug intervention, so ITP patients were further stratified into two subgroups according to PLT, ITP patients with severe thrombocytopenia (PLT <30 × 109/L), and ITP patients with mild and moderate thrombocytopenia (PLT≥30 × 109/L). The level of serum fucosylation in ITP patients with severe thrombocytopenia was significantly lower than that in ITP patients with mild and moderate thrombocytopenia. With respect to IgG N‐glycans, there was no significant difference in the relative abundances of IgG N‐glycan peaks and IgG glycosylation between two subgroups (Table 3).
TABLE 3.
Serum and IgG N‐glycans in ITP patients with PLT≥30 × 109/L and PLT<30×109/L
| Peak or glycosylation | Serum | IgG | ||||
|---|---|---|---|---|---|---|
|
PLT≥30 × 109/L (n = 39) |
PLT<30 × 109/L (n = 22) |
P1 |
PLT≥30 × 109/L (n = 20) |
PLT<30 × 109/L (n = 14) |
P2 | |
| Peak 1 | 7.60 ± 2.37 | 6.96 ± 2.85 | 0.347 | 29.10 ± 7.83 | 27.42 ± 7.19 | 0.530 |
| Peak 2 | 1.12 ± 0.36 | 1.08 ± 0.44 | 0.683 | 4.57 ± 1.61 | 4.21 ± 1.72 | 0.535 |
| Peak 3 | 6.12 ± 1.39 | 5.47 ± 1.29 | 0.080 | 21.58 ± 2.84 | 22.15 ± 3.61 | 0.609 |
| Peak 4 | 5.36 ± 0.90 | 5.14 ± 0.80 | 0.330 | 14.51 ± 1.93 | 14.57 ± 2.88 | 0.947 |
| Peak 5 | 41.51 ± 4.42 | 43.62 ± 4.01 | 0.069 | 5.26 ± 1.26 | 5.17 ± 1.26 | 0.828 |
| Peak 6 | 19.32 ± 2.96 | 18.23 ± 2.79 | 0.162 | 22.10 ± 7.91 | 23.44 ± 5.14 | 0.553 |
| Peak 7 | 5.56 ± 1.12 | 4.95 ± 1.24 | 0.052 | 2.88 ± 1.29 | 3.05 ± 1.49 | 0.733 |
| Peak 8 | 8.04 ± 2.03 | 8.48 ± 2.94 | 0.490 | ‐ | ‐ | ‐ |
| Peak 9 | 1.71 ± 1.03 | 2.24 ± 1.29 | 0.080 | ‐ | ‐ | ‐ |
| Peak 9p | 1.04 ± 0.38 | 1.06 ± 0.54 | 0.842 | ‐ | ‐ | ‐ |
| Peak 10 | 0.27 ± 0.08 | 0.34 ± 0.14 | 0.051 | ‐ | ‐ | ‐ |
| Peak 11 | 2.16 ± 0.70 | 2.20 ± 0.76 | 0.817 | ‐ | ‐ | ‐ |
| Fucosylation | 46.40 ± 5.81 | 43.22 ± 5.71 | 0.043* | 94.74 ± 1.26 | 94.83 ± 1.26 | 0.828 |
| Galactosylation | 91.09 ± 2.65 | 91.73 ± 3.08 | 0.396 | 66.33 ± 8.97 | 68.37 ± 7.89 | 0.498 |
Data were expressed as means ± standard deviation (SD); PLT: platelet counts; P1: comparison of serum N‐glycans between ITP with PLT≥30 × 109/L and PLT<30 × 109/L; P2: comparison of IgG N‐glycans between ITP with PLT≥30 × 109/L and PLT<30 × 109/L. * p < 0.05, the difference is statistically significant.
3.3. Comparison of N‐glycans between serum and IgG
N‐glycans of serum and IgG were simultaneously analyzed in 35 healthy controls and 34 ITP patients. In order to be consistent with IgG N‐glycan peaks, we recalculated the relative abundances of the front 7 N‐glycan peaks in serum. The abundances of 7 N‐glycan peaks and 2 glycosylations were compared between serum and IgG in paired manner. In ITP patients, 6 out of 7 N‐glycan peaks were significantly changed. Among them, the abundances of IgG peak 1, peak 2, peak 3, and peak 4 were significantly increased, while the abundance of IgG peak 5 and peak 7 was significantly decreased, and no significant difference in peak 6 between IgG and serum was found. With regard to glycosylation, IgG fucosylation was increased and IgG galactosylation was decreased compared with serum (Figure 2). Healthy controls had the same changes with ITP patients except for peak 6 and peak 7. In healthy controls, the abundance of IgG peak 6 was significantly increased compared with serum, while the abundance of peak 7 did not show significant difference between IgG and serum (Supplementary data, Figure S2).
