Abstract
Circadian clocks evolved as an adaptation to the cyclic change of day and night. To precisely adapt to this environment, the endogenous period has to be adjusted every day to exactly 24 hours by a process called entrainment. Organisms can use several external cues, called zeitgebers, to adapt. These include changes in temperature, humidity, or light. The latter is the most powerful signal to synchronize the clock in animals. Research shows that a complex visual system and circadian photoreceptors work together to adjust animal physiology to the outside world. This review will focus on the importance of the visual system for clock synchronization in the fruit fly Drosophila melanogaster. It will cover behavioral and physiological evidence that supports the importance of the visual system in light entrainment.
Keywords: Drosophila melanogaster, Circadian Clock, Entrainment, Light
Introduction
The switch from day to night is one of the most ancient environmental cues every organism is confronted with. As an adaptation to this predictable change, circadian clocks have evolved in many organisms and allow them to predict dusk and dawn. Work over the last several decades has shown that the key mechanism underlying circadian rhythms is a transcriptional–translational feedback loop. In a simplified view, transcriptional activators (Clock [CLK] and Cycle [CYC]) activate the transcription of hundreds (if not thousands) of clock-controlled genes. These include other genes encoding important proteins for time-keeping (Period [PER] and Timeless [TIM]), which act as transcriptional inhibitors: once their protein concentrations peak toward the end of the night, they inhibit their own transcription and hence start a new cycle (reviewed by Hardin 1 and by Top and Young 2 ).
In the fruit fly, this molecular mechanism is expressed in a specialized area of the brain, the central clock, which comprises approximately 75 pairs of neurons. 3 These neurons orchestrate the behavioral output of the fly, which is characterized by a morning (M) peak, a siesta (S), and an evening (E) peak of activity (Figure 1A). Subpopulations of clock neurons are implicated in specific features of this behavioral output. The small ventral lateral neurons (sLNvs) are essential for the M peak,4,5 whereas 3 of the 6 lateral dorsal neurons (LNds) and the fifth sLNv are important for E activity.4,5 The dorsal neurons 1 (DN1s) and the lateral posterior neurons (LPNs) are implicated in temperature entrainment6,7 and were recently identified to regulate sleep and siesta,8-11 whereas the DN2 neurons are associated with temperature entrainment and temperature preference rhythms.12,13 The DN3 subgroup is not extensively studied yet; however, nocturnin expression in these neurons appears to contribute to light-evoked behaviors in the fly. 14 More recent work suggests, however, that complex interactions of these neurons regulate fly behavior.15-22
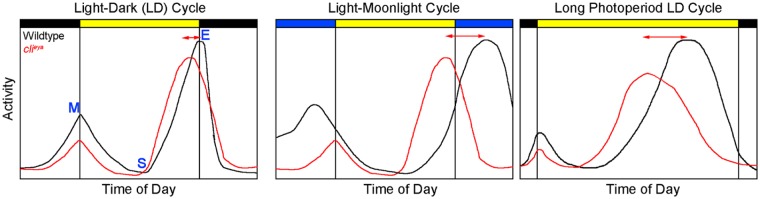
Figure 1.
Activity profiles of wild-type and eyes absent (clieya) flies at different light conditions. (A) Wild-type flies show a bimodal activity pattern in LD 12:12 with a morning (M) peak at lights-on and an evening (E) peak at lights-off. Both peaks are clearly separated by a siesta (S) during which flies tend to sleep. Eyes absent flies also show a bimodal activity pattern with a reduced M peak amplitude and an advanced E peak. (B) Moonlight simulation phase-advances the M peak and phase-delays the E peak in wild-type flies, whereas the activity profile of eyes absent flies remains unchanged compared to A. (C) Behavior of wild-type and eyes absent flies in long day conditions. Under long days, flies adjust their behavior by delaying the timing of the E peak. The timing of the peaks is uncoupling from lights-off in both cases. Flies without eyes shift the activity peak about 1.5 hours less than wild-type flies. LD indicates light–dark.
