Abstract
Background
In patients with RAS/BRAF wild-type metastatic colorectal cancer (mCRC), growing evidence supports anti-epidermal growth factor receptor (EGFR) retreatment, whereas little is known on the outcomes of anti-EGFR-based reinduction therapy during the upfront strategy.
Methods
We included patients enrolled in the Valentino study who had disease progression and received at least one dose of post-progression therapy. The Kaplan–Meier method and Cox proportional hazards regression were used for the survival analysis. When comparing the outcomes of anti-EGFR-based reinduction versus any second line, a propensity score–based matching was used.
Results
Liver-limited/single site of disease (P < .001 and P = .002), left-sidedness (P = .029), surgery of metastases (P = .003), early tumor shrinkage, and deeper responses (P = .018 and P = .036) were associated with the use of anti-EGFR-based reinduction versus any other second line. All patients treated with reinduction had an anti-EGFR-free interval of at least 3 months. In the propensity score–matched population, progression-free survival (PFS) was similar in the 2 treatment groups, the overall survival (OS) was significantly longer for patients treated with reinduction (P = .029), and the response rate was higher in patients treated with reinduction (P = .033). An oxaliplatin-free interval ≥12 months, left-sidedness, and molecular hyperselection beyond RAS/BRAF were associated with significantly better outcomes after anti-EGFR-based reinduction.
Conclusions
Reinduction strategies with anti-EGFR-based regimens are commonly used in clinical practice. Our data highlight the importance of clinical–molecular selection for re-treatments and the need for prospective strategy trials in selected populations.
Keywords: metastatic colorectal cancer, anti-EGFR, reinduction, chemotherapy
The optimal duration of an anti-EGFR-based first-line strategy is not well established for patients with RAS wild-type metastatic colorectal cancer (mCRC). This study assesses post-progression treatment outcomes in patients with RAS wild-type mCRC treated with initial FOLFOX-panitumumab followed by panitumumab-based maintenance in the frame of the Valentino trial.
Implications for Practice.
We aimed at assessing the post-progression treatment outcomes, with a special focus on anti-EGFR-based reinduction therapy, in patients with RAS wild-type mCRC treated with initial FOLFOX-panitumumab followed by panitumumab-based maintenance in the frame of the Valentino trial. In the subgroup of patients with doublet-free interval (DFI) >3 months and RAS/BRAF wild-type status, after applying a propensity score matching to compare patients receiving reinduction versus any anti-EGFR standard second-line therapy, we observed a similar PFS, but a significant OS and response rate difference in favor of reinduction therapy. This result suggests that the use of anti-EGFR-based reinduction in selected patients may allow achieving an optimal treatment sequencing and exploiting all available treatment options. Given the higher response rate observed, anti-EGFR-based reinduction may be considered when a tumor shrinkage is needed after the failure of the first-line therapy.
Introduction
In patients with RAS wild-type metastatic colorectal cancer (mCRC), the optimal duration of an anti-EGFR-based first-line strategy is not well established. The continuation of doublets plus an anti-epidermal growth factor receptor (EGFR) agent until disease progression is unfeasible in most patients because of cumulative toxicities—especially oxaliplatin-related neurotoxicity—and potential negative impact on quality of life.1 Post-induction strategies may be preplanned and vary from a full treatment holiday to deintensification with several maintenance options: single-agent anti- EGFRs, fluoropyrimidines, or their combination.2-6
Based on the on/off strategy adopted, several anti-EGFR-based re-treatment scenarios are feasible in clinical practice. Anti-EGFR “rechallenge” can be offered in later lines of therapy in patients with evidence of secondary resistance (ie, on-treatment disease progression [PD]) to prior anti-EGFR-based upfront therapy, followed by at least one line of intervening/non-cross resistant treatment to allow the decay of anti-EGFR-resistant tumor clones.7 When the anti-EGFR-based upfront strategy is stopped due to any reason other than PD (off-treatment PD), anti-EGFR treatment can be resumed after one or more intervening treatment lines in the chemorefractory setting (treatment “re-introduction”).8 Also, in patients treated with the first-line combination of chemotherapy plus an anti-EGFR agent, such a combinatorial regimen can be resumed after off-treatment PD during a treatment holiday (“reinduction” treatment). Notably, such “stop&go” anti-EGFR-based first-line strategies can be regarded as a reasonable option to potentially reduce continuous exposure to anti-EGFR agents and treatment-related skin toxicity, thus improving patients’ compliance and quality of life.1 However, whereas a growing body of evidence supports the re-treatment with anti-EGFR agents in later lines, little is known on the outcomes of anti-EGFR-based reinduction therapy as part of an upfront strategy.
Drawing from these considerations, we aimed at assessing post-progression treatment outcomes, with a special focus on anti-EGFR-based reinduction therapy, in patients with RAS wild-type mCRC treated with initial FOLFOX-panitumumab followed by panitumumab-based maintenance in the frame of the Valentino trial.