FIGURE 2.
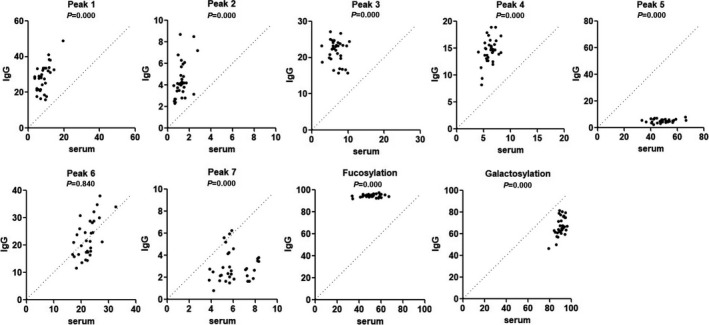
Comparisons of N‐glycan structure and glycosylation between serum and IgG in ITP patients (n = 34). The diagonal line represents the equal ratio between serum and IgG
3.4. Correlation between N‐glycans and platelet counts in ITP
For the significantly differential N‐glycans, we further investigated their correlations with platelet counts. The abundance of serum peak 5 was negatively correlated with platelet counts, while serum Peak 3 and fucosylation were positively correlated with platelet counts. In ITP patients with severe thrombocytopenia, the correlations between serum peak 5, serum fucosylation, and platelet counts were deepened and the absolute values of correlation coefficient were both over 0.5, but serum peak 3 was no longer associated with platelet counts (Table 4). Neither IgG N‐glycan peak nor IgG glycosylation was significantly correlated with platelet counts in ITP patients and ITP patients with severe thrombocytopenia (Supplementary data, Table S1).
TABLE 4.
Correlation between serum N‐glycans and platelet counts
| Correlation | Peak3 | Peak4 | Peak5 | Peak6 | Peak9p | Peak11 | Fucosylation |
|---|---|---|---|---|---|---|---|
| ITP (n = 61) | |||||||
| r | 0.268 | 0.228 | −0.315 | 0.097 | 0.052 | −0.051 | 0.329 |
| P | 0.036* | 0.078 | 0.013* | 0.456 | 0.690 | 0.695 | 0.010* |
| ITP with PLT <30 × 109/L(n = 22) | |||||||
| r | 0.375 | 0.158 | −0.563 | 0.335 | 0.060 | 0.029 | 0.548 |
| P | 0.086 | 0.483 | 0.006* | 0.128 | 0.789 | 0.898 | 0.008* |
Pearson correlation test; PLT, platelet counts. * p < 0.05, the difference is statistically significant.
3.5. Assessment of N‐glycans as diagnostic biomarkers in ITP
Receiver operating characteristic (ROC) curve was used to evaluate the diagnosis efficacies of significantly altered N‐glycans for discrimination of ITP patients from healthy individuals. In serum N‐glycans, the under area of curve (AUC) of peak 5 was the highest, 0.726 (95% CI 0.624–0.827), with 57.4% sensitivity, and 88.6% specificity for cutoff value 41.720. In IgG N‐glycans, the AUC of ‐ IgG peak 7 was the highest, 0.967 (95% CI 0.932–1.000), with 91.2% sensitivity and 91.4% specificity for cutoff value −5.272 (Figure 3). For discrimination of ITP patients with severe thrombocytopenia from ITP patients with mild and moderate thrombocytopenia, the AUC of serum fucosylation was 0.653 (95% CI 0.509–0.796), with a sensitivity of 68.2% and a specificity of 59.0% for cutoff value −45.299 (Figure 4).
FIGURE 3.
