Abstract
Study Objectives
To evaluate the effects of digital cognitive behavior therapy for insomnia (dCBT-I) delivered during pregnancy on subjective sleep outcomes, depressive symptoms, and anxiety symptoms through 6 months postpartum.
Methods
People up to 28 weeks gestation (N = 208) with insomnia were randomized to 6 weekly sessions of dCBT-I or standard care. We report follow-up data at 3 and 6 months postpartum. The primary outcome was insomnia symptom severity. Secondary sleep outcomes included global sleep quality and insomnia caseness. Mental health outcomes included depressive and anxiety symptom severity. We evaluated between-condition differences in change from baseline for each postpartum timepoint and categorical outcomes.
Results
dCBT-I participants did not experience significantly greater improvements in insomnia symptom severity relative to standard care participants, but they did experience higher rates of insomnia remission and lower rates of insomnia caseness at 6 months postpartum. dCBT-I participants experienced greater improvements in depressive symptom severity from baseline to both postpartum timepoints, and in anxiety symptom severity from baseline to 3 months postpartum. The proportion of participants with probable major depression at 3 months postpartum was significantly higher among standard care (18%) than dCBT-I (4%, p = 0.006) participants; this between-condition difference was pronounced among the subset (n = 143) with minimal depressive symptoms at baseline (18% vs 0%).
Conclusion
dCBT-I use during pregnancy leads to enduring benefits for postpartum insomnia remission. Findings provide strong preliminary evidence that dCBT-I use during pregnancy may prevent postpartum depression and anxiety, which is notable when considering the high frequency and importance of these problems.
Clinical Trials: ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02805998, NCT02805998.
Keywords: cognitive behavior therapy, insomnia, depression, anxiety, pregnancy, postpartum
Statement of Significance.
Insomnia is a pervasive and consequential problem during pregnancy and the postpartum period. Current findings suggest that digital cognitive behavior therapy for insomnia, when delivered during pregnancy, is associated with insomnia remission at 6 months postpartum. Moreover, this non-pharmacological, scalable, and acceptable intervention is associated with improved depressive and anxiety symptoms. Future work is needed to examine how to strengthen the effects for sleep and mental health outcomes, investigate whether benefits endure beyond 6 months postpartum, and conduct confirmatory efficacy research to evaluate whether digital cognitive behavior therapy for insomnia may prevent perinatal depression.
Introduction
Cognitive behavior therapy for insomnia (CBT-I) is an effective non-pharmacological intervention for insomnia [1], and is recommended by the American College of Physicians as the first line treatment [2]. Three randomized controlled trials show that CBT-I is effective for prenatal insomnia symptoms, whether delivered in-person or by an automated digital program [3–5]. To date, long-term follow-up data are lacking, with only one study providing follow-up findings up to 6 weeks postpartum; thus, it is unknown whether CBT-I treatment gains are maintained at 3 and 6 months postpartum. Critically, a longitudinal observational study found that sleep quality remained fairly stable from late pregnancy through 6 months postpartum [6]. This led us to hypothesize that improving insomnia symptoms during pregnancy would lead to sustained improvements in insomnia symptoms during the first 6 months postpartum.
Prenatal insomnia is associated with depressive and anxiety symptoms [6–8], and we previously reported that digital CBT-I improved depressive and anxiety symptoms at 10- and 18-week post-randomization relative to standard care. Further, in a non-perinatal population, digital CBT-I was associated with significantly greater reductions in depressive symptom severity and lower rates of moderate-to-severe depression at 1-year follow-up relative to control [9]. However, it is unknown whether digital CBT-I may prevent postpartum depressive symptoms.
In this paper, we report the long-term follow-up data from 3 and 6 months postpartum from a trial that randomized 208 pregnant people with elevated insomnia symptoms to digital CBT-I or standard care [3]. First, we hypothesized that participants randomized to digital CBT-I during pregnancy would have significantly greater improvements in insomnia symptoms from baseline to 3 and 6 months postpartum relative to participants randomized to standard care. Second, we hypothesized that participants randomized to digital CBT-I during pregnancy would have significantly greater improvements in depressive and anxiety symptoms from baseline to 3 and 6 months postpartum relative to participants randomized to standard care. Finally, we explored condition differences in rates of probable major depression in the full sample and among the subset of participants with minimal depressive symptoms at baseline and in rates of moderate-to-severe anxiety in the full sample and among the subset of participants with minimal to mild anxiety symptoms at baseline.
Methods
We provide a brief overview of study methods below, and greater detail is included in the original report [3].
Participants
Participants were recruited via flyers, a national health volunteer registry, social media advertisements, and word of mouth. Additionally, patients at a university hospital were recruited via messages sent through the electronic health record, direct mail, and via electronic flyers displayed in the obstetrics and gynecology waiting rooms.
