Abstract
Background
Older patients with cancer are at risk of physical decline and impaired quality of life during oncological treatment. Exercise training has the potential to reduce these challenges. The study aim was to investigate the feasibility and effect of a multimodal exercise intervention in older patients with advanced cancer (stages III/IV).
Patients and Methods
Eighty-four older adults (≥65 years) with advanced pancreatic, biliary tract, or non-small cell lung cancer who received systemic oncological treatment were randomized 1:1 to an intervention group or a control group. The intervention was a 12-week multimodal exercise-based program including supervised exercise twice weekly followed by a protein supplement, a home-based walking program, and nurse-led support and counseling. The primary endpoint was change in physical function (30-second chair stand test) at 13 weeks.
Results
Median age of the participants was 72 years (interquartile range [IQR] 68-75). Median adherence to the exercise sessions was 69% (IQR 21-88) and 75% (IQR 33-100) for the walking program. At 13 weeks, there was a significant difference in change scores of 2.4 repetitions in the chair stand test, favoring the intervention group (p < .0001). Furthermore, significant beneficial effects were seen for physical endurance (6-minute walk test), hand grip strength, physical activity, symptom burden, symptoms of depression and anxiety, global health status (quality of life), and lean body mass. No effects were seen for dose intensity, hospitalizations, or survival.
Conclusion
A 12-week multimodal exercise intervention with targeted support proved effective in improving physical function in older patients with advanced cancer during oncological treatment.
Keywords: advanced cancer, exercise, older patients, randomized controlled trial, resistance training, support
Older patients have a higher risk of physical and functional decline. This article reports a 12-week multimodal exercise-based and supportive intervention among older patients with advanced stage cancer who received systemic oncological treatment.
Implications for Practice.
Exercise is generally recommended during anticancer treatment to maintain physical function and to increase psychological well-being. Although current evidence is mainly based on younger and middle-aged patients, this study showed that a multimodal exercise and support intervention increased physical function in older patients (≥65 years) with advanced cancer during oncological treatment. Hence, these new findings strengthen the basis for existing recommendations and highlight the importance of focusing on and supporting older patients with cancer in a physically active lifestyle and engagement in exercise training.
Introduction
Older patients are at high risk of physical and functional decline in the trajectory of cancer treatment, which negatively affects quality of life (QoL).1,2 Older patients with cancer and cancer survivors experience more limitations in their daily activities, lower QoL, and a higher prevalence of geriatric syndromes than older people without a history of cancer.3,4
Cancer incidence and mortality increase with age for most cancers.5 With the aging population, the number of older patients with cancer and cancer survivors is predicted to rise in the future,6,7 with an estimated doubling of new cancer cases among older adults (≥65 years) worldwide from 6.7 million in 2012 to 14.2 million in 2035.7 Older patients experience age-related physiological decline in organ and immune function8-10 and in muscle mass,11 which in turn increase their susceptibility to experience toxicity with cancer treatment.12 Furthermore, the increased prevalence of preexisting comorbidities that occurs with aging heightens the risk of disability during the cancer trajectory.13
Patients with cancer are generally recommended to engage in aerobic and resistance exercise on a regular basis and most importantly to avoid physical inactivity.14 Apart from the overall general health benefits, exercise is important to prevent physical decline and loss of muscle mass during cancer treatment.14 Numerous randomized controlled trials (RCTs) have provided evidence to suggest exercise training (e.g., cardiovascular training, resistance training, balance, and flexibility exercises) as safe and effective strategies to maintain muscle mass and physical performance,14-16 psychological well-being, and QoL14-17 and to some extent to reduce cancer- and treatment-related symptoms and adverse effects.14,16,18 Beneficial effects of exercise have been documented among patients with different cancers, even though most of the conducted studies have focused on specific cancer diagnoses (e.g., breast, colorectal, or prostate cancer) in early stages.16,19,20 However, most of the evidence arises from trials conducted among younger and middle-aged patients, whereas older patients remain underrepresented.21,22 Cancer cachexia and sarcopenia are frequently seen among patients with advanced cancer,23 and these conditions are further problematic in older patients because of limited physical reserves, including age-related decline in muscle mass.11
Exercise is challenging for many older patients with cancer because of age-related barriers such as musculoskeletal disorders and decreased mobility, habits, and lifestyle (e.g., motivational challenges), and practical obstacles.24 We hypothesized that a combined exercise and supportive intervention would maintain levels of physical function and capacity; thus the aim of the current study was to investigate the feasibility and effect of a 12-week multimodal exercise-based and supportive intervention among older patients with advanced (stages III/IV) pancreatic cancer (PC), biliary tract cancer (BTC), or non-small cell lung cancer (NSCLC) who received systemic oncological treatment.
Materials and Methods
Applied methods and results from the current RCT are described according to the CONSORT statement recommendations for reporting randomized trials.25
Design
A two-armed single center RCT was designed in collaboration with patient representatives. A protocol of the study “Patient Activation through Counseling, Exercise and Mobilization - Pancreatic, Biliary tract and Lung cancer (PACE-Mobil-PBL)” has been published elsewhere26 and is summarized in the following.
Participants
Between April 2018 and March 2020, patients were eligible for inclusion if they had been diagnosed with unresectable and advanced PC, BTC, or NSCLC within 12 weeks and had received or were scheduled to receive first-line palliative systemic treatment (chemotherapy, immunotherapy, or targeted therapy), were ≥65 years old, had an Eastern Cooperative Oncology Group performance status score ≤2, and were able to speak and read Danish to provide informed consent to participate. All patients were treated at the Department of Oncology at Copenhagen University Hospital, Herlev and Gentofte Hospital, Denmark.
Exclusion criteria included any physical, mental, or cognitive conditions that hindered participation based on safety and/or feasibility concerns as assessed by the treating oncologist, including uncontrolled and/or extensive brain metastases, unstable medical disease, or bone metastases that were assessed to pose a risk of pathological fractures from exercising.