A key feature of circadian clocks is their ability to adjust to the switch from day to night by a process called entrainment. To do so, the central clock can use external stimuli, called zeitgebers, which will allow the animal to predict dawn or dusk (reviewed by Golombek and Rosenstein 23 ). The most potent zeitgeber is light, which can be received via the visual system of the fly or via the circadian photoreceptor cryptochrome (CRY). The latter is expressed within the Drosophila clock neurons (most lateral and some dorsal neurons), as well as some other brain areas and the visual system,24,25 and its function has been extensively studied. On illumination, CRY degrades TIM via the proteasome pathway and thereby “resets” the clock at the beginning of the day under regular light–dark (LD) conditions (reviewed by Foley and Emery 26 ). However, not all clock cells express CRY and flies deficient for CRY are still able to entrain to LD cycles, demonstrating the importance of the visual system for clock synchronization.27-29 This is further supported by the observation that both, CRY expression and the visual system, need to be ablated before a fly fails to entrain to LD cycles. 30 Over the past several years, a number of studies have deepened our understanding of how entrainment via the visual system impacts Drosophila at both behavioral and physiological levels. These studies are the focus of this review.
Subpopulations of Photoreceptor Cells Contribute to Clock Synchronization
The visual system of Drosophila consists of 7 eye structures: 3 ocelli, 2 Hofbauer-Buchner (HB) eyelets, and 2 compound eyes. 31 The most prominent organs are the compound eyes, which are comprised of approximately 800 identical structural units, which are called ommatidia. Each ommatidium contains 8 photoreceptor cells (R). R1-6 are located in the periphery, span the whole length of the ommatidium, and send their axons into the lamina. R7 and R8 are located in the center, with R7 in the distal and R8 in the proximal part of the eye. Both cells send their projections to the medulla (reviewed by Montell 32 ). Each receptor cell has a microvillar structure called rhabdomere. It contains the light-absorbing molecules (rhodopsins, Rh) as well as the molecular machinery to convert light information into action potentials. In short, a photon activates the rhodopsin by converting cis-retinal into all-trans retinal. This change leads to the activation of the heterotrimeric G-protein phospholipase C (encoded by the norpA gene) and to the opening of Trp and Trpl channels. When opened, these channels allow Na+ and Ca2+ to enter and depolarize the cell, which leads to the release of histamine at the synapse. 32 The compound eyes express 5 different rhodopsins in a well-defined manner: R1-6 express Rh1, whereas the central receptor cells express Rh3 to Rh6. In approximately 30% of the ommatidia (pale subtype), R7 express Rh3 and R8 express Rh5. In 70% of the ommatidia (yellow subtype), R7 express Rh4 and R8 express Rh6. 33 An exception to this rule is the dorsal rim area, in which both R7 and R8 express Rh3. 34
The first evidence of the compound eyes being involved in light entrainment comes from photoreceptor mutants. Under equinox conditions, wild-type flies show a bimodal activity pattern with an M peak at lights-on and an E peak at lights-off (Figure 1A). Flies lacking compound eyes (clieya) or flies deficient in norpA show an advanced timing of the E peak and a decreased M peak amplitude.35,36 They further lack the ability to appropriately entrain to different photoperiods36-38 (Figure 1C).
