Abstract
Background and aims:
Worldwide, type 2 diabetes mellitus accounts for a considerable burden of disease, with an estimated global cost of >800 billion USD annually. For this reason, the search for more effective and efficient therapeutic anti-diabetic agents is continuing. Coumarins are naturally derived and synthetic molecules with a wide variety of biological actions. The most common application of these molecules in medicine is for their thrombostatic activity. This study aims to give an overview of the current knowledge about the applicability of these chemical products in the therapeutic strategy against diabetes and its complications.
Methods:
For this purpose, we searched internet databases for publications and abstracts in English that investigated the effects of coumarins or coumarin-like agents with potential anti-diabetic activity.
Results:
The result is that a variety of these agents have proven in in vitro, in silico, and simple animal models to possess properties that may reduce the glucose absorption rate in the intestines, increase the level of insulin, increase the cellular uptake of glucose or reduce the gluconeogenesis. In addition, some of these agents also reduced the level of glycation of peptides in diabetic animal models and showed antioxidant properties.
Conclusion:
In conclusion, we can summarize that coumarins and their related derivatives may be potential antidiabetic agents. Useful formulations with appropriate pharmacokinetic and pharmacodynamic properties must be developed and tested for their efficacy and toxicity in comprehensive animal models before they can enter clinical trials.
Keywords: Coumarin, type 2 diabetes mellitus, diabetes complications, glucose metabolism
Introduction
Type 2 diabetes mellitus is a chronic disease characterized by the heterogeneous disturbance of mainly glucose metabolism in the body, ultimately leading to chronic hyperglycemia. 1 The disorder is caused mostly through impaired insulin action, followed frequently by impaired insulin secretion. 1 According to a recent comprehensive review of epidemiological data, in 2017 almost 500 million people suffered worldwide from this disorder, reflecting a global prevalence rate of >6% and with some regions showing figures above 20%. 2 The International Diabetes Federation estimated that the total cost of people suffering from this disease was about 850 billion USD for the same year. 3 Finally, the disease and its complications annually account for a mortality rate of up to 10%, 4 with more than 7 million reported deaths in the period of 2000 to 2016 in 108 countries. 5
The general opinion now is that a high postprandial blood glucose level 6 is an important risk factor for the development and progression of type 2 diabetes mellitus and its complications and that lowering this level should be one of the targets of treatment. 7 This level depends on the amount and rate of glucose absorbed in the intestine and the uptake by the liver and skeletal muscles. 8 The rate of absorption in the intestines can be limited through an inhibition of the digestion of the polysaccharides and disaccharides as well as limiting epithelial transport through specific glucose transporters in the intestinal epithelial cell membranes. 8
Transepithelial glucose transport in the intestines is facilitated by a sodium glucose cotransporter (SGLT)1 at the apical cell membrane and a glucose transporter (GLUT)2 at the basolateral membrane. 9 Inhibition of these could lead to colonic discomfort due to a higher than normal glucose availability to the microorganisms in the colon. 10 Nevertheless, a mild inhibition may slow the absorption of glucose in the intestines and hence flatten the postprandial glucose curve. 11
Studies show that a number of synthetic as well as naturally occurring coumarins can inhibit the enzymes α-glucosidase or α-maltase, which convert the disaccharides into simple monosaccharides like glucose. The inhibitory effect on α-glucosidase can be described to the structure of coumarin-like compounds especially the C-C or C-O-C biaryl, terpene sidechain linkage, or the cyclobutane ring. 12 Several in silico docking experiments have confirmed this hypothesis.13-15 However, the abundance of studies have been performed in in vitro and animal models, while research on human subjects is not available and the applicability as true antidiabetic agents has yet to be confirmed.
In addition, type 2 diabetes mellitus is generally characterized as a condition with a disturbed action of insulin on the cellular uptake of glucose with or without a diminished insulin availability. 16 Insulin release by the pancreas is predominately nutrient triggered. 17 Advanced research has also identified other molecular mechanisms that may initiate insulin release. These are, among others, the release of glucagon-like peptide (GLP)-1 during meals 18 and the inhibition of KATP channels by sulfonyl(thio)urea derivatives that are used as oral antidiabetics. 19 Glucagon-like peptide 1 augments the release of insulin by the pancreas after meals through a separate mechanism (i.e., not yet fully understood),20,21 but may involve an increased sensitivity of the beta-cells of the pancreas for glucose. 22 The glucose lowering effect of insulin is mainly through the promotion of peripheral cellular uptake of glucose with the aid of GLUT4 transporters.23,24
The complications of diabetes are mostly the consequence of advanced glycation end products 25 or oxidative processes.26,27 Examples of these are the cataract found in the eyes of diabetics, which is predominantly the consequence of glycation of the normally highly transparent lens proteins 28 and the diabetic retinopathy and nephropathy, which are more the consequence of oxidative damage to the blood vessels in these organs.29,30
Consequently, therapeutic strategies for this metabolic disorder include inhibition of glucose processing and absorption in the intestines, 31 stimulation of hepatic and muscle uptake of glucose by insulin,32,33 supplementing insulin, 34 stimulating insulin release, 35 decreasing peripheral insulin resistance, 36 inhibition of glucose reabsorption in the kidneys, 37 and prevention of the glycation and oxidation of biologically active proteins.26,38 Up till now, no single best agent for therapy has been found, so the search for effective and more efficient pharmacological agents is continuing.
Coumarin (Figure 1) is a 1, 2-benzopyrone class of molecule that is abundantly found in many plants. 39 The molecule and its related compounds have been evaluated for a variety of pharmacological properties such as antimicrobial, 40 anti-inflammatory, 41 antidiabetic, 42 and antioxidant 43 activity, as well as a significant influence on physiological processes like enzyme inhibitory activity. 44 In plants, coumarins are often found in the form of glycosides and esters. Most of the time they are in a free form and many of them can sublimate. Coumaric compounds are lactones of 2-coumaric acid (2-hydroxy-Z-cinnamic acid) and are constructed by a benzene ring fused to an α-pyrone ring (Figure 1). 45 Based on their chemical diversity and complexity, natural coumarins are subdivided in various classes like, but not limited to, simple coumarins, isocoumarins, furanocoumarins, pyranocoumarins (angular and linear), biscoumarins, and phenylcoumarins. 46
Figure 1.
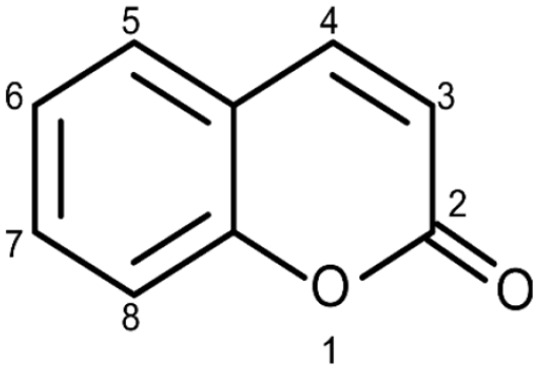
Basic structure of coumarin.