Patients were approached, informed, and recruited to the study by the primary investigator (M.K.M.). Verbal and written consent for participation were obtained from all participants.
Randomization
After inclusion and full baseline assessment, participants were randomized 1:1 to the intervention group (IG) or to the control group (CG). Randomization was stratified for diagnosis and was carried out through the randomization module in the Research Electronic Data Capture (REDCap) application27 by the primary investigator (M.K.M.). All technical procedures related to the randomization setup, including generation of randomization lists and block sizes, were conducted (and only known) by the program’s statistician (A.T.).
Intervention
During standard first-line oncological treatment, participants in the IG received a 12-week multimodal intervention consisting of four components. The first component was group-based exercise (mainly progressive resistance training) twice weekly in sessions of 60 minutes. The progressive resistance training program consisted of seven exercises targeting all major muscle groups. All sessions were supervised by physiotherapists, and programs were individually tailored to each participant. With an aim to increase adherence, medical appointments related to the oncological treatment were coordinated with the exercise sessions. Furthermore, all participants received a replacement training program that included a few effective exercises that could be performed at home if they were prevented from attending an exercise session. Secondly, a protein supplement was served after each supervised exercise session. Third, an individualized walking program using a pedometer (step counts) was provided to help maintain or increase level of daily activity. Each week an evaluation and new goal setting related to step counts were conducted in collaboration between the primary investigator and each participant, with the aim to find a realistic goal that the participant felt confident and motivated to achieve. Finally, two nurse-led structured counseling sessions were conducted by the primary investigator of the study (M.K.M.) at the start of the program and midway during the intervention period that included a holistic assessment of the participants’ life situation and of current symptoms that could challenge adherence to the intervention. Advice and counseling based on identified needs (e.g., symptom management) were offered immediately after the nurse-led sessions and continuously during the 12-week program, and appropriate referrals or contacts were provided. A detailed description of the components of the intervention is provided in Table 1.
Table 1.
Components of the intervention
| 12-week multimodal exercise-based intervention | ||||
|---|---|---|---|---|
| Supervised exercise training (60min) | Nutritional supplement | Unsupervised exercise | Nurse-led support and counseling | |
| Content |
Warm up (~15min): light CVT (e.g., stationary bicycling or relay games).
Balance and flexibility exercises (e.g., walking with changing directions, walking on toes and heels, or balance board). PRT (~35min) exercises: chest press, abdominal crunch, leg press, leg curl, leg extension, lower back, and low row. Number of repetitions for PRT: 15 RMa (session 1-2), 12 RMa (session 3-13), 10 RMa (session 14-24). Number of sets: 2 (session 1-6), 3 (session 7-24). Rest period between PRT sets: ~60 seconds. Relaxation (~10min), e.g., body awareness exercises, and stretching. |
Protein drink or protein bar.
200-300 calories, 12-18 grams of protein. |
Walking program based on step counts (Garmin Vivofit 3 activity tracker).
Individualized goal setting and evaluation. |
Individualized counseling and support based on a holistic assessment (physical, functional, psychological/emotional, social, and existential/spiritual) of each participant’s life situation and potential challenges.
Advice and referrals provided based on identified needs. |
| Frequency | 2 times a week. | 2 times a week after each supervised exercise session. | Continuously. Evaluation and goal setting once weekly. | Two organized sessions at week 1 + week 6/7 + continuously. |
| Setting | Hospital. | Hospital. | Home (walking). Hospital (goal setting and evaluation). | Hospital. |
The weight that can be lifted, using correct technique, 15, 12, and 10 times, respectively, before repetition failure.
Abbreviations: CVT, cardiovascular training; PRT, progressive resistance training; RM, repetition maximum.
Control Group
Participants in the CG received standard oncological treatment and were not asked to refrain from exercise. Step counting for three consecutive days was monitored at baseline and at 13 weeks. Furthermore, after completion of the 12-week intervention period, participants in the CG were retrospectively asked about physical activities performed during the 12-week period.
Data Collection
All medical and sociodemographic data, as well as data on current physical activity using the Saltin-Grimby Physical Activity Level Scale,28 were collected at baseline using medical charts and questionnaires. The primary endpoint was change in physical function from baseline to week 13 measured with the 30-second chair stand test (30s-CST).29 Secondary endpoints included feasibility and changes in the following: physical capacity (6-minute walk test [6MWT]29), strength (handgrip strength test30), body composition (whole-body dual-energy x-ray absorptiometry [DXA] scans and bioelectrical impedance assessment [BIA]), symptom burden (M.D. Anderson Symptom Inventory version 131), QoL (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30, version 3.032), symptoms of depression and anxiety (Hospital Anxiety and Depression Scale version 3.033), and physical activity (step counts using a Garmin Vivofit 3 activity tracker). Furthermore, treatment toxicities (National Cancer Institute Common Terminology Criteria for Adverse Events version 434) and hospital admissions were investigated.
Relative dose intensity of first-line chemotherapy was evaluated retrospectively by calculating for each drug the percentage of the actual cumulative dose delivered relative to the intended cumulative dose until treatment discontinuation. Survival was defined as time from randomization to death from any cause or censoring at time of last follow-up (August 19, 2020).
Further details of endpoints and assessment methods can be found in the protocol article.26
Timing of Data Collection
All baseline measurements were conducted before randomization. Physical tests and patient-reported outcome measures (PROMs) were conducted and collected at baseline and at week 7 (midway), week 13 (postintervention), and week 17 (follow-up). Body composition and step count assessments were conducted at baseline and at week 13. For the 4-month project period, treatment toxicities were assessed at every treatment cycle, and hospitalizations were documented from medical records.
Blinding Procedures
Assessments of the primary outcome and other physical tests were conducted by blinded physiotherapists. Because of the nature of the study, it was not possible to blind the participants. Furthermore, the study coordinator and physiotherapists in charge of the exercise program were not blinded.