Methods
Study Population
The Valentino study (NCT02476045) was a multicenter, randomized, open-label phase II trial that investigated the progression-free survival (PFS) non-inferiority of maintenance with single-agent panitumumab (arm B) versus panitumumab plus 5-FU/LV (arm A) following an induction treatment with panitumumab plus FOLFOX-4 in patients with RAS wild-type unresectable mCRC.3 The trial enrolled 229 (arm A/B: 117/112) patients. For this exploratory analysis, we included all randomized patients who had a disease progression event and received at least one dose of any post-progression therapy at investigators’ discretion and as per clinical practice. Early tumor shrinkage (ETS) and depth of response (DoR) were centrally reviewed as previously reported.9 Extended molecular data beyond RAS and BRAF mutational status—the “PRESSING panel” including HER2/MET amplifications, gene fusions, PIK3CA/PTEN mutations, RAS mutations with low mutant allele fraction, and microsatellite instability—were retrospectively assessed as previously reported.10 Institutional review board and ethics committee approval was obtained from all participating centers. All the patients provided written informed consent before any study-related procedures. The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
PFS was defined as the time from treatment initiation to disease progression or death from any cause. Overall survival (OS) was defined as the from treatment initiation to death from any cause. In the absence of events, PFS and OS times were censored at the last date when the patients were known to be alive. Time to progression (TTP) in the Valentino trial was defined as the time from randomization to disease progression. Doublet-free interval (DFI) in the Valentino trial was defined as the time from the last dose administration of the induction regimen to disease progression. Anti-EGFR-free interval was defined as the time from last dose administration of panitumumab in the Valentino trial to disease progression. To examine differences between groups, the Chi-square test or the Fisher exact test were used for categorical variables, as appropriate, whereas the nonparametric Kruskal–Wallis test was used for continuous variables. To summarize continuous variables, the median value with the corresponding range and/or interquartile range (IQR) were provided. The reverse Kaplan–Meier method was used for follow-up time assessment. The Kaplan–Meier method and Cox proportional hazards regression were used for survival analysis. Logistic regression was used to assess the probability of experiencing a RECIST response (ie, complete response [CR]+partial response [PR] as per RECIST v.1.1) conditional on the post-progression treatment.
To deal with systematic differences in the distribution of baseline characteristics when comparing survival outcomes of patients receiving post-progression reinduction and those receiving post-progression second line, a propensity score–based approach (propensity score matching analysis using the nearest neighbor method with a 1:1 ratio and a caliper of 0.1) was used with the aim of minimizing the effects of confounding factor.11 Stratification on the matched pairs was used to estimate the treatment effect on survival outcomes in the propensity score matching analysis. Conditional logistic regression was used in the propensity score matching analysis to estimate the treatment effect. As a sensitivity analysis, the effect of the treatment group on survival outcomes and tumor response was assessed in the non-matched (raw) population. A maximally selected rank statistics method applied to OS was used to define an optimal cut-off value to stratify patients who received a reinduction based on the DFI.12
The threshold for statistical significance was set to a P-value of .05 and all statistical tests were 2-sided. Statistical analyses were performed using the R software (version 3.5.0) and the survival, survminer, maxstat, dplyr, and MatchIt packages.
