Abstract
Study Objectives
Pediatric obstructive sleep apnea (OSA) affects cardiac autonomic regulation, altering heart rate variability (HRV). Although changes in classical HRV parameters occur after OSA treatment, they have not been evaluated as reporters of OSA resolution. Specific frequency bands (named BW1, BW2, and BWRes) have been recently identified in OSA. We hypothesized that changes with treatment in these spectral bands can reliably identify changes in OSA severity and reflect OSA resolution.
Methods
Four hundred and four OSA children (5–9.9 years) from the prospective Childhood Adenotonsillectomy Trial were included; 206 underwent early adenotonsillectomy (eAT), while 198 underwent watchful waiting with supportive care (WWSC). HRV changes from baseline to follow-up were computed for classical and OSA-related frequency bands. Causal mediation analysis was conducted to evaluate how treatment influences HRV through mediators such as OSA resolution and changes in disease severity. Disease resolution was initially assessed by considering only obstructive events, and was followed by adding central apneas to the analyses.
Results
Treatment, regardless of eAT or WWSC, affects HRV activity, mainly in the specific frequency band BW2 (0.028–0.074 Hz). Furthermore, only changes in BW2 were specifically attributable to all OSA resolution mediators. HRV activity in BW2 also showed statistically significant differences between resolved and non-resolved OSA.
Conclusions
OSA treatment affects HRV activity in terms of change in severity and disease resolution, especially in OSA-related BW2 frequency band. This band allowed to differentiate HRV activity between children with and without resolution, so we propose BW2 as potential biomarker of pediatric OSA resolution.
Clinical Trial Registration
Childhood Adenotonsillectomy Trial, NCT00560859, https://sleepdata.org/datasets/chat.
Keywords: biomarker, causal mediation analysis, frequency domain analysis, heart rate variability, obstructive sleep apnea, resolution, treatment
Statement of Significance.
Pediatric obstructive sleep apnea (OSA) is a frequent respiratory condition that has been linked to increased cardiovascular risk. Changes in classic heart rate variability (HRV) parameters after adenotonsillectomy for pediatric sleep apnea treatment have been previously identified, but these changes are not causally attributable to treatment outcomes. Furthermore, HRV approaches to assess the resolution of sleep apnea have not been explored. This research study shows that changes in specific HRV parameters specifically identify OSA treatment outcomes. Besides, HRV activity in BW2, a specific apnea-related spectral band, differentiates children with and without OSA resolution.
Introduction
Pediatric obstructive sleep apnea (OSA) is a highly prevalent sleep respiratory disorder, affecting between 1% and 5% of the general pediatric population [1]. It is characterized by disruptions in the normal ventilation during sleep due to intermittent complete upper airway obstruction (apnea) and/or prolonged partial obstruction (hypopnea) [2]. Since its initial description, OSA in children has been linked to increased cardiovascular risk, as illustrated by impaired autonomic regulation, right and left ventricular hypertrophy, increased systemic and pulmonary blood pressure, and nocturnal cardiac strain [1].
The currently accepted gold standard for pediatric OSA diagnosis is overnight polysomnography (PSG) [3, 4]. During this test, children spend the night in a sleep laboratory where up to 32 biomedical signals are recorded, including the electrocardiogram (ECG). Those signals are evaluated following consensus-based guidelines to obtain indices of respiratory disturbance, with the obstructive apnea-hypopnea index (OAHI) being the most commonly used [2, 3]. OAHI is used to establish OSA presence and severity, by reflecting the number of obstructive events per hour of sleep (e/h) [3]. Apneic events occurring in absence of inspiratory effort are considered as reflecting central events [5]. Although the clinical significance of central apneas in children is under discussion, a central apnea index (CAI) ≥ 1 e/h is common and should be incorporated into the clinical report [6]. Both central and mixed events (include both central and obstructive components) are often added to OAHI to form the overarching apnea-hypopnea index (AHI), which is employed to define the presence and severity of the obstructive and central sleep apnea (OSAO+C) [7]. Adenotonsillectomy (AT) is often the first line of treatment, and commonly results in improved OAHI or even OSA resolution in a large proportion of the children [8, 9]. In addition, significant reductions in the CAI after AT have also been reported [6].
The Childhood Adenotonsillectomy Trial (CHAT) was a prospective randomized trial designed to evaluate the efficacy of early adenotonsillectomy (eAT) versus a strategy of watchful waiting with supportive care (WWSC) for OSA treatment [10, 11]. Interestingly, the original CHAT study showed that half of the children who underwent WWSC experimented OSA resolution, while only a proportion of those undergoing eAT normalized their sleep studies [11]. These findings have led several investigators to analyze the CHAT database based on OSA resolution rather than rely on the treatment arm [12–14]. Accordingly, changes in heart rate emerged because of OSA improvements independent of the intervention, suggesting that it is the change in disease severity that induced changes in cardiac activity [13, 14]. Likewise, increased awareness to the cardiovascular risks associated with pediatric OSA has led several researchers to analyze the effects of treatment on the ECG [13–16], on pulse rate [17, 18], and on heart rate variability (HRV) [19–22].
