Abstract
Objectives:
To assess the outcomes of Botulinum Toxin-A (BoNT-A) to the external urethral sphincter (EUS) in dysfunctional voiding (DV) refractory to standard urotherapy and bowel management.
Methods:
Our criteria to diagnose DV in women included neurologically normal individuals with lower urinary tract symptoms, dilated proximal urethra on voiding cystourethrogram, and high detrusor pressure (PdetQmax > 20 cm H2O) associated with increased electromyography activity during voiding in urodynamic study (UDS). A total of 16 female patients with a median age of 36 years (5-60 years) received BoNT-A from June 2014 to December 2015. Patients below and above 10 years of age received 100 units and 200 units of BoNT-A to EUS, respectively. Patients were followed up till 6 months.
Results:
Mean AUA (American Urological Association) symptom score decreased significantly from 11.75 ± 6.14 to 5.06 ± 5.1 and 4.25 ± 3.4 at day 14 and day 45 after BoNT-A, respectively (P < .0001). There were no significant improvements in maximal flow (Qmax) on uroflowmetry (UFM) and detrusor pressure at maximal flow (PdetQmax) in UDS. Significant reduction in post-void residual (PVR) from 69.31 ± 77.3 to 17.50 ± 22.3 mL at day 14 (P = .007) was observed, although the reduction was not significant at day 45. Although minor adverse effects were reported, none were serious or life-threatening.
Conclusions:
Our study showed that BoNT-A plays a role in improvement of urinary symptoms and reduces PVR at D14 in DV, but showed no improvement in UFM and urodynamic parameters, albeit with limited numbers and limited follow-up.
Keywords: Botulinum Toxin-A, dysfunctional voiding, spinning top urethra, urinary tract infection, urodynamic study
Introduction
International Continence Society (ICS) defines dysfunctional voiding (DV) as intermittent or fluctuating flow rate due to involuntary intermittent contractions of the periurethral striated muscle during voiding in neurologically normal individuals. 1
In tertiary care centers, DV constitutes up to 40% of referrals in the Pediatric Urology department. 2 In urology centers treating adults as well, DV is usually noted in 0.5% to 2% of the patients. 3 It is thought to be often acquired in the childhood, during toilet training when the children learn to control urethral sphincteric activity, but can also appear in older ages, with higher frequency in the female sex. 4
Where patients are refractory to the conservative measures (urotherapy, bowel care, anticholinergics/α-blockers, and biofeedback), botulinum neurotoxin-A (BoNT-A) is a therapeutic option which results in significant subjective and objective improvements. Neuromodulation being the other option is still in its infancy in India.
Unfortunately, there are very limited worldwide data and no Indian data are available on the use of BoNT-A in refractory DV as there is no universal agreement regarding the diagnostic features and monitoring of DV. We present our results of BoNT-A to external urethral sphincter (EUS) in patients with refractory DV.
Methods
Study design
A total of 16 female patients were included after fulfilling our diagnostic criteria in this prospective, single-group assignment interventional study done from June 2014 to May 2015 with a follow-up period of 6 months till December 2015. Approval from the Institutional Review Board was obtained.
Diagnosis
Diagnosis of DV was established by history, clinical examination, imaging (ultrasound abdomen, voiding cystourethrogram [VCUG]), uroflowmetry (UFM) and urodynamic study (UDS). Magnetic resonance imaging of spine was done in all pediatric patients to exclude occult neurological disease.