Inclusion criteria were: (1) self-reported pregnancy up to 28 weeks gestation, (2) 18 years of age or older, (3) met DSM-5 criteria for insomnia disorder as determined by the Sleep Condition Indicator [10, 11] (participants experiencing symptoms ≥1 month were eligible in contrast to DSM-5 criteria requiring symptom duration ≥3 months in order to include participants whose symptoms onset during pregnancy) or experiencing elevated insomnia symptom severity as determined by a total score ≥11 on the Insomnia Severity Index [12, 13], and (4) had regular access to a web-enabled computer, tablet, or smart phone. Exclusion criteria were: (1) probable major depression as determined by a total score ≥15 on the Edinburgh Postnatal Depression Scale (EPDS [14]), (2) self-reported bipolar disorder, (3) self-reported history of psychosis, (4) active suicidality defined as scoring >1 on EPDS item 10 that assesses thoughts of self-harm, or report of a specific suicide plan or recent attempt, and (5) shift work employee.
Design
Participants were randomly assigned to receive digital CBT-I or standard care with a waitlist control. The study received ethical approval from the University of California, San Francisco Institutional Review Board and participants provided electronic informed consent. The trial was registered on ClinicalTrials.Gov (NCT02805998).
Interventions
Digital CBT-I
The digital CBT-I program, Sleepio, has been described in detail in other publications [15, 16]. In brief, digital CBT-I was delivered through 6 weekly sessions that are accessed via website or iOS app. Treatment content included sleep restriction, stimulus control, cognitive therapy, relaxation techniques, and sleep hygiene and education [17].
Standard care
The control condition reflected standard care for prenatal insomnia patients. No limits were placed on receiving non-study treatment, including medication or psychotherapy. Participants randomized to standard care received a free voucher code to Sleepio upon study completion at 6 months postpartum.
Outcomes
Subjective sleep outcomes
Insomnia symptom severity was measured by the Insomnia Severity Index (ISI) [12, 13]. Total scores ≤7 indicate no clinically significant insomnia, 8−14 indicate subthreshold insomnia, 15−21 indicate moderate severity insomnia, and ≥22 indicate severe insomnia. Remission was defined as a total ISI ≤7 [4]. Global sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI), a 19-item measure comprised of seven components assessing sleep duration, disturbance, latency, efficiency, quality, days of dysfunction due to sleepiness, and needing medication to sleep [18]. Components are summed to create a PSQI global sleep quality score ranging from 0 to 21; higher scores indicate worse global sleep quality, and scores above 5 indicate poor sleep. To determine whether participants met diagnostic criteria for insomnia, we used the Sleep Condition Indicator (SCI). Consistent with previous research [19], we added a ninth item to assess early morning awakening, which is a symptom of insomnia disorder in the DSM-5.
Mental health outcomes
Depressive symptom severity was assessed using the Edinburgh Postnatal Depression Scale (EPDS) [14]. The EPDS is a 10-item self-report measure that omits depressive symptoms that can be conflated with normal perinatal symptoms. Total scores range from 0 to 30, with higher scores indicating higher symptom severity. Scores of 15 or greater suggest probable major depression among pregnant people, and scores of 13 or greater suggest probable major depression among postpartum people [14, 20]. In clinical practice, EPDS scores of 10 or greater typically indicate that further evaluation is warranted to determine whether acute intervention is needed [19, 21]. Anxiety symptom severity was assessed using the Generalized Anxiety Disorder Scale (GAD-7) [22]. Scores range from 0 to 21 with higher scores indicating higher anxiety symptom severity. Scores of 0−4 suggest minimal anxiety, 5−9 suggest mild anxiety, 10−14 suggest moderate anxiety, and 11−21 suggest severe anxiety.
Use of non-study treatments
At each timepoint, participants were asked about their use of the following aids to improve sleep: sleep medication prescribed by a doctor; combination sleep aid and pain reliever; over-the-counter or store-bought sleep aid; alternative therapy or herbal supplement; therapy or counseling; alcohol, beer, or wine; eyemask or ear plugs; other. Participants were also asked about their use of the following aids to improve mood: antidepressant medication, therapy or counseling; support group; alternative therapy or herbal supplements; other. Response options were “rarely or never,” “a few nights a month,” “a few nights a week,” and “every night/almost every night.”
All study measures were self-report and collected via online survey systems (i.e. Qualtrics).
Randomization
The randomization sequence was generated by an independent investigator using Sealed Envelope with block sizes of 4, 6, and 8 [23]. The randomization sequence and block sizes were concealed from study investigators and staff. Randomization was not stratified by any baseline characteristic. Once a participant completed baseline measures, study staff requested the allocation assignment from the independent investigator.
Procedures
Eligibility determination
Eligibility was determined by a three-step process including a screening survey, completion of at least four of seven daily sleep diaries, and an orientation session conducted by phone. More details are included in the main outcomes paper [3].