HRV analysis evaluates the state of the autonomic nervous system (ANS) [23]. In patients with OSA, the heart responds to respiratory events with progressive bradycardia followed by abrupt tachycardia, although such patterns can be highly variable depending on the duration and severity of each of the respiratory events [24–27]. These characteristics patterns are the basis for the study of the treatment effects on HRV trends. For example, Isaiah et al. [19] analyzed changes in HRV related to pediatric OSA treatment employing the CHAT database to conduct a causal mediation analysis (CMA). This analysis allows for evaluation as to whether the treatment has a measurable effect, while detecting potential causal pathways through which treatment influences changes in HRV [28]. Although several differences in HRV emerged after treatment, these changes were deemed as not causally attributable to changes in OAHI or in oxyhemoglobin desaturation index (ODI) following treatment [19]. However, Isaiah et al. [19] only evaluated the classical HRV spectral bands [29], that is, very low frequency (VLF, 0–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.4 Hz) bands, as all the previous studies in which treatment effects on HRV were assessed [19–22]. In a previous study [30], we showed that the classical spectral bands have limitations that restrict the information relevant to pediatric OSA, and identified novel specific spectral bands that improved disease characterization. Furthermore, although HRV changes after treatment have been evaluated earlier, the ability of HRV classical and novel spectral band changes to assess resolution of both OSA and OSAO+C has not been evaluated.
Therefore, we hypothesized that specific changes in the pediatric OSA-related HRV spectral bands will occur, which are causally attributable to disease resolution, regardless of OSA resolution occurred after eAT or WWSC. Accordingly, we incorporated two specific objectives: (1) to evaluate the treatment effects on HRV indices including both classical and novel OSA spectral bands, and (2) to assess HRV potential ability to reflect resolution, not only when considering OSA context, but also in the OSAO+C context.
Methods
Subjects and signals under study
The multicenter CHAT study is a large database initially designed to analyze the effects of eAT versus WWSC for pediatric OSA treatment. The rationale, design, and primary outcomes for the CHAT study have been previously reported [10, 11], and all data are available at https://sleepdata.org/datasets/chat. The study investigators recruited children between 5 and 9.9 years with OSA symptoms for a baseline nocturnal laboratory-based PSG. We included 404 subjects who were randomly assigned to eAT or WWSC (206 vs. 198 children), met the inclusion criteria (for more details see reference [11]), and completed a follow-up laboratory-based PSG 7 months later.
In this study, we evaluated disease resolution regardless of the treatment arm. First, as in the original CHAT study, we assessed OSA resolution, considering resolution as those subjects in whom at follow-up an OAHI ≤ 2 e/h and an obstructive apnea index (OAI) ≤ 1 e/h were present [11] (i.e. 252 resolution vs. 152 no resolution subjects). Furthermore, we considered the importance of central apneas in OSAO+C, by defining more stringent rules for disease resolution based on the overall AHI and the apnea index (AI). Thus, those children who presented an AHI ≤ 2 e/h and an AI ≤ 1 e/h at follow-up were considered as OSAO+C resolved (157 resolution vs. 247 no resolution subjects). Table 1 shows the demographic and relevant clinical data for these groups at baseline.
Table 1.
Baseline clinical and demographic data from children included in the CHAT study for both resolution approaches
| OSA | OSAO+C | |||||
|---|---|---|---|---|---|---|
| Resolution | No resolution | P | Resolution | No resolution | P | |
| Subjects (n) | 252 | 152 | — | 157 | 247 | — |
| Age (years) | 6.00 [2.50] | 7.00 [2.50] | 0.07 | 6.00 [2.00] | 6.00 [3.00] | 0.85 |
| Males (n) | 119 (47.6%) | 77 (50.7%) | 0.50 | 73 (46.5%) | 123 (49.8%) | 0.52 |
| BMIz | 0.69 [1.93] | 1.47 [2.07] | <0.001 | 0.66 [2.11] | 1.03 [2.03] | 0.002 |
| AHI (e/h) | 4.80 [5.14] | 8.23 [7.59] | <0.001 | 4.79 [5.90] | 6.61 [6.35] | <0.001 |
| OAHI (e/h) | 3.61 [4.56] | 7.28 [7.44] | <0.001 | 3.77 [5.55] | 5.22 [6.25] | 0.02 |
| AI (e/h) | 2.18 [2.35] | 2.36 [3.32] | 0.07 | 1.95 [2.41] | 2.35 [2.91] | 0.03 |
| OAI (e/h) | 1.00 [1.74] | 1.32 [2.95] | 0.40 | 1.09 [1.96] | 1.09 [2.18] | 0.44 |
| Race (n) | 0.01 | 0.19 | ||||
| White | 105 (41.7%) | 41 (27.0%) | 57 (36.3%) | 89 (36.0%) | ||
| Black | 118 (46.8%) | 97 (63.8%) | 78 (49.7%) | 137 (55.5%) | ||
| Other | 29 (11.5%) | 14 (9.2%) | 22 (14.0%) | 21 (8.5%) | ||
| Heart rate (bpm) | 84.00 [14.00] | 85.00 [13.00] | 0.10 | 84.00 [13.00] | 84.00 [12.75] | 0.95 |
| Tonsil size > 2+ (n) | 197 (78.2%) | 106 (69.7%) | 0.20 | 126 (80.3%) | 177 (71.7%) | 0.06 |
Data are showed as median [interquartile range] or n (percentage).
eAT, early adenotonsillectomy; WWSC, watchful waiting with supportive care; BMIz, body mass index z-score; AHI, apnea-hypopnea index; OAHI, obstructive apnea-hypopnea index; AI, apnea index; OAI, obstructive apnea index; OSA, obstructive sleep apnea; OSAO+C, obstructive and central sleep apnea.
OSA resolution was defined as those subjects who showed an OAHI ≤ 2 e/h and an OAI ≤ 1 e/h at follow-up. OSAO+C resolution was defined as those children who presented an AHI ≤ 2 e/h and an AI ≤ 1 e/h at follow-up.
Statistically significant differences (p < 0.05) have been highlighted in bold.
All the nocturnal PSGs were performed following the 2007 American Academy of Sleep Medicine recommendations [3]. The comprehensive pre-processing protocol from ECG acquisition to HRV extraction is provided in the Online Supplement.