Statistical Analysis
In a study including patients with osteoarthritis, a difference of 2.6 repetitions in the 30s-CST was regarded as a clinically important change based on patients’ evaluation of changes in strength, endurance, and agility in performing activities.35 In former studies focused on patients with advanced cancer, an SD of approximately 3 has been found.36,37 To be able to detect a between-group difference of 2.6 repetitions in changes in the 30s-CST, with a power of 90% and a type I error rate of 5%, 29 participants were needed in each group based on a two-sample t test. With an estimated drop-out rate of 40%, we planned to recruit 50 patients for each group. Baseline characteristics were reported as medians and interquartile ranges (IQRs) for quantitative variables and as numbers and percentages for categorial variables. Data on physical tests, body composition, PROMs, side effects, and hospitalizations were summarized for all assessment times as raw means and SDs or medians and IQRs. Analyses of within group changes from baseline and between-group comparison of changes were performed using nonparametric tests. The log-rank test was used to compare the survival time between groups. The prodlim R package was used to obtain estimates and 95% confidence intervals (CIs) for the median survival time. All statistical analyses and the sample size calculation were conducted using R.38 The significance level of all tests was set at .05. We recommend that p values related to secondary outcomes be interpreted with some caution because of the risk of false positive findings.
Registrations and Approvals
The study protocol was approved by the Regional Ethics Committee for the Capital Region of Denmark (J.nr: H-18001096) and by the Danish Data Protection Agency (J.nr: 2012-58-0004). The study was registered at ClinicalTrials.gov (NCT03411200) on January 26, 2018.26
Results
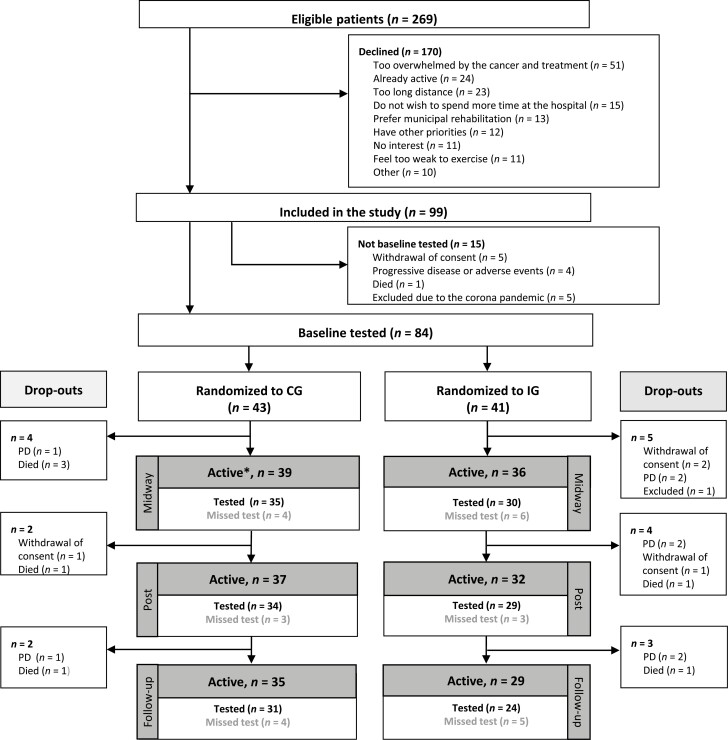
Of 269 eligible patients invited for study participation, 99 patients (37%) accepted. Reasons for declining participation are illustrated in Figure 1. A total of 15 included participants were not baseline tested or randomized because of withdrawal of consent (n = 5), progressive disease or adverse events (n = 4), death (n = 1), and exclusion due to restrictions related to the COVID-19 pandemic (n = 5). Thus, 84 participants (57% women) were tested at baseline, randomized, and included in the analyses. From baseline to postintervention testing, 15 participants left the study because of progressive disease (n = 5), death (n = 5), withdrawal of consent (n = 4), and exclusion due to safety concerns (n = 1). The study flow is illustrated in Figure 1.
Figure 1.
Study flow. Flow diagram showing the number of included participants, randomization, completed tests, and drop-outs. ∗, Active: number of participants that were still included in the study at the time of each assessment and thus should have been tested. Abbreviations: CG, control group; IG, intervention group; PD, progressive disease.
Baseline characteristics are shown in Table 2. The median age of the participants was 72 years (IQR 68-75). Most participants were diagnosed with NSCLC (46%) and PC (43%), and 11% were diagnosed with BTC. Baseline characteristics for the two groups were well-balanced.
Table 2.
Medical and demographic characteristics of the participants
| Variables | Intervention group (n=41), n(%) | Control group (n=43), n(%) |
|---|---|---|
| Sex | ||
| Female | 22 (53.7) | 26 (60.5) |
| Male | 19 (46.3) | 17 (39.5) |
| Age, median (IQR) | 72.1 (67.3-74.5) | 71.5 (68.5-75.3) |
| Diagnosis | ||
| Pancreatic cancer | 17 (41.5) | 19 (44.2) |
| Biliary tract cancer | 5 (12.2) | 4 (9.3) |
| Non-small cell lung cancer | 19 (46.3) | 20 (46.5) |
| Cancer stage | ||
| Locally advanced | 6 (14.6) | 6 (14.0) |
| Metastatic | 35 (85.4) | 37 (86.1) |
| Time since cancer diagnosis, in days, median (IQR) | 43 (29-53) | 35 (27-54) |
| Current treatment (first-line) | ||
| Chemotherapy | 30 (73.2) | 31 (72.1) |
| Immunotherapy | 6 (14.6) | 8 (18.6) |
| Chemotherapy/immunotherapy (combination) | 2 (4.9) | 2 (7.0) |
| Targeted therapy | 3 (7.3) | 2 (7.0) |
| Charlson comorbidity score | ||
| 0 | 14 (34.1) | 12 (27.9) |
| 1 | 10 (24.4) | 10 (23.3) |
| 2 | 11 (26.8) | 13 (30.2) |
| 3 | 4 (9.8) | 6 (14.0) |
| ≥4 | 2 (4.8) | 2 (4.6) |
| Performance status | ||
| 0 | 18 (43.9) | 27 (62.8) |
| 1 | 19 (46.3) | 15 (34.9) |
| 2 | 4 (9.8) | 1 (2.3) |
| Body Mass Index, median (IQR) | 23.0 (21.3-25.5) | 23.9 (20.8-26.8) |
| Living situation | ||
| Co-living | 25 (61.0) | 32 (74.4) |
| Living alone | 16 (39.0) | 11 (25.6) |
| Employment status | ||
| Retired | 35 (85.4) | 38 (88.4) |
| Working (full or part time) | 6 (14.6) | 5 (11.6) |
| Saltin-Grimby Physical Activity Level Scale | ||
| Level 1 (sedentary) | 12 (29.3) | 4 (9.3) |
| Level 2 (light) | 13 (31.7) | 17 (39.5) |
| Level 3 (moderate) | 11 (26.8) | 15 (34.9) |
| Level 4 (active) | 5 (12.2) | 7 (16.3) |
Abbreviation: IQR, interquartile range.