Baseline measures and randomization
Participants completed baseline measures on the Qualtrics online survey system. Upon completion, study staff requested the condition assignment from the independent investigator who generated the randomization sequence. Participants were informed of their condition assignment by phone call and email.
Follow-up assessments
The current report focuses on follow-up data from 3 and 6 months postpartum. Participants were sent $10 electronic gift cards for completing each assessment.
Statistical analysis
We followed intention to treat principles whereby participants were included based on randomization regardless of participation or adherence. The objectives of the statistical analysis were to compare changes in sleep and mental health outcomes from baseline to 3 months postpartum and 6 months postpartum in participants randomized to digital CBT-I versus standard care. We analyzed each outcome separately using linear mixed effects models. Each mixed effects model included terms for the number of months between baseline and the 3 and 6 months postpartum timepoints, intervention group, interactions of the two time variables with intervention group, and a random intercept to accommodate the repeated measures of participants. The two time by intervention group interactions measured the difference in outcome changes to each of the postpartum visits between the intervention groups. We simultaneously assessed the two time by intervention group interactions using a likelihood ratio test and present simultaneous 95% confidence intervals for the two interaction effects using a Bonferroni approach; we present 97.5% confidence intervals for the two interaction effects.
To characterize the clinical significance of findings, we also calculated predicted prevalances of participants with remitted insomnia (i.e. ISI ≤ 7), poor global sleep quality (i.e. PSQI > 5), insomnia caseness (i.e., determined by the SCI), probable major depression (i.e., EPDS ≥ 13), and moderate-to-severe anxiety (i.e. GAD-7 ≥ 10) at 3 and 6 months postpartum. We calculated odds ratios that assess the magnitude of differences in prevalence between the intervention groups at each postpartum visit using logistic regression models fit using a generalized estimating equations approach to accommodate the repeated measures. Analogous to the mixed effects models, the logistic models included terms for the 3- and 6-month postpartum visits, intervention group, and the time by intervention group interaction. We present simultaneous 95% confidence intervals for the odds ratio estimates at the two postpartum visits using a Bonferroni approach, that is, two 97.5% confidence intervals for the two odds ratio estimates.
To explore whether digital CBT-I may prevent perinatal depression or anxiety, we examined incidence of probable major depression at 3 and 6 months postpartum among the subset of participants with no to minimal depressive symptoms at baseline (EPDS < 10, n = 143), and the incidence of moderate-to-severe anxiety at 3 and 6 months postpartum among the subset of participants with minimal to mild anxiety symptoms at baseline (GAD-7 < 10; n = 158). The number needed to treat was calculated using the cliniccalc.com calculator, which uses incidence rates from each condition as inputs. The number needed to treat corresponds with the number of patients who would need to receive digital CBT-I in order to prevent one case of probable major depression or moderate-to-severe anxiety.
We compared use of non-study treatments between groups using chi-square tests. Due to small cell sizes, responses were recoded to “rarely or never” vs “a few nights a month or more.”
Results
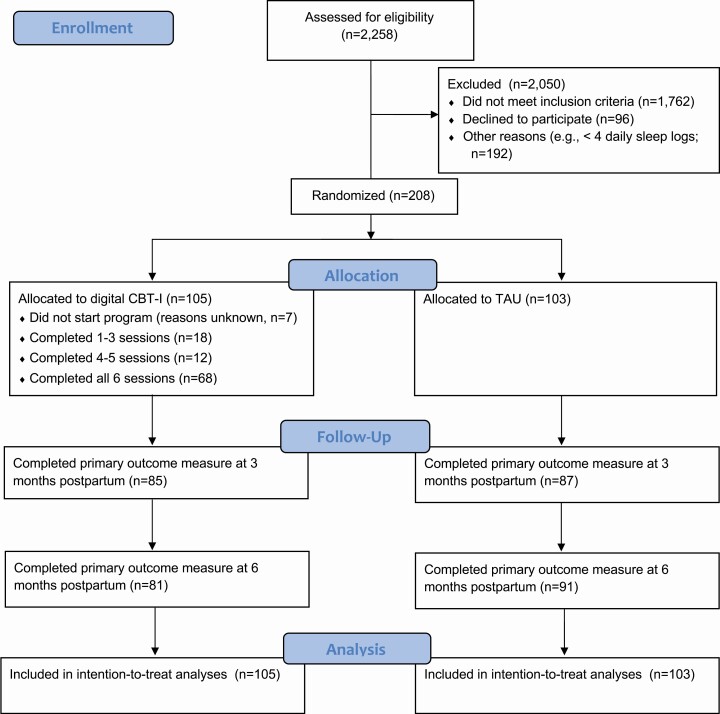
Participant characteristics are presented in Table 1. See Figure 1 for a participant flow diagram. Table 2 presents estimated monthly change in the continuous sleep and mental health outcomes from enrollment to 3 and 6 months postpartum between the digital CBT-I and standard care conditions, and differences in changes based on fits of linear mixed effects models. Table 3 presents estimated prevalences and odds ratios for binary sleep and mental health outcomes, as well as outcome counts, at the 3 and 6 months postpartum visits by condition from logistic regression models.