Results
Patients’ Characteristics
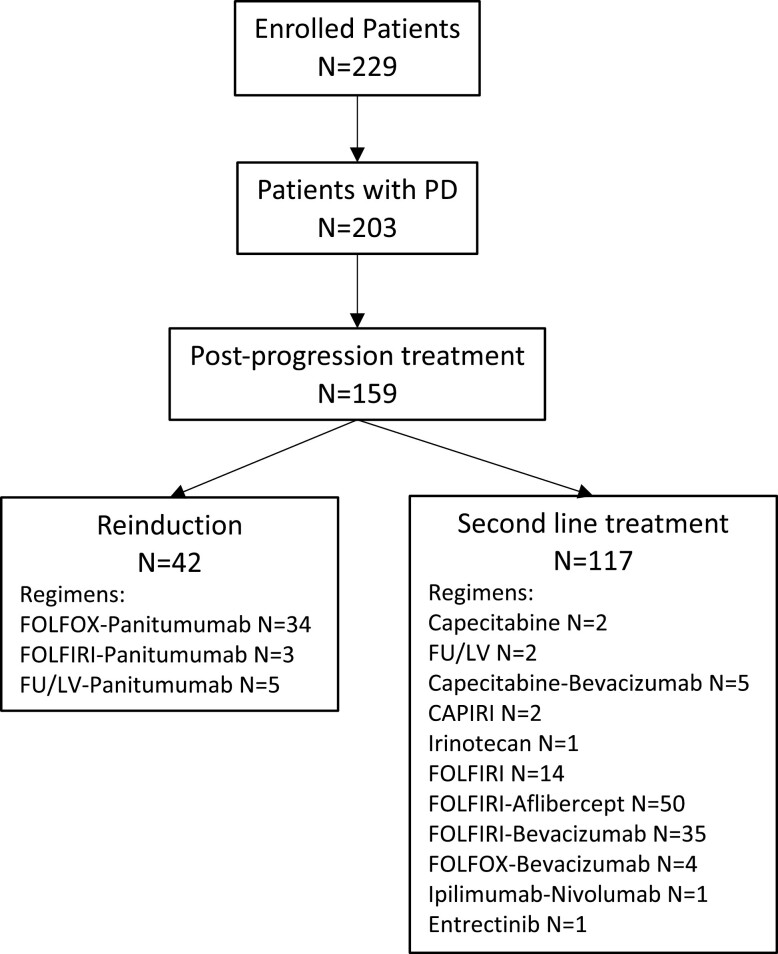
At the data cut-off date of January 14, 2021, and at a median follow-up of 48.4 months (IQR: 42.2-54.2), a total of 203 patients experienced a PD on or after study therapy and 159 of them (78.3%) received a post-progression systemic treatment: among these, 42 patients (26.4%) received an anti-EGFR-based reinduction, whereas 117 patients (73.6%) received an anti-EGFR-free standard second-line treatment. The diagram of patients’ flow is illustrated in Figure 1. Notably, 5 patients in the reinduction group were treated with FU/LV plus panitumumab alone due to performance status worsening/patients’ preferences, whereas 3 switched to FOLFIRI/panitumumab due to oxaliplatin-related allergic reactions or grade 2-3 neurotoxicity. When looking at the baseline characteristics prior to randomization, patients with liver-limited disease (P < .001), a single metastatic site (P = .002), and left-sided tumors (P = .029) received more frequently an anti-EGFR-based reinduction therapy (Table 1). An anti-EGFR-free interval of at least 3 months was observed in 100% of patients in the reinduction group and in 29 out of 117 patients (24.8%) receiving alternative second-line regimens (P < .001). Although the prevalence of patients with BRAF mutations was quite low in the study dataset, none of these received a reinduction. When analyzing on-treatment post-randomization outcomes, we observed that surgery of metastases (P = .003) and ETS (P = .018) were associated with the subsequent use of an anti-EGFR-based reinduction therapy (Table 1). Furthermore, patients who received an anti-EGFR-based reinduction had shown a better median DoR to the initial strategy compared with patients who received an anti-EGFR-free standard second-line treatment (P = .036).
Figure 1.
Flow chart showing the process of patients’ selection.
Table 1.
Patients’ characteristics according to the post-progression treatment group.
| Characteristics | Non-anti-EGFR-based second-line (N = 117) N (%) | Anti-EGFR-based reinduction (N = 42) N (%) | P a |
|---|---|---|---|
| Baseline | |||
| Age, years | .247 | ||
| Median | 64 | 61 | |
| IQR | 55-70 | 51-69 | |
| Sex | .445 | ||
| Female | 41 (35.0) | 12 (28.6) | |
| Male | 76 (65.0) | 30 (71.4) | |
| ECOG PS | .827 | ||
| 0 | 90 (76.9) | 33 (78.6) | |
| 1 | 27 (23.1) | 9 (21.4) | |
| Prior adjuvant treatment | .672 | ||
| No | 97 (82.9) | 36 (85.7) | |
| Yes | 20 (17.1) | 6 (14.3) | |
| Primary tumor resected | .091 | ||
| No | 36 (30.8) | 19 (45.2) | |
| Yes | 81 (69.2) | 23 (54.8) | |
| Liver-limited disease | <.001 | ||
| No | 83 (70.9) | 16 (38.1) | |
| Yes | 34 (29.1) | 26 (61.9) | |
| Synchronous metastases | .336 | ||
| No | 31 (26.5) | 8 (19.0) | |
| Yes | 86 (73.5) | 34 (81.0) | |
| Metastatic sites, N | .002 | ||
| 1 | 57 (48.7) | 32 (76.2) | |
| >1 | 60 (51.3) | 10 (23.8) | |
| Primary tumor sidedness | .029 | ||
| Left | 95 (81.2) | 40 (95.2) | |
| Right | 22 (18.8) | 2 (4.8) | |
| BRAF mutation | .326 | ||
| No | 112 (95.7) | 42 (100) | |
| Yes | 5 (4.3) | 0 (0) | |
| PRESSING panel | .092 | ||
| Negative | 74 (67.9) | 32 (82.1) | |
| Positive | 35 (32.1) | 7 (17.9) | |
| Not available | 8 | 3 | |
| Treatment arm | .455 | ||
| A | 59 (50.4) | 24 (57.1) | |
| B | 58 (49.6) | 18 (42.9) | |
| On-treatment | |||
| Secondary resection | .003 | ||
| No | 104 (88.9) | 29 (69.0) | |
| Yes | 13 (11.1) | 13 (31.0) | |
| ETS | .018 | ||
| No | 45 (41.3) | 7 (19.4) | |
| Yes | 64 (58.7) | 29 (80.6) | |
| Not applicable | 8 | 6 | |
| DoR | .036 | ||
| Median | −44.5 | −50.1 | |
| IQR | −54 to −21.5 | −61.7 to −40.5 | |
| Not applicable | 8 | 6 | |
| Anti-EGFR-free interval ≥ 3 months | <.001 | ||
| No | 88 (75.2) | 42 (100%) | |
| Yes | 29 (24.8) | 0 (0%) |
Bold values denote statistical significance.
