Abstract
MRL Diagnostics and Meridian Diagnostics have recently designed herpes simplex virus type 2 (HSV-2)-specific enzyme immunoassays for HSV-2 antibody detection. Blood donor sera were assayed for HSV-2 antibodies by both methods. The sensitivity, specificity, and efficiency were 97.9, 95.4, and 95.9% for the MRL assay and 83.2, 98.2, and 95.5% for the Meridian assay, respectively.
Detection of disease caused by herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) has been complicated by the lack of availability of consistently good diagnostic testing. Although culture is definitive in making a diagnosis, the timing of culture is critical for success. Culture during periods of active disease produces optimal recovery rates (6, 8). Reliance on culture for the detection of genital ulcer disease caused by herpes simplex viruses may result in underdiagnosis of the condition (7). Direct antigen detection techniques are not reliable since they appear to be only 50% as sensitive as optimal viral isolation procedures (5). Although PCR detection of viral shedding has been described as a much more sensitive method than culture for the detection of viral shedding, this method is not currently available for clinical diagnosis (2). Supplementing culture with direct fluorescent antibody staining specific for HSV-1 or HSV-2 may yield a diagnosis even when the culture is negative (12), but many institutions do not offer this method of diagnostic testing. Other diagnostic tests, including Pap smears, Giemsa-stained preparations, and many of the point-of-care antigen detection assays, do not differentiate between HSV-1 and HSV-2 infections. Since the type of HSV implicated in disease has ramifications for prognosis (9, 14), it is important to specify the HSV subtype.
Early application of type-specific serologic testing for HSV-1 and HSV-2 has been shown to be of benefit in testing first-time, recurrent, and asymptomatic infections as a means to definitive diagnosis and appropriate patient and spouse counseling (10). A seronegative status may be seen in patients with acute infection or in those at risk for acquiring infection, while a seropositive status is seen in patients with latent or recurrent infections. Until recently, enzyme immunoassays (EIAs) utilized either whole virus antigen preparations or type-specific antigenic determinants with extensive HSV-1 and HSV-2 immunologic response cross-reactivity (4). Since HSV-1 and HSV-2 share many common antigenic determinants (11, 13), these assays cannot be used reliably to differentiate HSV-1- from HSV-2-infected individuals. Western blot assays, although capable of differentiating antibodies against HSV-1 and -2, are expensive and are not readily available to most clinical laboratories (1). The identification of type-specific glycoproteins G1 (gG1; HSV-1-specific antigen) and G2 (gG2; HSV-2-specific antigen) led to the development of bulk protein production for type-specific assays. Recently, two manufacturers, MRL Diagnostics Inc. (Cincinnati, Ohio) and Meridian Diagnostics (Cypress, Calif.) have made available Food and Drug Administration-approved HSV-2 type-specific EIA kits for use in clinical laboratories. No head-to-head comparative testing of these two assays has been performed until now.
Serum from 532 blood donor specimens was obtained from the Central Kentucky Blood Center, Lexington, Ky., and frozen in 2-ml aliquots at −70°C until testing. Serologic evaluation of HSV-2 antibodies was performed using glycoprotein G2 type-specific EIA techniques. Assay kits from Meridian Diagnostics Inc. utilized 100 μl of a 1:21 dilution of serum for inoculation into gG2-coated wells in a 96-well plate with incubations at 37°C. Assay kits from MRL Diagnostics utilized 100 μl of a 1:101 dilution of serum inoculated into gG2-coated wells in a 96-well plate with incubations at room temperature. The assays were performed according to the manufacturers' specifications. Absorbed antibodies were quantitated using an automated ELx800 universal microplate reader (Bio-Tek Instruments Inc., Winooski, Vt.) at a 405-nm wavelength for the Meridian assays and a 450-nm wavelength for the MRL assays. For both assays, absorbance cutoff values were those established by validation studies with a mean absorbance value. Those with greater than 0.99 times the reference absorbance value were interpreted as positive, those with 0.91 to 0.99 times the reference absorbance value were interpreted as equivocal, and those with less than 0.91 times the reference absorbance value were interpreted as negative. All samples whose results by both tests were in agreement were interpreted as true positives or true negatives for the assays. Fifty-three (10%) of all concurring HSV-2 results (42 HSV-2 negative and 11 HSV-2 positive) were confirmed by immunoblotting.