Table 1.
Participant characteristics at baseline prior to randomization
| Participant characteristic, M (SD) or n (%) |
Treatment as usual (n = 103) |
Digital CBT-I (n = 105) |
Test statistic | P-value |
|---|---|---|---|---|
| Age | 33.21 (3.98) | 33.90 (3.38) | t(206) = –1.35 | 0.18 |
| White race | 65 (63.1%) | 73 (69.5%) | χ 2(1) = 0.96 | 0.33 |
| Hispanic ethnicity | 10 (9.7%) | 5 (4.8%) | χ 2(1) = 1.90 | 0.17 |
| Graduated four year college | 88 (85.4%) | 92 (87.6%) | χ 2(1) = 0.21 | 0.64 |
| Income ≥ $100,000 | 70 (68.0%) | 71 (67.6%) | χ 2(1) = 0.003 | 0.96 |
| Married or cohabiting | 96 (93.2%) | 100 (95.2%) | χ 2(1) = 0.40 | 0.53 |
| Gestational age at screening (weeks) | 18.07 (6.27) | 17.10 (6.41) | t(206) = 1.11 | 0.27 |
| Primiparous | 66 (64.1%) | 46 (43.8%) | χ 2(1) = 8.59 | 0.003 |
| Sleep outcomes | ||||
| Insomnia symptom severity | 14.19 (3.72) | 14.52 (3.26) | t(206) = –0.68 | 0.50 |
| Global sleep quality | 9.47 (3.02) | 9.52 (2.74) | t(206) = –0.14 | 0.89 |
| Insomnia caseness | 41 (39.8%) | 54 (51.4%) | χ 2(1) = 2.83 | 0.09 |
| Mental health outcomes | ||||
| Depressive symptom severity | 7.24 (3.77) | 7.79 (4.05) | t(206) = –1.01 | 0.31 |
| Anxiety symptom severity | 4.81 (3.23) | 5.43 (3.55) | t(206) = –1.32 | 0.19 |
Note. With permission, this table is reprinted from a previously published article [3]. The following measures were used to assess sleep and mental health outcomes: ISI for insomnia symptom severity; PSQI for global sleep quality; SCI for insomnia caseness; EPDS for depressive symptom severity; GAD-7 for anxiety symptom severity.
Figure 1.
CONSORT participant flow diagram.
Table 2.
Estimated changes in outcomes per month from baseline to 3 and 6 months postpartum by condition and differences in changes based on fits of linear mixed effects models
| Outcome | Change period | Digital CBT-I |
Standard care | 97.5% CI | Overall p-value |
|---|---|---|---|---|---|
| M | M | ||||
| Insomnia symptom severity | Baseline−3 months postpartum | −0.67 | −0.53 | −0.36, 0.08 | 0.08 |
| Baseline−6 months postpartum | −0.53 | −0.36 | −0.33, 0.00 | ||
| Global sleep quality | Baseline−3 months postpartum | −0.26 | −0.15 | −0.26, 0.04 | 0.14 |
| Baseline−6 months postpartum | −0.21 | −0.13 | −0.19, 0.03 | ||
| Depressive symptom severity | Baseline−3 months postpartum | −0.24 | 0.03 | −0.45, −0.10 | 0.002 |
| Baseline−6 months postpartum | −0.18 | −0.04 | −0.27, −0.01 | ||
| Anxiety symptom severity | Baseline−3 months postpartum | −0.18 | 0.08 | −0.41, −0.10 | 0.001 |
| Baseline−6 months postpartum | −0.11 | −0.01 | −0.22, 0.01 |
Note. The following measures were used to assess sleep and mental health outcomes: ISI for insomnia symptom severity; PSQI for global sleep quality; EPDS for depressive symptom severity; GAD-7 for anxiety symptom severity.
Table 3.