Adherence
Median adherence to the supervised exercise sessions was 69% (IQR 21%-88%), and the completion of exercise sessions when attending was 94%. The median adherence to the walking program was 75% (IQR 33%-100%). Of 84 randomized participants, 63 completed the postintervention assessment (34 of 43 participants in CG and 29 of 41 participants in IG).
All participants were provided with basic advice on nutrition and fatigue management at the nurse-led counseling session at time of inclusion. During the study period, 151 additional interventions from the nurse (M.K.M.) were carried out. Type and frequencies of these interventions are shown in supplemental online Table 1.
Endpoints
Primary Endpoint
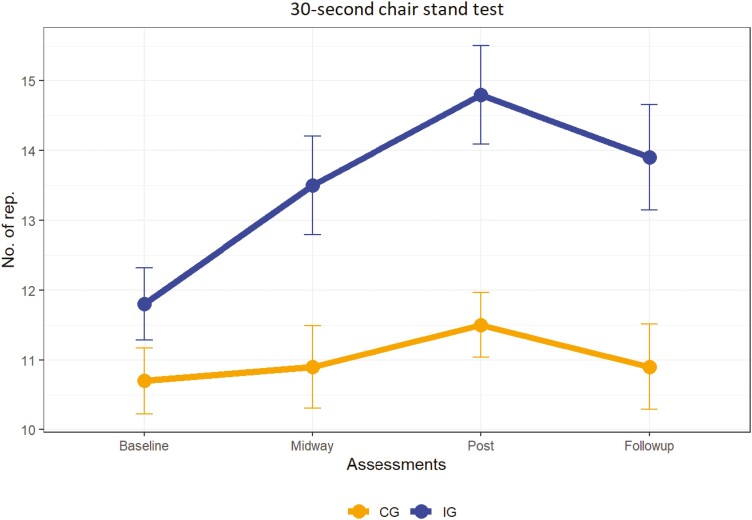
For physical function and lower body extremity strength measured with the 30s-CST at the postintervention assessment (week 13), there was a statistically significant difference between groups in change scores of 2.4 repetitions (SE 0.6) in favor of the IG (p < .0001; Table 3; Fig. 2). Whereas no significant change from baseline to week 13 was seen in the CG (change of 0.4 repetitions, SD 2.5, p = .489), a significant improvement of 2.8 repetitions (SD 2.2) was documented for the IG (p < .0001).
Table 3.
Results from baseline to 13-week assessment
| Variables | Control group, Mean (SD) | Control group, in-group change Mean (SD) | p valuea | Intervention group,Mean (SD) | Intervention group, in-group change Mean (SD) | p valuea | Between-group differences in change Mean (SE) | p valueb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 43) | 13 weeks (n = 34) | Baseline (n = 41) | 13 weeks (n = 29) | |||||||
| Physical tests | ||||||||||
| 30s-CST, no. | 10.7 (3.1) | 11.5 (2.7) | 0.4 (2.5) | .489 | 11.8 (3.3) | 14.8 (3.8) | 2.8 (2.2) | <.0001 | 2.4 (0.6) | <.0001 |
| 6MWT, meters | 434.4 (92.9) | 438.5 (117.2) | −13.6 (65.7) | .550 | 463.7 (98.1) | 503.5 (91.4) | 27.8 (62.5) | .004 | 41.4 (16.2) | .002 |
| Hand grip strength, kg | 30.8 (9.1) | 30.6 (8.1) | −0.5 (3.1) | .543 | 28.4 (9.3) | 31.6 (8.3) | 1.9 (4.5) | .019 | 2.4 (1.0) | .029 |
| Body composition, DXA | ||||||||||
| Total body mass, kg | 69.4 (15.8) | 69.1 (16.9) | 0.4 (2.7) | .432 | 68.1 (12.8) | 70.3 (13.2) | 1.4 (3.2) | .036 | 1.1 (0.7) | .188 |
| Lean body mass, kg | 47 (9.2) | 46.4 (9.1) | 0.4 (1.9) | .278 | 47.3 (8.1) | 48.7 (9.1) | 1.3 (1.5) | .0001 | 0.9 (0.4) | .033 |
| Fat mass, kg | 22.4 (9.4) | 22.7 (10) | −0.002 (2.4) | .918 | 20.8 (8.1) | 21.6 (7.6) | 0.2 (2.5) | .759 | 0.2 (0.6) | .893 |
| BMC, g | 2,432.7 (575.9) | 2,407.6 (569.7) | 1.5 (53) | .483 | 2,490.5 (612.1) | 2,468.5 (631) | −14.4 (39.7) | .069 | −15.9 (12.1) | .358 |
| BMD, g/cm2 | 1.1 (0.2) | 1.1 (0.2) | 0 (0) | .602 | 1.1 (0.2) | 1.1 (0.2) | 0 (0) | .204 | −0.008 (0.005) | .218 |
| Body composition, BIA | ||||||||||
| Lean body mass, kg | 42.9 (10.5) | 41.9 (8.8) | 0.6 (6) | .537 | 44.1 (8.5) | 44.4 (9.6) | −0.3 (3.8) | .675 | −0.9 (1.3) | .687 |
| Fat mass, kg | 18.5 (10.2) | 18.9 (11.1) | −0.4 (4) | .695 | 17.2 (8.8) | 17.4 (8.5) | 0.6 (3.5) | .681 | 1.0 (1.0) | .474 |