For the determination of coumarin in drugs, colorimetric, fluorometric, and liquid chromatographic methods are used. Preparative liquid chromatography and gel filtration are methods of isolating and purifying coumarins and their heterosides. They are photosensitive and when they are characterized by UV light absorption (320-380 nm), they can result in blue, green, and yellow fluorescence. 46 The smell of coumarin is specific, sweet, and spicy, often like vanilla. Generally, they may have a bitter taste. 45
The International Union of Pure and Applied Chemistry name of Coumarin is 2H-chromen-2-one. 47 The general molecular formula of the molecule is C9H6O2 and it has a relatively low molecular weight of 146.6 g/mol (Figure 1). 45 The melting point of the substance is 71°C (344 K), the boiling point is 301.71°C (574.86 K), and its density is 0.935 g/cm3. The molecule is soluble in hot water, methanol, ethanol, ether, chloroform, and other organic reagents. 46
Despite the wide availability of coumarins and their lead compounds and metabolites in natural products, 48 their medical application until now has been mostly limited to the anticoagulant activity of warfarin derived from dicoumarol and its analogs. 49 Warfarin (Figure 2) and its derivatives acenocoumarol (Figure 3) and fenprocoumon (Figure 4) are widely known for their thrombostatic properties. 50
Figure 2.
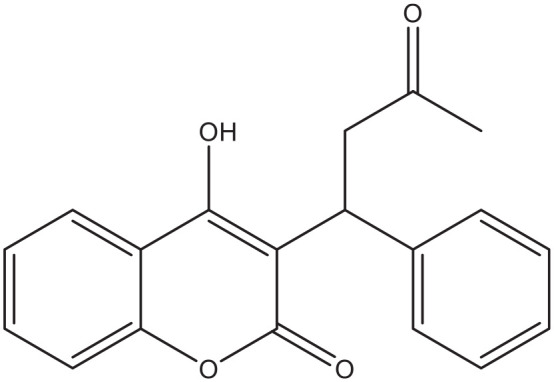
Warfarin.
Figure 3.
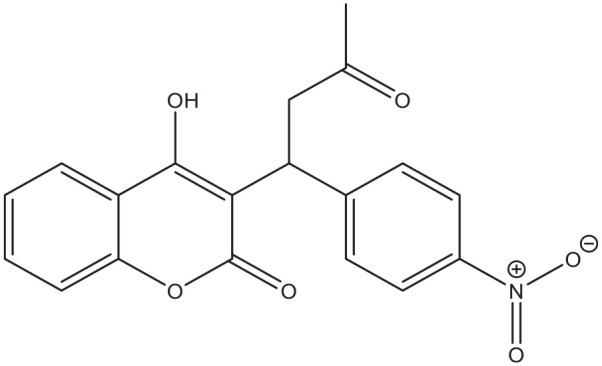
Acenocoumarol.
Figure 4.
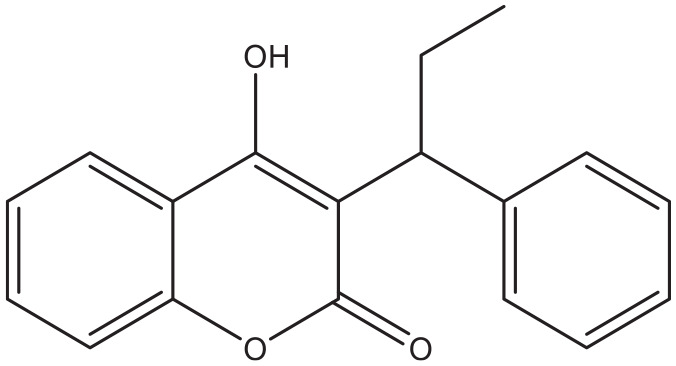
Fenprocoumon.
However, recent studies support the search for coumarins or related compounds with potential blood glucose lowering properties. For instance, several Structure-Activity Relationship studies showed that coumarins have the potential structure to bind to the active site of the enzymes α-glucosidase,13,46,51 a key step in the digestion of disaccharides and absorption of glucose by the intestines, and aldose reductase 52 which is involved in the synthesis of advanced glycation end products, leading to complications in diabetes mellitus.
Furthermore, investigators successfully bound a coumarin to glucagon-like peptide (GLP)-1, with the result that the half-life of this intestinal peptide with insulin release stimulating properties increased significantly in rats. 53 As mentioned earlier, GLP-1 augments the release of insulin by the pancreas after meals. The protein normally has a very short half life of only a few minutes. 22 In addition, coumarins reduced the glycation of peptides, 54 a metabolic step leading to most of the complications in diabetes.26,38 However, all available evidence has been derived from in vitro, in silico, and some animal studies.
The aim of this review is to present an overview of possible applications of coumarins or related compounds in the therapeutic strategies against type 2 diabetes mellitus and its complications. For this purpose, we reviewed the literature from the year 2000 onward. Preliminary searches revealed that before 2000, very few or no investigations were performed in this area. Despite this, older reports containing a clear lead toward the applicability of compounds were also included. The reported articles present examples of coumarins or their derivatives with different possible mechanisms of action against diabetes.
Methods
An extensive search of the PubMed, Medline, and Google Scholar databases was conducted to identify relevant reports concerning coumarins and different pathogenetic aspects of diabetes mellitus including intestinal processing of glucose, absorption of glucose, regulation of glucose metabolism, insulin secretion, insulin sensitivity, and complications of diabetes. The search strings included “coumarin AND glucose,” “coumarin AND diabetes mellitus,” “coumarin AND nephropathy,” “coumarin AND neuropathy,” and “coumarin AND retinopathy.” A preliminary search was performed in November 2020. The actual search was performed from 18th to 25th December 2020. Additional searches were performed in April and June 2021. We reviewed all publications in English and those having English abstracts. Articles reporting coumarin or coumarin related compounds with a possible antidiabetic activity in in vitro, in vivo, and in silico models were included. Articles cited in the reference lists of identified publications were considered as a potential source of information. Studies of raw material with a possible coumarin content that did not mention the exact (groups of) molecules or a specific mechanism of action were excluded. The compounds have been grouped according to their possible mechanisms in 3 categories, carbohydrate digestion and glucose absorption, cellular glucose uptake, and complications of Diabetes Mellitus. All figures were drawn with Chem Office 2016—ChemDraw.
Results
Results have been grouped by their possible mechanism of action. Table 1 gives an overview of the reported compounds, their possible mechanism of action, their source and the references citing them. Figures 5 to 14 show the chemical structures of the most cited and promising compounds.
Table 1.