Over the past years, several experiments have addressed the importance of specific photoreceptors for clock synchronization. Key experiments included the investigation of nature-like light conditions: simulation of moonlit nights shifted the E peak of activity and caused an increase in the nocturnal activity levels. 39 The compound eyes are essential for this behavioral transition. In particular, flies lacking Rh1 or Rh6 showed strongly reduced nocturnal activity in this condition39,40 (Figure 1B). Similar results were obtained by investigating twilight conditions.41,42 Even though these experiments demonstrated the contribution of specific rhodopsins to light synchronization, they did not address the question of whether photoreceptor subtypes are sufficient to entrain the circadian clock via the visual system. To answer this question, the Rouyer lab rescued norpA in specific photoreceptor subtypes in a norpAP24 cry02 mutant background. 43 These mutants failed to re-entrain to phase-shifted LD cycles. Rescue of norpA in all but the rh5-positive photoreceptors (and the ocelli) allowed the fly to re-synchronize to an altered light regime. Notably, the rescue appeared to be more efficient in phase advancing the clock than phase delaying it. 43 The key to these experiments was using low light intensities as flies lacking norpA and cry (norpAP41 cryb, similar to the mutants above) are still able to entrain to LD cycles of higher intensities.28,44 This suggested a norpA-independent pathway in the retina for clock synchronization. Most interestingly, this independent pathway was localized to the Rh5 and Rh6 expressing photoreceptors: quadruple mutants for norpA, rh5, rh6, and cry fail to re-entrain to phase-shifted LD cycles. 44 Recent work also demonstrated that the Rh5 and Rh6-positive receptors dominate the residual electroretinogram response of norpA mutants and suggested the importance of PLC21C, the second phospholipase C of the fly genome, as the target of these rhodopsins. 45
Besides the norpA-independent pathway, Rh6-expressing cells appear to be key neurons that drive many circadian behaviors. Flies lacking Rh6 fail to appropriately adjust to high light intensities and fail to appropriately adjust to long photoperiods.37,46 These data suggest that the Rh6-expressing photoreceptors might be similar to the mammalian retinal ganglion cells, as both are essential for high light intensity entrainment. This notion is further supported by a recent study demonstrating that these cells not only act as photoreceptors but also as interneurons via the histamine receptor HisCl. 47 Flies lacking histamine receptors display a deficiency to re-entrain to shifted LD-cycles, which could be improved by rescuing HisCl in rh6-positive photoreceptors.
However, Rh6 is not only expressed in the compound eyes but also in the Hofbauer-Buchner eyelets. These photoreceptors are located underneath the retina and are comprised of 4 receptor cells. They are the remainders of the larval eyes, Bolwig organ, which undergoes complex reorganization during pupal development. 48 The eyelets appear to be histaminergic as well as cholinergic and contribute to light synchronization. 49 As mentioned previously, flies deficient of norpA and cry are able to re-synchronize to high light intensity; however, additional blocking of neurotransmitter output from rh5-GAL4 positive neurons (the driver is expressed in the eyelets) strongly reduced this ability. 50 Also, the inability to adjust to high light intensities appears to be due to the eyelets; flies lacking compound eyes (clieya) but retaining eyelets can adapt to high light intensities, whereas the additional removal of Rh6 (clieya; rh6 1 ) renders the flies blind to high light intensities. 46
The Photoreceptors Target Different Subsets of Drosophila Clock Neurons
The photoreceptors of the compound eyes terminate in different layers of the fly brain. R1-6 terminate in the lamina where they connect to interneurons which innervate the medulla. Both inner receptor cells directly innervate the medulla where they lie near the fibers of the pigment dispersing factor (PDF) neurons. 32 The HB-eyelets directly innervate the accessory medulla where they terminate close to the sLNvs48,51,52 (Figure 2A-I). This anatomy suggested a direct connection of the visual system with the PDF neurons. This is indeed true for the HB-eyelet: GRASP (GFP Reconstitution Across Synaptic Partners) implicated that the HB-eyelets synaptically connect to both, the sLNvs and the lLNvs. 53 Exciting the HB eyelets lead to increases in Ca2+ and cAMP in the sLNvs. The activation of the sLNvs likely leads to an increased release of PDF, which adjusts PER cycling in downstream target cells like the DN1 and LNd neurons. 46 In contrast, the lLNvs are receptive to bath-applied histamine, 53 suggesting that histamine released by the HB-eyelets could impact the lLNvs. This does not imply a direct connection, but trans-tango experiments suggest that the eyelet also directly connects to the lLNvs (Figure 2J-L). In contrast, the compound eyes do not appear to directly connect to the PDF neurons, and GRASP experiments did not show any GFP signal in the medulla suggesting interneurons being involved in signal transduction from the compound eyes to the clock neurons. However, electrophysiological evidence suggests a connection between the compound eyes and the lLNvs. Recordings from these lLNv neurons with the retina attached to the brain significantly altered their firing pattern. 55 This change in firing pattern was mediated via cholinergic interneurons in the lamina. Similar results were obtained by a more recent study 56 that further extended our knowledge by recording from not only the lLNvs but also other clock neuron subgroups. The surprising finding was that most clock neuron clusters (sLNvs, lLNvs, fifth sLNv, CRY + LNds, DN1a, DN2, and DN3a) fired action potentials on light stimulation when the preparations included the compound eyes. Removing the compound eyes abolished the changes in physiology, suggesting that the eyes are able to activate an array of clock neurons. All of these neurons share projections into the accessory medulla, an important pacemaker center. Ablating this area lead to the loss of photosensitivity of the clock cells, 56 suggesting that visual information is integrated in this area.
