Abstract
Several strategies for discovering drugs from unexplored natural products continue to strengthen research and development with current commercial evidence supporting their applications. We assessed the effects of the hydroethanolic extract of Acridocarpus smeathmannii root (HEASR) against phenylhydrazine (PHZ)-induced haematotoxicity, biochemical changes, and oxidative stress in male Wistar rats. Groups 1 and 2 controls received normal saline (10 mL/kg/day) and PHZ (60 mg/kg, day 4 and 5), respectively, via oral gavage. Groups 3, 4, and 5 were administered dexamethasone (DXM, 0.014 mg/kg/day, p.o.), HEASR1 (50 mg/kg/day, p.o.) and HEASR2 (200 mg/kg/day, p.o.), respectively. Groups 6, 7, and 8 received HEASR2 (200 mg/kg/day), DXM (0.014 mg/kg/day), or their combination, respectively, and further received PHZ (60 mg/kg/day) intervention on day 4 and 5 only. Treatments lasted for 7 days. Phenylhydrazine toxicity manifested as lowered haemoglobin, white blood cells, lymphocytes, red blood cells, and platelet levels by 45.86%, 53.47%, 75.69%, 46.89%, and 30.29%, respectively, in rats. This was accompanied by an increase in serum alanine (ALT; 108.25%) and aspartate (AST; 78.79%) aminotransferases, urea (84.36%), total cholesterol (81.55%), and triglycerides (123.42%) levels. Similarly, malondialdehyde levels and serum cyclooxygenase-2 activity were elevated (P < 0.05) in the rats liver and spleen, respectively. Just HEASR alone, or in combination with DXM, preserved haematological and biochemical parameters, cyclooxygenase-2 activity, and corticosterone levels during PHZ intoxication and restored renal histopathological alterations in rats. The HEASR was found to contain high flavonoid and phenolic phytochemicals and demonstrated better in vitro antioxidants inhibitory action.
Keywords: Acridocarpus smeathmannii (DC.), phenylhydrazine, haematotoxicity, blood chemistry, oxidative stress, phytochemicals
Introduction
The development of therapeutic agents from natural products has renewed the world interest and has stimulated scientists on the benefits of medicinal plant research as an alternative scientific tool for the treatment of diseases.1-3 Despite the aforementioned, there are unexplored and yet to be screened herbs for phytochemical and biologic activities; however, with high throughput screening methods, these numbers are vast reducing globally.4,5
The Malpighiaceae is a medium-sized family of tropical and subtropical flowering plants comprising about 1200 to 1300 species divided into 77 genera.6,7 Acridocarpus smeathmannii (DC.) Guill. & Perr. (Malpighiaceae) is found in tropical West Africa. 8 Its common names in Africa include Elerujo, Gborígborí (Nigeria), Fu kéréfu, Fu tabéfu (Senegal), Golonge, Kamaiir (Sierra Leone), and Alasaayo (Ghana). 9 A. smeathmannii root is used locally for the management of blood disorders, infertility, pains, and others.3,10 Botanical and anatomical descriptions for A. smeathmannii have been documented in literature. 6
Haemolytic disorder (HD) causes anaemia, which is affecting people of all ages and posing a great threat to the global health care. The global prevalence of anaemia for the general population is on the increase, and it is estimated that 1.62 billion people are affected by this disease. 11 Chemical exposures, including the administration of some drugs have been found to alter the lifespan of red blood cells (RBCs) in the body,12,13 and HD is a part of the clinical syndrome associated with such intoxication.14,15 Several chemicals can cause haemolysis via interaction with sulfhydryl groups, the inhibition of various enzymes, immune mechanisms, and the fragmentation of erythrocytes as they pass through the platelet (PLT)-fibrin mesh or by unknown or poorly defined mechanisms. 11 The aforementioned described an HD where erythrocytes have a shortened life span. 13 Phenylhydrazine (PHZ) has been described as a suitable substance for inducing HD and studying anaemia mechanisms. 11 Phenylhydrazine-induced toxic anaemia offers a model for research into the pathogenesis of HD and the influence of anaemia on other physiological processes or the course of associated diseases. 13 Haemolytic disorder reduces the capacity of oxygen transfer and increases blood iron, which causes a series of changes in the body.15,16 Oxidative stress on erythrocytes is considered an important mechanism of haemolysis. 17 Phenylhydrazine increases reactive oxygen species (ROS) by increasing lipid peroxidation metabolites (malondialdehyde, MDA) and decreases antioxidant status, respectively.18,19 There are 2 major reasons for this study. First, there has been no report on the modulatory effect of A. smeathmannii on PHZ toxicity in rodents. Second, its phytochemical compositions and in vitro actions have not been reported.
Therefore, this study assessed the modulatory effects of the hydroethanolic extract of A. smeathmannii root (HEASR) on haematological, biochemical, and oxidative stress parameters in PHZ-induced toxicity in male Wistar rats in order to ascertain its chemopreventive benefit. In addition, the chemical compositions were determined.
Materials and Methods
Drugs and chemicals
Dexamethasone (DXM) was purchased from Jiangsu Pengyao Pharmaceutical INC., Yixing Jiansu, Longchi Road, China. Phenylhydrazine was purchased from Sigma Aldrich®, St Louis, MI, USA. All other chemicals and reagents used are of analytical grade.
Preparation of plant extract
Fresh roots of A. smeathmannii were collected, authenticated (LUH 6638), prepared, and reconstituted as described in our previous study. 12
In vitro antioxidant assays
The free radical scavenging activity of HEASR in 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was measured as described previously by Pérez-Jiménez et al. 20 The nitric-oxide (NOSAs) and hydroxyl radical scavenging activities (HORSAs) of HEASR were assayed by the methods described by Ahlemeyer and Krieglstein 21 and Özyürek et al, 22 respectively. The lipid peroxidation and ferric reducing antioxidant power (FRAP) activities of the extract were assayed by the methods described by Singh et al 23 and Firuzi et al, 24 respectively.
Phytochemical analyses of A. smeathmannii root
The total phenols content was determined by the Folin-Ciocalteu procedure described by Skerget et al. 25 The total flavonoids content was determined by the aluminum chloride colorimetric assay. 26 Saponins, tannins, alkaloids, terpenoids, and glycosides determination followed the methods of Borokini and Omotayo. 26
Proximate analysis of A. smeathmannii root
Proximate analysis of HEASR followed the method of Paez et al. 27 The chemical composition of A. smeathmannii root was determined using the Association of Official Analytical Chemists (AOAC International) methods. 27 The moisture, total ash, crude fibre, total fat, and protein were assayed, and carbohydrate was obtained by the difference. The energy content of the seed was calculated using the Bradbury’s equation (cloud base in feet = 400 times (temperature/dew point split). 27
Experimental animals
A total of 48 male Wistar albino rats (weighing 108-175 g) were obtained from a commercial private colony in Badagry, Lagos State, Nigeria. The experimental protocols were approved by Health Research Ethics Committee, College of Medicine University of Lagos (CMUL/HREC/09/18/424) and conformed to studies involving experimental animals and the procedures as documented.28,29
Induction of acute HD
Phenylhydrazine was administered to induce acute HD in rats following an oral administration at a dose of 60 mg/kg body weight (dissolved in normal saline) to achieve lower haematological concentration and haemoglobin (HGB) levels within 48 hours according to the methods of Lee et al 30 with slight modifications. Phenylhydrazine-induced HD, biochemical changes, and oxidative stress parameters were used to assess the modulation of haematopoietic function in the treated rats via mechanisms of actions involving corticosteroid pathway in vivo.
Experimental design and treatment
Rats in the different group (6 per group) received treatments as follow: Group 1 (negative control) received normal saline (10 mL/kg/day p.o.) daily for 7 days. Group 2 (toxicant control) received PHZ (60 mg/kg/day, p.o.) on days 4 and 5 only. Groups 3, 4, and 5 were administered DXM (0.014 mg/kg/day p.o.), HEASR1 (50 mg/kg/day p.o.), and HEASR2 (200 mg/kg/day p.o.), respectively, daily for 7 days. Group 6, 7, and 8 received HEASR2 (200 mg/kg/day), DXM (0.014 mg/kg/day), DXM (0.014 mg/kg/day p.o.) + HEASR2 (200 mg/kg/day), respectively, daily for 7 days. Furthermore, PHZ (60 mg/kg/day) intoxication was achieved via oral administration to Groups 6, 7, and 8 only on days 4 and 5, respectively, in addition to extract (HEASR) and DXM treatments. Both HEASR and DXM were administered prior to and throughout PHZ intoxication. More so, food and water intakes and faeces defecated were measured daily.