Discordant results obtained using the two manufacturers' kits were resolved using the MRL HSV-1 and HSV-2 immunoblot immunoglobulin G (IgG) assay as the definitive diagnostic test method. For this assay, donor antibodies were bound to the HSV common antigen, gG1, and gG2, which were immobilized on nitrocellulose membrane strips. The bound antibodies were visualized using alkaline phosphatase-conjugated goat anti-human IgG reacted with bromo-chloro-indolyl phosphatase and nitroblue tetrazolium for color development. The resulting band reactivity was then interpreted by comparison to the gG2 antigen control band staining intensity. In order for the assay to be interpretable, the IgG control band had to be identifiable. In addition, the presence of the HSV common antigen band was required for a positive interpretation of the gG1 and gG2 band staining.
All 532 blood donor specimens were tested using both the MRL Diagnostics and the Meridian Diagnostics HSV-2-specific assays. Of those tested, 409 (76.9%) were negative by both assays, while 77 (14.5%) were positive by both assays. Forty-two of the repeatedly positive specimens and 11 of the repeatedly negative specimens were randomly selected for testing by HSV-1 and HSV-2 recombinant immunoblotting. The results for all 42 of the positive specimens and all 11 of the negative specimens found to be concordant by EIA were confirmed by this third assay. Forty-six specimens produced discrepant results on initial EIA testing. Each discordant specimen was tested by immunoblot assay, which was considered the “gold standard” for interpretation. The results of this testing are summarized in Table 1.
TABLE 1.
HSV-2 EIA discrepant results
| Assay result
|
Discrepancy classificationb | No. of specimens with discrepancy results | Comment | ||
|---|---|---|---|---|---|
| MRL | Meridian | Resolveda | |||
| − | + | + | MRL FN | 2 | |
| + | − | + | Meridian FN | 16 | |
| + | − | − | MRL FP | 7 | |
| + | − | − | MRL FP | 8 | Very weak gG2 on blot |
| + | − | − | MRL FP | 4 | No common antigen |
| EQc | − | − | MRL FP | 1 | No common antigen |
| − | + | − | Meridian FP | 7 | |
| − | EQ | − | Meridian FP | 1 | |
Result resolved by immunoblot assay.
FN, false negative; FP, false positive.
EQ, equivocal result.
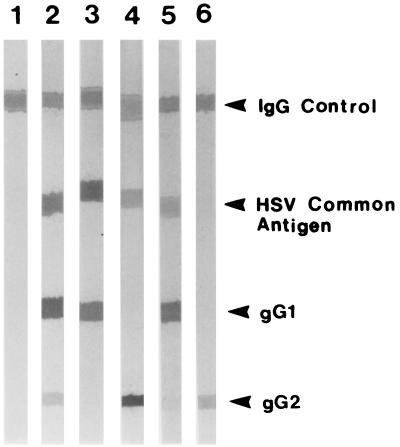
The Meridian Diagnostics kit produced 79 true-positive (14.8%), 429 true-negative (80.6%), 16 false-negative (3.0%), and 8 false-positive (1.5%) test results. One of the eight false-positive test results was equivocal. The MRL Diagnostics assay had 93 true-positive (17.4%), 417 true-negative (78.4%), and only 2 false-negative (0.4%) results but produced 20 false-positive results (3.8%), including 1 equivocal. Analysis of the immunoblot staining patterns for the MRL false-positive specimens revealed some interesting results (Fig. 1). Seven of the 20 MRL false positives (35% of the false positives; 1.3% of all specimens tested) produced no HSV-2-specific band in immunoblots (Fig. 1, lanes 1 and 3). Six of these seven specimens were seropositive for HSV-1. An additional eight specimens (40% of the false positives; 1.5% of all specimens tested) stained for antibodies at both the common antigen band and the gG2 band, but the gG2 band staining intensity was insufficient to permit a positive interpretation according to the manufacturer's guidelines (Fig. 1, lane 5). All eight of these specimens demonstrated HSV-1-specific gG1 band staining. Five specimens (25% of the false positives; 0.9% of all specimens tested) produced distinct HSV-2 gG2 bands but lacked antibody binding to the HSV common antigen band (Fig. 1, lane 6).
FIG. 1.