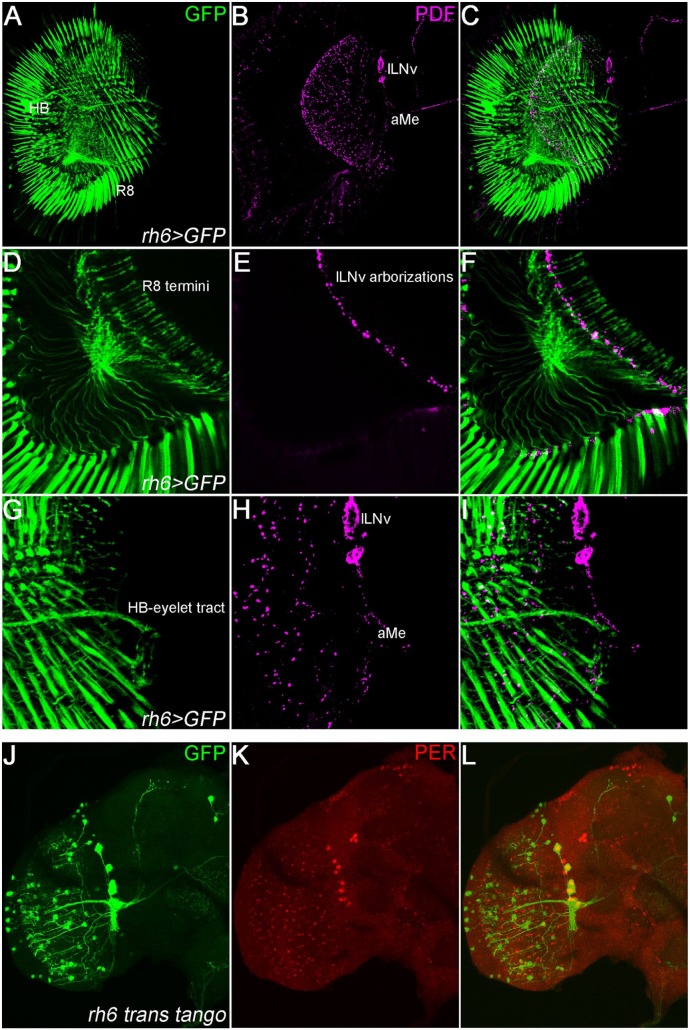
Figure 2.
(A-I) Termini of rhodopsin 6 expressing photoreceptors reside near PDF-neuron arborizations. (A-C) Brain and compound eyes of rh6-GAL4 UAS-GFP flies stained against GFP (green) and PDF (magenta). Rh6 is expressed in 70% of R8 which directly innervate the medulla. The HB-eyelet expresses Rh6 and sends its projections toward the accessory medulla (aMe). (D-F) Single layer from the posterior part of the brain. R8 termini (GFP, green) innervate the medulla, where they are near the fibers of the PDF neurons (magenta). (G-I) Z-stack of 4 layers in the anterior part of the brain. The HB-eyelet directly innervates the accessory medulla (aMe) (adapted from Schlichting et al 46 ). (J-L) Rh6-GAL4 positive neurons connect to sLNvs and lLNvs using trans-tango. Rh6-GAL4/trans-tango QUAS-GCamp6 flies were stained against GFP (green) and Period (red). Trans-tango was recently developed to investigate synaptic partners of neurons of choice (for details see Talay et al 54 ). Neurons expressing GCamp (green, labeled with GFP antibody) lie downstream of rh6-expressing photoreceptors. As expected, downstream neurons include several medulla neurons likely involved in visual information transduction. Co-labeling with anti-PER reveals the sLNvs and also the lLNvs as direct targets, whereas other clock-neurons appear to be indirect targets of the photoreceptor cells. HB indicates Hofbauer-Buchner; PDF, pigment dispersing factor; PER, Period.