Acute oral toxicity test
Acute oral toxicity study was conducted using the limit dose test of up and down procedure of Organization for Economic Co-operation and Development (OECD/OCDE) 31 Test Guidelines on Acute Oral Toxicity. All observations were systematically recorded with individual records being maintained for each rat. 29
Biochemical assessments
Serum total cholesterol and triglyceride levels, as well as liver and renal biomarkers were estimated using commercial kits obtained from Randox Laboratories Ltd. (Crumlin, UK). High-density lipoprotein was estimated according to the method of Warnick and Albers 32 while serum low-density lipoprotein was determined following the precipitation method described by Wieland and Seidel. 33 Total protein concentration was determined using the Biuret reaction. 34 The method of Rahman et al 35 was followed in estimating the level of reduced glutathione. The level of superoxide dismutase (SOD) and catalase (CAT) activities, and lipid peroxidation level was determined by the method of Zhou and Prognon, 36 Iwase et al, 37 and Shahidi and Zhong, 38 respectively.
Haematological assessments
Full blood counts were determined using a fully automated haematology analyser (Pentra-XL 80, Horiba ABX, USA). 39
Necropsy
The animals were sacrificed by cervical dislocation 24 hours after the last treatment. Blood samples were collected by cardiac puncture into plain bottles and centrifuged at 4200 r/min at room temperature for 5 minutes to separate serum. The liver, kidney, and spleen were removed, cleared of adhering tissues, weighed, and processed further. Both sera and respective organ homogenates were used for biochemical analysis. 13
Measurement of COX-2 activity and corticosterone level
Cyclooxygenase-2 (COX-2) activity and corticosterone level in serum were assessed using enzyme-linked immunosorbent assay (ELISA) kits. Briefly, triplicate samples were tested twice per plate [intra assay: coefficient of variation (CV), CV < 8% and inter-assay: CV < 10%] in each case and expressed as Units/L. The optical density of each well was determined according to the manufacturer’s instructions.40,41
Statistics
Results were expressed as mean ± standard error of the mean (SEM). Differences between groups were determined by one-way analysis of variance (ANOVA) using Statistical Package for Social Sciences (SPSS, 20.0) software for windows. The Student’s t-test was used to compare the differences between the initial and final blood concentration means of each treatment during the repletion. An intergroup comparison using the least significant difference followed by Dunnett’s post hoc test was used. P < .05 was considered significant.
Results
Phytochemical analysis
Preliminary qualitative phytochemical screening of HEASR revealed the presence of flavonoids, terpenoids, tannins, saponins, glycosides, phenolic compounds, and alkaloids. Results of the quantitative phytochemical analysis revealed the following: total flavonoids (67.03 ± 0.44 gallic acid equivalent [GAE]/100 g), terpenoids (18.13 ± 1.41 mg/100 g), tannins (21.89 ± 0.71 mg/100 g), saponins (13.77 ± 1.06 mg/100 g), glycosides (34.03 ± 1.13 mg/100 g), total phenol (57.93 ± 0.56 mg GAE/100 g), and alkaloids (6.81 ± 1.13 mg/100 g), respectively. Thus, the descending order of this bioactive compounds present in HEASR is total flavonoids >total phenols >glycosides >tannins >terpenoids >saponins >alkaloids.
In vitro antioxidants activities
Results of in vitro antioxidant activity showed inhibitory concentrations (IC50) against standard controls, ascorbic acid (AA, µg/mL) and GAE (µg/mL) presented in Table 1. The HEASR demonstrated antioxidant activity IC50 for nitric oxide scavenging radical activity (NOSA), DPPH, HORSA and lipid peroxidation scavenging activity (LPOSA), and FRAP, respectively.
Table 1.
Quantitative phytochemical analysis of in vitro antioxidant activity of HEASR.
| Compounds | Minimum inhibitory concentrations (IC50) | ||||
|---|---|---|---|---|---|
| NOSA | DPPH | HORSA | LPOSA | FRAP | |
| HEASR | 30.880 ± 0.490 | 30.661 ± 0.410 | 29.830 ± 0.730 | 28.631 ± 0.544 | 0.123 ± 0.003 |
| AA (µg/mL) | 26.380 ± 1.360 | 20.910 ± 0.440 | 27.042 ± 1.281 | 26.642 ± 0.191 | 0.103 ± 0.001 |
| GAE (µg/mL) | 24.513 ± 0.520 | 20.011 ± 1.830 | 30.401 ± 1.332 | 25.540 ± 0.321 | 0.101 ± 0.007 |
Results expressed mean ± SEM. n = 3 (triplicate). Abbreviations: AA, ascorbic acid; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; FRAP, ferric-reducing antioxidant power; GAE, gallic acid equivalent; HEASR, hydroethanolic extract of A. smeathmannii root; HORSA, hydroxyl radical scavenging activity; LPOSA, lipid peroxidation scavenging activity; NOSA, nitric oxide scavenging radical activity.
Proximate compositions of A. smeathmannii
The percentage proximate compositions of A. smeathmannii recorded in triplicate are carbohydrate (61.62 ± 0.49) >crude fat (14.07 ± 0.43) >protein (10.08 ± 0.05) >moisture (7.29 ± 0.033) >ash (5.81 ± 0.02) >crude fibre (1.13 ± 0.03).
Acute oral toxicity test
There was no mortality observed 24 hours after treatment at 2000 mg/kg via oral gavage, although behavioural as well as morphological changes were marked. The animals showed hyperactivity, grooming, rearing, circular movement, and dullness in at least 5 animals in the first 2 hours. However, the effects diminished completely 2 weeks postadministration.
Haematological indices
Phenylhydrazine lowered (P < .05) white blood cell (WBC), lymphocyte (LYMP), LYMP%, HGB, RBC, and PLT by 53.47%, 75.69%, 60.76%, 45.86%, 46.89%, and 30.29%, respectively, when comparing Table 2 with initial baseline level (Table 1). In addition, there were increased (P < .05) WBC and HGB in the treated rats when HEASR2 and HEASR2 + DXM treated rats by 46.17%, 65.95%, and 63.49%, 65.94%, respectively, compared with control PHZ group. Similarly, RBC improved in HEASR (68.29%), DXM (33.71%), and HEASR + DXM (86.29%) treated rats that received PHZ intervention. More so, PHZ + DXM but not PHZ + HEASR2 (200 mg/kg) improved PLTs by 39.48% when compared with control PHZ group.
Table 2.
Effects of HEASR on haematological parameters in normal and phenylhydrazine-treated male rats.