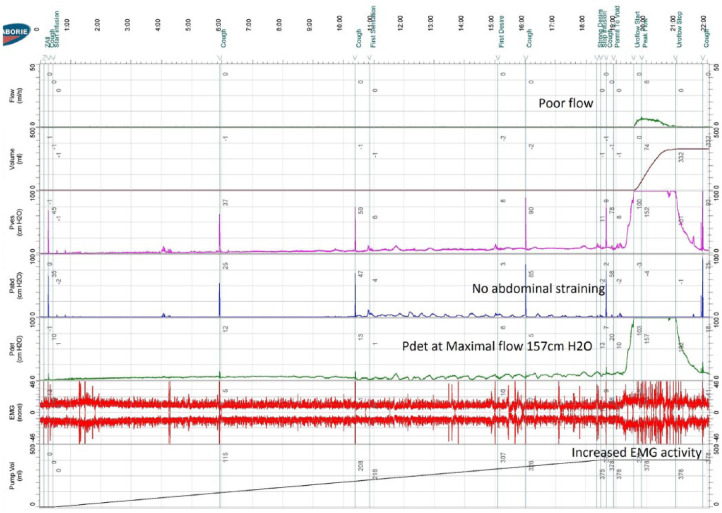
Although there are no exact criteria to diagnose DV, our diagnostic criteria included neurologically normal individuals with lower urinary tract symptoms, dilated proximal urethra (spinning top urethra) on VCUG, and high detrusor pressure (PdetQmax > 20 cm H2O) associated with increased electromyography (EMG) activity during voiding in UDS (Figure 1).
Figure 1.
Classical features of dysfunctional voiding on urodynamic study.
The inclusion criteria were as follows: female patients aged above 5 years diagnosed with DV, who had persistent symptoms or ongoing complications despite urotherapy and bowel care for a period of 4 weeks.
Voiding cystourethrogram was done in all cases. A tiny lead clip (5 mm size) was placed at meatus, prior to cystogram, to help us know the exact location of meatus on VCUG. We have also measured the length of dysfunctional segment (which we have termed so) on VCUG image in our hospital database software to help us guide the depth of BoNT-A injection into the EUS (Figure 2).
Figure 2.
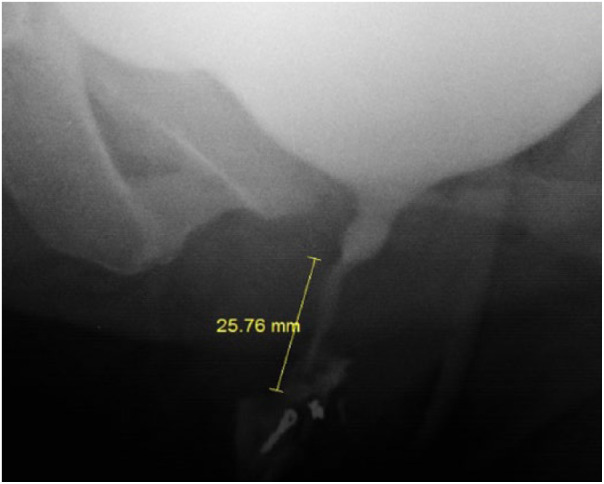
Length of the dysfunctional segment on VCUG.
We used Laborie Delphis urodynamics machine (model UDS-94-BT) with UDS double lumen 10F rectal catheter and UDS double lumen 5F to 8F urethral catheter. Surface electrodes were used for EMG recordings. Pediatric UDS was done under intravenous sedation with propofol and fentanyl in the presence of an anesthesiologist.
Urotherapy counseling was done by our procedure room staff using PowerPoint presentations and every patient received a public information brochure on urotherapy. Bowel care was with oral laxatives and enemas if needed. Those who had persistent symptoms or ongoing complications despite urotherapy and bowel care were considered for BoNT-A to EUS.
Technique of injection
Each vacuum dried vial of BoNT-A was reconstituted with 6 mL of sterile 0.9% sodium chloride solution. Under appropriate anesthesia (usually intravenous sedation) in operation theater, we injected BoNT-A to the EUS in lithotomy position as per the following dosage schedule:
Subjects below 10 years of age: 100 units;
Subjects above 10 years of age: 200 units.