Examples of coumarins with effects hinting toward a possible antidiabetic action.
| Compound | Observed effect | Source | Research method | References |
|---|---|---|---|---|
| 3-(5′-Methyl-2′-aryl-3′-[thiazol-2′-yl amino] thiazolidin-4′-one) coumarin | Hypoglycemic | Synthetic | In vivo/animal | Kini and Ghate 73 |
| 3-coumarincarbohydrazides | Inhibition of α-glucosidase and pancreatic lipase | Synthetic | In vitro/in silico | Xu et al 51 |
| 3-coumarincarbohydrazones | Inhibition of α-glucosidase | Synthetic | In vitro/in silico | Taha et al 13 |
| 4-hydroxy Pd-C-III coumarin | Inhibition of α-glucosidase Inhibition of Protein Tyrosine Phosphatase 1B |
Semi-synthetic | In vitro | Ali et al 63 |
| Coumarin | Prolonging half life of GLP1 Enhanced glycolysis and decreased gluconeogenesis Increasing insulin and decreasing glucose in diabetic rats Reduced protein glycation |
Natural/semisynthetic | In vitro/in vivo/animals | Han et al 53 , Abul Qais and Ahmad 54 , Pari and Rajarajeswari 74 |
| Coumarin diglycoside | Stimulation of insulin release | Natural | In vitro/cell culture | Cao et al 72 |
| Coumarin-3-carboxylic acid derivatives | Decreased gluconeogenesis | Semi-synthetic | In vitro/cell culture | Ji et al 95 |
| Coumarin-cyclic imide conjugates | Increased cellular uptake of glucose | Synthetic | In vitro/cell culture | Reddy et al 82 |
| Decursinol | Inhibition of α-glucosidase | Natural | In vitro | Ali et al 63 |
| Esculin | Increased cellular uptake of glucose | Natural | In vitro/cell culture/in vivo/animals | Mo et al 87 |
| Ficusin | Increased cellular uptake of glucose | Natural | In vivo/animals/in silico | Irudayaraj et al 83 |
| Flavonoid-coumarin hybrids | Inhibition of α-glucosidase/Increased glucose uptake by hepatic cellular cells | Synthetic | In vitro/cell culture | Sun et al 56 |
| Fraxetin | Increased cellular uptake of glucose | Natural/semisynthetic | In vitro/cell culture/in vivo/animals | Mo et al 87 |
| Isofraxidin | Lowers triglyceride and total cholesterol content of liver cells. Possible enhanced sensitivity for insulin | Natural | In vitro/cell culture/animals | Li et al 76 |
| Isorutarine | Inhibition of α-glucosidase | Natural | In silico | Mishra et al 67 |
| Osthole | Increased cellular uptake of glucose Increased bone fromation |
Natural | In vitro/cell culture/in vivo/animals | Lee et al 85 , Alabi et al 86 , Mo et al 87 , Gao et al 97 |
| Praeruptori | Inhibition of SGLT1 | Natural | In vitro/cell culture | Oranje et al 69 |
| Pteryxin | Selective inhibition of SGLT1 and Lowering of intracellular triglyceride content | Natural | In vitro/cell culture | Oranje et al 69 |
| Quinazolinone-coumarin hybrids | Inhibition of α-glucosidase | Natural | In vitro/in silico | Menteşe et al 55 |
| Scoparone | Inhibition of glucose induced proliferation of mesangial cells | Natural | In vitro/cell culture | Wang et al 100 |
| Scopoletin | Inhibition of α-glucosidase and reducing the postprandial glucose level Increased sensitivity for insulin |
Natural | In vitro/in vivo/animal | Jang et al 60 , Jang et al 61 |
| Selaginolide A | Inhibition of Protein Tyrosine Phosphatase 1B | Natural | In vitro/cell culture | Nguyen et al 80 |
| Skimming | Decreased glomerulosclerosis | Natural | In vivo/animals | Sen et al 101 |
| Umbelliferone | Increased cellular uptake of glucose | Semi -Synthetic | In vivo/animals | Naowaboot et al 84 , Ramesh and Pugalendi 94 |
| Decreased gluconeogenesis |
Figure 5.
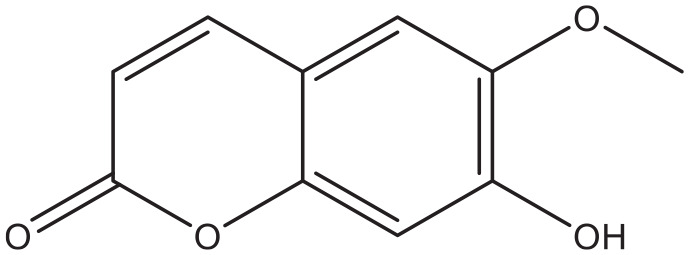
Scopoletin.
Figure 14.
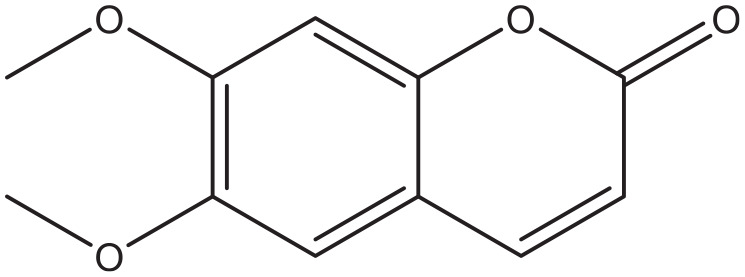
Scoparone.
Coumarins and intestinal carbohydrate digestion and absorption of glucose
Investigators synthesized about 17 coumarin derivatives that showed varying degrees of inhibition on α-glucosidase with IC50 values ranging between 1.10 ± 0.01 and 36.46 ± 0.70 μM compared with acarbose having IC50 values 39.45 ± 0.10 μM in an in vitro assay. 13 In another study, synthetic coumarin derivatives inhibited α-glucosidase in a reversible fashion. The study further showed that the coumarins formed hydrogen bonds with LYS293 on the enzyme to lower the binding affinity for the saccharose in silico. 51 Furthermore, coumarin derivatives like N′-{2-(2-[3,4-dichlorobenzyl]-4-oxoquinazolin-3[4H]-yl)acetyl}-2-oxo-2H-chromene-3-carbohydrazide and N′-{2-(2-[4-bromobenzyl]-4-oxoquinazolin-3[4H]-yl)acetyl}-2-oxo-2H-chromene-3-carbohydrazide showed pancreatic lipase and α-glucosidase inhibition properties. 55 In other studies, synthesized coumarin hybrids showed inhibition of the activity of α-glucosidase, α-amylase, and α-galactosidase, while some of them stimulated glucose uptake by cultured hepatic cells. 56 Other coumarin hybrids showed a higher affinity for α-glucosidase than the standard acarbose. 57 Molecular docking and in silico studies revealed, as mentioned earlier in the introduction, that the mechanism of action was probably based on the reversible binding on the active sites of the enzymes.46,51,57-59
In addition to these (semi) synthetic coumarins, naturally occurring compounds also showed in vitro inhibition activity on the above-mentioned enzymes. Scopoletin (Figure 5) is a coumarin that is widespread in the plant world. Investigators showed that this compound inhibited α-glucosidase in vitro and reduced the postprandial glucose level in mice. 60 The latter effect is possibly the result of phosphatidylinositol-3-kinase induced expression of the plasma membrane GLUT4. 61
Coumarins extracted from the flowers of Edgeworthia gardneri significantly inhibited the in vitro activity of both α-amylase and α-glucosidase. 62 At least 2 coumarins, 4-hydroxy Pd-C-III, and decursinol (Figure 6) isolated from Angelica decursiva also showed α-glucosidase enzyme inhibition, 63 while a cinnamon extract with abundant coumarin derivatives inhibited the enzyme in vitro and lowered the postprandial glucose excursion in diabetic rats. 64 Recent reports also confirmed the α-glucosidase inhibition properties of other plant-derived pyranocoumarins.65,66 With computer aided drug design technology, researchers identified the best active coumarin derivatives to inhibit lysosomal α-glucosidase. They found that isorutarine, among a few others, showed a good in silico inhibition of the enzyme. In addition they showed that substitution with hydrophobic groups at the C4 and C5 positions of the coumarin ring increased the activity, while substitutions at the C1, C2, C3, C6, C7, and C8 on the contrary showed a decreased activity of the compounds. 67
Figure 6.