| FBC | NS (10 mg/kg) | PHZ (60 mg/kg) | DXM (0.014 mg/kg) | HEASR1 (50 mg/kg) | HEASR2 (200 mg/kg) | PHZ + HEASR2 | PHZ + DXM | PHZ + HEASR2 + DXM |
|---|---|---|---|---|---|---|---|---|
| WBC | 11.67 ± 0.6 | 10.10 ± 0.3 | 13.03 ± 0.8 | 9.47 ± 0.5 | 7.10 ± 0.3 | 13.19 ± 0.4 | 12.27 ± 0.3 | 12.27 ± 0.5 |
| WBCf | 10.10 ± 0.3 | 4.70 ± 0.2* | 13.70 ± 0.5 | 8.83 ± 0.3 | 6.27 ± 0.3 | 6.87 ± 0.6* # | 7.80 ± 0.2* # | 6.33 ± 0.6* # |
| LYMP | 6.23 ± 0.2 | 6.50 ± 0.3 | 7.80 ± 0.2 | 7.27 ± 0.4 | 5.93 ± 0.2 | 6.07 ± 0.3 | 4.93 ± 0.6 | 3.83 ± 0.3 |
| LYMPf | 5.77 ± 0.7 | 1.58 ± 0.2* | 5.20 ± 0.4 | 5.37 ± 0.8 | 5.83 ± 0.6 | 5.60 ± 0.3 # | 5.20 ± 0.3 # | 3.23 ± 0.2 # |
| MID | 2.37 ± 0.2 | 0.80 ± 0.1 | 1.53 ± 0.1 | 1.30 ± 0.1 | 0.70 ± 0.1 | 1.05 ± 0.1 | 3.37 ± 0.2 | 3.90 ± 0.1 |
| MIDf | 2.00 ± 0.2 | 1.13 ± 0.2 | 0.63 ± 0.1 | 1.37 ± 0.1 | 0.50 ± 0.1 | 1.23 ± 0.1 | 1.67 ± 0.1* | 1.17 ± 0.1* |
| GRAN | 3.07 ± 0.2 | 2.80 ± 0.1 | 3.70 ± 0.2 | 0.90 ± 0.2 | 0.47 ± 0.2 | 3.64 ± 0.1 | 3.70 ± 0.2 | 4.53 ± 0.3 |
| GRANf | 2.35 ± 0.1 | 2.00 ± 0.1 | 1.87 ± 0.2* | 2.17 ± 0.1* | 0.93 ± 0.3* | 2.03 ± 0.1 | 1.93 ± 0.1* | 1.93 ± 0.1* |
| LYMP% | 52.33 ± 3.4 | 63.97 ± 4.1 | 59.80 ± 2.3 | 77.67 ± 6.4 | 85.13 ± 3.2 | 50.47 ± 2.3 | 40.83 ± 1.7 | 29.13 ± 2.4 |
| LYMP%f | 35.03 ± 2.1 | 25.10 ± 1.9* | 30.33 ± 2.6 | 47.63 ± 1.3 | 57.20 ± 3.0 | 25.63 ± 1.3* | 36.83 ± 1.5 # | 27.87 ± 1.3 |
| MID% | 21.90 ± 1.4 | 8.30 ± 1.0 | 11.97 ± 1.3 | 13.13 ± 1.6 | 8.53 ± 1.1 | 8.42 ± 1.5 | 28.07 ± 1.2 | 33.23 ± 2.3 |
| MID%f | 19.40 ± 2.1 | 27.23 ± 1.5* | 18.27 ± 1.8 | 12.00 ± 1.3 | 16.23 ± 2.1* | 27.23 ± 1.9* | 27.20 ± 2.2 | 26.77 ± 1.7 |
| GRAN% | 26.43 ± 2.1 | 27.73 ± 1.7 | 28.23 ± 1.2 | 49.20 ± 2.4 | 26.33 ± 1.3 | 29.43 ± 1.1 | 31.00 ± 2.0 | 37.63 ± 1.2 |
| GRAN%f | 45.60 ± 3.3 | 47.50 ± 2.5 | 51.40 ± 2.6 | 44.57 ± 1.8 | 26.57 ± 1.6 | 47.13 ± 2.1 | 35.97 ± 1.7 | 45.37 ± 2.2 |
| HGB | 12.87 ± 0.7 | 12.80 ± 0.4 | 13.03 ± 0.5 | 13.13 ± 1.1 | 12.63 ± 0.7 | 13.73 ± 0.7 | 15.77 ± 0.5 | 19.10 ± 1.4 |
| HGBf | 11.03 ± 1.0 | 6.93 ± 0.7* | 9.70 ± 0.8 | 12.23 ± 1.1 | 11.97 ± 1.5 | 11.63 ± 0.6 # | 11.33 ± 0.5 # | 11.50 ± 0.7 # |
| RBC | 6.45 ± 0.3 | 6.59 ± 0.3 | 6.55 ± 0.2 | 6.67 ± 0.2 | 6.46 ± 0.2 | 6.63 ± 0.3 | 8.27 ± 0.2 | 8.77 ± 0.3 |
| RBCf | 5.05 ± 0.2 | 3.50 ± 0.2* | 4.65 ± 0.3 | 6.42 ± 0.3 | 4.91 ± 0.3 | 5.89 ± 0.3 # | 4.68 ± 0.3 # | 6.52 ± 0.3 # |
| HCT | 46.63 ± 2.3 | 45.00 ± 1.8 | 46.53 ± 1.4 | 39.83 ± 2.7 | 38.33 ± 1.6 | 48.00 ± 2.1 | 53.97 ± 1.9 | 55.10 ± 2.8 |
| HCTf | 22.80 ± 1.5 | 35.60 ± 1.2 | 25.10 ± 1.0* | 52.93 ± 1.4 | 41.30 ± 1.3 | 39.53 ± 1.3 | 28.20 ± 1.6* | 36.30 ± 1.3* |
| MCV | 72.80 ± 2.4 | 68.37 ± 1.6 | 71.17 ± 2.1 | 88.23 ± 1.8 | 88.50 ± 1.4 | 72.40 ± 2.7 | 65.67 ± 2.2 | 63.23 ± 1.7 |
| MCVf | 75.03 ± 1.3 | 102.43 ± 1.7* | 96.37 ± 2.3 | 82.57 ± 1.7 | 86.83 ± 2.4 | 101.80 ± 1.7* | 111.50 ± 2.5* | 103.60 ± 3.2* |
| MCH | 19.90 ± 1.1 | 19.40 ± 0.8 | 19.87 ± 0.6 | 19.77 ± 1.3 | 19.70 ± 1.8 | 20.67 ± 1.3 | 19.10 ± 1.7 | 22.55 ± 1.0 |
| MCHf | 22.60 ± 1.5 | 32.53 ± 1.3* | 43.93 ± 2.4* | 19.03 ± 1.7 | 25.63 ± 1.2 | 29.93 ± 1.5 | 55.77 ± 2.6* # | 32.78 ± 1.8 |
| MCHC | 27.60 ± 0.8 | 28.43 ± 0.6 | 27.97 ± 0.6 | 32.97 ± 0.7 | 33.10 ± 0.6 | 28.57 ± 0.7 | 29.13 ± 0.9 | 35.45 ± 1.3 |
| MCHCf | 30.80 ± 1.2 | 31.67 ± 1.7 | 41.73 ± 1.4* | 23.10 ± 1.1 | 29.23 ± 1.3 | 29.40 ± 2.6 | 48.43 ± 2.1* | 31.67 ± 0.8 |
| RDWCV | 18.90 ± 1.3 | 17.47 ± 1.1 | 16.70 ± 1.5 | 18.30 ± 2.1 | 19.37 ± 1.0 | 18.13 ± 1.1 | 16.13 ± 1.4 | 18.93 ± 1.9 |
| RDWCVf | 20.70 ± 1.3 | 19.70 ± 1.6 | 19.50 ± 1.2 | 23.33 ± 1.8 | 21.63 ± 2.0 | 20.77 ± 1.2 | 20.43 ± 1.5 | 20.97 ± 1.5 |
| RDWSD | 45.03 ± 1.6 | 39.33 ± 1.4 | 39.57 ± 1.8 | 41.40 ± 1.3 | 42.20 ± 1.1 | 42.30 ± 1.6 | 31.37 ± 1.4 | 38.80 ± 1.1 |
| RDWSDf | 50.50 ± 2.4 | 51.57 ± 2.1* | 47.83 ± 2.8 | 61.93 ± 3.0 | 56.50 ± 3.7 | 54.43 ± 3.1 | 54.03 ± 2.8* | 55.03 ± 2.3* |
| PLT | 741.67 ± 5.3 | 961.67 ± 5.9 | 728.33 ± 8.2 | 658.67 ± 7.4 | 617.33 ± 6.3 | 854.00 ± 5.5 | 936.00 ± 8.1 | 957.33 ± 4.8 |
| PLTf | 928.33 ± 7.2 | 670.33 ± 6.4* | 766.67 ± 10.3 | 828.67 ± 14.2 | 930.00 ± 10.1 | 783.67 ± 6.8 | 935.00 ± 9.4 # | 702.00 ± 5.6 |
| MPV | 7.97 ± 0.4 | 7.67 ± 0.4 | 8.60 ± 0.2 | 7.07 ± 0.3 | 6.60 ± 0.3 | 8.23 ± 0.2 | 7.87 ± 0.5 | 7.47 ± 0.3 |
| MPVf | 8.57 ± 0.3 | 9.83 ± 0.6 | 9.43 ± 0.8 | 8.80 ± 0.5 | 8.80 ± 0.3 | 8.57 ± 0.6 | 8.13 ± 0.7 | 8.90 ± 0.2 |
| PDW | 15.37 ± 1.0 | 15.30 ± 0.8 | 15.47 ± 1.4 | 10.27 ± 0.7 | 9.73 ± 1.0 | 15.20 ± 1.5 | 15.60 ± 0.7 | 15.63 ± 1.1 |
| PDWf | 15.80 ± 1.5 | 15.70 ± 1.1 | 15.60 ± 1.3 | 15.50 ± 1.3 | 15.87 ± 0.9* | 15.67 ± 1.2 | 15.40 ± 1.4 | 15.73 ± 1.3 |
| PCT | 0.59 ± 0.1 | 0.67 ± 0.3 | 0.62 ± 0.3 | 0.47 ± 0.2 | 0.41 ± 0.2 | 0.67 ± 0.2 | 0.68 ± 0.4 | 0.69 ± 0.3 |
| PCTf | 0.61 ± 0.3 | 0.66 ± 0.2 | 0.68 ± 0.5 | 0.68 ± 0.2 | 0.67 ± 0.3 | 0.67 ± 0.4 | 0.60 ± 0.3 | 0.61 ± 0.2 |
Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control initial blood count level. Subscript ‘f’ indicates final full blood count level of rat. #P < .05 or ##P < .001 when compared to phenylhydrazine-treated group. Abbreviations: FBC, full blood count; NS, normal saline. DXM, dexamethasone; GRAN (%), granulocytes; HCT (%), haematocrit; HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; HGB (g/dL), haemoglobin; LYMP ×103 (/mL), lymphocyte; MCH (pg/dL), mean corpuscular haemoglobin; MCHC (g/dL), mean corpuscular haemoglobin concentration; MCV (fL), mean corpuscular volume; MID (%), minimum inhibitory dilution; MPV (fL), mean platelet volume; PCT (%), plateletcrit; PDW, plate volume distribution width; PHZ, phenylhydrazine; PLT, platelet; RBC ×106/mL, red blood cell; RDWCV (%), red blood cell volume distribution width-CV; RDWSD (%), red blood cell volume distribution width-SD; WBC ×103 (/mL), white blood cell.