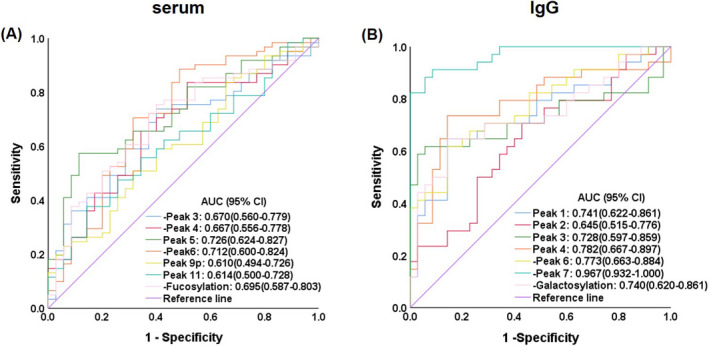
Receiver operating characteristic (ROC) curve in prediction of clinically significant discrimination of ITP patients from healthy individuals. (A) The ROC of significantly altered serum N‐glycans. (B) The ROC of significantly altered IgG N‐glycans
FIGURE 4.
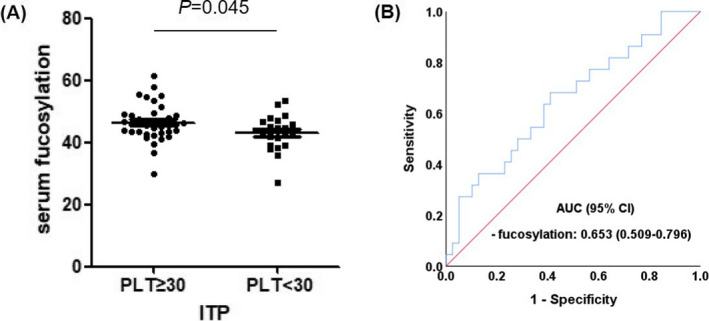
Comparison of serum fucosylation between ITP patients with PLT<30 × 109/L (n=22) and ITP patients with PLT≥30 × 109/L (n = 39) and ROC curves. (A) Comparison of serum fucosylation between ITP patients with PLT<30 × 109/L and ITP patients with PLT≥30 × 109/L. (B) ROC curves of serum fucosylation for discriminating ITP patients with PLT<30 × 109/L from ITP patients with PLT≥30 × 109/L
4. DISCUSSION
Protein glycosylation, the most common post‐translational modification, finely tunes the function of proteins. Addition of different N‐glycans to IgG plays an important role in regulating the pro‐inflammatory or anti‐inflammatory activities of IgG. For example, high level of sialylation in IgG Fc fragment decreased inflammatory activity of IgG, 2 whereas lack of core fucose residues improved cytotoxic activity of IgG. 22 Studies on the glycosylation alteration of antibodies could assist in understanding the exact underlying pathophysiological mechanism 23 , 24 and possibly provide novel biomarkers or therapeutic targets for autoimmune and inflammatory diseases including ITP.
The exact pathophysiological mechanism of ITP is not yet fully understood and its diagnosis is exclusive. In order to explore the potential of N‐glycans as auxiliary diagnostic biomarkers for ITP, we analyzed N‐glycan profiling both serum and IgG in ITP patients and healthy controls, and found alterations of N‐glycan structure and glycosylation in ITP. 6 of 12 serum N‐glycan peaks, 6 of 7 IgG N‐glycan peaks, serum fucosylation, and IgG galactosylation were significantly different between ITP patients and healthy controls. In terms of diagnostic efficacy for discriminating ITP patients from healthy individuals, IgG N‐glycans were collectively better than serum N‐glycans. Of the significantly altered N‐glycans, the diagnostic efficacy of IgG peak 7 (bigalactosylated, core‐α‐1,6‐fucosylated biantennary, NA2FB) was the best, and the AUC of – IgG peak 7 was 0.967. IgG peak 7 had the potential for auxiliary diagnosis of ITP, but its usefulness as a diagnostic biomarker of ITP should be further evaluated. Larger sample size and secondary thrombocytopenic disorders such as SLE and Evan's syndrome should be included in the validation investigation.