Abbreviations: DoR, depth of response; ETS, early tumor shrinkage; IQR, interquartile range.
TTP to first-line strategy in the Valentino trial was significantly longer for patients who subsequently received an anti-EGFR-based reinduction (median: 15.6 months, IQR: 9.7-22.5) compared with patients who received any second-line treatment (median: 10.0 months, IQR: 6.9-14.2, Kruskal–Wallis test P < .001). Similarly, patients receiving a reinduction experienced a longer DFI (median: 11.5 months, IQR: 5.4-18.5, range: 3.1-37.7) compared with patients receiving a second-line treatment (median: 5.0 months, IQR: 2.2-9.3, range: 0.1-22.9, Kruskal–Wallis test P < .001).
Clinical Outcomes According to Post-progression Treatment
To properly compare the survival outcomes of patients treated with reinduction versus second-line therapy, we excluded the patients in the second-line therapy cohort with BRAF-mutated tumors and/or with a DFI less than 3 months (total patients in the second-line cohort considered: 75). Table 2 reports the exposure to the different agents received in further lines of treatment after the progression to the anti-EGFR-based reinduction or to the non-anti-EGFR-based second line. The post-progression median follow-up was 29.5 months (IQR: 22.7-37.7).
Table 2.
Agents received in further lines according to post-progression treatment group.
| Agent | Non-anti-EGFR-based second-line (N = 75)aN (%) | Anti-EGFR-based reinduction (N = 42) N (%) | P b |
|---|---|---|---|
| Irinotecan | <.001 | ||
| No | 60 (80.0) | 20 (47.6) | |
| Yes | 15 (20.0) | 22 (52.4) | |
| Oxaliplatin | .709 | ||
| No | 69 (92.0) | 40 (95.2) | |
| Yes | 6 (8.0) | 2 (4.8) | |
| Fluoropyrimidine | <.001 | ||
| No | 57 (76.0) | 18 (42.9) | |
| Yes | 18 (24.0) | 24 (57.1) | |
| Bevacizumab or Aflibercept | <.001 | ||
| No | 68 (90.7) | 22 (52.4) | |
| Yes | 7 (9.3) | 20 (47.6) | |
| Later anti-EGFR retreatment | .825 | ||
| No | 55 (73.3) | 30 (71.4) | |
| Yes | 20 (26.7) | 12 (28.6) | |
| Regorafenib | .212 | ||
| No | 57 (76.0) | 36 (85.7) | |
| Yes | 18 (24.0) | 6 (14.3) | |
| Trifluridine/Tipiracil | .222 | ||
| No | 53 (70.7) | 34 (81.0) | |
| Yes | 22 (29.3) | 8 (19.0) | |
| Other | .124 | ||
| No | 62 (82.7) | 39 (92.9) | |
| Yes | 13 (17.3) | 3 (7.1) |
Patients with BRAF wild-type tumors and with a DFI ≥ 3 months.
Bold values denote statistical significance.
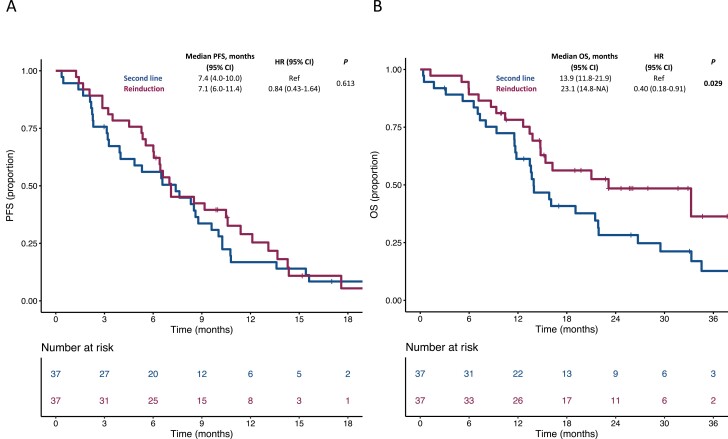
We then used a 1:1 propensity score–based matching considering the randomly allocated maintenance treatment arm, previous adjuvant therapy, primary tumor sidedness, and secondary resection of metastases with curative intent for the estimation of the propensity score. A total of 37 patients for each treatment cohort were matched. The distribution of propensity scores before and after matching in the 2 cohorts is illustrated in Supplementary Figure S1. In the propensity score–matched population, PFS was similar in patients treated with anti-EGFR-based reinduction (median: 7.1, 95% confidence interval [CI]: 6.0-11.4) compared with patients receiving any second-line therapy (median: 7.4, 95% CI: 4.0-10.0; hazard ratio [HR]: 0.84, 95% CI: 0.43-1.64; P = .613; Figure 2A). Conversely, OS was significantly longer for patients treated with reinduction (median: 23.1, 95% CI: 14.8-NA) compared with patients receiving any second-line therapy (median: 13.9, 95% CI: 11.8-21.9; HR: 0.40, 95% CI: 0.18-0.91; P = .029; Figure 2B). Similar results were observed in the sensitivity analyses in the unmatched population (Supplementary Figure S2).