Liver function parameters
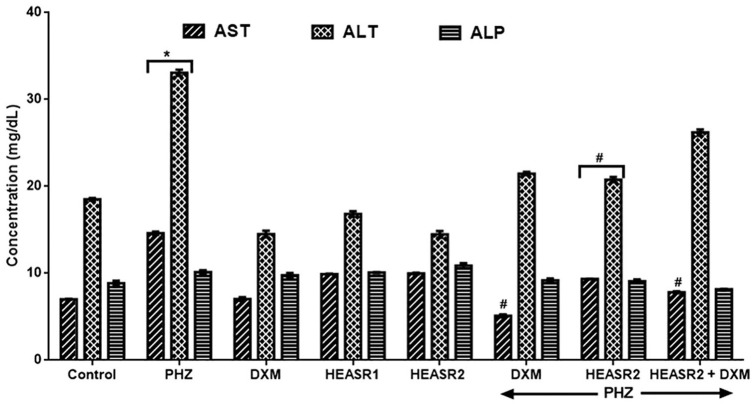
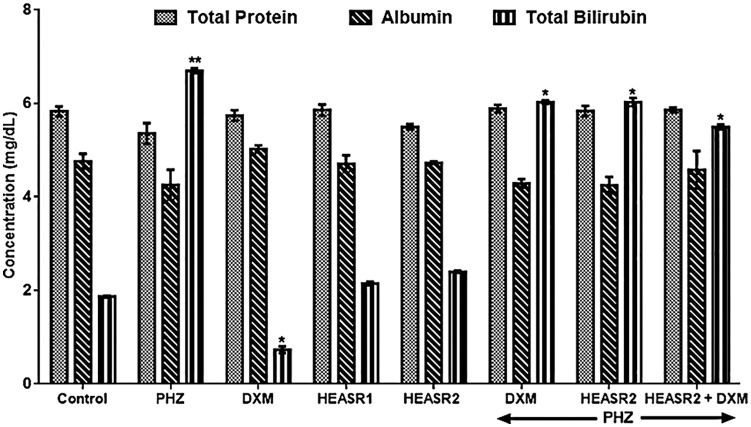
Phenylhydrazine induced increase (P < .05) in serum ALT and AST levels by 108.25% and 78.79%, respectively, when compared with control normal saline group (Figure 1). Phenylhydrazine + HEASR2 (200 mg/kg) and PHZ + DXM and PHZ + DXM + HEASR2 administration, however, lowered ALT levels by 36.06%, 62.98%, and 46.49%, respectively, in rats. In addition, PHZ + HEASR2 (200 mg/kg) decreased AST levels by 37.20% in the treated rats. Although, PHZ administration resulted in elevated alkaline phosphatase (ALP) levels, this was not significant when compared with control normal saline group. Both DXM and HEASR (50 or 200 mg/kg) modulated hepatic enzyme function, although insignificantly in the treated animals. Phenylhydrazine produced elevated serum total bilirubin level when compared with control normal saline group (Figure 2) which remained elevated in the treated rats.
Figure 1.
Effect of HEASR on liver function in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 when compared to control normal saline group. #P < .05 when compared to control phenylhydrazine group. ALP indicates alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DXM, dexamethasone; HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine.
Figure 2.
Effect of HEASR on serum total protein, albumin, and total bilirubin in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. ALB indicates albumin; DXM, dexamethasone; HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine; TBIL, total bilirubin; TP, total protein.
Renal function parameters
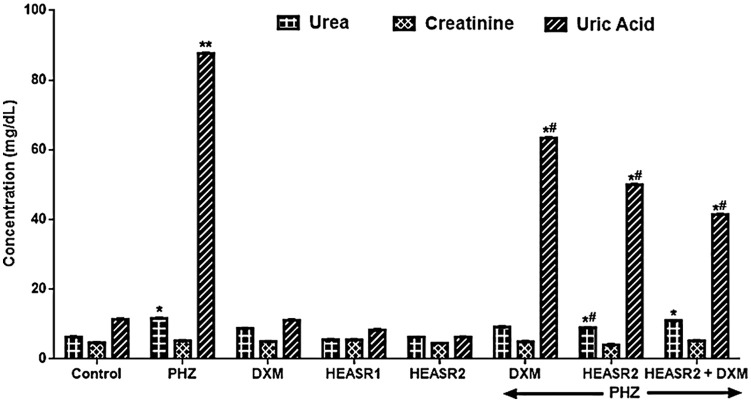
Phenylhydrazine administration caused elevated urea and uric acid levels by 84.36% (P < .05) and 669.59% (p < .001) when compared with control normal saline group (Figure 3). Phenylhydrazine + HEASR2 (200 mg/kg) and PHZ + DXM lowered urea levels by 23.14% and 21.22%, respectively, compared with control PHZ group. Urea levels remained significantly reduced by 46.42% in PHZ + DXM + HEASR2 (200 mg/kg) treated rats against control normal saline group. Phenylhydrazine + HEASR2, PHZ + DXM, and PHZ + DXM + HEASR2 showed reduced (P < .05) levels of uric acid by 27.66%, 42.87%, and 67.74%, respectively, compared with control PHZ group. Dexamethasone, HEASR2, and HEASR + DXM administrations reduced creatinine levels by 27.66%, 42.97%, and 52.73%, respectively, in the treated rats.
Figure 3.
Effect of HEASR on blood urea nitrogen, creatinine, and uric acid levels in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 or ##P < .001 when compared to control phenylhydrazine group. DXM indicates dexamethasone; HEASR: hydroethanolic extract of A. smeathmannii root; HEASR1: 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2: 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine.
Lipid peroxidation level
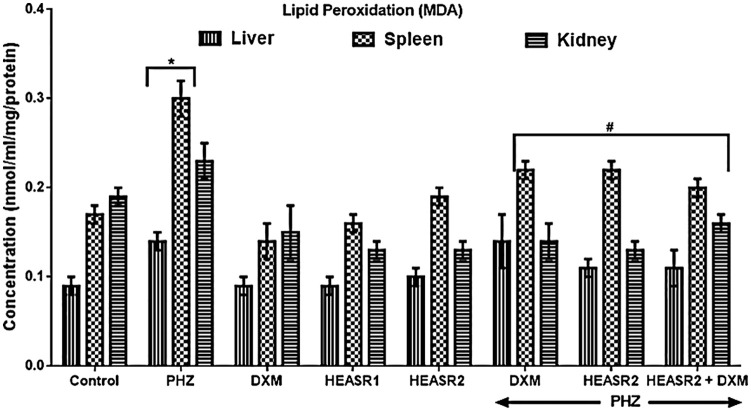
Results in Figure 4 showed that PHZ induction increased (P < .05) MDA levels in the liver and spleen by 55.56% and 76.47%, respectively, compared with control normal saline group. Phenylhydrazine + HEASR2 (200 mg/kg) or PHZ + DXM lowered hepatic MDA similarly by 21.43% when compared with control PHZ group. Both HEASR2 (200 mg/kg) and DXM when administered reduced (P < .05) MDA levels in the spleen by 26.67%, and 33.33%, respectively, when compared with control PHZ group.