In female subjects, 1 mL to each site was given periurethrally to the length of dysfunctional segment (measured on VCUG) using a 24-gauge needle at 1-, 3-, 5-, 7-, 9-, and 11-o’clock positions of the urethral meatus without using a cystoscope. Metal probe was used to assess the direction of the urethra initially (Figure 3) and then inject accordingly without inadvertent spillage of BoNT-A intraluminally. Care was taken to inject more into the dorsal aspect of EUS as the EUS is thick dorsally.
Figure 3.
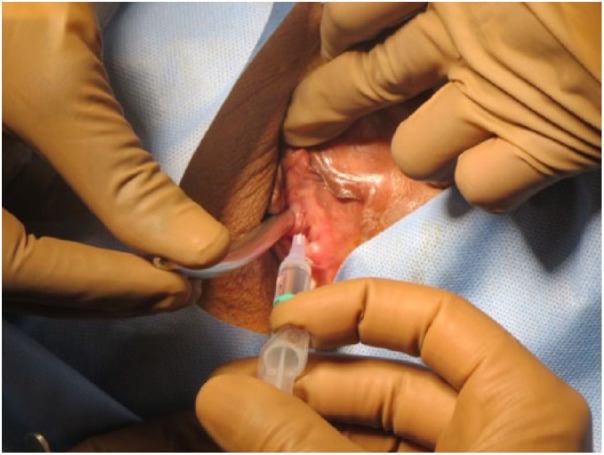
Botulinum Toxin-A being injected to external urethral sphincter.
No prophylactic antibiotics were given.
Follow-up protocol
Table 1 shows the follow-up protocol.
Table 1.
Follow-up protocol (6 months [180 days]).
| Time period, d | Evaluation |
|---|---|
| 14 ± 3 | History, clinical examination, 3-day bladder diary, PVR on USG, UFM, and urine culture |
| 45 ± 3 | History, clinical examination, 3-day bladder diary, USG-KUB, UDS, VCUG, and urine culture |
| 90 ± 4 | History, clinical examination, 3-day bladder diary and urine culture |
| 180 ± 7 | History, clinical examination, 3-day bladder diary and urine culture |
Abbreviations: PVR, post-void residual; UDS, urodynamic study; UFM, uroflowmetry; USG-KUB, ultrasound—kidney, ureter, and bladder; VCUG, voiding cystourethrogram.
Evaluation of treatment outcome
The following outcomes were evaluated:
Objective improvement using AUA (American Urological Association) symptom score at day 14 and day 45;
Improvement in Qmax on UFM at D14 and D45;
Reduction in post-void residual (PVR) at D14 and D45;
Decrease in PdetQmax on UDS at D45.
Statistical methods
Data were analyzed using standard Statistical Software “Statistical Package for the Social Sciences” (SPSS) version 20. Paired-sample t test was used to compare means of before injection and after injection. Here, the normality assumption was not fully satisfied especially for the PVR parameters, so we also used Wilcoxon signed rank test which showed the same results. The results were tested for significance at P < .05.
Results
A total of 16 female patients with a median age of 36 years (5-60 years) and mean age of 33.93 years (SD ±17.69) were analyzed during the study period. Average glomerular filtration rate at presentation was 100.4 mL/min. Six patients did not come for follow-up clinical visit after 45 days, although they were available for follow-up telephonically till 6 months. The remaining 10 patients were available for a minimum of 6 months of follow-up as per the protocol.
Nine patients (56%) had coexistent bowel dysfunction diagnosed based on grossly loaded bowel on plain X-ray abdomen with/without clinical history of constipation. These patients were treated for constipation accordingly by oral laxatives and/or soap water enema once daily.
All patients except one (94%) had a dilated proximal urethra (spinning top deformity) on VCUG. The average length of dysfunctional segment on VCUG was 18.1 mm.
Two (2/15) patients had poor bladder compliance and 3 (3/15) patients had detrusor overactivity on UDS at presentation.
Primary outcomes
The primary outcomes are summarized in Table 2.