Despite these advances in our understanding, it was still unclear which of these light responses drive the rhythmic behavior of the fly. Early evidence suggested the PDF-neurons as key players: cry01 pdf01 mutants lack the evening peak of activity which suggested that eye-mediated signaling is integrated into the system via PDF.57,58 Similarly, Rh6-positive neurons (R8 from the compound eyes and/or the HB-eyelets) target the sLNvs in equinox conditions, where they interfere with the clock machinery and most likely PDF release into the dorsal brain.45,46 In long day conditions, the compound eyes contact the lLNvs where they trigger PDF release in the accessory medulla, which signals directly to the E cells.37,53 Moreover, silencing or ablating the PDF neurons reproduced the advanced E peak phenotype of flies lacking compound eyes, whereas changing the firing pattern of DN1s and/or LNds had different effects on behavior.9,36,37,59,60 All of these experiments point to a prominent role of the PDF neurons in light synchronization via the visual system, which parallels what is known about mammalian mechanisms. 61 However, flies lacking CRY and PDF are still able to entrain to LD cycles, suggesting that other neurons can receive light information from the compound eyes. How these connections contribute to light synchronization in wild-type flies is still unknown.
In summary, recent research has provided a substantial body of evidence on the ways light information is integrated in the Drosophila clock neuron network. Key players include Rhodopsin 6 expressing photoreceptors as well as PDF neurons, as ablating these neurons largely reproduces the behavior of eyes absent (clieya) mutants. However, a PDF-independent pathway allows the animal to synchronize to LD cycles in its absence. This pathway likely includes another rhodopsin (Rh7), which appears to be expressed within the clock neuron network and subpopulations of R8 of the compound eyes.62,63
Acknowledgments
I want to thank Dr Katharine Abruzzi and Dr Shlesha Richhariya for comments on an earlier version of this manuscript. M.S. was funded by the Howard Hughes Medical Institute (HHMI) and a fellowship of the German research foundation (DFG, SCHL2135). Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. I also want to thank Dr. Michael Rosbash and Dr. Charlotte Helfrich-Förster for their support and two anonymous reviewers for their very helpful comments on a previous version of this manuscript.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.S. was sponsored by the Howard Hughes Medical Institute (HHMI) and a fellowship of the German Research Foundation (DFG).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Matthias Schlichting performed experiments, wrote and revised the manuscript.
ORCID iD: Matthias Schlichting  https://orcid.org/0000-0002-0822-0265
https://orcid.org/0000-0002-0822-0265
References
- 1. Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Top D, Young MW. Coordination between differentially regulated circadian clocks generates rhythmic behavior. Cold Spring Harb Perspect Biol. 2018;10:a033589. doi: 10.1101/cshperspect.a033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helfrich-Forster C, Yoshii T, Wulbeck C, et al. The lateral and dorsal neurons of Drosophila melanogaster: new insights about their morphology and function. Cold Spring Harb Symp Quant Biol. 2007;72:517-525. [DOI] [PubMed] [Google Scholar]
- 4. Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862-868. [DOI] [PubMed] [Google Scholar]
- 5. Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869-873. [DOI] [PubMed] [Google Scholar]
- 6. Yoshii T, Heshiki Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, Tomioka K. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci. 2005;22:1176-1184. [DOI] [PubMed] [Google Scholar]
- 7. Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115-126. [DOI] [PubMed] [Google Scholar]