Figure 4.
Effect of HEASR on lipid peroxidation (MDA) in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 when compared to control normal saline group. #P < .05 when compared to control phenylhydrazine group. DXM indicates dexamethasone, HEASR: hydroethanolic extract of A. smeathmannii root; HEASR1: 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2: 200 mg/kg of hydroethanolic extract of A. smeathmannii root; MDA, malondialdehyde (nmol/mg protein); PHZ, phenylhydrazine.
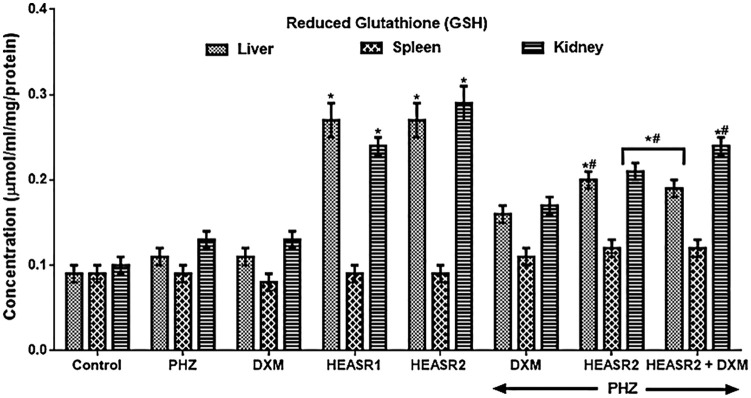
Reduced glutathione level
HEASR1 and HEASR2 produced a dose-dependent increase in renal glutathione (GSH) level by 140% and 190% in rats (Figure 5). More so, animals that received DXM, HEASR, and HEASR + DXM treatments following PHZ intoxication had improved GSH levels in the liver (45.45%, 81.82%, and 72.73%), spleen (22.22%, 33.33%, and 33.33%), and kidney (30.77%, 61.54%, and 84.62%), respectively.
Figure 5.
Effect of HEASR on reduced glutathione (GSH) level in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 or ##P < .001 when compared to control phenylhydrazine group. DXM indicates dexamethasone; GSH, reduced glutathione (µmol/mL/mg protein); HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine.
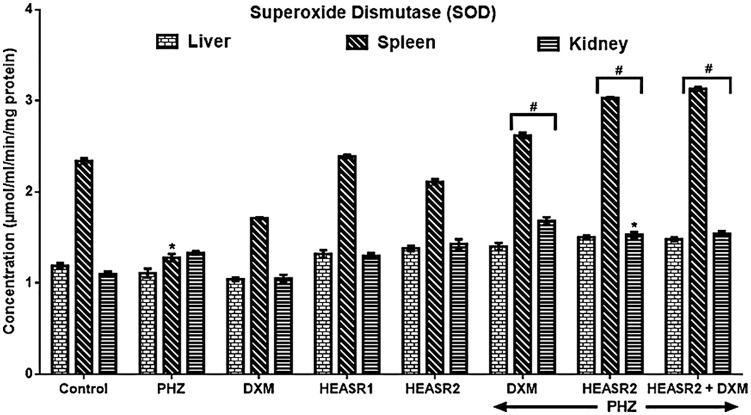
SOD activity
The administration of PHZ lowered (P < .05) SOD activity in the spleen by 45.30% in normal rats (Figure 6). Hydroethanolic extract of A. smeathmannii root and DXM increased SOD activities in the liver, spleen, and kidney by 26.143%, 104.69%, 26.31%, and 35.14%, 136.72%, and 15.04%, respectively, in the treated rats compared with PHZ group. Similarly, HEASR + DXM improved SOD activities (liver, spleen, and kidney) by 33.33%, 144.53%, and 15.79%, respectively.
Figure 6.
Effect of HEASR on superoxide dismutase (SOD) level in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 or ##P < .001 when compared to control phenylhydrazine group. DXM indicates dexamethasone; HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine; SOD: superoxide dismutase (µmol/mL/min/mg protein).
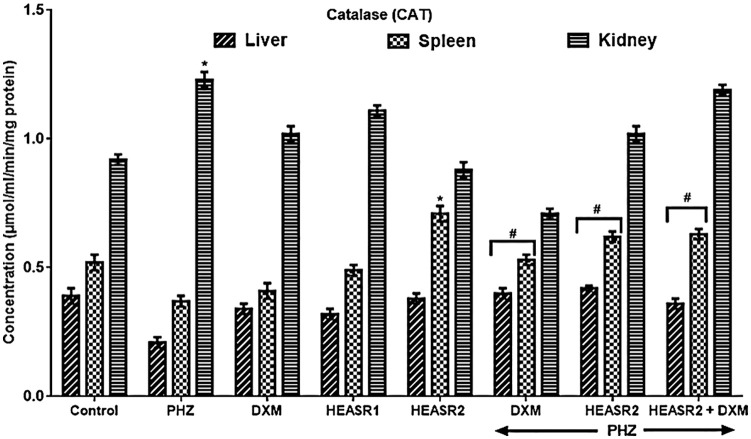
CAT activity
Results in Figure 7 show decreased CAT activity in liver and spleen by 46.15% and 28.85%, respectively, in the control PHZ rats, whereas renal CAT was elevated (35.87%). The administrations of HEASR, DXM, and their combination improved liver and spleen CAT by 90.47%, 100, and 71.43%, and 43.24%, 67.57%, and 70.27%, respectively.
Figure 7.
Effect of HEASR on catalase (CAT) level in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 or ##P < .001 when compared to control phenylhydrazine group. CAT indicates catalase (µmol/mL/min/mg protein); DXM, dexamethasone, HEASR: hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ: phenylhydrazine.
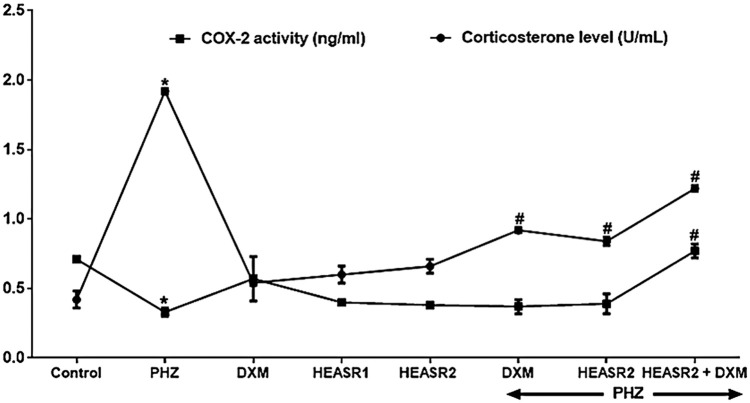
Cyclooxygenase activity and corticosterone level
Phenylhydrazine induced acute HD to produce an elevated (P < .01, 357.14%) serum COX-2 activity while reducing (P < .05, 53.52%) corticosterone levels compared with control normal saline group (Figure 8). Phenylhydrazine + HEASR2 and PHZ + DXM lowered COX-2 activity by 52.08% and 56.25%, respectively, compared with control PHZ group. In addition, HEASR2 (200 mg/kg) given alone and in combination with DXM following PHZ administration improved corticosterone levels in the treated rats.
Figure 8.
Enzyme-linked immunosorbent assay for the quantitative measurement of rat COX-2 activity and corticosterone level in serum of normal and phenylhydrazine-treated rats. Results are expressed as mean ± SEM, n = 6. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 or ##P < .001 when compared to control phenylhydrazine group. COX-2 indicates cyclooygenase-2; DXM, dexamethasone; HEASR: hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2, 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ: phenylhydrazine.