Figure 2.
Kaplan–Meier estimates for PFS (A) and OS (B) in the propensity score–matched population according to post-progression treatment. Blue lines indicate patients receiving any second-line therapy, whereas purple lines indicate patients receiving an anti-EGFR-based reinduction. OS, overall survival; PFS, progression-free survival.
In terms of anti-tumor activity, a higher response rate was observed in the propensity score–matched population for patients receiving anti-EGFR-based reinduction (18/37, 48.6%) compared with patients receiving any second line (8/37, 21.6%; odds ratio [OR]: 3.00, 95% CI: 1.09-8.25; P = .033). Similar results were observed in the sensitivity analysis in the unmatched population (21/42, 50% vs 17/75, 22.7%; OR: 3.41, 95% CI: 1.52-7.68; P = .003).
Survival Outcomes of Anti-EGFR-based Reinduction in Specific Subgroups
We then focused on the subgroup of patients treated with anti-EGFR-based reinduction therapy. No differences in survival outcomes (Supplementary Figure S3) or response rate (P > .999) were observed according to the randomly allocated maintenance treatment arm in the Valentino study.
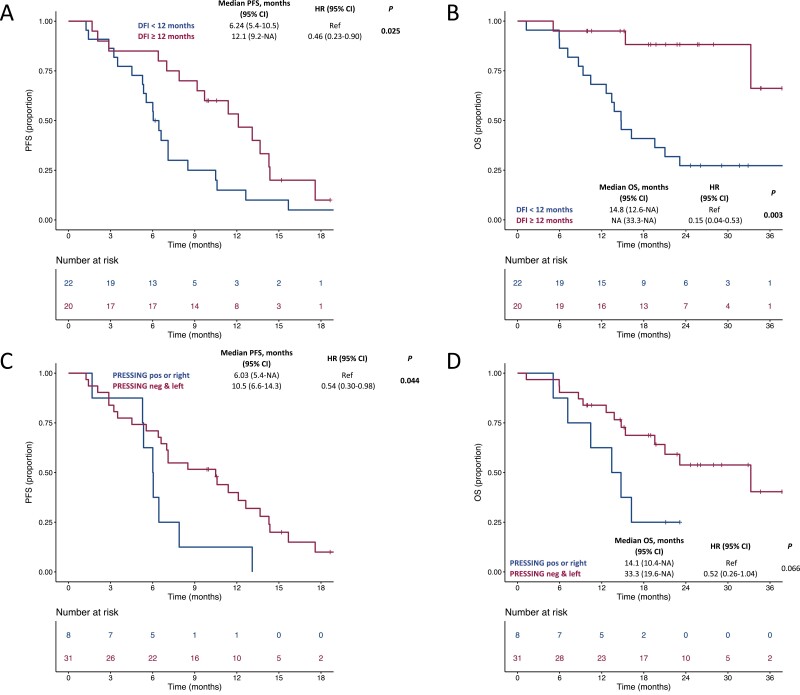
We first explored if the magnitude of the DFI was associated with better survival outcomes to the reinduction of the anti-EGFR-based therapy, and observed a 15% decrease in the hazard of PFS every 3 months increase in DFI (HR: 0.85, 95% CI 0.74-0.97; P = .016 and a 27% decrease in the hazard of OS every 3 months increase in DFI (HR: 0.73, 95% CI: 0.57-0.94; P = .014). We then sought to determine an optimal cut-off for DFI. The optimal cut-off value identified was 11.7 months (Supplementary Figure S4). Therefore, we divided the patients into 2 cohorts based on a DFI <12 months versus ≥ 12 months. Patients with a DFI ≥12 months experienced a better PFS (HR: 0.46, 95% CI: 0.23-0.90; P = .025) and OS (HR: 0.15, 95% CI: 0.04-0.53; P = .003; Figure 3A and B, respectively) in comparison to patients with an DFI <12 months. No difference in terms of response rate was observed according to DFI (P = .537). We also explored the association of survival outcomes to the reinduction of the anti-EGFR-based therapy with an extended molecular status beyond RAS and BRAF (PRESSING status) and sidedness. In patients with PRESSING negative and left-sided tumors, we observed a better PFS (HR: 0.54, 95% CI: 0.30-0.98; P = .044) as well as a trend toward a better OS (HR: 0.52, 95% CI: 0.26-1.04; P = .066) in comparison to patients with PRESSING positive or right-sided tumors (Figure 3C and D, respectively). No difference in terms of response rate was observed according to PRESSING status and sidedness (P = .480).