- 8. Guo F, Holla M, Diaz MM, Rosbash M. A circadian output circuit controls sleep-wake arousal in Drosophila. Neuron. 2018;100:624-635. doi: 10.1016/j.neuron.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 9. Guo F, Yu J, Jung HJ, et al. Circadian neuron feedback controls the Drosophila sleep: activity profile. Nature. 2016;536:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ni JD, Gurav AS, Liu W, et al. Differential regulation of the sleep homeostat by circadian and arousal inputs. Elife. 2019;8:e40487. doi: 10.7554/eLife.40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamaze A, Kratschmer P, Chen KF, Lowe S, Jepson JEC. A wake-promoting circadian output circuit in Drosophila. Curr Biol. 2018;28:3098-3105. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko H, Head LM, Ling J, et al. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr Biol. 2012;22:1851-1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Picot M, Klarsfeld A, Chelot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci. 2009;29:8312-8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagoshi E, Sugino K, Kula E, et al. Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci. 2010;13:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yao Z, Bennett AJ, Clem JL, Shafer OT. The Drosophila clock neuron network features diverse coupling modes and requires network-wide coherence for robust circadian rhythms. Cell Rep. 2016;17:2873-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlichting M, Díaz MM, Xin J, Rosbash M. Neuron-specific knockouts indicate the importance of network communication to rhythmicity. Elife. 2019;8:31613223. doi: 10.7554/eLife.48301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delventhal R, O’Connor RM, Pantalia MM, et al. Dissection of central clock function in through cell-specific CRISPR-mediated clock gene disruption. Elife. 2019;8:e48308. doi: 10.7554/eLife.48308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee A, Lamaze A, De J, et al. Reconfiguration of a multi-oscillator network by light in the Drosophila circadian clock. Curr Biol. 2018;28:2007-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dissel S, Hansen CN, Ozkaya O, Hemsley M, Kyriacou CP, Rosato E. The logic of circadian organization in Drosophila. Curr Biol. 2014;24:2257-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins B, Kaplan HS, Cavey M, et al. Differentially timed extracellular signals synchronize pacemaker neuron clocks. PLoS Biol. 2014;12:e1001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bulthuis N, Spontak KR, Kleeman B, Cavanaugh DJ. Neuronal activity in Non-LNv clock cells is required to produce free-running rest: activity rhythms in Drosophila. J Biol Rhythms. 2019;34:249-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063-1102. [DOI] [PubMed] [Google Scholar]
- 24. Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol. 2008;508:952-966. [DOI] [PubMed] [Google Scholar]
- 25. Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms. 2008;23:296-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foley LE, Emery P. Drosophila cryptochrome: variations in Blue [published online ahead of print 10 October, 2019]. J Biol Rhythms. doi: 10.1177/0748730419878290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669-679. [DOI] [PubMed] [Google Scholar]
- 28. Stanewsky R, Kaneko M, Emery P, et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681-692. [DOI] [PubMed] [Google Scholar]
- 29. Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY Is a deep brain circadian photoreceptor. Neuron. 2000;26: 493-504. [DOI] [PubMed] [Google Scholar]
- 30. Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249-261. [DOI] [PubMed] [Google Scholar]
- 31. Hofbauer A, Buchner E. Does Drosophila have seven eyes? Naturwissenschaften. 1989;76:335-336. doi: 10.1007/bf00368438. [DOI] [Google Scholar]
- 32. Montell C. Drosophila visual transduction. Trends in Neurosciences. 2012;35:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rister J, Razzaq A, Boodram P, et al. Single-base pair differences in a shared motif determine differential Rhodopsin expression. Science. 2015;350:1258-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267-279. [DOI] [PubMed] [Google Scholar]
- 35. Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms. 1993;8:67-94. [DOI] [PubMed] [Google Scholar]
- 36. Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377-391. [DOI] [PubMed] [Google Scholar]
- 37. Schlichting M, Weidner P, Diaz M, et al. Light-mediated circuit switching in the Drosophila neuronal clock network. Curr Biol. 2019;29:3266-3276. [DOI] [PubMed] [Google Scholar]
- 38. Kistenpfennig C, Nakayama M, Nihara R, Tomioka K, Helfrich-Forster C, Yoshii T. A tug-of-war between cryptochrome and the visual system allows the adaptation of evening activity to long photoperiods in Drosophila melanogaster. J Biol Rhythms. 2018;33:24-34. [DOI] [PubMed] [Google Scholar]