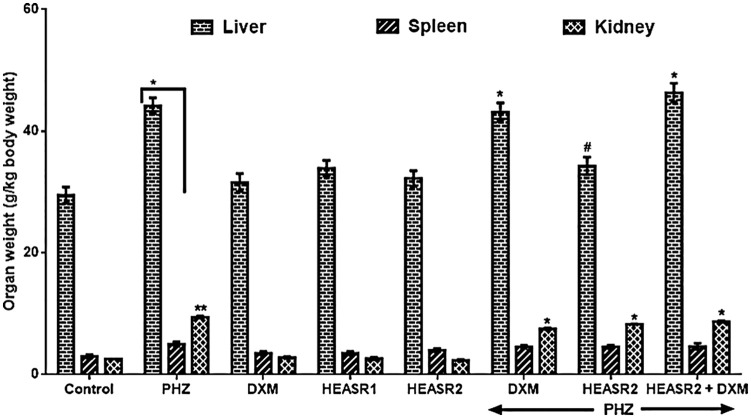
Organ weight
Phenylhydrazine administration elevated liver, kidney, and spleen weights (g/kg body weight) in rats by 49.78%, 67.22%, and 269.41%, respectively, when compared with control normal saline group (Figure 9). Hydroethanolic extract of A. smeathmannii root (200 mg/kg) administered alone reduced hepatic weight in the intoxicated rats by 22.36% (Figures 10 to 12).
Figure 9.
Effect of HEASR on organ weights in normal and phenylhydrazine-treated male Wistar rats. Results are expressed as mean ± SEM, n = 6. Unit: g/kg body weight. *P < .05 or **P < .001 when compared to control normal saline group. #P < .05 when compared to control phenylhydrazine group. DXM indicates dexamethasone; HEASR, hydroethanolic extract of A. smeathmannii root; HEASR1, 50 mg/kg of hydroethanolic extract of A. smeathmannii root; HEASR2: 200 mg/kg of hydroethanolic extract of A. smeathmannii root; PHZ, phenylhydrazine.
Figure 10.
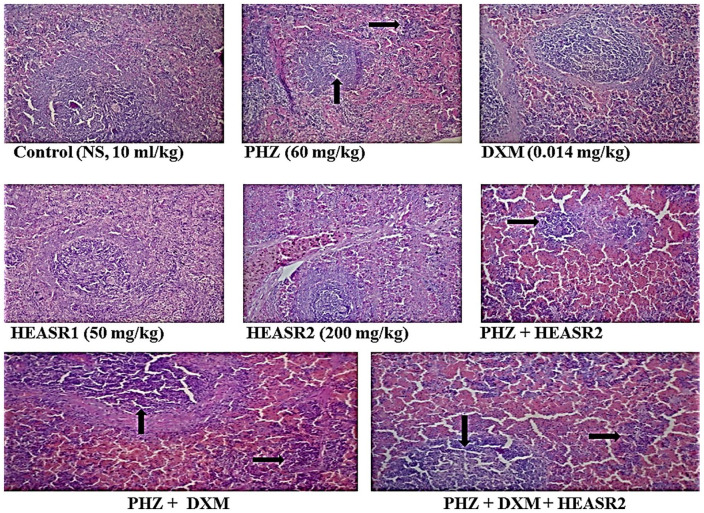
Splenic tissue of rat shows lymphoid aggregates which form follicles seen in control (normal saline, 10 mL/kg, p.o.) (NA); phenylhydrazine (PHZ, 60 mg/kg) (splenic congestion; see arrow); dexamethasone (DXM, 0.014 mg/kg); HEASR1 (50 mg/kg) (NA); HEASR2 (200 mg/kg) (NA); PHZ + HEASR2 (splenic congestion; see arrow); PHZ + DXM (splenic congestion; see arrow); PHZ + DXM + HEASR2 (splenic congestion; see arrow). HEASR indicates hydroethanolic extract of A. smathmannii Root (H & E, mag. ×400); NA, no abnormality.
Figure 12.
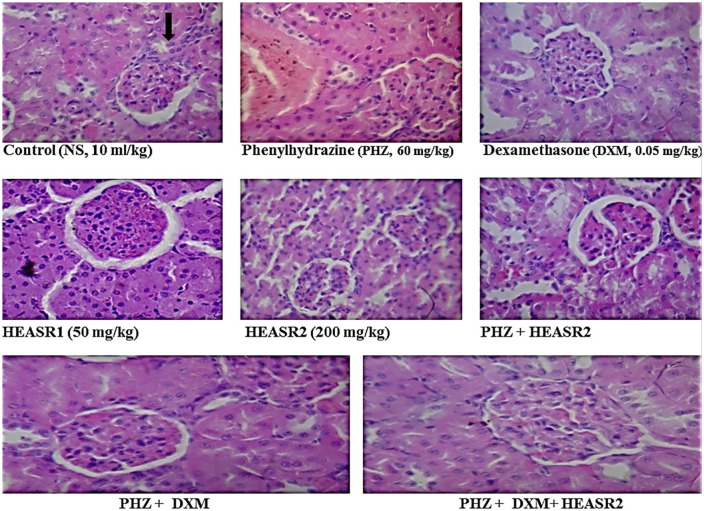
The sections of rat kidney tissue show normocellular glomerular tufts disposed on a background containing renal tubules of control (normal saline, 10 mL/kg, p.o.) (NA); phenylhydrazine (PHZ, 60 mg/kg) (vascular congestion; see arrow); dexamethasone (DXM, 0.014 mg/kg); HEASR1 (50 mg/kg) (NA); HEASR2 (200 mg/kg) (NA); PHZ + HEASR2 (NA); PHZ + DXM (NA); PHZ + DXM + HEASR2 (NA). HEASR indicates hydroethanolic extract of A. smathmannii Root. (H & E, mag. ×400); NA: no abnormality.
Figure 11.
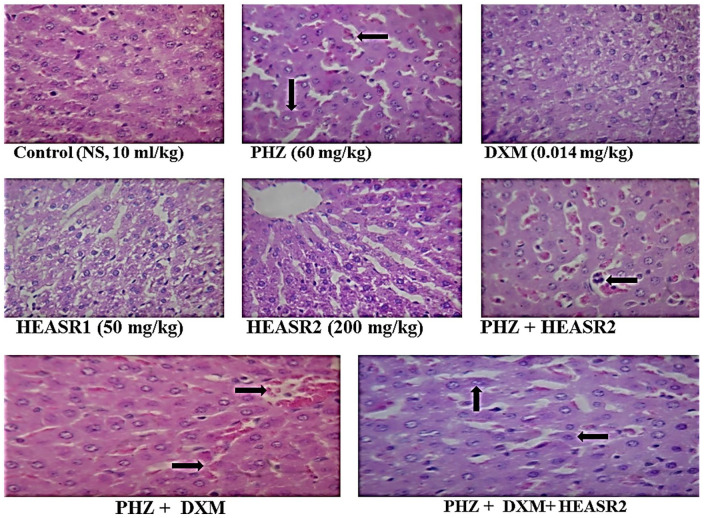
The sections of rat liver tissue show normocellular glomerular tufts disposed on a background containing renal tubules of control (normal saline, 10 mL/kg, p.o.) (NA); phenylhydrazine (PHZ, 60 mg/kg) (hepatic venous and sinus congestion; see arrow); dexamethasone (DXM, 0.014 mg/kg); HEASR1 (50 mg/kg) (NA); HEASR2 (200 mg/kg) (NA); PHZ + HEASR2 (hepatic venous and sinus congestion; see arrow); PHZ + DXM (hepatic venous and sinus congestion; see arrow); PHZ + DXM + HEASR2 (hepatic venous and sinus congestion; see arrow). HEASR indicates hydroethanolic extract of A. smathmannii Root (H & E, mag. ×400); NA, no abnormality.