Estimated prevalences and odds ratios for binary sleep and mental health outcomes at three and six months postpartum by condition from logistic regression
| Outcome | Timepoint | Digital CBT-I |
Standard care | OR | 97.5% CI | P | ||
|---|---|---|---|---|---|---|---|---|
| % | n | % | n | |||||
| Insomnia remission | 3 months postpartum | 46.13 | 39 | 34.15 | 31 | 0.61 | 0.30, 1.22 | 0.11 |
| 6 months postpartum | 53.00 | 42 | 35.15 | 32 | 0.48 | 0.24, 0.96 | 0.02 | |
| Poor sleep quality | 3 months postpartum | 67.88 | 55 | 75.18 | 61 | 1.43 | 0.66, 3.11 | 0.30 |
| 6 months postpartum | 62.95 | 49 | 70.92 | 63 | 1.44 | 0.69, 3.00 | 0.27 | |
| Insomnia caseness | 3 months postpartum | 13.85 | 12 | 22.25 | 19 | 1.78 | 0.72, 4.42 | 0.16 |
| 6 months postpartum | 11.12 | 9 | 24.99 | 23 | 2.66 | 1.02, 6.93 | 0.02 | |
| Probable major depression | 3 months postpartum | 3.96 | 3 | 18.47 | 15 | 5.49 | 1.38, 21.85 | 0.006 |
| 6 months postpartum | 5.54 | 4 | 10.73 | 10 | 2.05 | 0.55, 7.67 | 0.22 | |
| Moderate-to-severe anxiety | 3 months postpartum | 3.83 | 3 | 17.04 | 14 | 5.16 | 1.26, 21.18 | 0.009 |
| 6 months postpartum | 6.80 | 5 | 11.23 | 10 | 1.73 | 0.50, 6.00 | 0.32 |
Note. Insomnia remission was defined as ISI ≤ 7; poor sleep quality was defined as PSQI > 5; insomnia caseness was defined by the SCI; probable major depression was defined as EPDS ≥ 13; moderate-to-severe anxiety was defined as GAD-7 ≥ 10.
Subjective sleep outcomes
Insomnia symptoms
Average ISI scores decreased from baseline to the 3- and 6-month timepoints in both of the intervention conditions. The decreases for the digital CBT-I condition were greater than for the standard care condition, but the likelihood ratio test that assessed the simultaneous changes to the two postpartum time points was not statistically significant at the 5% level, p = 0.08 (Table 2). Participants randomized to digital CBT-I had higher rates of insomnia remission compared to those randomized to the standard care condition at 6 months postpartum (approximately 53% vs 35%, p = 0.02; Table 3).
Global sleep quality
Findings were similar for PSQI scores. Although participants randomized to digital CBT-I had greater improvements in PSQI scores, the likelihood ratio test that assessed the simultaneous changes to the two postpartum time points was not statistically significant at the 5% level, p = 0.14 (Table 2). Accordingly, estimated prevalences of poor global sleep quality did not differ between conditions at 3 or 6 months postpartum (Table 3).
Insomnia caseness
Estimated prevalences of insomnia caseness were greater for standard care participants than for digital CBT-I participants, but only the estimated odds ratio at the 6 month visit was statistically significant (approximately 25% vs 11%, p = 0.02; Table 3).
Mental health outcomes
Depressive symptoms
The likelihood ratio test of the two differences in change from baseline was statistically significant (p = 0.002). Relative to participants randomized to standard care during pregnancy, participants randomized to digital CBT-I experienced significantly greater improvements in EPDS scores from baseline to 3 months postpartum; that is, improvements were twelvefold that of the standard care condition (monthly reduction of 0.24 vs monthly increase of 0.03). At 6 months postpartum, between-condition differences in EPDS improvement remained significant but were slightly attenuated: average improvement in EPDS scores in the digital CBT-I condition was over fourfold that of the standard care condition (monthly reduction of 0.18 vs 0.04). See Table 2.
Prevention of probable major depression
Among the full sample, the estimated prevalences of probable major depression were lower among participants randomized to digital CBT-I than participants randomized to standard care, but only the odds ratios at the 3 month timepoint were statistically significant (3.96% vs 18.47%; Table 3).
Among the subset of participants with minimal depressive symptoms at baseline (EPDS < 10; n = 143), 0% of digital CBT-I participants reported probable major depression at 3 months postpartum compared to 18% (n = 12) of standard care participants, corresponding with a number needed to treat of 5.6 (χ 2(1) = 12.45, Fisher’s exact p < 0.001). At 6 months postpartum, 0% of digital CBT-I participants reported probable major depression relative to 10% (n = 7) of standard care participants, corresponding with a number needed to treat of 10 (χ 2(1) = 6.22, Fisher’s exact p = 0.015).
Anxiety symptoms
The likelihood ratio test of the two differences in change from baseline was statistically significant (p = 0.001). From baseline to 3 months postpartum, participants in the digital CBT-I condition demonstrated improvements in GAD-7 scores, whereas participants in the standard care condition demonstrated worsening GAD-7 scores. From baseline to 6 months postpartum, participants in the digital CBT-I condition demonstrated improvements in GAD-7 scores, whereas participants in the standard care condition demonstrated little change in GAD-7 scores (Table 2). At 3 months, approximately 4% (n = 3) of digital CBT-I participants reported moderate-to-severe anxiety (i.e. GAD-7 ≥ 10) relative to 17% (n = 14) of standard care participants (p = 0.009). Differences in moderate-to-severe anxiety did not statistically differ at 6 months postpartum (Table 3).