- 39. Bachleitner W, Kempinger L, Wulbeck C, Rieger D, Helfrich-Forster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:3538-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schlichting M, Grebler R, Peschel N, Yoshii T, Helfrich-Forster C. Moonlight detection by Drosophila’s endogenous clock depends on multiple photopigments in the compound eyes. J Biol Rhythms. 2014;29:75-86. [DOI] [PubMed] [Google Scholar]
- 41. Schlichting M, Grebler R, Menegazzi P, Helfrich-Forster C. Twilight dominates over moonlight in adjusting Drosophila’s activity pattern. J Biol Rhythms. 2015;30:117-128. [DOI] [PubMed] [Google Scholar]
- 42. Schlichting M, Menegazzi P, Helfrich-Forster C. Normal vision can compensate for the loss of the circadian clock. Proc Biol Sci. 2015;282: 1846. doi: 10.1098/rspb.2015.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saint-Charles A, Michard-Vanhee C, Alejevski F, Chelot E, Boivin A, Rouyer F. Four of the six Drosophila rhodopsin-expressing photoreceptors can mediate circadian entrainment in low light. J Comp Neurol. 2016;524:2828-2844. [DOI] [PubMed] [Google Scholar]
- 44. Szular J, Sehadova H, Gentile C, et al. Rhodopsin 5– and Rhodopsin 6–mediated clock synchronization in Drosophila melanogaster is independent of retinal phospholipase C-β signaling. Journal of Biological Rhythms. 2012;27:25-36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ogueta M, Hardie RC, Stanewsky R. Non-canonical phototransduction mediates synchronization of the Drosophila melanogaster circadian clock and retinal light responses. Curr Biol. 2018;28:1725-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schlichting M, Menegazzi P, Rosbash M, Helfrich-Förster C. A distinct visual pathway mediates high light intensity adaptation of the circadian clock in Drosophila. J Neurosci. 2019:1497-1418. doi: 10.1523/jneurosci.1497-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alejevski F, Saint-Charles A, Michard-Vanhee C, et al. The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles. Nat Commun. 2019;10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helfrich-Forster C, Edwards T, Yasuyama K, et al. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 2002;22:9255-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veleri S, Rieger D, Helfrich-Forster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms. 2007;22:29-42. [DOI] [PubMed] [Google Scholar]
- 51. Yasuyama K, Okada Y, Hamanaka Y, Shiga S. Synaptic connections between eyelet photoreceptors and pigment dispersing factor-immunoreactive neurons of the blowfly Protophormia terraenovae. J Comp Neurol. 2006;494:331-344. [DOI] [PubMed] [Google Scholar]
- 52. Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443-1453. [DOI] [PubMed] [Google Scholar]
- 53. Schlichting M, Menegazzi P, Lelito KR, et al. A neural network underlying circadian entrainment and photoperiodic adjustment of sleep and activity in Drosophila. J Neurosci. 2016;36:9084-9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Talay M, Richman EB, Snell NJ, et al. Transsynaptic mapping of second-order taste neurons in flies by trans-tango. Neuron. 2017;96:783-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muraro NI, Ceriani MF. Acetylcholine from visual circuits modulates the activity of arousal neurons in Drosophila. J Neurosci. 2015;35:16315-16327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li M-T, Cao L-H, Xiao N, et al. Hub-organized parallel circuits of central circadian pacemaker neurons for visual photoentrainment in Drosophila. Nat Commun. 2018;9:4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci. 2009;12:1431-1437. [DOI] [PubMed] [Google Scholar]
- 58. Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS One. 2011;6:e18974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo F, Chen X, Rosbash M. Temporal calcium profiling of specific circadian neurons in freely moving flies. Proc Natl Acad Sci U S A. 2017;114:E8780-E8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A PDF neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791-802. [DOI] [PubMed] [Google Scholar]
- 61. Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916: 172-191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 62. Ni JD, Baik LS, Holmes TC, Montell C. A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature. 2017;545:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grebler R, Kistenpfennig C, Rieger D, et al. Drosophila Rhodopsin 7 can partially replace the structural role of Rhodopsin 1, but not its physiological function. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017;203: 649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]