HRV frequency analysis
In a previous study, we identified three pediatric OSA-specific bands in HRV spectrum: [30] BW1 (0.001–0.005 Hz), BW2 (0.028–0.074 Hz), and BWRes (0.04 Hz around the adaptive respiratory peak) (see Online Supplement for more details). In this study, we used the CHAT database to compute the relative power (RP) in these spectral bands, along with the classical spectral HRV indices (RPs in VLF, LF, and HF, as well as LF/HF ratio) to evaluate treatment effects. The choice of RP is based on its ability to assess HRV activity in any given frequency range, as shown by previous pediatric OSA studies [19–22, 31, 32].
Mediation analysis
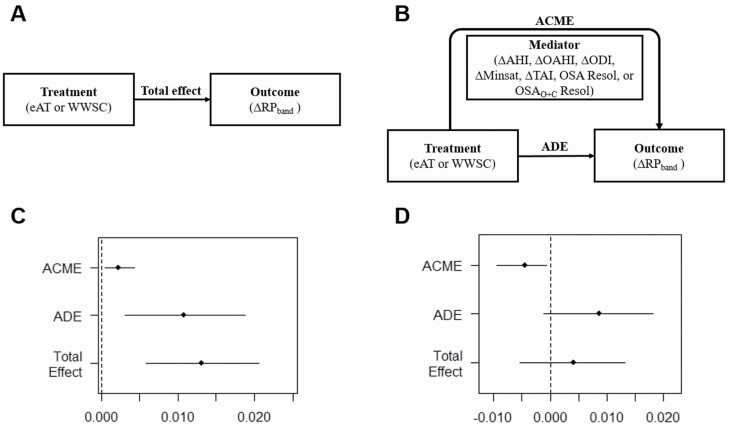
We conducted a CMA to evaluate whether variations in HRV outcomes were causally attributable to treatment [28]. The general framework of CMA for this study is shown in Figure 1. The software developed by Tingley et al. [33] was used to assess CMA. This software allows the decomposition of the intervention total effect (Figure 1, A) into two components (Figure 1, B). The first one is the average causal mediation effect (ACME) and represents the indirect effects of the treatment on the outcome (HRV indices) through a given mediator. Accordingly, ACME evaluates the relationships between the after-treatment variations occurring in HRV and the variations in variables representing the disease severity, that is, the mediators. The second one is the average direct effect (ADE), which reflects the treatment effect unlinked to the mediator considered [28, 33], thus evaluating how the treatment affects HRV through any other factor different from the mediator evaluated in each case [28, 33].
Figure 1.
Framework of the causal mediation analysis performed. (A) Typical estimation of the causal total effect measures the change in the outcome (ΔRPband) due to the intervention (regardless of eAT or WWSC). (B) Causal mediation analysis identifies the causal pathways (mediators) through which the intervention influences the change in the outcome. The average causal mediation effect (ACME) measures the change in the outcome due to the change in the mediator because of the treatment. The average direct effect (ADE) measures the change in the outcome unlinked to the mediator. In this study, these effects are averaged over both trial arms assuming no interaction between them. Both ACME and ADE jointly conform the total effect. (C) Representation for an outcome and a mediator of the effects estimated and the 95th percentile confidence intervals, where all of them are in the same direction. In this example, the estimations do not include the 0 value (no effect at all), so they are considered statistically significantly different from 0. (D) Representation for an outcome and a mediator of the effects estimated and the 95% of confidence intervals, with ACME showing an opposite direction to the ADE and Total Effect. In this case, the ACME does not include the 0 value, but ADE and total effect does, so only ACME is considered statistically significantly different from 0. eAT, early adenotonsillectomy; WWSC, watchful waiting with supportive care; RP, relative power; ΔRPband = RPband_follow-up − RPband_baseline; ΔAHI, change in the apnea-hypopnea index; ΔOAHI, change in the obstructive apnea-hypopnea index; ΔODI, change in the oxyhemoglobin desaturation index 3%; ΔMinsat, change in the minimum saturation level; ΔTAI, change in the total arousals index; OSA resol, obstructive sleep apnea resolution; OSAO+C resol, obstructive and central sleep apnea resolution.
As can be observed in Figure 1, D, if ACME and ADE are in the opposite direction, with higher values of ADE, a traditional analysis of only total effect would mask ACME effects. This possibility highlights the importance of performing a CMA, with the ACME as the means to reveal causal pathways, that otherwise would be undetectable [28, 34].
In this study, the intervention is represented by one of the treatment arms (either eAT or WWSC), the outcomes are the changes in RPs after the treatment (ΔRPband = RPband_follow-up − RPband_baseline), and five continuous and two binary mediators were considered. The selected five continuous mediators included the variations in AHI, OAHI, ODI, and minimum saturation (Minsat), directly related to the disease severity, and total arousal index (TAI), which has proven to reflect sleep disturbance associated with OSA [35]. For binary mediators, we wanted to evaluate the causal mediation effect of the disease resolution, and thus selected OSA and OSAO+C resolution as mediators.
We conducted a preliminary analysis to assess whether treatment effects on classical and OSA-related frequency bands depends on the treatment approach (eAT or WWSC). Agreeing with previous studies, we found that effects on HRV did not differ between treatment arms (Supplementary Tables S4–S10). Accordingly, we assumed that there is no-interaction between the type of treatment and the mediating variables, yielding an averaged effect for the two treatment arms [33].
To give a causal interpretation to the average mediation effect, a key assumption, named “sequential ignorability,” is needed, whereby two ignorability assumptions are made sequentially [28, 31]. First, the treatment assignment is assumed to be independent of the potential outcomes and mediators, a condition naturally satisfied in randomized trials such as the CHAT study [23, 28]. The second assumption states that according to the treatment assigned and potential pretreatment confounders, the mediator is independent [28, 31]. This implies that all baseline confounders have been controlled. To handle this assumption, we included in the mediation analysis the following well-established baseline confounders: age, race, sex, body mass index (z-score), average heart rate, and tonsil size. Furthermore, the software allows for performing a sensitivity analysis to measure to what extent the sequential ignorability can be violated until the conclusions are reversed. The higher the margin required to breach the assumption, the greater the robustness of the conclusions [28, 33, 36]. Full methodological details for the mediation and sensitivity analysis are available in the Online Supplement.