Immunoblot confirmatory testing for HSV-1 and HSV-2. Lane 1, negative control. This specimen is negative for HSV-1 and HSV-2 antibodies. The IgG band is clearly demonstrated and indicates that the kit reagents have worked properly. Lane 2, HSV-1- and HSV-2-seropositive control. The specimen demonstrates staining for the IgG and HSV common antigen bands in addition to that for the gG1 and gG2 bands. The staining intensity of the gG2 band in this positive control demonstrates the minimum band intensity required for a specimen to be considered seropositive. Lane 3, HSV-1-seropositive and HSV-2-seronegative donor specimen. Lane 4, HSV-1-seronegative and HSV-2-seropositive donor specimen. Lane 5, MRL HSV-2 false positive. Despite its presence, the gG2 band in this specimen does not exhibit staining intensity equal to or greater than that of the gG2 band in the positive control (see lane 2). This specimen must therefore be interpreted as seronegative for HSV-2. All such specimens seen were HSV-1 seropositive. Lane 6, MRL HSV-2 false positive. Despite the very pronounced presence of a gG2 band, this specimen is defined as negative for HSV-2 antibodies since it lacks staining for the HSV common antigen band.
The diagnostic sensitivity, specificity, positive and negative predictive values, and overall efficiency of the assays were calculated using the equations defined by Bayes' theorem (15). The equations and the calculated values are summarized in Table 2.
TABLE 2.
Performance characteristics of Meridian and MRL HSV-2 type-specific EIAsa
| Assay | Sensitivity
|
Specificity
|
Negative predictive value
|
Positive predictive value
|
Efficiency
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | No. of specimens [TP/(TP + FN)] | % | No. of specimens [TN/(TN + FP)] | % | No. of specimens [TN/(TN + FN)] | % | No. of specimens [TP/(TP + FP)] | % | No. of specimens [(TP + TN)/(TP + TN + FP + FN)] | |
| Meridian | 83.2 | 79/(79 + 16) | 98.2 | 429/(429 + 8) | 96.4 | 429/(429 + 18) | 90.8 | 79/(79 + 8) | 95.5 | (79 + 429)/532 |
| MRL | 97.9 | 93/(93 + 2) | 95.4 | 417/(417 + 20) | 99.5 | 417/(417 + 2) | 82.3 | 93/(93 + 20) | 95.9 | (93 + 417)/532 |
TP, true positive; FP, false positive; TN, true negative; FN, false negative.
The HSV-2 EIAs from the two companies demonstrated very different performance characteristics. While the Meridian kit demonstrated excellent specificity (98.2%), its sensitivity was lacking (83.2%). Sixteen of the 95 positive samples (16.8% of the true positives) were missed by this assay. In comparison, the MRL test demonstrated good specificity (95.4%) and excellent sensitivity (97.9%).
Still, there were 20 false positives (3.8% of all specimens tested) found with the MRL HSV-2 kit. Seven specimens (1.3% of all specimens tested) lacked any evidence of gG2 band staining, with six of these specimens demonstrating HSV-1 antibodies. Although a faint band at the specific gG2 site was seen for 8 of the 20 false-positive specimens (40% of the MRL false positives), the staining intensity was insufficient to be considered truly positive according to the immunoblot package insert instructions. While these bands may represent low-level positivity for anti-HSV-2 antibodies, they might also represent nonspecific antibody binding, since all of these specimens also had anti-HSV-1 antibodies. An additional five specimens (0.9% of all tested) demonstrated a band at gG2 but lacked the HSV common antigen band required for a positive interpretation. In a discussion with technical assistance personnel at MRL, it was confirmed that approximately 1% of all the specimens they have assayed have produced this same result, representing an apparent cross-reactivity to a non-HSV-2 antigenic stimulant. The findings of the current study are in agreement with those of the MRL technical personnel, with 0.9% of our specimens demonstrating this type of false-positive test result.
Any HSV-2 EIA that produces a significant number of false-positive results will have a negative impact on patients from both a social and an emotional standpoint. Extreme care must be taken when reporting test results, especially when the outcome is to assign a diagnosis of an incurable sexually transmitted disease. The Centers for Disease Control and Prevention recommend that positive screening tests for other incurable diseases such as hepatitis C virus be confirmed using a second, very specific assay before reporting the results for a patient (3). It is certainly reasonable that a diagnosis of HSV-2 would also fall under this same recommendation.