Figure 3.
Kaplan–Meier estimates for PFS and OS in patients receiving an anti-EGFR-based reinduction according to DFI (panels A and B, respectively) and PRESSING/sidedness (panels C and D, respectively). In panels A and B, blue lines indicate patients with a DFI<12 months, whereas purple lines indicate patients with a DFI ≥12 months. In panels C and D, blue lines indicate patients with PRESSING positive or right-sided tumors, whereas purple lines indicate patients with PRESSING negative and left-sided tumors. DFI, doublet-free interval; OS, overall survival; PFS, progression-free survival.
Discussion
In this post-hoc analysis of the Valentino study carried out in patients with RAS wild-type mCRC treated with an anti-EGFR-based upfront strategy, the use of an anti-EGFR-based reinduction after a non-preplanned treatment holiday was restricted to BRAF wild-type disease and almost exclusively in left-sided tumors. Notably, these clinical–molecular criteria are commonly used in clinical practice to select the optimal candidates for EGFR inhibition. Moreover, when compared with any other second-line therapy, the use of reinduction was adopted only in patients with an anti-EGFR-free treatment interval of at least 3 months and was more frequent in patients with on-treatment evidence of greater benefit from the initial strategy (ie, those with ETS, higher DoR, longer TTP to first-line strategy, and resection of metastases). Although such parameters are well-known good prognostic factors,9 it must be pointed out that a recent study failed to show any association of such on-treatment markers of efficacy with the outcome of either anti-EGFR re-introduction or rechallenge in later lines.8 Despite such evidence, we acknowledge that the promising OS achieved after the start of reinduction, as well as the observed response rate of 48.6% observed in the propensity score–matched population, may have been deeply impacted by the selection of patients with greater sensitivity to both chemotherapy and targeted therapy. On the contrary, the choice of non-cross-resistant second-line regimen, mostly FOLFIRI with or without antiangiogenic agents, may be driven by resistance to the specific drugs used in the first-line strategy. The retrospective nature of our work prevents us from drawing conclusions about the different efficacy of reinduction versus any anti-EGFR-free second-line therapy. In fact, the lack of randomization did not allow to exclude that the clinical outcomes reported with administered therapies might have been affected by unbalances in prognostic factors. Although acknowledging these biases, to compare more properly the outcomes of the 2 post-progression treatment groups, we selected only RAS and BRAF wild-type patients with DFI ≥3 months (thus mirroring a scenario of retained treatment sensitivity) and we applied a propensity score–based matching considering the variables related to treatment sensitivity and prior surgery of metastases after induction.
Our results showed a lack of significant differences between the 2 groups in terms of PFS but a clinically meaningful and statistically significant OS and response rate difference in favor of reinduction therapy. Such a discrepancy may reflect, as already highlighted, selection biases related to the specific treatment choices and therefore should be interpreted with caution. As a possible explanation, the use of anti-EGFR-based reinduction in selected patients may allow maximizing the efficacy of the first-line strategy and optimizing the treatment sequences, with the option of irinotecan/anti-VEGF-based non-cross-resistant regimens after failure of reinduction. Notably, about half of the patients treated with reinduction were able to receive irinotecan-based chemotherapy plus antiangiogenic drugs during the subsequent disease course. Finally, given the higher response rate observed after anti-EGFR reinduction and the relatively low response rate to other available second-line options, the former strategy may be chosen when a tumor shrinkage is needed after the first disease progression (eg, high disease burden, symptomatic progression, goal of repeated surgery of metastases).
The hallmark retrospective analysis of the FIRE-3 study showed that the treatment sequences might be more important than overall exposure to single agents and, in patients with RAS wild-type mCRC, the strategy of upfront anti-EGFR-based therapy followed by an anti-VEGF-based one may maximize the survival outcomes.13 However, such a strategy is not fully generalizable to the subgroup of patients clinically eligible for reinduction. In parallel, oxaliplatin-based chemotherapy reinduction is a valuable strategy in the upfront treatment algorithm.14,15 This option is valid also when bevacizumab-based regimens are used in the context of a “stop&go” chemotherapy strategy associated with sustained angiogenesis inhibition.16,17
When focusing on the subgroup of patients with RAS and BRAF wild-type, left-sided mCRC treated with an anti-EGFR-based upfront therapy, how should clinicians select the optimal post-progression regimen? If the anti-EGFR-based therapy was discontinued—relatively early—in order to perform resection of metastases, reinduction may be a valuable option based on patients’ and disease characteristics and prior response patterns, potentially including pathological response in resected metastases.18 On the contrary, in patients with off-treatment PD during a treatment break or FU/LV maintenance, several options are available. If the reason for discontinuation of the anti-EGFR agent is a patient preference, severe skin toxicity, or impaired quality of life, then alternative regimens may be the most reasonable option.