Statistical analysis
The software evaluates if the effects estimated are statistically different from 0, that is, no effect at all (see Figure 1, C and D). Those HRV indices that show statistically significant ACMEs when using OSA and OSAO+C resolution as mediators (i.e. those in which resolution showed a causally attributable effect on HRV), were subsequently assessed for statistically significant differences between resolution and no resolution groups (p-value <0.05 after false discovery rate correction). The Mann–Whitney U-test was applied after observing that these indices did not meet neither normality nor homoscedasticity distributions.
For the sake of the completeness of the analyses, the Online Supplement includes details on the differences in the power spectral densities for each treatment arm at baseline and follow-up (Supplementary Figure S3), RPs differences for each band under study (Supplementary Table S1 and Figure S4) and a correlation study between RPs and common clinical variables (Supplementary Tables S2 and S3).
Results
Causal mediation analysis
Table 2 shows ACME and ADE effects, along with their corresponding 95th percentile confidence intervals (CIs) for each mediator (see Supplementary Table S11 for associated p-values). ΔRPBW2 was the only parameter that reached statistically significant ACMEs for all the mediators. In this setting, the treatment effect on ΔRPBW2 was mediated in the negative direction by all the mediators evaluated, as reflected by the negative values of ACMEs obtained. Similarly, RPBW2 was the only parameter that showed a significant ACME with OSA resolution. Besides, ΔRPBW2 was the only parameter that showed some non-statistically significant ADEs, which occurred three times: when analyzing ΔMinsat and both resolution mediators (OSA and OSAO+C). As the ADE reflects the treatment effect unlinked to the mediator, the absence of statistically significant ADE together with a statistically significant ACME reflects the importance of these mediators in BW2.
Table 2.
Findings from the causal mediation analysis assessing treatment effects on change in heart rate variability relative powers (follow-up–baseline) through different mediators
| Mediator | ΔRPVLF | ΔRPLF | ΔRPHF | ΔLF/HF | ΔRPBW1 | ΔRPBW2 | ΔRPBWRes | |
|---|---|---|---|---|---|---|---|---|
| ΔAHI | ACME (95% CI) |
−0.001 (−0.008 to 0.006) |
−0.007* (−0.011 to −0.002) |
0.005 (−0.001 to 0.011) |
−0.033
(−0.062 to −0.007) |
0.002 (−0.001 to 0.005) |
−0.011* (−0.017 to −0.007) |
0.003 (−0.001 to 0.008) |
| ADE (95% CI) |
0.047* (0.024 to 0.069) |
0.018* (0.008 to 0.027) |
−0.066* (−0.086 to −0.047) |
0.204* (0.131 to 0.280) |
0.011* (0.003 to 0.018) |
0.015* (0.006 to 0.024) |
−0.045* (−0.060 to −0.031) |
|
| ΔOAHI | ACME (95% CI) |
−0.001 (−0.007 to 0.005) |
−0.006
(−0.010 to −0.002) |
0.004 (−0.002 to 0.010) |
−0.029
(−0.059 to −0.004) |
0.003
(0.001 to 0.006) |
−0.011* (−0.016 to −0.006) |
0.002 (−0.002 to 0.010) |
| ADE (95% CI) |
0.047* (0.025 to 0.069) |
0.017 (0.008 to 0.027) |
−0.065* (−0.083 to −0.045) |
0.200* (0.125 to 0.280) |
0.010 (0.003 to 0.018) |
0.015* (0.006 to 0.023) |
−0.044* (−0.059 to −0.030) |
|
| OSAO+C resol | ACME (95% CI) |
−0.001 (−0.006 to 0.003) |
−0.001 (−0.004 to 0.001) |
0.002 (−0.002 to 0.007) |
−0.015
(−0.040 to −0.003) |
0.001 (−0.001 to 0.003) |
−0.003
(−0.005 to −0.001) |
0.001 (−0.003 to 0.004) |
| ADE (95% CI) |
0.046* (0.027 to 0.068) |
0.012 (0.003 to 0.022) |
−0.063* (−0.082 to −0.045) |
0.193* (0.115 to 0.263) |
0.012* (0.005 to 0.019) |
0.006 (−0.003 to 0.016) |
−0.043* (−0.056 to −0.029) |
|
| OSA resol | ACME (95% CI) |
−0.002 (−0.012 to 0.008) |
−0.001 (−0.005 to 0.004) |
0.001 (−0.007 to 0.010) |
−0.033 (−0.072 to 0.008) |
0.002 (−0.002 to 0.005) |
−0.005
(−0.010 to −0.001) |
−0.001 (−0.007 to 0.005) |
| ADE (95% CI) |
0.048* (0.026 to 0.068) |
0.012 (0.002 to 0.022) |
−0.062* (−0.082 to −0.043) |
0.199* (0.120 to 0.280) |