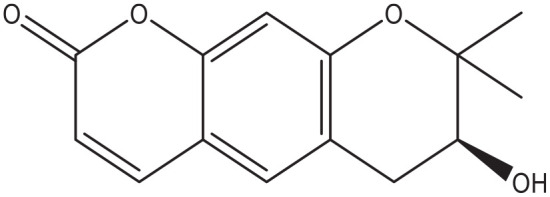
Decursinol.
A relatively early investigation already showed the inhibitory activity of coumarin-like compounds on sodium-dependent glucose absorption in brush border membrane vesicles isolated from rat small intestine. 68 Moreover, in a well-carried out comprehensive in vitro study, several naturally derived pyranocoumarin compounds like pteryxin (Figure 7) and praeruptorin showed significant and selective SGLT1 inhibition in cultured adapted Chinese Hamster Ovary and Caco-2 intestinal cells, 69 with a potential effect on transepithelial intestinal glucose absorption. It is noteworthy that pteryxin also demonstrates favorable effects on fat metabolism, since it suppressed the triglyceride content in both cultured adipocytes and hepatocytes, 70 a factor that could reduce resistance to insulin action.
Figure 7.
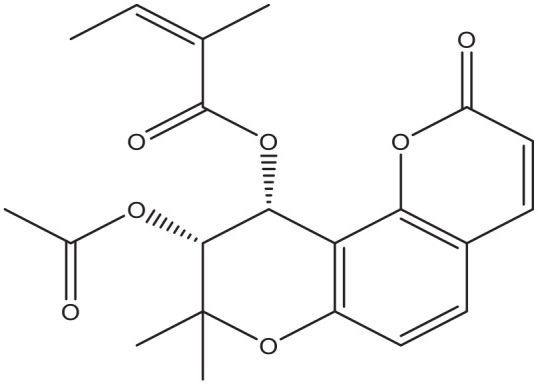
Pteryxin.
Finally, in a study carried out with an extract of various herbs containing an abundance of coumarin, an inhibition of transepithelial glucose transport was observed in a cell culture model of the intestine. 71 Unfortunately, it was not determined whether this effect could be specifically ascribed to the action of the coumarins, but the mechanism hinted toward interference with the SGLT-1 and GLUT-2 transporters.
Possible effects of coumarins on glucose uptake
Lately, coumarins stimulating or prolonging the release of insulin have been identified. The 6-Prenylcoumarin-7-O-b-D-apiofuranosyl-(1!6)-b-D-glucopyranoside coumarin derivative induced at least 2 times more insulin release from cultured pancreatic islet cells compared to glucose alone, 72 but the study did not reveal any mechanism of action. Synthetic 3-(5′-Methyl-2′-aryl-3′-[thiazol-2ʺ-yl amino] thiazolidin-4′-one) coumarin derivatives led to hypoglycemic effects in diabetic Wistar rats. 73 However, it is not entirely clear from the report whether this could be ascribed to an increased insulin activity. Moreover, researchers conjugated glucagon-like peptide 1 with coumarin and achieved a longer half-life for the peptide and consequently prolonged the insulin increasing effect 53 with this molecular binding. In another study, investigators showed that coumarin itself increased the insulin level and reduced the level of glucose in plasma of diabetic rats possibly through increased activity of the glycolytic enzyme hexokinase and the hepatic shunt enzyme glucose-6-phophate dehydrogenase or decreased activity of the gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-biphosphatase. 74
Most of the identified compounds work through enhancement of the sensitivity to insulin on a cellular level in fat, liver, or muscle tissue. Free fatty acids among other factors contribute to the development of insulin resistance. 75 The coumarin Isofraxidin altered lipid metabolism in cultured hepatic cells and mice with induced liver steatosis and decreased plasma free fatty acids through enhanced phosphorylation of adenosine monophosphate (AMP)-activated protein kinase among other actions. 76 Researchers also synthesized several coumarins which antagonized aldehyde dehydrogenase 1A1 that improved palmitic acid-induced impairment of glucose consumption in cultured hepatic cells, 77 a process that may result in a possible increased sensitivity for insulin. The observed glucose lowering activity of coumarin that has been previously mentioned, might be partly the result of an enhanced sensitivity to insulin since apart from the increased insulin level, a significant increase in the levels of the glycolytic enzyme hexokinase and the hepatic shunt enzyme glucose-6-phophate dehydrogenase was observed in the liver of the animals treated with the compound. 74
Currently, Protein Tyrosine Phosphatase 1B inhibitors are being considered as potential antidiabetic agents. This enzyme has a negative feedback on the insulin signaling pathway, hence inhibition of this phosphatase may result in an increased sensitivity for insulin.78,79 The coumarin selaginolide A demonstrated inhibitory activity on this enzyme as well as on α-glucosidase 80 and in this way may have a beneficial effect on diabetes. Docking studies with dihydroxanthyletin-type coumarins—(+)-trans-decursidinol showed that they preferably bound with the active site of Protein Tyrosine Phosphatase 1B. 81
Coumarin-cyclic imide conjugates increased the glucose absorption rate to a level that is comparable to metformin in cultured hepatic carcinoma and kidney cells. 82 The furanocoumarin ficusin significantly improved the cellular uptake through the translocation and activation of GLUT4 in the plasma membranes of adipose tissue of diabetic rats. 83 In addition to this, it also lowered serum fat and demonstrated antioxidant activity, 2 processes that enhance the sensitivity to insulin. 83 A 7-hydroxycoumarin, also known as Umbelliferone (Figure 12), significantly reduced the elevated blood glucose level and insulin resistance, and increased liver glycogen in diabetic rats, probably due to increased mobilization of GLUT4. 84 The naturally occurring coumarins osthole (Figure 10) and scopoletin (Figure 5) significantly induced AMP-activated protein kinase, which is favorable for preventing and treating type 2 diabetes mellitus. They also increased the level of translocation of GLUT4 to plasma membranes, increased the glucose uptake in skeletal muscle and fat cells, and reduced the plasma glucose level in diabetic mice.61,85 Furthermore, osthole increased the absorption of glucose in cultured fibroblasts. 86 A similar effect was observed in dexamethasone induced diabetic mice with the coumarin esculin. 87 Esculin (Figure 8), fraxetin (Figure 9), and osthole (Figure 10) also were able to promote glucose uptake in cultured normal and insulin resistance-induced myotubes. 87 Moreover, coumarins demonstrate antioxidant activity, an action that increases the sensitivity for insulin.88,89
Figure 12.
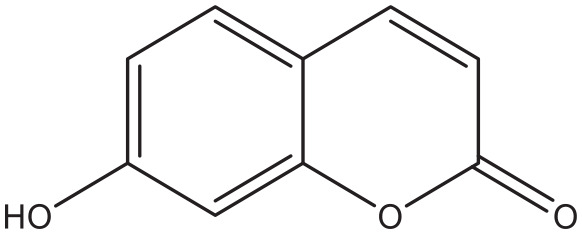
Umbelliferone.