The significantly altered glycosylation of ITP patients was different in serum and IgG, which may be caused by the differently altered N‐glycan structures in serum and IgG. For IgG, ITP patients exhibited increased agalactosylation (peak 1 and peak 2) and decreased digalactosylation (peak 6 and peak 7), which resulted in a decrease in the level of IgG galactosylation. Low IgG galactosylation was also observed in a number of autoimmune and inflammatory diseases such as RA, 9 SLE, 7 Crohn's disease (CD), ulcerative colitis (UC), 8 and ANCA‐associated vasculitis, 25 and may be not a disease‐specific marker. As with other autoimmune disorders, inflammation plays an important role in the development of ITP. 26 It was reported that low galactosylation had increased pro‑inflammatory activity of IgG. So, we speculate the decrease in IgG galactosylation may be a reflection of inflammatory state of ITP. However, two other ITP related researches did not detect a decrease in IgG galactosylation. 17 , 18 This inconsistence may be caused by different glycosylation analysis method, different patients background, and small sample size. For serum, the N‐glycans with core fucose (peak 3, peak 4, and peak 6) were decreased, while the N‐glycans without core fucose (peak 5 and peak 11) were increased, which resulted in a decrease in serum fucosylation of ITP patients. Furthermore, ITP patients with low serum fucosylation were more likely to develop severe thrombocytopenia, and the AUC of ‐ fucosylation for distinguishing ITP patients with severe thrombocytopenia from those with mild and moderate thrombocytopenia was 0.653. The decrease in serum fucosylation was also observed in multiple sclerosis. 12 IgG with low fucosylation has an increase in the pro‑inflammatory activity, whether the decrease in fucosylation of serum proteins was also an indicator of elevated inflammatory state has not been known.
About the correlation between N‐glycans and platelet counts in ITP, serum N‐glycans showed a better relevance with platelet counts than IgG N‐glycans. In ITP patients with severe thrombocytopenia who were in high bleeding risk, serum fucosylation and serum peak 5 showed correlations with platelet counts, and the absolute values of correlation coefficient were both over 0.5. Because the decrease in platelet counts is associated with bleeding risk, the low serum fucosylation and high peak 5 abundance may predict bleeding. The assessment of bleeding risk for ITP patients is very important because it determines the initiation of treatment and choice of therapeutic regime. However, the assessment of bleeding risk for ITP is challenging and need new biomarkers. In the future study, we will combine the abundance of serum peak 5, serum fucosylation, and platelet counts to assess the bleeding risk in ITP.
When comparing serum and IgG N‐glycan profiling, we found the structure abundances of most N‐glycan peaks changed, whether in ITP patients or in healthy controls. When comparing significantly altered N‐glycan peaks of ITP patients between serum and IgG, only three were common, peak 3, peak 4, and peak 6. The abundances of peak 4 and peak 6 of ITP patients were both decreased in serum and IgG compared with healthy controls; however, peak 3 was in the opposite direction, lower in serum while higher in IgG when comparing with healthy controls. The significantly altered glycosylation pattern of ITP patients was also different in serum and IgG. All of these suggested the alterations of N‐glycan structure and glycosylation of IgG were in general different from serum in ITP patients, and we should discuss serum and IgG separately when we talk about N‐glycan alterations in ITP.
There are two limitations in our study: (1) The sample size is small; (2) the differentially diagnostic capacity of N‐glycans for distinguishing ITP from secondary thrombocytopenia was not included. These will be improved in the future study. In summary, our study has addressed for the first time both serum and IgG N‐glycan profiling of ITP patients. The significant modifications of N‐glycan structure and glycosylation in serum and IgG were found, but they were in general different. IgG peak 7 may be a potential biomarker for auxiliary diagnosis of ITP; however, further studies are required to confirm its clinical utility.
CONFLICT OF INTEREST
All authors claim that there is no conflict of interest including a desire for financial gain, prominence, professional advancement, or a successful outcome.
AUTHOR CONTRIBUTIONS
Wei Wang and Xuewen Xu make the same contribution to this article.
AUTHORSHIP
All authors have accepted responsibility for the entire content of this article and approved its submission.
ETHICS APPROVAL
The study was approved by the Institutional Review Boards at the leading study center (EHBHKY2020‐02–012).
Supporting information
Supplementary Material
Wang W, Xu X, Huang C, Gao C. N‐glycan profiling alterations of serum and immunoglobulin G in immune thrombocytopenia. J Clin Lab Anal.2022;36:e24201. doi: 10.1002/jcla.24201
Wei Wang and Xuewen Xu have contributed equally to this work.