Prevention of moderate-to-severe anxiety. Among the subset of participants with minimal to mild anxiety symptoms at baseline (GAD-7 < 10; n = 158), 1% (n = 1) of digital CBT-I participants reported moderate-to-severe anxiety at 3 months postpartum compared to 15% (n = 12) of standard care participants, corresponding with a number needed to treat of 7.1 (χ 2(1) = 10.14, Fisher’s exact p = 0.002). At 6 months postpartum, 4% (n = 3) of digital CBT-I participants reported moderate-to-severe anxiety relative to 7% (n = 6) of standard care participants, corresponding with a number needed to treat of 33.3 (χ 2(1) = 0.69, Fisher’s exact p = 0.50)
Use of non-study treatments
Use of non-study treatments data were available from 170 (82%) participants at 3 months postpartum and 169 (81%) participants at 6 months postpartum. More participants in the standard care condition used a prescription sleep medication at least a few nights per month at 6 months postpartum than participants in the digital CBT-I condition, but this difference was only marginally significant (n = 11, 12.1% and n = 3, 3.8%, χ 2(1) = 3.76, p = 0.05). There were no statistically significant differences in the use of other non-study treatments to improve sleep or mood at either timepoint.
Discussion
The first major finding from this investigation is that participants who received digital cognitive behavior therapy for insomnia while they were pregnant experienced higher rates of insomnia remission and lower rates of insomnia caseness at approximately 6 months postpartum relative to participants who received standard care. These findings are consistent with research among non-perinatal populations demonstrating the long-term benefits of digital CBT-I [24]. In the current study, participants randomized to digital CBT-I did not report higher rates of good sleep quality relative to participants randomized to standard care. Thus, digital CBT-I may be more helpful for improving insomnia-related distress and impairment than for improving postpartum sleep quality, which may be driven by infant responsibilities, such as feeding, changing, and soothing [25].
However, there were no statistically significant between-condition differences in insomnia symptom improvement from baseline to either postpartum timepoint. This dovetails with previous research suggesting that the benefits of CBT on insomnia symptoms attenuate in the initial postpartum period. For example, a previous trial that found that digital CBT-I did not outperform digital sleep education at 6 weeks postpartum for either insomnia symptom severity or overall sleep quality [5]. A trial that compared telephone- and email-delivered CBT to an attention-matched control among pregnant women not selected for insomnia found that between-group differences in insomnia symptom severity diminished in the first year postpartum, but were significant by 2 years postpartum [26]. Thus, it is possible that tools learned in CBT-I might be less pertinent in the early postpartum period and become relevant again later in the postpartum period when maternal and infant sleep are not as tightly coupled. Indeed, less than half of participants in another trial reported that digital CBT-I was as helpful for postpartum sleep [27]. A meta-analysis found that non-pharmacological sleep interventions, such as massage, aromatherapy, and behavioral-educational programs delivered to mothers during the postpartum period improved maternal sleep quality, particularly when the intervention was delivered at 0−3 weeks postpartum [28]. However, postpartum participants have competing demands on their time and energy, and a sleep intervention delivered prior to birth may be of particular interest. Additionally, because insomnia is a risk factor for adverse outcomes, such as preterm birth [29] and elevated postpartum depression symptoms [6], it is important to treat promptly.
Each of the aforementioned studies that evaluated the effect of prenatal CBT-I on postpartum insomnia symptoms utilized a remote delivery format (i.e. digital and telephone). It is possible that clinician-led CBT-I would have produced larger effect sizes. At the same time, remote delivery options maximize access, which may be particularly important at a time when patients are increasingly opting for telehealth. Further research is needed to evaluate how to enhance the effect of CBT-I during the perinatal period, such as tailoring CBT-I for perinatal-specific sleep disturbances (e.g. discomfort during pregnancy, infant sleep during the postpartum period) and offering booster sessions.
The second major finding from this investigation is that digital CBT-I delivered during pregnancy was consistently associated with greater improvements in postpartum depressive and anxiety symptoms relative to standard care. Differences were clinically meaningful at 3 months postpartum—18% of standard care participants reported probable major depression versus 4% of digital CBT-I participants, an over fourfold difference. The number-needed-to-treat indicated that approximately six people would need to receive digital CBT-I to prevent depression. At 3 months postpartum, 17% of standard care participants reported moderate-to-severe anxiety versus 4% of digital CBT-I participants.
We found that the depression prevention effect of digital CBT-I was robust among participants who began the study with minimal depressive symptoms. Among these participants, the rates of probable major depression were 18% for standard care participants versus 0% among digital CBT-I participants. These findings are consistent with research in a non-perinatal sample that found depression prevention effects of digital CBT-I among the subset of participants who began the study with minimal depressive symptoms [9]. Among these participants, 18.8% of participants randomized to digital sleep hygiene education experienced moderate-to-severe depression at 1-year follow-up, relative to 9.6% of participants randomized to digital CBT-I. Interestingly, it appears that our perinatal sample experienced more marked depression prevention benefits than Cheng et al.’s (2019) non-perinatal sample; however, the use of different outcome measures preclude a direct comparison.