Figure 10.
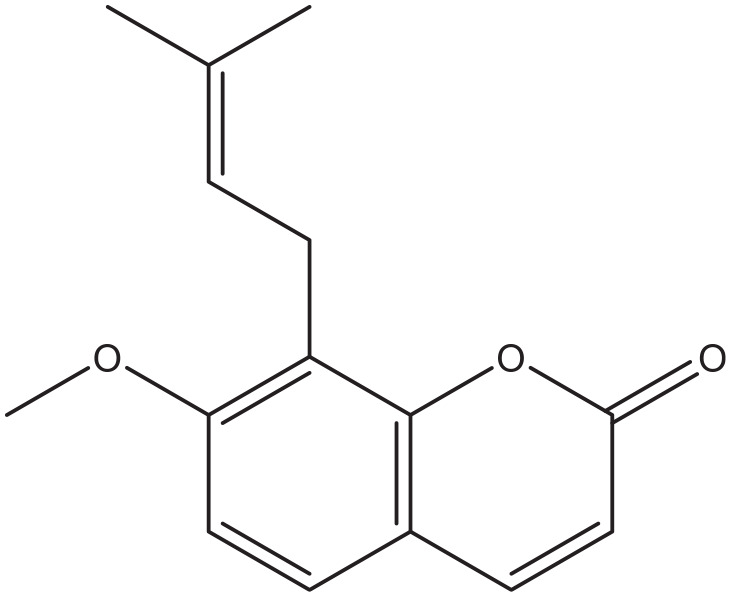
Osthole.
Figure 8.
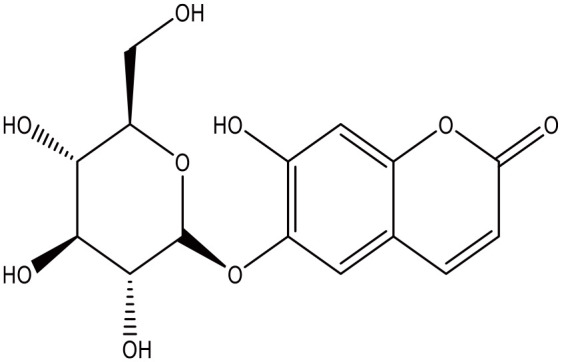
Esculin.
Figure 9.
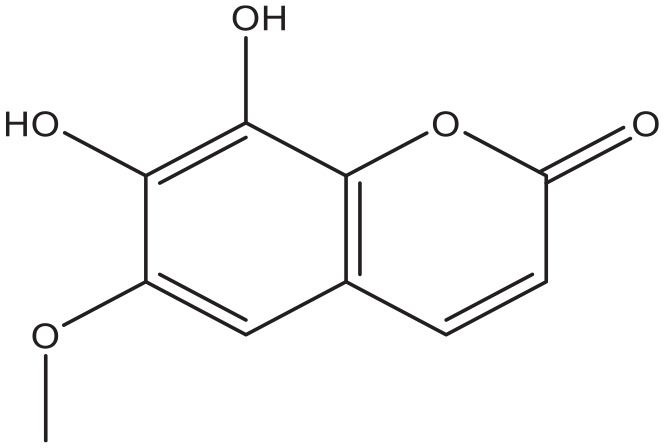
Fraxetin.
Novel production of glucose 90 in the liver 91 and other organs like the kidney 92 may also contribute to an increased glucose level in plasma. 93 Coumarin itself significantly reduced the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase in diabetic rats. 74 Umbelliferone decreased blood glucose and HbA1c, as well as the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase. 94 In addition, it elevated plasma insulin and liver glycogen as well as glucokinase and glucose-6-phosphate dehydrogenase in diabetic rats. 94 Coumarin-3-carboxylic acid derivatives inhibited the uptake of lactate by several tumoral cell lines. 95 Hepatic conversion of lactate is one of the known mechanisms for gluconeogenesis. 96
Coumarins and complications of diabetes
In a very recent in vitro study, coumarin showed up to 80% reduction of the glycation of human serum albumin by glucose and methylglyoxal. 54 On one hand, this is a favorable action, but we must realize that the monitoring of therapy of diabetes also depends on the amount of glycated hemoglobin (HbA1C). A lower level of this product induced by coumarin might falsely implicate a successful therapy, when we realize that these molecules are abundant in food and supplements and potentially might lower the glycation of peptides, including hemoglobin and albumin. Like most natural products, coumarins have also proven to possess antioxidative properties. Isofraxidin, 76 coumarin, 43 ficusin (Figure 11), 83 umbelliferone, 77 and coumarin-3-carboxamides and their hybrids with alpha-lipoic acid (Figure 13) 88 are examples of coumarins or their derivatives with antioxidant capability. Investigators demonstrated an increased bone formation effect of the coumarin-like osthole in mice, 97 which is possibly capable of reversing the osteolysis found in the periodontal space of diabetics. 98 This is a well known complication of the disorder.
Figure 11.
Ficusin.
Figure 13.
Alpha-lipoic acid.
Coumarins derived from the plants Urtica dentata, 99 Artemisia capillaries, 100 and Hydrangea paniculate 101 showed the potency to slow down the progression of diabetic nephropathy. The total coumarin content of Urtica dentata prevented the high glucose-induced proliferation and hypertrophy of a mesangial cell line, a preliminary process in the development of the nephropathy. 99 In addition they significantly reduced albuminuria and serum creatinine in streptozotocin-induced diabetic rats. 99 The coumarin scoparone, found in Artemisia capillaries, also inhibited the glucose induced proliferation of cultured mesangial cells. 100 Finally, treatment with the compound skimming found in Hydrangea paniculate, led to a lower degree of glomerulosclerosis compared to losartan treated streptozotocin-induced diabetic rats. 101 The mechanism of this process has not been elucidated yet.
Conclusion
Diabetes is a very disabling disorder and its prevalence is projected to have a considerable burden on the world economy. The current treatment strategies vary from changes in lifestyle to medication, and surgical intervention of complications. However, they all stop short of curing the disease. Consequently, the search for more effective and efficient therapeutic strategies, especially new drug agents, is continuing. In the past decades, a substantial number of natural and (semi)synthetic coumarins have been acquired. Among these, there are a number with proven efficacy in in vitro and in silico studies as well as in some animal models.
The abundance of the coumarins mentioned in this review inhibit the intestinal enzyme α-glucosidase. Examples of these are scopoletin, decursinol, and flavonoid hybrids of coumarin. Inhibition of this enzyme blocks the formation of glucose in the intestines and therefore limits the absorption rate of the carbohydrate. The coumarins pteryxin and praeruptorin inhibit sodium coupled glucose transport across the intestinal epithelium. Several coumarins have been found that are able to increase the secretion of or the sensitivity for insulin. Examples of these are coumarin, ficusin, esculin, fraxetin, and osthole. Some other compounds like coumarin, umbelliferone, and coumarin-3-carboxylic acid derivatives have the potential to inhibit gluconeogenesis. Finally the coumarins scoparone and skimming have demonstrated properties that might be useful in the management of diabetic nephropathy.