0.011 (0.003 to 0.019) |
0.009 (−0.001 to 0.018) |
−0.041 (−0.055 to −0.026) |
|
| ΔODI | ACME (95% CI) |
−0.001 (−0.006 to 0.005) |
−0.005
(−0.009 to −0.002) |
0.005
(0.001 to 0.010) |
−0.027
(−0.051 to −0.008) |
0.002 (−0.001 to 0.004) |
−0.008* (−0.012 to −0.004) |
0.003 (0.001 to 0.007) |
| ADE (95% CI) |
0.046* (0.025 to 0.070) |
0.016* (0.007 to 0.025) |
−0.066* (−0.084 to −0.048) |
0.198* (0.127 to 0.271) |
0.011 (0.004 to 0.019) |
0.012 (0.003 to 0.020) |
−0.045* (−0.059 to −0.031) |
|
| ΔMinsat | ACME (95% CI) |
−0.002 (−0.006 to 0.002) |
−0.001 (−0.005 to 0.001) |
0.004
(0.001 to 0.008) |
−0.010 (−0.027 to 0.002) |
0.001 (−0.001 to 0.002) |
−0.002
(−0.005 to −0.001) |
0.003 (−0.001 to 0.007) |
| ADE (95% CI) |
0.048* (0.027 to 0.067) |
0.013 (0.003 to 0.022) |
−0.064* (−0.083 to −0.047) |
0.180* (0.110 to 0.256) |
0.012 (0.005 to 0.020) |
0.006 (−0.003 to 0.016) |
−0.045* (−0.059 to −0.030) |
|
| ΔTAI | ACME (95% CI) |
−0.001 (−0.006 to 0.005) |
−0.008* (−0.012 to −0.004) |
0.007
(0.002 to 0.013) |
−0.028
(−0.056 to −0.004) |
0.002
(0.001 to 0.005) |
−0.011* (−0.016 to −0.006) |
0.005
(0.001 to 0.009) |
| ADE (95% CI) |
0.046* (0.026 to 0.067) |
0.019* (0.010 to 0.028) |
−0.067* (−0.086 to −0.049) |
0.199* (0.129 to 0.268) |
0.011 (0.003 to 0.018) |
0.015* (0.006 to 0.023) |
−0.047* (−0.061 to 0.033) |
RP, relative power; VLF, very low frequency; LF, low frequency; HF, high frequency; AHI, apnea-hypopnea index; OAHI, obstructive AHI; OSAO+C resol, obstructive and central sleep apnea resolution; OSA resol, obstructive sleep apnea resolution; ODI, oxyhemoglobin desaturation index 3%; Minsat, minimum saturation level; TAI, total arousals index; ACME, average causal mediation effect; ADE, average direct effect; CI, confidence interval.
Statistically significant ACME effects (p < 0.05) have been highlighted in bold.
*P < 0.001.
ΔRPVLF was the only outcome that did not reach any statistically significant ACME with any of the mediators. Like ΔRPBW2, the treatment effect on ΔRPLF and ΔLF/HF was mediated in the negative direction by ΔAHI, ΔOAHI, ΔODI, and ΔTAI. OSAO+C resolution behaved in a similar way, but only for ΔLF/HF. In contrast, ΔODI, ΔMinsat, and ΔTAI mediated the remaining HRV parameters in the positive direction (increases of RP at follow-up).
An accessory sensitivity analysis was conducted for those outcomes that showed statistically significant ACMEs with the different mediators. ΔRPBW2 had the lowest probability to violate the sequential ignorability assumption for most of the mediators, with the highest absolute sensitive parameter (|ρ|) among all of them (see Supplementary Figures S6–S13 and Table S12).
OSA and OSAO+C resolution
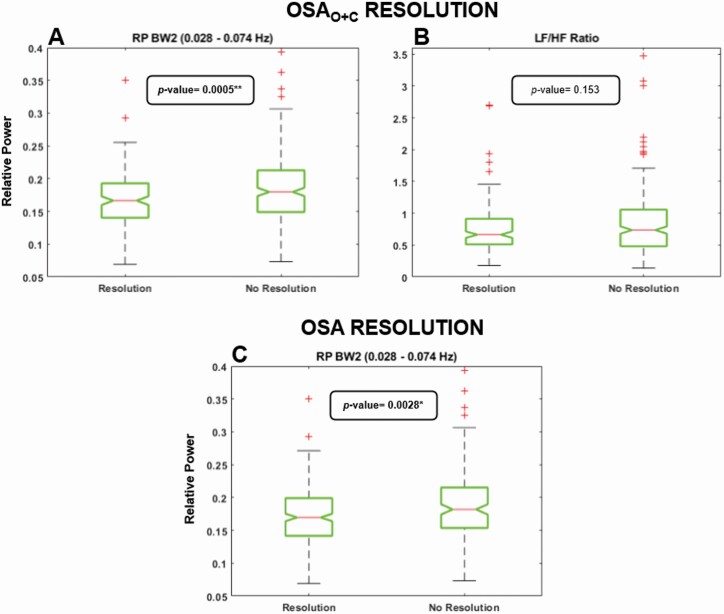
We assessed differences at follow-up between OSA resolution and no resolution subjects for RPBW2, as well as OSAO+C resolution and no resolution subjects for RPBW2 and LF/HF. Results are shown in Figure 2. RPBW2 was the only parameter that allowed for differentiation between subjects who had disease resolution, showing statistically significant differences between resolved and non-resolved groups.
Figure 2.
Differences at follow-up between children with and without resolution considering OSAO+C and OSA resolution approaches. Only parameters that showed statistically significant ACME with resolution mediators were evaluated. (A) Differences in RPBW2 considering OSAO+C resolution. (B) Differences in LF/HF ratio considering OSAO+C resolution. (C) Differences in RPBW2 considering OSA resolution.
Discussion
In this study, changes in HRV after OSA intervention were causally attributable to treatment effects. Furthermore, BW2 activity (0.028–0.074 Hz), which was previously identified as the HRV frequency band of highest interest in the pediatric OSA context, allowed for differentiation between children whose OSA resolved and those who did not. These results highlight BW2 as a potential biomarker of OSA resolution.