In our work, significant longer post-reinduction PFS and OS were achieved in patients with DFI ≥12 months and PRESSING negative/left-sided cancers. Therefore, molecular hyperselection beyond RAS/BRAF and treatment-free intervals may help clinicians to choose an anti-EGFR-based reinduction in clinical practice. However, it should also be pointed out that the role of DFI and PRESSING panel were investigated retrospectively and these conclusions (despite being clinically sound) should be interpreted with caution. Notably, the lack of differences in response rate to reinduction according to PRESSING status and sidedness may be related to the high sensitivity to FOLFOX chemotherapy, as well as the small sample size and the lack of central review of post-study scans.
In our study, none of the patients treated with reinduction had on-treatment PD during the panitumumab-based maintenance. Indeed, the acquired resistance to anti-EGFR treatment is associated with the emergence of several genomic alterations (most commonly RAS/BRAF/EGFR ectodomain mutations), that are initially present in undetectable tumor subclones and then become predominant and clinically detectable under the selective pressure of EGFR inhibition.19,20 Consistently, the published phase II clinical trials investigating different anti-EGFR-based maintenance strategies (including the Valentino study) did not schedule the reinduction with the initial anti-EGFR-based regimen in case of PD during the maintenance phase. Recently, the Panama trial (NCT01991873), which scheduled FOLFOX/panitumumab reinduction following maintenance therapy with FU/LV with or without panitumumab, suggested that the effectiveness of this strategy is restricted to patients in the anti-EGFR-free arm with FU/LV monotherapy.
It has also been shown that resistant clones decay in a time-dependent fashion after an intervening non-cross-resistant second-line treatment, therefore providing the biological rationale for the activity of anti-EGFR retreatment in later lines.21 Therefore, in patients with acquired resistance to EGFR inhibition during the upfront treatment strategy, a treatment cross-over to an alternative biological agent may be the optimal option and anti-EGFR re-treatment in later lines is preferable. However, in patients with off-treatment progression after an upfront anti-EGFR-based strategy, there is uncertainty whether immediate reinduction may be truly superior to “re-introduction” after intervening treatment lines.
Beyond the above-mentioned limitations of our work, we also acknowledge the heterogeneity of (1) the reasons for the interruption of first-line, anti-EGFR-based treatment; (2) the duration of exposure to initial anti-EGFR-based therapy; and (3) the lack of per-protocol recommendations about the post-progression treatment.
In conclusion, reinduction of an anti-EGFR-based first-line regimen is frequently—but empirically—used in the clinical practice for patients with RAS wild-type metastatic colorectal cancer. Our data support a promising outcome of this strategy when patients are carefully selected from a clinical and molecular point of view and also support the design of prospective trials investigating stop&go strategies in the context of anti-EGFR-based first-line treatment.
Supplementary Material
Contributor Information
Giovanni Fucà, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Alessandra Raimondi, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Michele Prisciandaro, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Sara Lonardi, Department of Oncology, Veneto Institute of Oncology IOV - IRCCS, Padua, Italy.
Chiara Cremolini, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Margherita Ratti, Oncology Unit, Oncology Department, ASST of Cremona, Cremona, Italy.
Matteo Clavarezza, Medical Oncology Unit, Ente Ospedaliero Ospedali Galliera, Genoa, Italy.
Roberto Murialdo, Department of Internal Medicine (Di.M.I.), University of Genoa and IRCCS AOU San Martino-IST, Genoa, Italy.
Andrea Sartore-Bianchi, Niguarda Cancer Center, Grande Ospedale Metropolitano Niguarda, Milan, Italy; Oncology and Hemato-oncology Department, University of Milan, Milan, Italy.
Valeria Smiroldo, Medical Oncology and Hematology Unit, Humanitas Cancer Center, IRCCS Humanitas Research Hospital, Rozzano, Italy.
Rosa Berenato, Medical Oncology Unit A.O. Papardo and Department of Human Pathology, University of Messina, Messina, Italy.
Patrizia Racca, Colorectal Cancer Unit, Oncology Department, AOU Città della Salute e della Scienza di Torino, Turin, Italy.
Francesca Bergamo, Department of Oncology, Veneto Institute of Oncology IOV - IRCCS, Padua, Italy.
Salvatore Corallo, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Maria Di Bartolomeo, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Filippo de Braud, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy; Oncology and Hemato-oncology Department, University of Milan, Milan, Italy.
Federica Morano, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Filippo Pietrantonio, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Funding
None declared.