Unfortunately, up till now these studies have been limited to in vitro and in silico biochemical and simple animal studies. No clinical trial has yet been performed. The absence of these as well as the absence of detailed studies addressing biochemical or physiological and pharmacological mechanisms in suitable animals still limit the prospect of one of these agents to be developed into a useful drug against type 2 diabetes mellitus. There is thus still a long way to go to formulate clinical applicable agents from this group with high efficiency and low toxicity. However, the future of these compounds as potential antidiabetic agents looks promising.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SR designed the study, assisted in the search of the databases, complemented the chemical part of the study, assisted in the draft of the manuscript and designed the figures and table. RB performed the initial searches, selected the articles in cooperation with SR, complemented the biomedical part of the manuscript and drafted the first version. Both authors critically reviewed and approved the final manuscript and take full responsibility for the content of the manuscript.
Research Ethics and Patient Consent: Review board approval or patient consent were not required due to the retrospective nature of the study.
ORCID iDs: Sara Ranđelović  https://orcid.org/0000-0001-5259-6780
https://orcid.org/0000-0001-5259-6780
Robbert Bipat  https://orcid.org/0000-0001-8711-4737
https://orcid.org/0000-0001-8711-4737
References
- 1. Kerner W, Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:384-386. [DOI] [PubMed] [Google Scholar]
- 2. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [DOI] [PubMed] [Google Scholar]
- 4. Setacci C, De Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg. 2009;50:263-273. [PubMed] [Google Scholar]
- 5. Ling W, Huang Y, Huang YM, Fan RR, Sui Y, Zhao HL. Global trend of diabetes mortality attributed to vascular complications, 2000-2016. Cardiovasc Diabetol. 2020;19:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan R. Postprandial blood glucose. Diabetes Care. 2001;24:775-778. [DOI] [PubMed] [Google Scholar]
- 7. Bastyr EJ, Stuart CA, Brodows RG, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. IOEZ Study Group. Diabetes Care. 2000;23:1236-1241. [DOI] [PubMed] [Google Scholar]
- 8. Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909-1914. [DOI] [PubMed] [Google Scholar]
- 9. Takata K. Glucose transporters in the transepithelial transport of glucose. J Electron Microsc (Tokyo). 1996;45:275-284. [DOI] [PubMed] [Google Scholar]
- 10. Anonymous. Alpha glucosidase inhibitors. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 11. Zhang BW, Li X, Sun WL, et al. Dietary flavonoids and acarbose synergistically inhibit α-glucosidase and lower postprandial blood glucose. J Agric Food Chem. 2017;65:8319-8330. [DOI] [PubMed] [Google Scholar]
- 12. Menezes JCJMDS, Diederich MF. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur J Med Chem. 2019;182:111637. [DOI] [PubMed] [Google Scholar]
- 13. Taha M, Shah SAA, Afifi M, et al. Synthesis, α-glucosidase inhibition and molecular docking study of coumarin based derivatives. Bioorg Chem. 2018;77:586-592. [DOI] [PubMed] [Google Scholar]
- 14. Asgari MS, Mohammadi-Khanaposhtani M, Kiani M, et al. Biscoumarin-1,2,3-triazole hybrids as novel anti-diabetic agents: design, synthesis, in vitro α-glucosidase inhibition, kinetic, and docking studies. Bioorg Chem. 2019;92:103206. [DOI] [PubMed] [Google Scholar]
- 15. Channa Basappa V, Hamse Kameshwar V, Kumara K, Achutha DK, Neratur Krishnappagowda L, Kariyappa AK. Design and synthesis of coumarin-triazole hybrids: biocompatible anti-diabetic agents, in silico molecular docking and ADME screening. Heliyon. 2020;6:e05290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olefsky JM, Nolan JJ. Insulin resistance and non-insulin-dependent diabetes mellitus: cellular and molecular mechanisms. Am J Clin Nutr. 1995;61:980S-986S. [DOI] [PubMed] [Google Scholar]
- 17. Chang W-C, Wu SC, Xu KD, Liao B-C, Wu JF, Cheng A-S. Scopoletin protects against methylglyoxal-induced hyperglycemia and insulin resistance mediated by suppression of advanced glycation endproducts (AGEs) generation and anti-glycation. Molecules. 2015;20:2786-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994;107:1848-1855. [DOI] [PubMed] [Google Scholar]
- 19. Sulis PM, Dambrós BF, Mascarello A, et al. Sulfonyl(thio)urea derivative induction of insulin secretion is mediated by potassium, calcium, and sodium channel signal transduction. J Cell Physiol. 2019;234:10138-10147. [DOI] [PubMed] [Google Scholar]
- 20. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [DOI] [PubMed] [Google Scholar]
- 21. Drucker DJ. Mechanisms of action and therapeutic application of Glucagon-like peptide-1. Cell Metab. 2018;27:740-756. [DOI] [PubMed] [Google Scholar]
- 22. Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol Metab. 1997;273:E981-E988. [DOI] [PubMed] [Google Scholar]
- 23. Posner BI. Insulin signalling: the inside story. Can J Diabetes. 2017;41:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Begum N, Leitner W, Reusch JE, Sussman KE, Draznin B. GLUT-4 phosphorylation and its intrinsic activity. Mechanism of Ca(2+)-induced inhibition of insulin-stimulated glucose transport. J Biol Chem. 1993;268:3352-3356. [PubMed] [Google Scholar]
- 25. Wautier JL, Zoukourian C, Chappey O, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Investig. 1996;97:238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heidari F, Rabizadeh S, Rajab A, et al. Advanced glycation end-products and advanced oxidation protein products levels are correlates of duration of type 2 diabetes. Life Sci. 2020;260:118422. [DOI] [PubMed] [Google Scholar]
- 27. Bajaj S, Khan A. Antioxidants and diabetes. Indian J Endocrinol Metab. 2012;16:S267-S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chitra PS, Chaki D, Boiroju NK, et al. Status of oxidative stress markers, advanced glycation index, and polyol pathway in age-related cataract subjects with and without diabetes. Exp Eye Res. 2020;200:108230. [DOI] [PubMed] [Google Scholar]
- 29. Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017;49:837-844. [DOI] [PubMed] [Google Scholar]
- 30. Dehdashtian E, Mehrzadi S, Yousefi B, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20-33. [DOI] [PubMed] [Google Scholar]
- 31. Ikeda T, Iwata K, Murakami H. Inhibitory effect of metformin on intestinal glucose absorption in the perfused rat intestine. Biochem Pharmacol. 2000;59:887-890. [DOI] [PubMed] [Google Scholar]
- 32. Khan A, Pessin J. Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia. 2002;45:1475-1483. [DOI] [PubMed] [Google Scholar]