| Total body water, liters | 37.1 (7.7) | 36.9 (7.2) | 0.7 (2.9) | .183 | 37.5 (5.9) | 38.5 (7.3) | 0.6 (1.9) | .216 | −0.1 (0.7) | .738 |
| Activity, steps per day | 5,563 (2,447) | 4,992 (2,513) | −955 (2,031) | .015 | 4,590 (3,170) | 6,641 (2,687) | 1,575 (2,284) | .001 | 2,529 (567) | <.0001 |
| EORTC-QLQ-C30 | ||||||||||
| Global health status | 70.9 (18.6) | 70.8 (22) | −3.2 (19.7) | .741 | 57.3 (22.1) | 69.3 (16.5) | 9.8 (22.4) | .046 | 13 (5.3) | .020 |
| Physical functioning | 78.1 (17.1) | 81 (14.8) | −1 (14.4) | .988 | 75.9 (19.6) | 85.5 (12.4) | 5.5 (14.7) | .036 | 6.5 (3.7) | .055 |
| Role functioning | 71.7 (26.6) | 73 (24.6) | −4.9 (23.8) | .310 | 63 (29) | 75.3 (23.4) | 8 (26.2) | .162 | 13 (6.3) | .075 |
| Emotional functioning | 78.9 (17.9) | 84.1 (17.7) | 4.7 (16.7) | .193 | 80.1 (20.2) | 90.8 (11.4) | 8.9 (15.7) | .007 | 4.3 (4.1) | .211 |
| Cognitive functioning | 84.5 (20.4) | 87.7 (16.1) | −0.5 (14.5) | .833 | 85.8 (20.3) | 88.5 (16.1) | 0 (15.4) | .522 | 0.5 (3.8) | .718 |
| Social functioning | 88 (16.4) | 89.7 (16.4) | 0 (16.9) | .828 | 75.6 (26.6) | 90.8 (17) | 13.8 (31.8) | .063 | 13.8 (6.3) | .073 |
| Fatigue | 39.8 (22.4) | 34.6 (22.2) | 0 (18.1) | .807 | 46.3 (25) | 31 (18.9) | −10 (22.1) | .055 | −10 (5.1) | .104 |
| Nausea and vomiting | 9.7 (12.7) | 4.9 (11.3) | −2 (14.7) | .843 | 18.3 (22.6) | 5.2 (11) | −9.2 (23) | .071 | −7.2 (4.8) | .173 |
| Pain | 23.3 (21.6) | 14.2 (18.9) | −3.4 (19.6) | .562 | 23.6 (21.4) | 6.9 (13) | −13.2 (23.3) | .014 | −9.8 (5.4) | .082 |
| Dyspnea | 24 (26.6) | 26.5 (34.6) | 6.9 (30.5) | .194 | 30.9 (36) | 32.2 (22.7) | 4.6 (34.2) | .885 | −2.3 (8.1) | .988 |
| Insomnia | 18.6 (29.4) | 17.6 (26.3) | 3.9 (17.9) | .227 | 26 (33.8) | 18.4 (21.1) | −4.6 (29.2) | .276 | −8.5 (6) | .296 |
| Appetite loss | 31.8 (32.5) | 10.8 (19.6) | −17.6 (27.5) | .001 | 33.3 (35) | 9.2 (19.7) | −21.8 (33.7) | .002 | −4.2 (7.7) | .743 |
| Constipation | 20.9 (26.2) | 17.6 (26.3) | −1 (38.9) | .961 | 19.5 (25.8) | 10.3 (20.1) | −10.3 (33.5) | .140 | −9.4 (9.2) | .312 |
| Diarrhea | 12.4 (20.6) | 19.6 (24.8) | 7.8 (26) | .083 | 28.5 (33) | 5.7 (15.6) | −19.5 (27.5) | .002 | −27.4 (6.8) | .0002 |
| Financial difficulties | 2.3 (11.3) | 1 (5.7) | −2 (14.1) | .586 | 4.9 (14.1) | 5.7 (15.6) | 1.1 (14) | .766 | 3.1 (3.6) | .474 |
| MDASI | ||||||||||
| Symptoms (core) | 1.6 (0.9) | 1.5 (1.4) | 0.1 (1.3) | .620 | 2.4 (1.6) | 1.5 (1.2) | −0.7 (1.5) | .020 | −0.8 (0.4) | .044 |
| Symptom interference | 2.2 (1.9) | 2.3 (2.5) | 0.4 (2.3) | .776 | 2.8 (2.3) | 1.6 (1.9) | −1.1 (2) | .010 | −1.5 (0.5) | .009 |
| HADS | ||||||||||
| Anxiety | 4 (2.6) | 3.4 (2.7) | −0.6 (2.9) | .306 | 4.1 (2.9) | 2.1 (1.8) | −2.3 (3) | .001 | −1.8 (0.7) | .033 |
| Depression | 2.9 (2.5) | 2.8 (2.2) | 0.4 (2.4) | .234 | 3.5 (3) | 1.6 (2.1) | −1.8 (2.3) | .001 | −2.2 (0.6) | .0004 |
Wilcoxon test.
Mann-Whitney U test.
Abbreviations: 30s-CST, 30-second chair stand test; 6MWT, 6-minute walk test; BIA, bioelectrical impedance assessment; BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; EORTC-QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HADS, Hospital Anxiety and Depression Scale; MDASI, M.D. Anderson Symptom Inventory.