Table 2.
Primary outcomes of the study.
| Parameters | Before BoNT-A |
After BoNT-A |
||||||
|---|---|---|---|---|---|---|---|---|
| n | 14 d | 45 d | 180 d | |||||
| n | n | n | ||||||
| AUA symptom score | 16 | 11.75 ± 6.14 | 16 | 5.06 ± 5.1 (P < .001) |
12 | 4.25 ± 3.4 (P < .001) |
NA | NA |
| Qmax on UFM, mL/s | 16 | 14.7 ± 8.2 | 16 | 15.01 ± 6.3 (P = .084) |
9 | 16.36 ± 5.5 (P = .085) |
NA | NA |
| PdetQmax on UDS, mm Hg | 8 | 61.25 ± 25.6 | NA | NA | 8 | 56.75 ± 20.4 (P = .234) | NA | NA |
| PVR, mL | 16 | 69.31 ± 77.3 | 16 | 17.50 ± 22.3 (P = .007) |
10 | 25.30 ± 22.4 (P = .128) | NA | NA |
| UTI episodes | 14 | 2.6 ± 1.62 | NA | NA | NA | NA | 14 | 0.87 ± 1.25 (P < .001) |
Abbreviations: AUA, American Urological Association; BoNT-A, botulinum toxin A; PVR, post-void residual; UTI, urinary tract infection; NA, not applicable.
Mean AUA symptom score improved significantly (P < .0001) from 11.75 ± 6.14 (prior to BoNT-A) to 5.06 ± 5.1 and 4.25 ± 3.4 at day 14 and day 45 after BoNT-A, respectively.
There was no significant improvement in maximal flow (Qmax) on UFM from 14.70 ± 8.2 to 15.01 ± 6.3 mL/s at day 14 and 16.36 ± 5.5 mL/s. Although 8 of 15 (53.3%) patients had abnormal flow pattern (interrupted/staccato) on UFM at presentation, the flow pattern was normal in 12 of 15 (80%) patients at 15 days. One patient was on clean intermittent catheterisation (CIC) and hence UFM was not done.
There was significant reduction in PVR from 69.31 ± 77.3 mL (prior to BoNT-A) to 17.50 ± 22.3 mL at day 14 (P = .007), but the reduction in PVR at day 45 (25.30 ± 22.4 mL) was not significant (P = .128).
Although our follow-up protocol had mandatory UDS at 45 days after BoNT-A, only 9 patients agreed for UDS after 45 days of BoNT-A. Of the 9 patients, 1 patient did not void during UDS prior to BoNT-A, but voided with PdetQmax of 45 cm Normal saline (NS) during UDS only after BoNT-A.Hence, his PdetQmax value was not included for statistical analysis. In the remaining 8 patients, no significant decrease in PdetQmax (61.25 ± 25.6 to 56.75 ± 20.5) was observed at 45 days after BoNT-A. The EMG activity was increased in 6 of the 13 patients who voided during UDS prior to BoNT-A. After BoNT-A, 4 of the 13 patients continued to have increased EMG activity.
Of the 14 patients who had recurrent urinary tract infection (UTI), number of UTI episodes 6 months before and after BoNT-A (P < .0001) reduced from 2.6 ± 1.62 to 0.87 ± 1.25. Although this was statistically significant, the clinical inference from this finding is still ambiguous in view of short follow-up of 6 months to assess UTI.
Secondary outcomes
Three of our patients had vesicoureteral reflux (VUR) prior to BoNT-A (2 unilateral and 1 bilateral), but there was no change in the grade of VUR on VCUG after 45 days of BoNT-A.