The depression findings have the potential to have high public health impact. Approximately one in seven women experience perinatal depression [30]. Perinatal depression is associated with a myriad of acute and long-lasting consequences, such as adverse birth outcomes [21, 31], increased risk of suicide [30], impairments in parenting [32], and psychological consequences for children including increased risk of internalizing (e.g. depression) and externalizing (e.g. ADHD) disorders [32, 33]. In 2019, the United States Preventive Services Task Force recommended that pregnant people at risk for depression receive counseling interventions [34]. To date, preventive interventions have mainly focused on pregnant people with elevated depressive symptoms or a history of depression [35]. Increasingly, evidence shows that prenatal insomnia is associated with increased risk for perinatal depression [36], and researchers have suggested that insomnia symptoms are a viable target for reducing or preventing perinatal depressive symptoms [36–38]. Prenatal insomnia may be a low-stigma target for promoting mental health during this critical lifecycle phase because insomnia may be viewed as a normal symptom of pregnancy. In contrast, many perinatal women report that stigma is a barrier to seeking help for depression [39, 40]. More research is needed to investigate whether, how, and for whom digital CBT-I prevents perinatal depression in an adequately powered confirmatory efficacy trial. If replicated, these findings would suggest that pregnant people with insomnia should be referred for digital CBT-I to prevent depression. A factor that increases the potential public health impact of this approach is the scalability of digital CBT-I.
Limitations
There were some important limitations to the current research. First, the purpose of this project was to investigate a scalable intervention; thus, all outcomes were based on self-report symptom measures instead of diagnostic clinical interviews or objective measures of sleep. Second, there are limits to generalizability as the majority of the sample was wealthy, white, and educated. Third, participants were only followed through 6 months postpartum. There is a need for a longer follow-up given that depression beyond the immediate postpartum period has negative consequences for child outcomes [33]. Fourth, participants were permitted to receive non-study treatment for sleep and mood, including medication and psychotherapy. Standard care participants reported a statistically significant higher use of prescription sleep medication at the post-intervention timepoint [3], and a marginally significant higher use at 6 months postpartum, which may have attenuated the between-groups differences in insomnia symptom change. Finally, it is important to note that the current study included participants who experienced insomnia symptoms for at least 1 month, which precludes generalizability to other studies using the DSM-5 criterion requiring symptom duration ≥3 months.
Summary
In conclusion, we found that participants who received a non-pharmacological intervention for insomnia while they were pregnant continued to experience improvements in insomnia symptoms at 6 months postpartum, and improvements in depressive and anxiety symptoms at 3 and 6 months postpartum. Additionally, findings from this trial provide strong preliminary evidence that digital CBT-I delivered during pregnancy may prevent postpartum depression and anxiety. Digital CBT-I has the potential to be a low-stigma, scalable means to have a favorable impact on the enormous public health problem of perinatal insomnia, anxiety, and depression.
Acknowledgments
Alison Hartman, Esperanza Castillo, and Brianne Taylor were project coordinators and assisted with recruitment, enrollment, and data collection. Danielle Roubinov, PhD, generated the randomization sequence. Alinne Barrera, PhD, served as an independent safety officer. We are deeply grateful for the participants who volunteered their time for this research despite the significant competing demands on their time and energy during the transition to parenthood.
Disclosure Statement
The research was supported by the UCSF California Preterm Birth Initiative transdisciplinary post-doctoral fellowship, funded by Marc and Lynne Benioff and the Bill & Melinda Gates Foundation. This research was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR001872. Dr. Felder received salary support from the National Center for Complementary and Integrative Health (K23AT009896) and National Institute of Mental Health (T32MH019391). Dr. Prather received salary support from the National Heart, Lung, and Blood Institute (R01HL142051). Dr. Neuhaus received salary support from the National Center for Advancing Translational Sciences (KL2 TR001870). Big Health Ltd. donated the voucher codes for the digital CBT-I intervention (Sleepio).
Dr Felder and Dr Neuhaus report no financial arrangements or connections that are pertinent to the submitted manuscript.
Dr Felder, Dr Epel, Dr Neuhaus, and Dr Prather report no non-financial interests that could be relevant to the submitted manuscript. Dr Epel is an advisor to Meru Health, a digital platform for mental health. Dr Krystal discloses the following financial interests pertinent to the submitted manuscript: he is an owner of Big Health options; he is a paid consultant to Big Health. Dr Krystal also has received research grants support from the following: Janssen Pharmaceuticals, Axsome Pharmaceutics, Reveal Biosensors, The Ray and Dagmar Dolby Family Fund, and the National Institutes of Health. He has also received consulting support from the following: Adare, Axsome Therapeutics, Eisai, Evecxia, Ferring Pharmaceuticals, Galderma, Harmony Biosciences, Idorsia, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Millenium Pharmaceuticals, Merck, Neurocrine Biosciences, Neurawell, Pernix, Otsuka Pharmaceuticals, Sage, and Takeda. Dr Prather is a paid consultant for Fitbit. He has received grant support from: National Institutes of Health, Headspace, Inc, and Eisai Co., Ltd.