Figure 2.
Results of the 30-second chair stand test across assessments. Results are shown as means with SEMs. Abbreviations: CG, control group; IG, intervention group; rep., repetitions.
Secondary Endpoints
For the 30s-CST, there were also statistically significant differences in change scores between groups at week 7 of 1.7 repetitions (SE 0.5, p = .001) and at the follow-up assessment at week 17 of 2.4 repetitions (SE 0.7, p = .001). Results for the 30s-CST across assessments are shown in Figure 2. All results from baseline to assessments at weeks 7 and 17 are shown in supplemental online Tables 2 and 3. In the following sections, only results from the postintervention assessment at week 13 will be presented. All results for the 13-week assessment are shown in Table 3.
Physical Tests and Body Composition.
For the 6MWT, there was a statistically significant between-group difference in change scores of 41.4 meters (SE 16.2, p = .002), favoring the IG. Furthermore, a statistically significant difference in change scores in the handgrip strength test was documented of 2.4kg (SE 1.0, p = .029), in favor of the IG. For measures of body composition, there was a statistically significant difference in change of lean body mass of 0.9kg (SE 0.4) assessed with DXA scans, favoring the IG (p = .033). Participants in the IG had a significant increase in lean body mass of 1.3kg (SD 1.5, p = .0001), whereas no significant within group changes were documented in the CG. There were no significant differences between groups in change scores related to total body mass, bone mineral content, or bone mineral density. Furthermore, no significant differences were seen for total body water or for any other BIA measure. For physical activity, there was a statistically significant difference in change scores for daily steps of 2,529 steps (SE 567, p < .0001), favoring the IG.
Patient-Reported Outcome Measures.
For QoL measures, there were no significant differences in change scores for most domains. However, there was a statistically significant difference in favor of the IG for changes in global health status of 13 points (SE 5.3, p = .020). For symptoms of depression and anxiety, there were statistically significant differences between groups in anxiety scores (–1.8 points, SE 0.7, p = .033) and depression scores (–2.2 points, SE 0.6, p = .004) favoring the IG. In symptom burden, between-group differences in change scores were documented for core symptoms with a difference of –0.8 points (SE 0.4, p = .044) and for symptom interference with a difference of –1.5 points (SE 0.5, p = .009), both favoring the IG.
Clinical Outcomes
The median number of days hospitalized during the project period was 1 (IQR 0-6.5) for the CG and 1 (IQR 0-3) for the IG (p =.847). There were no statistically significant differences in number of hospitalized days between groups. For toxicity, there were no significant differences between groups regarding any adverse events (diarrhea, nausea, vomiting, constipation, anorexia, fatigue, stomatitis, itching, allergy, and neuropathy). The most frequently reported grade 3 and 4 adverse event was infection, which was documented in 19% of participants in the CG and in 20% of participants in the IG.
Furthermore, no significant differences were seen for dose intensity of any chemotherapy regimens (supplemental online Table 4). From time of randomization until last follow-up (August 19, 2020), 47 deaths occurred, with most being cancer-related (n = 45). The median overall survival was 14.6 months (95% CI, 11.7-21.0) for the CG and 14.0 months (95% CI, 9.2-24.6) for the IG, with no significant differences (p = .818).
Safety
Five grade 1 (bruises and feeling sick/dizzy) and one grade 2 (swollen knee) adverse events arose. Three grade 3 events occurred, of which two were possibly related to the exercise intervention: one participant fell in the waiting room before exercising and sustained a distal radius fracture (not related), one experienced back pain in days following exercise and was diagnosed with osteoporotic spinal compression (possibly related to/triggered by exercise), and one experienced pain and instability in a knee after initiating the program and was later diagnosed with spinal stenosis (knee problems possibly related to exercise). In the first two cases, the participants returned to the program and finished with no further problems. In the last case, the participant with spinal stenosis was excluded from the program and referred to specialized back rehabilitation.
Activity Levels of Participants in the Control Group
The activity level for participants in the CG dropped from baseline to postintervention with a mean decrease of 955 steps per day (SD 2,031, p = .015), compared with an increase in the IG of 1,575 steps per day (SD 2,284, p = .001). Half of the participants in the CG (50%) reported having performed physical activity corresponding to ≥30 minutes of moderate intensity activity per day during the intervention period.
Discussion
The results from this RCT showed that a 12-week multimodal exercise program was effective in improving physical function in older patients with advanced PC, BTC, or NSCLC during oncological treatment. Improvements demonstrated in the 30s-CST also reached levels of clinically relevant importance.35 The sit-to-stand movement is regarded as a prerequisite for activities of daily living, and living independently and maintaining daily activities are associated with higher QoL in older patients with cancer.39 In the current study, significant increased scores in global health status (QoL) were documented for the IG from baseline to week 13, and these improvements were of clinical relevance with a small to medium effect.40 Only a few prior studies have investigated effects of exercise interventions among older patients with cancer during oncological treatment. A systematic review conducted by Forbes et al.,41 which aimed to summarize effects of physical activity and nutrition interventions on health-related QoL among older adults (≥60 years) with cancer, included 13 activity-based and mixed (i.e., combined activity and nutrition) intervention studies. The studies were heterogenous, and most studies focused on patients with breast or prostate cancer (n = 8). Effects of QoL measures were mixed and inconsistent across studies.41 Another systematic review by our research group42 investigated evidence for effects of exercise interventions in older patients (≥65 years) with cancer during oncological treatment, and only four studies were identified. Although some beneficial effects were seen for physical function, muscle strength, and activity level, differences between studies and mixed results made the overall results inconclusive.42 Thus, our current study is one of the first sufficiently powered exercise-based RCTs focusing on older patients with advanced cancer during oncological treatment.