Treatment effects on HRV
CMA revealed that all the assessed mediators directly impact at least one of the outcomes. Although ΔLF/HF showed higher absolute ACME values, the statistical significance of the effect was higher in ΔRPBW2 for most of these parameters. This is due to the different range of values covered by LF/HF. For all other outcomes, the ACMEs in ΔRPBW2 were the highest for almost all the mediators, only surpassed when assessing ΔMinsat in ΔHF. It is noteworthy that ΔRPBW2 was the only parameter that reached statistically significant ACMEs for all the mediators, highlighting that pediatric OSA treatment effects are primarily reflected in this frequency range. Furthermore, the results obtained in the sensitivity analysis, along with the fact that major potential confounding variables at baseline were controlled, increase the robustness of the conclusions derived for ΔRPBW2 [36].
OSA and OSAO+C resolution
The goal of OSA treatment is obviously resolution of the disease, but AT is not necessarily accompanied by resolution and conversely no treatment can be accompanied by OSA resolution [11, 37]. Evaluation of OSA resolution using the same criteria as the original CHAT study showed that it only causally impacts ΔRPBW2. Using a stricter definition of resolution, we observed that OSAO+C resolution again causally impacts ΔRPBW2, but also ΔLF/HF. After assessing differences between children whose OSA resolved and those who did not, only BW2 was significantly affected (Figure 2). The absence of differences in LF/HF could be explained by the presence of a greater statistically significant ADE in the opposite direction that would be masking the resolution effects, which means that LF/HF could be affected by unknown causes in the opposite direction to OSAO+C resolution effects on LF/HF. On the other hand, BW2 reached statistical significance only for the ACME, with both resolution criteria mediating the effects in the negative direction. This can be observed in Figure 2, with both resolution groups showing lower values of RP. Larger differences obtained for OSAO+C resolution may be due to the association of central apneas with changes in heart rate and blood pressure of children [5], along with the fact that the BW2 spectral band was originally established based on differences between OSAO+C severity groups [30]. These results support the conclusions from our previous work [30] about the utility and importance of the BW2 frequency range when HRV is analyzed in the pediatric OSA context. In addition to the potential clinical utility of BW2 observed, it is important to point out that its implementation can be easily implemented on commonly HRV analysis tools, such as Physionet’s HRV toolkit [38], Kubios HRV sotware [39], or PhysioNet Cardiovascular Signal Toolbox [40]. A slight code adaptation in the frequency range defined for HRV analysis is sufficient to include this (and the two others) novel HRV frequency bands.
Comparison with previous work
Before the randomized trial CHAT, several studies analyzed the effect of treatment on the HRV. Şaylan et al. [20] conducted a prospective trial with 15 OSA and 15 healthy children, showing that altered HRV in pre-operative patients apparently did not change after AT. However, Kaditis et al. [16] analyzed R-R intervals in 21 patients pre- and post-AT, and observed a reduction in sympathetic tone, as reflected by changes in the LF spectral band [23]. Conversely, Muzumdar et al. [21] observed an increment in both LF and HF activity in 18 OSA children, along with a reduction in the LF/HF ratio after AT. Recently, Kirk et al. [22] reported changes in sympathetic-parasympathetic balance around arousal events in 12 obese children suffering from OSA. However, no overall treatment effect was found in the HRV parameters after 12 months of noninvasive ventilation [22]. From these studies of classical HRV parameters, the current study agrees with the increment in the LF activity after AT (Supplementary Figure S4) as reported by Muzumdar et al. [21]. Despite some methodological differences with these previous studies, the major difference resides in the marked cohort size along with the fact that the data are based on a multicenter approach, thereby favoring the generalization and robustness of our results.
Finally, we need to compare our results with the study conducted by Isaiah et al. [19], who also evaluated treatment effects on HRV in the CHAT population. They reported an absence of causal mediation effects, despite the use of a very similar CMA approach. Although we included OSA-related spectral bands in our study with the BW2 band showing the highest treatment-related effects, we also observed treatment effects for all the classical parameters except VLF. We believe that the differences between Isaiah et al. and the present study are primarily due to a different interpretation of the results: Our estimates of causal mediation effects were based on the ACME, while Isaiah et al. reached their conclusions based on the proportion mediated. The proportion mediated assesses the magnitude of ACME relative to the average total effect [28]. Although such approach is an interesting way to evaluate the mediation effects, Figure 1, D shows that if ADE is contrary to the ACME, or is non-statistically significant, it can result in a proportion mediated that will not achieve statistical significance. Under such circumstances, the findings would lead Isaiah et al. to conclude that the effects are not causally attributable to the treatment, hiding ACME effects. Thus, a re-evaluation of their results in the context of classical HRV parameters focused on the ACME is presented here.
Limitations and outlook
Despite the potential usefulness of our findings, some limitations deserve mention. First, a healthy control group to compare with children after resolution was not available. Although BW2 spectral band activity allowed to differentiate children with and without resolution, it would be interesting to include a control non-snoring group to evaluate if there are residual differences between subjects whose OSA resolved at follow-up and control subjects. Indeed, it has been previously shown that even when the sleep study traditional severity measures and particularly the OAHI return to the normative range, many children can exhibit persistent respiratory effort during sleep after AT [41].
Then, despite the statistically significant differences observed between resolution and no resolution subjects for RPBW2 in both approaches, resolution predictive ability was not satisfactory, achieving around 0.6 area under the receiver operating characteristic curve for both OSA and OSAO+C resolution (Supplementary Table S13). Efforts to improve the predictive ability adding a quality of life score did not result in higher performance (Supplementary Table S13). Thus, further investigation is required to find the causes for which strong causal effects are not reflected in high diagnostic performance, thus improving the clinical usefulness of RPBW2 in OSA context.