References
- 1. Trauer JM, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 2. Qaseem A, et al. ; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 3. Felder JN, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry. 2020;77(5):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manber R, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133(5):911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalmbach DA, et al. Objective sleep disturbance is associated with poor response to cognitive and behavioral treatments for insomnia in postmenopausal women. Sleep Med. 2020;73:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomfohr LM, et al. Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 2015;38(8):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osnes RS, et al. Insomnia late in pregnancy is associated with perinatal anxiety: a longitudinal cohort study. J Affect Disord. 2019;248:155–165. [DOI] [PubMed] [Google Scholar]
- 8. Suri R, et al. Prospective longitudinal study of predictors of postpartum-onset depression in women with a history of major depressive disorder. J Clin Psychiatry. 2017;78(8):1110–1116. [DOI] [PubMed] [Google Scholar]
- 9. Cheng P, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espie CA, et al. The sleep condition indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. 2014;4(3):e004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espie CA, et al. The sleep condition indicator: reference values derived from a sample of 200 000 adults. J Sleep Res. 2018;27(3):e12643. [DOI] [PubMed] [Google Scholar]
- 12. Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- 13. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 14. Cox JL, et al. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 15. Espie CA, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Espie CA, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry. 2019;76(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morin CM, et al. Insomnia: A Clinical Guide to Assessment and Treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 18. Buysse DJ, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 19. Espie CA, et al. The daytime impact of DSM-5 insomnia disorder: comparative analysis of insomnia subtypes from the Great British Sleep Survey. J Clin Psychiatry. 2012;73(12):e1478–e1484. [DOI] [PubMed] [Google Scholar]
- 20. Matthey S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309–315. [DOI] [PubMed] [Google Scholar]
- 21. Accortt EE, et al. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. 2015;19(6):1306–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spitzer RL, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 23. Create a blocked randomisation list. Sealed Envelope Ltd.; 2016. https://www.sealedenvelope.com/simple-randomiser/v1/lists
- 24. Luik AI, et al. Long-term benefits of digital cognitive behavioural therapy for insomnia: follow-up report from a randomized clinical trial. J Sleep Res. 2020;29(4):e13018. [DOI] [PubMed] [Google Scholar]
- 25. Insana SP, et al. A mixed-method examination of maternal and paternal nocturnal caregiving. J Pediatr Health Care. 2014;28(4):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bei B, et al. Improving perinatal sleep via a scalable cognitive behavioural intervention: findings from a randomised controlled trial from pregnancy to 2 years postpartum. Psychol Med. 2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalmbach DA, et al. Examining patient feedback and the role of cognitive arousal in treatment non-response to digital cognitive-behavioral therapy for Insomnia during Pregnancy. Behav Sleep Med. 2021:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owais S, et al. Non-pharmacological interventions for improving postpartum maternal sleep: a systematic review and meta-analysis. Sleep Med Rev. 2018;41:87–100. [DOI] [PubMed] [Google Scholar]
- 29. Felder JN, et al. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–581. [DOI] [PubMed] [Google Scholar]
- 30. Gavin NI, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. [DOI] [PubMed] [Google Scholar]
- 31. Grote NK, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stein A, et al. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. [DOI] [PubMed] [Google Scholar]
- 33. Goodman SH, et al. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14(1):1–27. [DOI] [PubMed] [Google Scholar]
- 34. Curry SJ, et al. Interventions to prevent perinatal depression US Preventive Services Task Force recommendation statement. JAMA. 2019;321(6):580–587. [DOI] [PubMed] [Google Scholar]
- 35. O’Connor E, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(6):588–601. [DOI] [PubMed] [Google Scholar]
- 36. Sedov ID, et al. Trajectories of Insomnia symptoms and associations with mood and anxiety from early pregnancy to the Postpartum. Behav Sleep Med. 2021;19(3):395–406. [DOI] [PubMed] [Google Scholar]
- 37. Dietch JR, et al. Insomnia and cognitive arousal are important potential targets to reduce perinatal depression risk. Sleep. 2021;44(6):zsab091. doi: 10.1093/sleep/zsab091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kalmbach DA, et al. A pathogenic cycle between insomnia and cognitive arousal fuels perinatal depression: exploring the roles of nocturnal cognitive arousal and perinatal-focused rumination. Sleep. 2021:zsab091. doi: 10.1093/sleep/zsab091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dennis CL, et al. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323–331. [DOI] [PubMed] [Google Scholar]
- 40. Kopelman RC, et al. Barriers to care for antenatal depression. Psychiatr Serv. 2008;59(4):429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]