Three patients received reinjections (200 units each) between 4 and 8 months after initial satisfactory results. The indications for reinjections were as follows. (1) One patient who had undergone right radical nephrectomy for emphysematous pyelonephritis continued to have persistently high PdetQmax in the follow-up UDS. (2) The second patient who did not require CIC after BoNT-A again developed the need for CIC after 5 months. She also had persistently high PdetQmax in the follow-up UDS. (3) The third patient who was continent after BoNT-A developed incontinence after 4 months of BoNT-A. She also had left grade V VUR and persistently high PdetQmax in the follow-up UDS.
No serious adverse events were reported after BoNT-A treatment, although minor events were noted (Table 3).
Table 3.
Adverse events in our patients after botulinum toxin A.
| S. no. | Adverse events | No. of patients |
|---|---|---|
| 1 | Fever | 1 |
| 2 | Headache | 1 |
| 3 | Urge incontinence | 1 |
| 4 | Stress incontinence | 1 |
| 5 | Burning micturition | 1 |
| 6 | Right shoulder pain | 1 |
| 7 | Left lower limb weakness | 1 |
Discussion
This study was done over a period of 18 months (including 6 months of follow-up). Although most of the studies on DV included children only, this study included both children and adults. The sample size in most of the studies is 10 to 25.5–8 Dysfunctional voiding is usually noted in 0.5% to 2% of outpatient department patients in tertiary urological centers. 3 Urotherapy needs to be taught and its compliance has to be checked at regular intervals. Convincing refractory patients for BoNT-A after primary therapy is a difficult task considering the duration of efficacy and the cost of BoNT-A. Our study included 16 female patients who continued to have persistent symptoms after urotherapy and bowel care.
We have used AUA symptom score to assess the subjective improvement after BoNT-A. We did not use the DV scoring system described by Farhat et al, 2 as it applies to children only. Most of our patients were adults; hence, to maintain the uniformity, we have used AUA symptom score. We have noted significant improvement in AUA symptom score from 11.7 to 5.1 at 14 days. ’t Hoen et al 8 reported 80% improvement in urinary incontinence in 20 children following BoNT-A to EUS. Mokhless et al 7 reported significant improvement in symptoms in 90% (9/10) of his patients. Radojicic et al 5 noted improvement in voiding symptoms in 85% (17/20) of the patients.
One patient in our study who was on daily CIC did not require CIC for a period of 5 months after BoNT-A. She received repeat injection after 5 months following which she resumed her normal voiding, without CIC. The study by Mokhless et al 7 included 10 patients with DV, of which 9 were on CIC, all 9 (100%) did not require CIC after BoNT-A.
Most of the papers on BoNT-A in DV have PVR as the only primary outcome measure.7–9 We noted a significant reduction in PVR at day 14, although the effect seemed to wear off by day 45. The main outcome measure in the study by ’t Hoen et al 8 , PVR, significantly decreased by 75% from a median of 47.5 to 0.0 mL after 6 to 12 weeks of BoNT-A. The study by Mokhless et al 7 reported marked improvement in PVR, which decreased from 325 ± 166 mL preoperatively to 36.67 ± 29 mL at 2 weeks and 62.5 ± 61 mL at 3 months. In the study by Radojicic et al 5 , 6 months after treatment, PVR significantly decreased in 17 of 20 patients by 0 to 130 mL (mean 45.75 ± 32.17 mL). Vricella et al 9 also reported significant improvement in mean PVR from 115 ± 83 vs 57 ± 61 mL before vs after treatment, respectively.
We did not notice significant improvement in Qmax, although we did notice improvement in flow patterns in 80% of our patients who had abnormal flow patterns prior to BoNT-A. Similar to our results, ’t Hoen et al 8 also did not notice significant improvement in Qmax but noticed improvement in flow patterns in 90% (18/20) of their patients. Radojicic et al 5 observed improvement in flow patterns in 85% (17/20) of the patients but did not mention on the changes in Qmax. In contrary to our study, Mokhless et al 7 observed significant improvement in Qmax from 2.17 ± 2.48 to 17.82 ± 8.13 mL/s at 2 weeks and 16.8 ± 10.2 mL/s at 1 month.