Results from assessments of body composition using DXA scans showed positive effects on lean body mass with a between-group difference of 0.9kg in favor of the IG. This is an important finding, as cancer cachexia and sarcopenia are common challenges among patients with PC, BTC, and NSCLC23,43 and as muscle wasting negatively affects physical function and QoL and predicts reduced survival among patients with cancer.44 The validity of DXA has been questioned particularly in very lean and obese people and can furthermore be affected by hydration status.45 In the current study, we used BIA to supplement data from DXA scans, to investigate hydration status across time points. As data showed no differences in changes of total body water between groups, the beneficial effects on lean body mass, as an estimate of muscle mass, is strengthened.
The prognosis of advanced PC, BTC, and NCSLC is overall poor, and the goal of treatment is symptom reduction, better QoL, and prolonged survival. Although some exercise-based trials have been conducted among patients with advanced NSCLC, very few have included patients with advanced PC and BTC. In a Cochrane systematic review, the effect of exercise among patients with advanced lung cancer (LC) (mean age ranged from 59 to 70 years) was investigated.46 In the six studies including 221 participants, beneficial effects were documented in physical capacity and LC-specific QoL. No effects were seen for cancer-related symptoms or psychological symptoms.46 In a three-armed RCT conducted by Wiskemann et al.,47 feasibility and effects of a 6-month supervised resistance training program and a 6-month unsupervised program were investigated in 65 patients (mean age 60 years) with PC or bile duct cancer.47 Beneficial effects were documented in some muscle groups, and the supervised program was more effective than the non-supervised.47 No effects were seen for physical functioning, body weight, or QoL domains at the postintervention assessment after 6 months; however, some beneficial effects related to QoL, physical functioning, physical activity, and cancer-related symptoms were documented after 3 months.48
Although beneficial effects were documented on physical and psychological outcomes, we found no effects on clinical outcomes (i.e., dose intensity, toxicity, hospitalizations, or survival). From observational studies, there is some evidence to support associations between higher activity levels and reduced risk of all-cause and cancer-specific mortality among patients with breast, colorectal and prostate cancer.49 However, results from RCTs are lacking.50-52 Recently, a systematic review investigating effects of exercise on mortality and recurrence in patients with cancer and cancer survivors was published.53 Even though only two of the eight included RCTs individually demonstrated beneficial effects of exercise on survival, results from meta-analyses showed that exercise significantly reduced risk of mortality (risk ratio [RR] = 0.76; 95% CI, 0.40-0.93) and risk of recurrence in cancer survivors (RR = 0.52; 95% CI, 0.29-0.92).53 Patients included in the current RCT had advanced PC, BTC, or NSCLC with expected short survival time. Thus, potential effects of exercise on cancer progression can perhaps not be expected in these populations.
Another systematic review looked at chemotherapy completion rates and included eight exercise-based RCTs.54 Although two studies reported beneficial effects of exercise on chemotherapy completion rate (including higher dose intensity and fewer dose adjustments) in patients with early-stage breast cancer, the remaining studies reported no differences.54 In the current study, no differences between groups were seen for dose intensity. However, as chemotherapy regimens and completion rates in an adjuvant breast cancer setting are very different from the regimens seen in our study, it is difficult to compare the results. Findings from prior studies have suggested associations between reduced lean body mass and increased risk of toxicity.44 As the exercise intervention in the current study had a beneficial effect on lean body mass, this could hypothetically have influenced incidence of toxicities and thus dose intensity. However, no effects were documented. Other studies are currently investigating associations between muscle mass and treatment completion and toxicity in larger patient populations,55,56 with the potential to improve decision-making algorithms for chemotherapy doses and possibly improve treatment tolerance through strengthening exercise strategies.
In the current study, 37% of eligible patients accepted participation, and 31% were baseline tested and randomized. As approximately two thirds of eligible participants declined, patients included in the study thereby represent a selected subgroup and might not be representative of the patient populations. Even though the proportion of decliners in our study was large, especially when considering the efforts of involving patients in the study design, our inclusion rate exceeded the inclusion rate documented by Wiskemann et al.,47 where 21% of eligible patients with PC or bile duct cancer were included and randomized. Furthermore, completion rates between the two studies were comparable with approximately 75% of randomized participants completing the postintervention assessment.47
Although the positive results from the current study could lead to adoption of the program in clinical practice, more studies are still recommended to be conducted, especially to explore potential effects on clinical outcomes in larger populations. Furthermore, in this study, multiple interventions from the nurse were conducted to support the participants during the intervention period. Thus, this individualized and comprehensive support must be considered when evaluating both feasibility and effects of the intervention. In a health economic perspective, investigating the feasibility of exercise interventions containing less additional support could also be considered to increase possibilities for implementation in clinical practice.
Strengths and Limitations
The current study is strengthened by a sufficiently powered sample size and validated outcome instruments. Furthermore, outcome assessors for the primary endpoint were blinded to the participants’ allocation. Participants were, however, not blinded because of the nature of the study, and this is an unavoidable limitation. The study design also entailed a risk of contamination as participants in the CG could exercise on their own or in other teams, including municipal rehabilitation. The activity level in the CG was not monitored during the entire intervention, but we did measure levels of physical activity at baseline and at postintervention using pedometers, and the participants’ activities were retrospectively registered.
Even though our results showed beneficial effect on PROMs (i.e., symptom burden, psychological symptoms, and QoL) and body composition, and no effects were seen for clinical outcomes, these results must be interpreted with caution as the study was not powered to these analyses. Furthermore, as comparison for several secondary outcomes were included in the study, there is a high risk of false positive results, and therefore significant results should be regarded as being mainly hypothesis-generating.
Conclusion
A 12-week multimodal intervention comprising progressive resistance training, home-based walking, protein supplements after exercise sessions, and targeted support and advice proved effective in improving physical function and lower body muscle strength in older patients with advanced pancreatic, biliary tract, or non-small cell lung cancer during oncological treatment. Furthermore, results on secondary endpoints showed improvements in psychological well-being and lean body mass, and the intervention did not affect dose intensity of chemotherapy, toxicities, hospitalizations, or survival.