Funding information
This study was funded by the Innovation Group Project of Shanghai Municipal Health Commission [2019CXJQ03]
DATA AVAILABILITY STATEMENT
All data included in this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Elias S, Kol I, Kahlon S, et al. Anti‐RhD antibody therapy modulates human natural killer cell function. Haematologica. 2021;106(7):1846‐1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quast I, Keller CW, Maurer MA, et al. Sialylation of IgG Fc domain impairs complement‐ dependent cytotoxicity. J Clin Invest. 2015;125(11):4160‐4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuhns S, Shu J, Xiang C, et al. Differential influence on antibody dependent cellular phagocytosis by different glycoforms on therapeutic Monoclonal antibodies. J Biotechnol. 2020;317:5‐15. [DOI] [PubMed] [Google Scholar]
- 4. Jo M, Kwon HS, Lee KH, et al. Engineered aglycosylated full‐length IgG Fc variants exhibiting improved FcgammaRIIIa binding and tumor cell clearance. Mabs. 2018;10(2):278‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peschke B, Keller CW, Weber P, et al. Fc‐galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement‐dependent cytotoxicity. Front Immunol. 2017;8:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonneveld ME, de Haas M, Koeleman C, et al. Patients with IgG1‐anti‐red blood cell autoantibodies show aberrant Fc‐glycosylation. Sci Rep. 2017;7(1):8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vučković F, Krištić J, Gudelj I, et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015;67(11):2978‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Šimurina M, de Haan N, Vučković F, et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology. 2018;154(5):1320‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su Z, Xie Q, Wang Y, et al. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ‐ESI‐MS. Int J Mol Sci. 2020;21(6):2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sjowall C, Zapf J, von Löhneysen S, et al. Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus. 2015;24(6):569‐581. [DOI] [PubMed] [Google Scholar]
- 11. Fokkink WJR, Selman MHJ, Dortland JR, et al. IgG Fc N‐glycosylation in Guillain‐Barré syndrome treated with immunoglobulins. J Proteome Res. 2014;13(3):1722‐1730. [DOI] [PubMed] [Google Scholar]
- 12. Cvetko A, Kifer D, Gornik O, et al. Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines. 2020;8(10):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou C, Huang C, Yan L, et al. Serum N‐glycan profiling as a diagnostic biomarker for the identification and assessment of psoriasis. J Clin Lab Anal. 2021;35(4):e23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kodama H, Yoneyama T, Tanaka T, et al. N‐glycan signature of serum immunoglobulins as a diagnostic biomarker of urothelial carcinomas. Cancer Med. 2021;10(4):1297‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee JW, Lee K, Ahn SH, et al. Potential of MALDI‐TOF‐based serum N‐glycan analysis for the diagnosis and surveillance of breast cancer. Sci Rep. 2020;10(1):19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conroy LR, Stanback AE, Young LEA, et al. In situ analysis of n‐linked glycans as potential biomarkers of clinical course in human prostate cancer. Mol Cancer Res. 2021. Jun 15. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu J, Deng J, Chen YK, et al. Study on the glycosylation specificity of the IgG antibody in ITP. Guangzhou Medical Journal. 2016;47(2):27‐29. [Google Scholar]
- 18. Schmidt DE, de Haan N, Sonneveld ME, et al. IgG‐Fc glycosylation before and after rituximab treatment in immune thrombocytopenia. Sci Rep. 2020;10(1):3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thrombosis and Hemostasis Group , Chinese Society of Hematology , Chinese Medical Association . Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). Chin J Hematol. 2020;41(8): 617‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang M, Zhao YP, Zhou FG, et al. N‐glycan based models improve diagnostic efficacies in hepatitis B virus‐related hepatocellular carcinoma. Int J Cancer. 2010;127(1):148‐159. [DOI] [PubMed] [Google Scholar]
- 21. Liu XE, Desmyter L, Gao CF, et al. N‐glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46(5):1426‐1435. [DOI] [PubMed] [Google Scholar]
- 22. Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N‐linked oligosaccharide improves binding to human Fcgamma RIII and antibody‐dependent cellular toxicity. J Biol Chem. 2002;277(30):26733‐26740. [DOI] [PubMed] [Google Scholar]
- 23. Kapur R, Kustiawan I, Vestrheim A, et al. A prominent lack of IgG1‐Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123(4):471‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bakchoul T, Walek K, Krautwurst A, et al. Glycosylation of autoantibodies: insights into the mechanisms of immunethrombocytopenia. Thromb Haemost. 2013;110(6):1259‐1266. [DOI] [PubMed] [Google Scholar]
- 25. Lardinois OM, Deterding LJ, Hess JJ, et al. Immunoglobulins G from patients with ANCA‐ associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS One. 2019;14(2):e0213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Ma S, Shao L, et al. Inflammation‐Related Gene Polymorphisms Associated With Primary Immune Thrombocytopenia. Front Immunol. 2017;8:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All data included in this study are available from the corresponding author on reasonable request.