Additionally, we did not evaluate each sleep stage separately when analyzing HRV. However, the absence of statistically significant correlations with sleep stage percentages (Supplementary Table S3), and the fact that no differences were observed for sleep stage percentages between resolved and non-resolved children suggests that the contributions of sleep stages may be relatively minor. However, influence of sleep stage in HRV has been well-reported in the literature [31, 42–44]. In this sense, a stratification of our results by sleep stages could be an interesting goal for future studies.
Finally, all the children included in the study were diagnosed through a laboratory-based PSG. While it is the gold standard approach for diagnosis of pediatric OSA, the high number of sensors required, along with spending the night in a laboratory far from home, make it especially intrusive and could lead to changes in sleep architecture and therefore be less representative of usual sleep [45, 46]. In this regard, several studies have shown the feasibility of performing home-based respiratory polygraphy in pediatric OSA [47, 48]. Thus, the reproducibility of the results obtained in the present study should be ascertained in the future by analyzing at-home pediatric OSA databases.
Conclusions
To the best of our awareness, this is the first study where changes in the HRV spectral content of pediatric OSA patients have been causally attributable to treatment effects. These causal mediation effects have been linked to some polysomnographic variables directly related to the underlying sleep pathology, such as AHI, OAHI, ODI, minimum saturation, arousal index, as well as strongly associated with disease resolution. The OSA-related BW2 frequency band (0.028–0.074 Hz) reflected the greatest effects among all the outcomes evaluated and was the only one causally mediated by OSA resolution. Furthermore, changes in the BW2 spectral band allowed for distinguishing subjects whose OSA resolved from those in whom persistent OSA was present, particularly when incorporating central apneas into the criteria used for resolution. Thus, HRV spectral analysis in this frequency range may provide a viable and simple follow-up tool for children, with a reduction in RP reporting an improvement in OSA severity. Hence, we propose the use of the BW2 spectral band as a potential biomarker of pediatric OSA and its resolution after treatment.
Supplementary Material
Acknowledgments
The Childhood Adenotonsillectomy Trial (CHAT) was supported by the National Institutes of Health (HL083075, HL083129, UL1-RR-024134, UL1 RR024989). The National Sleep Research Resource was supported by the National Heart, Lung, and Blood Institute (R24 HL114473, 75N92019R002).
Funding
This work was supported by “Ministerio de Ciencia, Innovación y Universidades—Agencia Estatal de Investigación” and “European Regional Development Fund (FEDER)” under projects DPI2017-84280-R and RTC-2017-6516-1, by “European Commission” and “FEDER” under project “Análisis y correlación entre la epigenética y la actividad cerebral para evaluar el riesgo de migraña crónica y episódica en mujeres” (“Cooperation Programme Interreg V-A Spain-Portugal POCTEP 2014–2020”), and by “CIBER en Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN)” through “Instituto de Salud Carlos III” co-funded with FEDER funds, as well as under the project SleepyHeart from 2020 valorization call. A. Martín-Montero was in receipt of a “Ayudas para contratos predoctorales para la Formación de Doctores” grant from the Ministerio de Ciencia, Innovación y Universidades (PRE2018-085219). F. Vaquerizo-Villar was in receipt of a “Ayuda para contratos predoctorales para la Formación de Profesorado Universitario (FPU)” grant from the Ministerio de Educación, Cultura y Deporte (FPU16/02938). Daniel Álvarez was supported by a “Ramón y Cajal” grant (RYC2019-028566-I) by the “Ministerio de Ciencia e Innovación—Agencia Estatal de Investigación” co-funded by ESF. L. Kheirandish-Gozal and D. Gozal were supported by National Institutes of Health (NIH) grant HL130984, the Leda J. Sears Foundation, and by a Tier 2 grant from the University of Missouri. D. Gozal is also supported by NIH grants HL140548, and AG061824.
Disclosure Statement
Financial disclosure: None. No potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Nonfinancial disclosure: None. All authors have declared no nonfinancial conflicts of interest related to the study submitted for publication.
Authors’ Contributions
Adrián Martín-Montero designed the study, analyzed and interpreted data, and drafted the manuscript. Gonzalo C. Gutiérrez Tobal designed the study, analyzed and interpreted data, and revised the manuscript. Leila Keirandish-Gozal contributed to study design and revised the manuscript. Fernando Vaquerizo-Villar analyzed and interpreted data and revised the manuscript. Daniel Álvarez interpreted data and revised the manuscript. Félix del Campo obtained funding, interpreted data, and revised the manuscript. David Gozal designed the study, acquired and interpreted data, and revised the manuscript. Roberto Hornero designed the study, analyzed and interpreted data, obtained funding, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data Availability
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1. Marcus CL, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. [DOI] [PubMed] [Google Scholar]
- 2. American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866–878. [DOI] [PubMed] [Google Scholar]
- 3. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Science; 2007. [Google Scholar]
- 4. Wise MS, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389–398. doi: 10.1093/sleep/34.3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Driscoll DM, et al. Central apnoeas have significant effects on blood pressure and heart rate in children. J Sleep Res. 2009;18(4):415–421. [DOI] [PubMed] [Google Scholar]
- 6. Boudewyns A, et al. Central apneas in children with obstructive sleep apnea syndrome: prevalence and effect of upper airway surgery. Sleep Med. 2016;25:93–97. [DOI] [PubMed] [Google Scholar]
- 7. Berry RB, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suen JS, et al. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995;121(5):525–530. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117(10):1844–1854. [DOI] [PubMed] [Google Scholar]