In our study, 2 patients had unilateral VUR and 1 had bilateral VUR. There was no downgrading/resolution of VUR in all 3 patients after BoNT-A. Of the 4 children with bilateral VUR, Mokhless et al 7 reported downgrading in VUR in 2 and the other 2 had complete resolution. One child with unilateral VUR showed complete resolution after BoNT-A.
Suskind et al 10 reported physical discomfort in 42.9% and anxiety in 27.7% in 314 patients who underwent UDS. Although our follow-up protocol had mandatory UDS at 45 days after BoNT-A, only 9 patients agreed for UDS after 45 days of BoNT-A. Of the 9 patients, 1 patient did not void during UDS prior to BoNT-A, but voided with PdetQmax of 45 cm NS during UDS only after BoNT-A. Hence, her PdetQmax value was not included for statistical analysis. In the remaining 8 patients, no significant decrease in PdetQmax was observed at 45 days after BoNT-A.
Sinha 11 mentioned in his review article that 90% of the children with DV show detrusor overactivity, a fact that explains the high prevalence of storage symptoms. In contrary, we noticed detrusor overactivity in only 20% (3/15) of our patients on UDS.
We used surface electrodes to monitor the EMG activity during UDS as it is painless, comfortable to the patients, and easy to monitor. The EMG activity was increased in 6 of 13 patients who voided during UDS prior to BoNT-A. After BoNT-A, 4 of 13 patients continued to have increased EMG activity. Steward et al 12 and many others have reported that concentric needle recordings detected more local activity than wire or surface electrodes. Hence, it could be said that the persistence of increased EMG activity even after BoNT-A could be due to the activity of other perineal muscles such as levator ani and anal sphincter.
The 2009 European consensus report 13 mentioned 26% improvement in detrusor leak point pressure (DLPP) in adults after BoNT-A injection to EUS.
The only study in children which has done UDS after BoNT-A to EUS is by Mokhless et al, 7 which reported significant reduction in DLPP from 66 ± 18 cm of H2O to 37 ± 4 cm of H2O.
The number of UTI episodes reduced from 2.6 ± 1.62 to 0.87 ± 1.25 in 6 months in 14 patients who had prior recurrent UTI. In view of small numbers and short follow-up of 6 months to assess UTI in this study, it would be difficult to conclude the positive role of BoNT-A in UTI. In the study by ’t Hoen et al of 20 children, 11 experienced recurrent UTI prior to BoNT-A. During 13 months median follow-up, 5 remained infection free, whereas the other 6 experienced only one episode of UTI. 8 In the study by Radojicic et al with a follow-up up to 14 months after BoNT-A, all 20 patients were without UTI and fever, whereas 5 were still on chemoprophylaxis as their urine cultures were positive. 5 Vricella et al 9 reported 4 of 7 patients (57%) with recurrent UTIs were able to discontinue antibiotic prophylaxis.
In this study, a dose of 200 units of BoNT-A was used. Various studies have used BoNT-A to EUS in the range of 50 to 300 units.6–8 No consensus has been reached about what dose will achieve the optimal effect without causing side effects.
Use of a metal probe guides us in orienting the needle so that all the BoNT-As are concentrated in the sphincter, avoiding spillage into the urethral lumen/bladder.
We did not use cystoscope during the injection; instead, we followed this simple step of using a metal probe, although Franco et al 6 and Vricella et al 9 used cystoscope in women to monitor any intraluminal spillage during injection.
Like in other studies, no serious adverse effects were reported in our study. One patient in our study complained of lower limb weakness, we do not know whether it was due to gluteus maximus paresis similar to the first report in 2015 by ’t Hoen et al. 8
Limitations of this study
This study had the following limitations. (1) The ideal study would be a randomized, placebo controlled trial. Challenges and limitations of trial design for patients with refractory DV include patient heterogeneity, the small number of patients in whom traditional urotherapy fails, and the lack of validated study tools to measure outcomes. (2) It is unclear at what point BoNT-A injection should be offered in the treatment algorithm and whether alternative modalities should initially be considered, eg, biofeedback techniques, electrical nerve stimulation, or sacral neuromodulation which are not universally available. (3) Long-term follow-up is required and (4) criteria for reinjections are ill-defined.