Conflict of Interest
Sara Lonardi: Amgen, Merck Serono, Eli Lilly & Co., AstraZeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, Servier, Merck Sharp & Dohme (C/A), Amgen, Merck, Serono, Bayer, Roche, Eli Lilly & Co., AstraZeneca, Bristol-Myers Squibb (RF [institutional]), Roche, Eli Lilly & Co., Bristol-Myers Squibb, Servier, Merck Serono, Pierre-Fabre, GSK, Amgen (H); Chiara Cremolini: Roche, Amgen, Bayer, Servier, Merck Serono (RF); Andrea Sartore-Bianchi: Amgen, Bayer, Merck Sharp & Dohme, Sanofi, Servier (C/A); Maria Di Bartolomeo: Bristol-Myers Squibb, Merck Sharp & Dohme, Servier, Eli Lilly & Co. (C/A), Eli Lilly & Co. (RF); Filippo de Braud: Amgen, Roche, Novartis (C/A); Filippo Pietrantonio: Amgen, Roche, AstraZeneca, Merck Serono, Servier, Bayer, Eli Lilly & Co. (C/A), Bristol-Myers Squibb, AstraZeneca (RF).
The other authors indicated no financial relationships. (C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent-holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: G.F. and F.P. Provision of study material or patients: A.R., M.P., S.L., C.C., M.R., M.C., R.M., A.S.-B., R.B., P.R., F.B., S.C., M.D.B., F.B., F.M., and F.P. Collection and/or assembly of data: G.F., A.R., M.P., S.L., C.C., M.R., M.C., R.M., A.S.-B., R.B., P.R., S.C., and F.M. Data analysis and interpretation: G.F., A.R., and F.P. Manuscript writing: G.F., A.R., M.P., S.L., C.C., M.R., M.C., R.M., A.S.-B., R.B., P.R., F.B., S.C., M.D.B., F.B., F.M., and F.P. Final approval of manuscript: All authors.
Data Availability
The data underlying this article will be shared at reasonable request to the corresponding author.
References
- 1. Raimondi A, Di Maio M, Morano F, et al. Health-related quality of life in patients with RAS wild-type metastatic colorectal cancer treated with panitumumab-based first-line treatment strategy: a pre-specified secondary analysis of the Valentino study. Eur J Cancer. 2020;135:230-239. [DOI] [PubMed] [Google Scholar]
- 2. Wasan H, Meade AM, Adams R, et al. ; COIN-B investigators . Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pietrantonio F, Morano F, Corallo S, et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1268-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Modest DP, Rivera F, Bachet JB, et al. Panitumumab-based maintenance after oxaliplatin discontinuation in metastatic colorectal cancer: a retrospective analysis of two randomised trials. Int J Cancer. 2019;145(2):576-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aranda E, García-Alfonso P, Benavides M, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) . First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263-272. [DOI] [PubMed] [Google Scholar]
- 6. Munemoto Y, Nakamura M, Takahashi M, et al. SAPPHIRE: a randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur J Cancer. 2019;119:158-167. [DOI] [PubMed] [Google Scholar]
- 7. Cremolini C, Rossini D, Dell’Aquila E, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5(3):343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossini D, Germani MM, Pagani F, et al. Retreatment with anti-EGFR antibodies in metastatic colorectal cancer patients: a multi-institutional analysis. Clin Colorectal Cancer. 2020;19(3):191-199.e6. [DOI] [PubMed] [Google Scholar]
- 9. Manca P, Corallo S, Randon G, et al. Impact of early tumor shrinkage and depth of response on the outcomes of panitumumab-based maintenance in patients with RAS wild-type metastatic colorectal cancer. Eur J Cancer. 2021;144:31-40. [DOI] [PubMed] [Google Scholar]
- 10. Morano F, Corallo S, Lonardi S, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol. 2019;37(33):3099-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics. 1992;48(1):73-85. [Google Scholar]
- 13. Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3718-3726. [DOI] [PubMed] [Google Scholar]
- 14. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer–a GERCOR study. J Clin Oncol. 2006;24(3):394-400. [DOI] [PubMed] [Google Scholar]
- 15. André T, Tournigand C, Mineur L, et al. Phase II study of an optimized 5-fluorouracil-oxaliplatin strategy (OPTIMOX2) with celecoxib in metastatic colorectal cancer: a GERCOR study. Ann Oncol. 2007;18(1):77-81. [DOI] [PubMed] [Google Scholar]
- 16. Hegewisch-Becker S, Graeven U, Lerchenmüller CA, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355-1369. [DOI] [PubMed] [Google Scholar]
- 17. Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843-1852. [DOI] [PubMed] [Google Scholar]
- 18. Cremolini C, Milione M, Marmorino F, et al. Differential histopathologic parameters in colorectal cancer liver metastases resected after triplets plus bevacizumab or cetuximab: a pooled analysis of five prospective trials. Br J Cancer. 2018;118(7):955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaz LA Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misale S, Yaeger R, Hobor S, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486(7404):532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siravegna G, Mussolin B, Buscarino M, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21(7):795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared at reasonable request to the corresponding author.