Discussion
Several strategies for discovering drugs from previously unexplored natural products have continued to strengthen research and development with current commercial evidence supporting their applications. 4 Ethnomedicinal survey of plants used in the management of anaemia and related conditions in Southern Nigeria have been reviewed.2,42 Suggestions are that only a few of the known plants have been explored. Different substances have been implicated in acute anaemia; however, the deleterious effects due to PHZ has become apparent during chemically induced haematological and biochemical alterations, as well as oxidative stress in rodents. 18 Thus, several mechanisms of induction of HD due to PHZ resulting in haematotoxicity and free radical generations and deleterious oxidation of components of erythrocytes have been reported.11,18
Dexamethasone has long been recognized to have effects on human bone marrow stromal cells and been suggested to be required for erythropoiesis.43,44 More so, the mechanistic intervention of DXM in experimental anaemia is known. 44 It is a glucocorticoid that specifically stimulates self-renewal of the early erythroid progenitor, the burst-forming unit erythroid (BFU-E) and can also increase the production of terminally differentiated erythroid cells.30,44 For instance, corticosteroids have been used to treat patients with Diamond-Blackfan anaemia (DBA), a rare congenital disorder characterized by disorder of blood and a selective decrease or absence of erythroid precursors in otherwise normocellular bone marrow. 45 Phenylhydrazine is an oxidant compound shown to inflict damage on the cell membrane producing gradual haematological alterations, inflammatory mediators, and increased red cells apoptosis.46,47
The oral acute toxicity test results from this study showed no mortality up to 2000 mg/kg orally. In line with the aforementioned, we recently reported the aphrodisiac potential and reproductive enhancing functions of A. smeathmannii containing octadecanoic acid ethyl ester as its most abundant bioactive component. 12 Studies have provided convergent reports on in vitro haemolysis resulting from loss of antioxidant capacity. However, HEASR demonstrated significant in vitro antioxidant activity in the NOSA, DPPH, HORSA, LPOSA, and FRAP models used in this study. More so, from the results obtained, HGB, RBC, and WBC increase might have prevented from PHZ intoxication. There is a link between oxidation and denaturation of HGB which presumably results from alterations in the cell membrane itself. 16 Interestingly, HEASR showed a tendency to improve haematological indices in this study. Phenylhydrazine haematoxicity in rats may be detected based on hepatic changes in the expression of a subset of genes which are mechanistically linked to haematotoxicity. 48 Treatments with HEASR2, DXM, or their combination showed a tendency to protect against increase in the levels of liver and renal toxicity biomarkers in rodents by moderately regulating AST, urea, and uric acid levels, respectively. Whether this effectiveness will translate into a clinically relevant situation requires further investigations.
Reports have it that direct-acting haemolytic agents attenuate lipids and proteins as major targets of their pro-oxidant actions. 19 A cross-linking of membrane proteins by disulfide exchange with precipitated HGB has been suggested to play a major role in decreasing deformability during PHZ exposure. 46 More so, PHZ generates ROS within both human and rat erythrocytes by increasing lipid peroxidation and decreasing GSH levels, respectively.18,49 Glutathione system, or CAT and superoxide activities are present in the erythrocytes which contribute considerably to the antioxidant capacity of the blood. 47 Therefore, when the condition of anaemia arises, this ability is compromised, resulting in oxidative stress and subsequently acute HD. The ROS which is produced in excess due to PHZ induction could lead to the oxidization of lipids, proteins, and DNA molecules, and this could eventually lead to the death of cells and organ damage. Reports have suggested that anaemia aggravates and contributes to oxidative stress in the body.18,47 Evidence abounds that oxidative damage to erythroid cells plays a fundamental role in haemolytic process due to ineffective erythropoiesis in the bone marrow and short survival of red blood cells in the circulation. 47 Studies have reported consistency in the tissue oxidative stress than in blood due to PHZ intoxication.13,16-19 Although an elevated MDA level was observed following an acute PHZ dosing in the liver and spleen, HEASR2 administration was able to lower hepatic MDA relative to the PHZ intoxicated rats. There have been suggestions that red cells susceptibility to haemolysis involves a failure to maintain the GSH level due to haemolytic process.48,50 Although, antioxidants (GSH, SOD, and CAT) were not directly measured in blood; however, studies have also reported consistency in the tissue antioxidants level in this regard due to PHZ intoxication.48,50 The administration of HEASR1 or HEASR2 caused elevation of hepatic GSH level in vital organs in rats in this study. Phenylhydrazine prevented the action of the antioxidant system by inducing free radical changes which in turn resulted in oxidative stress. Superoxide dismutase level in the PHZ group was lowered in the rat spleen which was reversed by HEASR2, DXM, and their combination in the treated rats. Similarly, an increase in CAT level was achieved in the liver and spleen at the early phase of PHZ intoxication. This may, in part, be due to the previous exposure to HEASR prior to PHZ exposure. Organ weight relative to animal weight was modulated in the treated rats, although, weight lost was not reversed in the treated rats.
More so, PHZ-induced erythrocyte deformity was suggested to decrease erythropoietin and corticosterone levels, and increase osmotic resistance and secondary tumour biomarkers to produce haematotoxicity in rodents. Recently, the effectiveness of recombinant human erythropoietin in an animal model of oxidative stress and genotoxicity was reported by Rjiba-Touati et al. 50 Although, our present report did not estimate erythropoietin level, an elevation of COX-2 activity usually accompanies tissue damage which in this study resulted from PHZ exposure. Phenylhydrazine induction of HD and associated oxidative stress did not only cause elevated serum COX-2 activity but also reduced corticosterone levels, respectively, in normal rats. Cyclooxygenase-2 is the second isoenzyme of the cyclooxygenase enzymes, the other (COX-1) being constitutive in the gastrointestinal tract; it catalyses arachiodionic acid metabolism following stimulation. 29 On the contrary, the glucocorticoids are compounds naturally regulated by hormones of the hypothamo-pituitary axis.44,45 Thus, corticosteroid acts as anti-inflammatory substance in the body.30,44 In addition, the haematopoietic system performance respond to corticosteroid stimulation, and this has been used for a decade before erythropoietin synthesis.44,45 Here, HEASR lowered COX-2 activity in PHZ-untreated rats and improved corticosterone levels in rats. Phenylhydrazine-induced haemolytic condition causes stimulation of erythropoiesis which is associated with increased functional activity of erythroid precursors and resulting in changes in the regulatory capacity of a haemopoietic milieu of the plasma. 51 Thus, the increase in corticosterone level may possibly be due to the stimulation of erythropoiesis primarily in human bone marrow progenitor cells. Our findings indicate that HEASR combined with DXM has a distinct functional effect and may have therapeutic benefit in combination. Thus, ethnorelevance of HEASR may be used in several cases of occupational exposure to PHZ with suggestions that the liver, kidney, and spleen are potential targets, thereby preventing HD. Splenomegaly of PHZ animals was not diminished by HEASR; however, HEASR or DXM alone and their combination ameliorated renovascular abnormality in rats. This indicates that further study may be required to explore the renoprotective effects of HEASR in rodents. Moreover, the protective effects demonstrated by HEASR following PHZ toxicity as observed in this study were predicated on the modulation of biochemical, haematological, as well as morphological characterizations. Still, further study may, in part, provide an explanation for the possible molecular mechanisms between bioactive compounds present in HEASR and PHZ, while investigating further into how bioactivity guided fractionation studies would yield a drug candidate.
Conclusion and Recommendations
Overall, results from this study show that HEASR demonstrated increased HGB, RBC, PLT, and WBC, and lowered liver toxicity biomarkers, lipid peroxidation plus COX-2 activity in annulling PHZ-induced haematological and biochemical alterations in rats. This was as a result of increased antioxidant activities both in vitro and in vivo, as well as increased corticosterone levels in the treated animals. In addition, HEASR ameliorated renal injury induced by PHZ. The molecular aspect of this interaction and study on toxicological profile are essential.
Acknowledgments
The technical assistance of Mr. Adenekan S of the Department of Biochemistry, University of Lagos, Lagos, Nigeria is gratefully acknowledged.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: OEK, OA, and AJA conceived and designed the study, drafted the first version of the manuscript, and critically revised and approved the final version.
Disclosure and Ethics: The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. There are no conflicts of interest to declare. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors also confirm that this article is unique and not under consideration for publication or already published in any other Journal, and no copyrighted material is reproduced.