- 33. Krook A, Wallberg-Henriksson H, Zierath JR. Sending the signal: molecular mechanisms regulating glucose uptake. Med Sci Sports Exerc. 2004;36:1212-1217. [DOI] [PubMed] [Google Scholar]
- 34. Cernea S, Raz I. Insulin therapy: future perspectives. Am J Ther. 2020;27:e121-e132. [DOI] [PubMed] [Google Scholar]
- 35. Gudat U, Bungert S, Kemmer F, Heinemann L. The blood glucose lowering effects of exercise and glibenclamide in patients with type 2 diabetes mellitus. Diabet Med. 1998;15:194-198. [DOI] [PubMed] [Google Scholar]
- 36. Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabet Med. 1999;16:477-481. [DOI] [PubMed] [Google Scholar]
- 37. Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140-151. [DOI] [PubMed] [Google Scholar]
- 38. Yang P, Feng J, Peng Q, Liu X, Fan Z. Advanced glycation end products: potential mechanism and therapeutic target in cardiovascular complications under diabetes. Oxid Med Cell Longev. 2019;2019:9570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sarker SD, Nahar L. Progress in the chemistry of naturally occurring coumarins. In: Kinghorn A, Falk H, Gibbons S, Kobayashi J. eds. Progress in the Chemistry of Organic Natural Products. Vol. 106. Springer; 2017;241-304. [DOI] [PubMed] [Google Scholar]
- 40. Alshibl HM, Al-Abdullah ES, Haiba ME, et al. Synthesis and evaluation of new coumarin derivatives as antioxidant, antimicrobial, and anti-inflammatory agents. Molecules. 2020;25:i: E3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang H, Shi Y, Zeng K, Zhao M, Tu P, Jiang Y. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry. 2020;177:112416. [DOI] [PubMed] [Google Scholar]
- 42. Lee SO, Choi SZ, Lee JH, et al. Antidiabetic coumarin and cyclitol compounds fromPeucedanum japonicum. Arch Pharm Res. 2004;27:1207-1210. [DOI] [PubMed] [Google Scholar]
- 43. Singh AK, Patel PK, Choudhary K, Joshi J, Yadav D, Jin JO. Quercetin and coumarin inhibit dipeptidyl peptidase-IV and exhibits antioxidant properties: in silico, in vitro, ex vivo. Biomolecules. 2020;10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu L, Wang X, Xu W, Farzaneh F, Xu R. The structure and pharmacological functions of coumarins and their derivatives. Curr Med Chem. 2009;16:4236-4260. [DOI] [PubMed] [Google Scholar]
- 45. Matos MJ, Santana L, Uriarte E, Abreu OA, Molina E, Yordi EG. coumarins—an important class of phytochemicals. In: Venket Rao A, Rao LG, eds. Phytochemicals - Isolation, Characterisation and Role in Human Health. InTech; 2015. [Google Scholar]
- 46. Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci. 2020;21:1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. PubChem. National Libary of Medicine, National Center for Biotechnology Information, Accessed June 17, 2021 https://pubchem.ncbi.nlm.nih.gov/#query=coumarin
- 48. Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res Int. 2013;2013:963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rohini K, Ps SRIKUMAR. Therapeutic role of coumarins and coumarin-related compounds. J Thermodyn Catal. 2014;05:1-3. [Google Scholar]
- 50. Bipat R. From rat poison to medicine: medical applications of coumarin derivatives. In: Rao V, Mans DRA, Rao L. eds. Phytochemicals in Human Health. IntechOpen; 2019;91-104. [Google Scholar]
- 51. Xu XT, Deng X-Y, Chen J, et al. Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur J Med Chem. 2020;189:112013. [DOI] [PubMed] [Google Scholar]
- 52. Okada Y, Miyauchi N, Suzuki K, et al. Search for naturally occurring substances to prevent the complications of diabetes. II. Inhibitory effect of coumarin and flavonoid derivatives on bovine lens aldose reductase and rabbit platelet aggregation. Chem Pharm Bull. 1995;43:1385-1387. [DOI] [PubMed] [Google Scholar]
- 53. Han J, Sun L, Huang X, et al. Novel coumarin modified GLP-1 derivatives with enhanced plasma stability and prolonged in vivo glucose-lowering ability. Br J Pharmacol. 2014;171:5252-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abul Qais F, Ahmad I. Mechanism of non-enzymatic antiglycation action by coumarin: a biophysical study. New J Chem. 2019;43:12823-12835. [Google Scholar]
- 55. Menteşe E, Karaali N, Akyüz G, Yılmaz F, Ülker S, Kahveci B. Synthesis and evaluation of α-glucosidase and pancreatic lipase inhibition by quinazolinone-coumarin hybrids. Chem Heterocycl Compd. 2016;52:1017-1024. [Google Scholar]
- 56. Sun H, Song X, Tao Y, et al. Synthesis & α-glucosidase inhibitory & glucose consumption-promoting activities of flavonoid-coumarin hybrids. Future Med Chem. 2018;10:1055-1066. [DOI] [PubMed] [Google Scholar]
- 57. Sherafati M, Mohammadi-Khanaposhtani M, Moradi S, et al. Design, synthesis and biological evaluation of novel phthalimide-Schiff base-coumarin hybrids as potent α-glucosidase inhibitors. Chem Pap. 2020;74:4379-4388. [Google Scholar]
- 58. Kazmi M, Zaib S, Ibrar A, et al. A new entry into the portfolio of α-glucosidase inhibitors as potent therapeutics for type 2 diabetes: design, bioevaluation and one-pot multi-component synthesis of diamine-bridged coumarinyl oxadiazole conjugates. Bioorg Chem. 2018;77:190-202. [DOI] [PubMed] [Google Scholar]
- 59. Salar U, Taha M, Khan KM, et al. Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies. Eur J Med Chem. 2016;122:196-204. [DOI] [PubMed] [Google Scholar]
- 60. Jang JH, Park JE, Han JS. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur J Pharmacol. 2018;834:152-156. [DOI] [PubMed] [Google Scholar]
- 61. Jang JH, Park JE, Han JS. Scopoletin increases glucose uptake through activation of PI3K and AMPK signaling pathway and improves insulin sensitivity in 3T3-L1 cells. Nutr Res. 2020;74:52-61. [DOI] [PubMed] [Google Scholar]
- 62. Zhao D-G, Zhou A-Y, Du Z, Zhang Y, Zhang K, Ma Y-Y. Coumarins with α-glucosidase and α-amylase inhibitory activities from the flower of Edgeworthia gardneri. Fitoterapia. 2015;107:122-127. [DOI] [PubMed] [Google Scholar]
- 63. Ali MY, Jannat S, Jung HA, Jeong HO, Chung HY, Choi JS. Coumarins from Angelica decursiva inhibit α-glucosidase activity and protein tyrosine phosphatase 1B. Chem Biol Interact. 2016;252:93-101. [DOI] [PubMed] [Google Scholar]
- 64. Shihabudeen HMS, Priscilla DH, Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab (Lond). 2011;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thant TM, Aminah NS, Kristanti AN, Ramadhan R, Phuwapraisirisan P, Takaya Y. A new pyrano coumarin from Clausena excavata roots displaying dual inhibition against α-glucosidase and free radical. Nat Prod Res. 2021;35:556-561. [DOI] [PubMed] [Google Scholar]