- 10. Redline S, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509–1517. doi: 10.5665/sleep.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcus CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isaiah A, et al. Polysomnography and treatment-related outcomes of childhood sleep apnea. Pediatrics. 2019;144(4):e20191097. [DOI] [PubMed] [Google Scholar]
- 13. Quante M, et al. ; Childhood Adenotonsillectomy Trial (CHAT). The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. Sleep. 2015;38(9):1395–1403. doi: 10.5665/sleep.4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baumert M, et al. The effect of adenotonsillectomy for childhood sleep apnoea on cardiorespiratory control. ERJ Open Res. 2016;2(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu X, et al. Effect of adenotonsillectomy for childhood obstructive sleep apnea on nocturnal heart rate patterns. Sleep. 2018;41(11). doi: 10.1093/sleep/zsy171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaditis AG, et al. Effects of adenotonsillectomy on R-R interval and brain natriuretic peptide levels in children with sleep apnea: a preliminary report. Sleep Med. 2011;12(7):646–651. [DOI] [PubMed] [Google Scholar]
- 17. Pavone M, et al. At-home pulse oximetry in children undergoing adenotonsillectomy for obstructive sleep apnea. Eur J Pediatr. 2017;176(4):493–499. [DOI] [PubMed] [Google Scholar]
- 18. Constantin E, et al. Pulse rate and pulse rate variability decrease after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2008;43(5):498–504. [DOI] [PubMed] [Google Scholar]
- 19. Isaiah A, et al. Treatment-related changes in heart rate variability in children with sleep apnea. Otolaryngol Head Neck Surg. 2020;162(5):737–745. [DOI] [PubMed] [Google Scholar]
- 20. Şaylan B, et al. Spectral and time-domain analyses of heart-rate variability in children with severe upper airway obstruction. Balkan Med J. 2011;28(2):148–150. [Google Scholar]
- 21. Muzumdar HV, et al. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 2011;139(5):1050–1059. [DOI] [PubMed] [Google Scholar]
- 22. Kirk VG, et al. Cardiovascular changes in children with obstructive sleep apnea and obesity after treatment with noninvasive ventilation. J Clin Sleep Med. 2020;16(12):2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acharya UR, et al. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031–1051. [DOI] [PubMed] [Google Scholar]
- 24. Gozal D, et al. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185(1):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vitelli O, et al. Autonomic imbalance during apneic episodes in pediatric obstructive sleep apnea. Clin Neurophysiol. 2016;127(1):551–555. [DOI] [PubMed] [Google Scholar]
- 26. Aljadeff G, et al. Heart rate variability in children with obstructive sleep apnea. Sleep. 1997;20(2):151–157. doi: 10.1093/sleep/20.2.151 [DOI] [PubMed] [Google Scholar]
- 27. Shouldice RB, et al. Detection of obstructive sleep apnea in pediatric subjects using surface lead electrocardiogram features. Sleep. 2004;27(4):784–792. doi: 10.1093/sleep/27.4.784 [DOI] [PubMed] [Google Scholar]
- 28. Imai K, et al. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309–334. [DOI] [PubMed] [Google Scholar]
- 29. Task Force of the ESC and the NSA of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 30. Martín-Montero A, et al. Heart rate variability spectrum characteristics in children with sleep apnea. Pediatr Res. 2020;89:1771–1779. doi: 10.1038/s41390-020-01138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baharav A, et al. Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res. 1999;9(6):345–351. [DOI] [PubMed] [Google Scholar]
- 32. Liao D, et al. Sleep-disordered breathing and cardiac autonomic modulation in children. Sleep Med. 2010;11(5):484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tingley D, et al. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38.26917999 [Google Scholar]
- 34. Cha J, et al. The effects of obstructive sleep apnea syndrome on the dentate gyrus and learning and memory in children. J Neurosci. 2017;37(16):4280–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scholle S, et al. Arousals and obstructive sleep apnea syndrome in children. Clin Neurophysiol. 2001;112(6):984–991. [DOI] [PubMed] [Google Scholar]
- 36. Imai K, et al. Identification, inference and sensitivity analysis for causal mediation effects. Stat Sci. 2010;25(1):51–71. [Google Scholar]
- 37. Chervin RD, et al. ; Childhood Adenotonsillectomy Trial. Prognosis for spontaneous resolution of OSA in children. Chest. 2015;148(5):1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mietus JE, et al. Heart Rate Variability Analysis with the HRV Toolkit; 2014. https://archive.physionet.org/tutorials/hrv-toolkit/. Accessed May 17, 2021.
- 39. Tarvainen MP, et al. Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–220. [DOI] [PubMed] [Google Scholar]
- 40. Vest AN, et al. An open source benchmarked toolbox for cardiovascular waveform and interval analysis. Physiol Meas. 2018;39(10):105004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinot JB, et al. Persistent respiratory effort after adenotonsillectomy in children with sleep-disordered breathing. Laryngoscope. 2018;128(5):1230–1237. [DOI] [PubMed] [Google Scholar]
- 42. Nisbet LC, et al. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. [DOI] [PubMed] [Google Scholar]
- 43. Walter LM, et al. Autonomic dysfunction in children with sleep disordered breathing. Sleep Breath. 2013;17(2):605–613. [DOI] [PubMed] [Google Scholar]
- 44. Liao D, et al. Sleep-disordered breathing in children is associated with impairment of sleep stage-specific shift of cardiac autonomic modulation. J Sleep Res. 2010;19(2):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kheirandish-Gozal L. What is “abnormal” in pediatric sleep? Respir Care. 2010;55(10):1366–1374. [PubMed] [Google Scholar]
- 46. Álvarez D, et al. Automated screening of children with obstructive sleep apnea using nocturnal oximetry: an alternative to respiratory polygraphy in unattended settings. J Clin Sleep Med. 2017;13(5):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alonso-Álvarez ML, et al. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest. 2015;147(4):1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiner E, et al. Home respiratory polygraphy is useful in the diagnosis of childhood obstructive sleep apnea syndrome. J Clin Med. 2020;9(7):2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.