ORCID iD: Oluwafemi Ezekiel Kale  https://orcid.org/0000-0003-2965-1723
https://orcid.org/0000-0003-2965-1723
References
- 1. Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007;4:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Awodele O, Popoola TD, Amadi KC, Coker HA, Akintonwa A. Traditional medicinal plants in Nigeria – remedies or risks. J Ethnopharmacol. 2013;150:614-618. [DOI] [PubMed] [Google Scholar]
- 3. Catarino L, Havik PJ, Romeiras MM. Medicinal plants of Guinea-Bissau: therapeutic applications, ethnic diversity and knowledge transfer. J Ethnopharmacol. 2016;183:71-94. [DOI] [PubMed] [Google Scholar]
- 4. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111-129. [DOI] [PubMed] [Google Scholar]
- 5. Akintonwa A, Awodele O, Afolayan G, Coker HA. Mutagenic screening of some commonly used medicinal plants in Nigeria. J Ethnopharmacol. 2009;125:461-470. [DOI] [PubMed] [Google Scholar]
- 6. Davis CC, Anderson WR. A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Am J Bot. 2010;97:2031-2048. [DOI] [PubMed] [Google Scholar]
- 7. Akindele AJ, Adeneye AA, Salau OS, Sofidiya MO, Benebo AS. Dose and time-dependent sub-chronic toxicity study of hydroethanolic leaf extract of Flabellaria paniculata Cav. (Malpighiaceae) in rodents. Front Pharmacol. 2014;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morton CV. A typification of some subfamily, sectional, and subsectional names in the family malpighiacae. Taxon. 1968;17:314-324. [Google Scholar]
- 9. Ainslie JR. A list of plants used in native medicine in Nigeria. Imperial Forestry, Institute Paper 7. Published 1937. [Google Scholar]
- 10. Van Andel TR, Croft S, Van Loon EE, Quiroz D, Towns AM, Raes N. Prioritizing West African medicinal plants for conservation and sustainable extraction studies based on market surveys and species distribution models. Biol Conserv. 2015;181:173-181. [Google Scholar]
- 11. Stevens GA, Bennett JE, Hennocq Q, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health. 2015;3:e528-e536. [DOI] [PubMed] [Google Scholar]
- 12. Berger J. Phenylhydrazine haematotoxicity. J Appl Biomed. 2007;5:125-130. [Google Scholar]
- 13. Kale OE, Awodele O, Akindele AJ. Acridocarpus Smeathmannii (DC.) Guill. & Perr. Root enhanced reproductive behaviour and sexual function in male Wistar rats: biochemical and pharmacological mechanisms. J Ethnopharmacol. 2019;230:95-108. [DOI] [PubMed] [Google Scholar]
- 14. Guillaud C, Loustau V, Michel M. Hemolytic anemia in adults: main causes and diagnostic procedures. Expert Rev Hematol. 2012;5:229-241. [DOI] [PubMed] [Google Scholar]
- 15. Awodele O, Akindele AJ, Adebowale GO, Adeyemi OO. Polycyclic aromatic hydrocarbon, haematological and oxidative stress levels in commercial photocopier operators in Lagos, Nigeria. Ghana Med J. 2015;49:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biswas S, Bhattacharyya J, Dutta AG. Oxidant induced injury of erythrocyte-role of green tea leaf and ascorbic acid. Mol Cell Biochem. 2005;276:205-210. [DOI] [PubMed] [Google Scholar]
- 17. Sochaski MA, Bartfay WJ, Thorpe SR, et al. Lipid peroxidation and protein modification in a mouse model of chronic iron overload. Metabolism. 2002;51:645-651. [DOI] [PubMed] [Google Scholar]
- 18. Luangaram S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P. Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem Toxicol. 2007;45:448-455. [DOI] [PubMed] [Google Scholar]
- 19. McMillan DC, Powell CL, Bowman ZS, Morrow JD, Jollow DJ. Lipids versus proteins as major targets of pro-oxidant, direct-acting hemolytic agents. Toxicol Sci. 2005;88:274-283 [DOI] [PubMed] [Google Scholar]
- 20. Pérez-Jiménez J, Arranz S, Tabernero M, et al. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: extraction, measurement and expression of results. Food Res Int. 2008;41:274-285. [Google Scholar]
- 21. Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Özyürek M, Bektaşoğlu B, Güçlü K, Apak R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal Chim Acta. 2008;616:196-206. [DOI] [PubMed] [Google Scholar]
- 23. Singh G, Maurya S, DeLampasona MP, Catalan CA. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem Toxicol. 2007;45:1650-1661. [DOI] [PubMed] [Google Scholar]
- 24. Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by ‘ferric reducing antioxidant power’ assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721:174-184. [DOI] [PubMed] [Google Scholar]
- 25. Skerget M, Kotnik P, Hadolin M, Hras AR, Simonis M, Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191-198. [Google Scholar]
- 26. Borokini TI, Omotayo FO. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plants Res. 2012;6:1106-1118. [Google Scholar]
- 27. Paez V, Barrett WB, Deng X, et al. AOAC SMPR(®) 2016.002. J AOAC Int. 2016;99:1122-1124. [DOI] [PubMed] [Google Scholar]
- 28. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cerebr Blood F Met. 2011;31:991-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kale OE, Awodele O, Akindele AJ. Subacute and subchronic oral toxicity assessments of Acridocarpus Smeathmannii (DC.) Guill. & Perr. root in Wistar rats. Toxicol Rep. 2019;6:161-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HY, Gao X, Barrasa MI, et al. PPAR-α and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature. 2015;522:474-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Organisation for Economic Co-operation and Development. Test No. 420: Acute Oral Toxicity – Fixed Dose Procedure. Paris, Paris: OECD Publishing; 2002. [Google Scholar]
- 32. Nevin KG, Rajamohan T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin Biochem. 2004;37:830-835. [DOI] [PubMed] [Google Scholar]
- 33. Bairaktari ET, Seferiadis KI, Elisaf MS. Evaluation of methods for the measurement of low-density lipoprotein cholesterol. J Cardiovasc Pharmacol Ther. 2005;10:45-54. [DOI] [PubMed] [Google Scholar]
- 34. Rito-Palomares M, Dale C, Lyddiatt A. Generic application of an aqueous two-phase process for protein recovery from animal blood. Process Biochem. 2000;35:665-673. [Google Scholar]
- 35. Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159-3165. [DOI] [PubMed] [Google Scholar]
- 36. Zhou JY, Prognon P. Raw material enzymatic activity determination: a specific case for validation and comparison of analytical methods – the example of superoxide dismutase (SOD). J Pharm Biomed Anal. 2006;40:1143-1148. [DOI] [PubMed] [Google Scholar]
- 37. Iwase T, Tajima A, Sugimoto S, et al. A simple assay for measuring catalase activity: a visual approach. Sci Rep. 2013;3:3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067-4079. [DOI] [PubMed] [Google Scholar]
- 39. Kale OE, Awodele O. Safety evaluation of Bon-santé cleanser® polyherbal in male Wistar rats. BMC Complement Altern Med. 2016;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scoditti E, Nestola A, Massaro M, et al. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis. 2014;232:17-24. [DOI] [PubMed] [Google Scholar]
- 41. Kobayashi N, Iwakami K, Kotoshiba S, et al. Immunoenzymometric assay for a small molecule, 11-deoxycortisol, with attomole-range sensitivity employing an scFv−enzyme fusion protein and anti-idiotype antibodies. Anal Chem. 2006;78:2244-2253. [DOI] [PubMed] [Google Scholar]
- 42. Amujoyegbe OO, Idu M, Agbedahunsi JM, Erhabor JO. Ethnomedicinal survey of medicinal plants used in the management of sickle cell disorder in Southern Nigeria. J Ethnopharmacol. 2016;185:347-360. [DOI] [PubMed] [Google Scholar]
- 43. Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang L, Prak L, Rayon-Estrada V, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vilsen B, Nielsen H. Reaction of phenylhydrazine with erythrocytes: cross-linking of spectrin by disulfide exchange with oxidized hemoglobin. Biochem Pharmacol. 1984;33:2739-2748. [DOI] [PubMed] [Google Scholar]
- 47. Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008;8:609-619. [DOI] [PubMed] [Google Scholar]
- 48. Rokushima M, Omi K, Araki A, et al. A toxicogenomic approach revealed hepatic gene expression changes mechanistically linked to drug-induced hemolytic anemia. Toxicol Sci. 2007;95:474-484. [DOI] [PubMed] [Google Scholar]
- 49. Claro LM, Leonart MS, Comar SR, do Nascimento AJ. Effect of vitamins C and E on oxidative processes in human erythrocytes. Cell Biochem Funct. 2006;24:531-535. [DOI] [PubMed] [Google Scholar]
- 50. Rjiba-Touati K, Ayed-Boussema I, Guedri Y, Achour A, Bacha H, Abid-Essefi S. Effect of recombinant human erythropoietin on mitomycin C-induced oxidative stress and genotoxicity in rat kidney and heart tissues. Hum Exp Toxicol. 2016;35:53-62. [DOI] [PubMed] [Google Scholar]
- 51. Zyuz’kov GN, Abramova EV, Dygai AM, Gol’dberg ED. Mechanisms of regulation of erythropoiesis during hemolytic anemia. Bull Exp Biol Med. 2004;138:334-337. [DOI] [PubMed] [Google Scholar]