Conclusions
The study showed that BoNT-A was helpful in relieving symptoms as evidenced by AUA score and reducing the PVR at day 14, but with no significant improvements in UFM and urodymanic parameters. Neurological causes have to be excluded prior to initiation of treatment. Concurrent urotherapy and bowel care are a must for the success of BoNT-A treatment. The effect of BoNT-A is transitory. However, it can break the vicious cycle of involuntary intermittent contractions of the periurethral striated muscle during voiding and the period when it is sustained can be used for retraining the patient in normal voiding. Long-term follow-up is vital to identify patients who require repeat injection. More numbers and longer duration of follow-up are further required to validate BoNT-A in DV.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: All authors made a substantial contribution to the concept and design, acquisition of data or analysis and interpretation of data, They drafted the article or revised it critically for important intellectual content and approved the version to be published.
References
- 1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. [DOI] [PubMed] [Google Scholar]
- 2. Farhat W, Bagli DJ, Capolicchio G, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol. 2000;164:1011–1015. [DOI] [PubMed] [Google Scholar]
- 3. Jorgensen TM, Djurhuus JC, Schroder HD. Idiopathic detrusor sphincter dyssynergia in neurologically normal patients with voiding abnormalities. Eur Urol. 1982;8:107–110. [DOI] [PubMed] [Google Scholar]
- 4. Everaert K, Van Laecke E, De Muynck M, et al. Urodynamic assessment of voiding dysfunction and dysfunctional voiding in girls and women. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:254–264. [DOI] [PubMed] [Google Scholar]
- 5. Radojicic ZI, Perovic SV, Milic NM. Is it reasonable to treat refractory voiding dysfunction in children with botulinum-A toxin? J Urol. 2006;176:332–336, discussion 336. [DOI] [PubMed] [Google Scholar]
- 6. Franco I, Landau-Dyer L, Isom-Batz G, et al. The use of Botulinum Toxin-A injection for the management of external sphincter dyssynergia in neurologically normal children. J Urol. 2007;178:1775–1779. [DOI] [PubMed] [Google Scholar]
- 7. Mokhless I, Gaafar S, Fouda K, et al. Botulinum A toxin urethral sphincter injection in children with nonneurogenic neurogenic bladder. J Urol. 2006;176:1767–1770. [DOI] [PubMed] [Google Scholar]
- 8. ’tHoen LA van den Hoek J Wolffenbuttel KP et al. Breaking the vicious circle: onabotulinum toxin A in children with therapy-refractory dysfunctional voiding. J Pediatr Urol. 2015;11:119.e1–119.e6. [DOI] [PubMed] [Google Scholar]
- 9. Vricella GJ, Campigotto M, Coplen DE, et al. Long-term efficacy and durability of botulinum-A toxin for refractory dysfunctional voiding in children. J Urol. 2014;191:1586–1591. [DOI] [PubMed] [Google Scholar]
- 10. Suskind AM, Clemens JQ, Kaufman SR, et al. Patient perceptions of physical and emotional discomfort related to urodynamic testing: a questionnaire-based study in men and women with and without neurologic conditions. Urology. 2015;85:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinha S. Dysfunctional voiding: a review of the terminology, presentation, evaluation and management in children and adults. Indian J Urol. 2011;27:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steward JE, Clemons JD, Zaszczurynski PJ, et al. Quantitative evaluation of electrodes for external urethral sphincter electromyography during bladder-to-urethral guarding reflex. World J. 2010;28:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55:100–119. [DOI] [PubMed] [Google Scholar]