- 66. Heydari Z, Mohammadi-Khanaposhtani M, Imanparast S, et al. Pyrano[3,2-c]quinoline derivatives as new class of α-glucosidase inhibitors to treat type 2 diabetes: synthesis, in vitro biological evaluation and kinetic study. Med Chem. 2019;15:8-16. [DOI] [PubMed] [Google Scholar]
- 67. Mishra N, Maurya AK, Mulpuru V. Discovery of novel coumarin analogs against the α-glucosidase protein target of diabetes mellitus: pharmacophore-based QSAR, docking, and molecular dynamics simulation studies. ACS Omega. 2020;5:32234-32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Welsch CA, Lachance PA, Wasserman BP. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698-1704. [DOI] [PubMed] [Google Scholar]
- 69. Oranje P, Gouka R, Burggraaff L, et al. Novel natural and synthetic inhibitors of solute carriers SGLT1 and SGLT2. Pharmacol Res Perspect. 2019;7:e00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nugara RN, Inafuku M, Takara K, Iwasaki H, Oku H. Pteryxin: a coumarin in Peucedanum japonicum Thunb leaves exerts antiobesity activity through modulation of adipogenic gene network. Nutrition. 2014;30:1177-1184. [DOI] [PubMed] [Google Scholar]
- 71. Farrell TL, Ellam SL, Forrelli T, Williamson G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: interactions with SGLT1 and GLUT2 transporters. Biofactors. 2013;39:448-456. [DOI] [PubMed] [Google Scholar]
- 72. Cao N-K, Zhu S-S, Chen Y-M, et al. A new prenylated coumarin diglycoside with insulin-release promoting activity from Clausena dunniana. J Asian Nat Prod Res. 2021;23:385-391. [DOI] [PubMed] [Google Scholar]
- 73. Kini D, Ghate M. Synthesis and oral hypoglycemic activity of 3-[5’-methyl-2’-aryl-3’-(thiazol-2ʺ-yl amino) thiazolidin-4’-one] coumarin derivatives. J Chem. 2011;8:386-390. [Google Scholar]
- 74. Pari L, Rajarajeswari N. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem Biol Interact. 2009;181:292-296. [DOI] [PubMed] [Google Scholar]
- 75. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607. [DOI] [PubMed] [Google Scholar]
- 76. Li J, Li X, Li Z, et al. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017;8:2886-2896. [DOI] [PubMed] [Google Scholar]
- 77. Liang D, Fan Y, Yang Z, et al. Discovery of coumarin-based selective aldehyde dehydrogenase 1A1 inhibitors with glucose metabolism improving activity. Eur J Med Chem. 2020;187:111923. [DOI] [PubMed] [Google Scholar]
- 78. Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 2002;1:696-709. [DOI] [PubMed] [Google Scholar]
- 79. Prabhakar PK, Sivakumar PM. Protein tyrosine phosphatase 1B inhibitors: a novel therapeutic strategy for the management of type 2 diabetes mellitus. Curr Pharm Des. 2019;25:2526-2539. [DOI] [PubMed] [Google Scholar]
- 80. Nguyen DT, To DC, Tran TT, Tran MH, Nguyen PH. PTP1B and α-glucosidase inhibitors from Selaginella rolandi-principis and their glucose uptake stimulation. J Nat Med. 2021;75:186-193. [DOI] [PubMed] [Google Scholar]
- 81. Ali MY, Jannat S, Jung HA, Choi JS. Insulin-mimetic dihydroxanthyletin-type coumarins from Angelica decursiva with protein tyrosine phosphatase 1B and α-Glucosidase inhibitory activities and docking studies of their molecular mechanisms. Antioxidants. 2021;10:292. https://pubmed.ncbi.nlm.nih.gov/33672051/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reddy DS, Kongot M, Singh V, et al. Coumarin tethered cyclic imides as efficacious glucose uptake agents and investigation of hit candidate to probe its binding mechanism with human serum albumin. Bioorg Chem. 2019;92:103212. [DOI] [PubMed] [Google Scholar]
- 83. Irudayaraj SS, Stalin A, Sunil C, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARγ expression in type 2 diabetic rats. Chem Biol Interact. 2016;256:85-93. [DOI] [PubMed] [Google Scholar]
- 84. Naowaboot J, Somparn N, Saentaweesuk S, Pannangpetch P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phyther Res. 2015;29:1388-1395. [DOI] [PubMed] [Google Scholar]
- 85. Lee W-H, Lin R-J, Lin S-Y, Chen Y-C, Lin H-M, Liang Y-C. Osthole enhances glucose uptake through activation of AMP-activated protein kinase in skeletal muscle cells. J Agric Food Chem. 2011;59:12874-12881. [DOI] [PubMed] [Google Scholar]
- 86. Alabi OD, Gunnink SM, Kuiper BD, Kerk SA, Braun E, Louters LL. Osthole activates glucose uptake but blocks full activation in L929 fibroblast cells, and inhibits uptake in HCLE cells. Life Sci. 2014;102:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mo Z, Li L, Yu H, Wu Y, Li H. Coumarins ameliorate diabetogenic action of dexamethasone via Akt activation and AMPK signaling in skeletal muscle. J Pharmacol Sci. 2019;139:151-157. [DOI] [PubMed] [Google Scholar]
- 88. Melagraki G, Afantitis A, Igglessi-Markopoulou O, et al. Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur J Med Chem. 2009;44:3020-3026. [DOI] [PubMed] [Google Scholar]
- 89. Borges Bubols G, da Rocha Vianna D, Medina-Remon A, et al. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13:318-334. [DOI] [PubMed] [Google Scholar]
- 90. Hers HG, Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617-653. [DOI] [PubMed] [Google Scholar]
- 91. Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885-909. [DOI] [PubMed] [Google Scholar]
- 92. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382-391. [DOI] [PubMed] [Google Scholar]
- 93. Shah AM, Wondisford FE. Tracking the carbons supplying gluconeogenesis. J Biol Chem. 2020;295:14419-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562-566. [DOI] [PubMed] [Google Scholar]
- 95. Ji H, Tan Y, Gan N, et al. Synthesis and anticancer activity of new coumarin-3-carboxylic acid derivatives as potential lactate transport inhibitors. Bioorg Med Chem. 2021;29:115870. [DOI] [PubMed] [Google Scholar]
- 96. Zhang X, Yang S, Chen J, Su Z. Unraveling the regulation of hepatic gluconeogenesis. Front Endocrinol. 2018;9:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gao L-N, An Y, Lei M, et al. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials. 2013;34:9937-9951. [DOI] [PubMed] [Google Scholar]
- 98. Catalfamo DL, Britten TM, Storch DL, Calderon NL, Sorenson HL, Wallet SM. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013;19:303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cao H, Ji Y, Li W, Liu Y, Fu R, Xiang M. Protective effects of the total coumarin fraction of Urtica dentata on experimental diabetic nephropathy in vitro and in vivo. Planta Med. 2015;81:1353-1360. [DOI] [PubMed] [Google Scholar]
- 100. Wang Y, Wang M, Chen B, Shi J. Scoparone attenuates high glucose-induced extracellular matrix accumulation in rat mesangial cells. Eur J Pharmacol. 2017;815:376-380. [DOI] [PubMed] [Google Scholar]
- 101. Sen Z, Weida W, Jie M, Li S, Dongming Z, Xiaoguang C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. 2019;57:385-395. [DOI] [PubMed] [Google Scholar]