While neither manufacturer's HSV-2 EIA test alone is ideal, the MRL EIA has superior performance characteristics and would serve as an excellent screening test due to its high sensitivity. Its lower specificity could be offset by the use of a good confirmatory test such as immunoblotting or Western blotting to identify the false-positive reactions. This combination of screening and confirmation would ensure optimal diagnostic sensitivity and specificity so that appropriate patient intervention and counseling could occur.
Acknowledgments
This work was supported by the Women's Health Initiative of the Chandler Medical Center Research Fund, University of Kentucky, Lexington.
REFERENCES
- 1.Ashley R L, Benedetti J, Corey L. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J Med Virol. 1985;17:153–166. doi: 10.1002/jmv.1890170208. [DOI] [PubMed] [Google Scholar]
- 2.Boggess K A, Watts H, Hobson A C, Ashley R L, Brown Z A, Corey L. Herpes simplex virus type 2 detection by culture and polymerase chain reaction and relationship to genital symptoms and cervical antibody status during the third trimester of pregnancy. Am J Obstet Gynecol. 1997;176:443–451. doi: 10.1016/s0002-9378(97)70513-1. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Morb Mortal Wkly Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 4.Coleman R M, Pereira L, Bailey P D, Dondero D, Wickliffe C, Nahmias A J. Determination of herpes simplex virus type-specific antibodies by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983;18:287–291. doi: 10.1128/jcm.18.2.287-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey L, Holmes K K. Genital herpes simplex virus infections: current concepts in diagnosis, therapy, and prevention. Ann Intern Med. 1983;98:973–983. doi: 10.7326/0003-4819-98-6-973. [DOI] [PubMed] [Google Scholar]
- 6.Guinan M E, MacCalman J, Kern E R, Overall J C, Spruance S L. The course of untreated recurrent genital herpes simplex infection in 27 women. N Engl J Med. 1981;304:759–763. doi: 10.1056/NEJM198103263041305. [DOI] [PubMed] [Google Scholar]
- 7.Koutsky L A, Stevens C E, Holmes K K, Ashley R L, Kiviat N B, Critchlow C W, Corey L. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N Engl J Med. 1992;326:1533–1539. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- 8.Krone M R, Tabet S R, Paradise M, Wald A, Corey L, Celum C L. Herpes simplex virus shedding among human immunodeficiency-negative men who have sex with men: site and frequency of shedding. J Infect Dis. 1998;178:978–982. doi: 10.1086/515666. [DOI] [PubMed] [Google Scholar]
- 9.Lafferty W E, Coombs R W, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection: influence of site of infection and viral type. N Engl J Med. 1987;316:1444–1449. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- 10.Munday P E, Vuddamalay J, Slomka M J, Brown D W G. Role of type specific herpes simplex virus serology in the diagnosis and management of genital herpes. Sex Transm Infect. 1998;74:175–178. doi: 10.1136/sti.74.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahmias A, Josey W E, Nabil Z M, Luce C F, Duffey A. Antibodies to Herpesvirus hominis types 1 and 2 in humans. I. Patients with genital herpetic infections. Am J Epidemiol. 1970;91:539–546. doi: 10.1093/oxfordjournals.aje.a121165. [DOI] [PubMed] [Google Scholar]
- 12.Nahmias A, DelBuno I, Pipkin J, Hutton R, Wickliffe C. Rapid identification and typing of herpes simplex virus types 1 and 2 by a direct immunofluorescence technique. Appl Microbiol. 1971;22:455–458. doi: 10.1128/am.22.3.455-458.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahmias A J, Dowdle W R. Antigenic and biologic differences in herpes virus hominis. Prog Med Virol. 1968;10:119–159. [PubMed] [Google Scholar]
- 14.Reeves W C, Corey L, Adams H G, Vontuer L A, Holmes K K. Risk of recurrence after first episodes of genital herpes: relation to HSV type and antibody response. N Engl J Med. 1981;305:315–319. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- 15.Winkel P, Statland B E. Interpreting laboratory results: reference values and decision making. In: Henry J B, editor. Clinical diagnosis and management by laboratory methods. 18th ed. Philadelphia, Pa: W. B. Saunders Co.; 1991. pp. 49–76. [Google Scholar]