Supplementary Material
Acknowledgments
We thank all participants for their efforts and contributions to the study. Thanks to all nurses, physiotherapists, and doctors at the Department of Oncology and Department of Physiotherapy and Occupational Therapy at Herlev and Gentofte Hospital for assistance and collaboration. Finally, we would like to thank Jesper Frank Christensen from Centre for Physical Activity Research (CFAS), Copenhagen University Hospital, Rigshospitalet, for advice during the project preparation phase. This work was supported by grants from the Novo Nordisk Foundation [NNF16OC0022338, NNF17OC0029756] and the Velux Foundation (salary to C.M.L.) [18310]. The Novo Nordisk Foundation and the Velux Foundation did not have any role in the study design, conduction of the study, data collection, data analyses, or in the drafting or approval of the manuscript.
Author Contributions
Conception/design: Marta K. Mikkelsen, Cecilia M. Lund, Anders Vinther, Anders Tolver, Julia S. Johansen, Inna Chen, Anne-Mette Ragle, Bo Zerahn, ELotte Engell-Noerregaard, Finn O. Larsen, Susann Theile, Dorte L. Nielsen, Mary Jarden
Provision of study material or patients: Marta K. Mikkelsen, Julia S. Johansen, Inna Chen, Lotte Engell-Noerregaard, Finn O. Larsen
Collection and/or assembly of data: Marta K. Mikkelsen, Anne-Mette Ragle, Bo Zerahn
Data analysis and interpretation: Marta K. Mikkelsen, Cecilia M. Lund, Anders Vinther, Anders Tolver, Dorte L. Nielsen, Mary Jarden
Manuscript writing: Marta K. Mikkelsen, Cecilia M. Lund, Anders Vinther, Dorte L. Nielsen, Mary Jarden
Final approval of manuscript: Marta K. Mikkelsen, Cecilia M. Lund, Anders Vinther, Anders Tolver, Julia S. Johansen, Inna Chen, Anne-Mette Ragle, Bo Zerahn, Lotte Engell-Noerregaard, Finn O. Larsen, Susann Theile, Dorte L. Nielsen, Mary Jarden
Disclosures
The authors indicated no financial relationships.
References
- 1. Miaskowski C, Wong ML, Cooper BAet al. Distinct physical function profiles in older adults receiving cancer chemotherapy. J Pain Symptom Manage 2017;54:263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkhus L, Harneshaug M, Šaltytė Benth Jet al. Modifiable factors affecting older patients’ quality of life and physical function during cancer treatment. J Geriatr Oncol 2019;10:904-912. [DOI] [PubMed] [Google Scholar]
- 3. Mohile SG, Xian Y, Dale Wet al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst 2009;101:1206-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker F, Haffer SC, Denniston M.. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer 2003;97:674-681. [DOI] [PubMed] [Google Scholar]
- 5. Harding C, Pompei F, Wilson R.. Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer 2012;118:1371-1386. [DOI] [PubMed] [Google Scholar]
- 6. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilleron S, Sarfati D, Janssen-Heijnen Met al. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer 2019;144:49-58. [DOI] [PubMed] [Google Scholar]
- 8. Weinstein JR, Anderson S.. The aging kidney: Physiological changes. Adv Chronic Kidney Dis 2010;17:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhutto A, Morley JE.. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care 2008;11:651-660. [DOI] [PubMed] [Google Scholar]
- 10. Pritz T, Weinberger B, Grubeck-Loebenstein B.. The aging bone marrow and its impact on immune responses in old age. Immunol Lett 2014;162:310-315. [DOI] [PubMed] [Google Scholar]
- 11. Frontera WR, Hughes VA, Fielding RAet al. Aging of skeletal muscle: A 12-yr longitudinal study. J Appl Physiol (1985) 2000;88:1321-1326. [DOI] [PubMed] [Google Scholar]
- 12. Shahrokni A, Wu AJ, Carter Jet al. Long-term toxicity of cancer treatment in older patients. Clin Geriatr Med 2016;32:63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Ferrucci L, Darer Jet al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255-263. [DOI] [PubMed] [Google Scholar]
- 14. Campbell KL, Winters-Stone KM, Wiskemann Jet al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 2019;51:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buffart LM, Kalter J, Sweegers MGet al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev 2017;52:91-104. [DOI] [PubMed] [Google Scholar]
- 16. Stout NL, Baima J, Swisher AKet al. A systematic review of exercise systematic reviews in the cancer literature (2005-2017). PM R 2017;9:S347-S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerritsen JK, Vincent AJ.. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2016;50:796-803. [DOI] [PubMed] [Google Scholar]
- 18. Nakano J, Hashizume K, Fukushima Tet al. Effects of aerobic and resistance exercises on physical symptoms in cancer patients: A meta-analysis. Integr Cancer Ther 2018;17:1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen YJ, Li XX, Ma HKet al. Exercise training for improving patient-reported outcomes in patients with advanced-stage cancer: A systematic review and meta-analysis. J Pain Symptom Manage 2020;59:734-749.e710. [DOI] [PubMed] [Google Scholar]
- 20. Nadler MB, Desnoyers A, Langelier DMet al. The effect of exercise on quality of life, fatigue, physical function, and safety in advanced solid tumor cancers: A meta-analysis of randomized control trials. J Pain Symptom Manage 2019;58:899-908.e897. [DOI] [PubMed] [Google Scholar]
- 21. Kilari D, Soto-Perez-de-Celis E, Mohile SGet al. Designing exercise clinical trials for older adults with cancer: Recommendations from 2015 Cancer and Aging Research Group NCI U13 Meeting. J Geriatr Oncol 2016;7:293-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loh KP, Lin PJ, Uth Jet al. Exercise for managing cancer- and treatment-related side effects in older adults. J Geriatr Oncol 2018;9:405-410. [DOI] [PubMed] [Google Scholar]
- 23. Sun L, Quan XQ, Yu S.. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer 2015;67:1056-1062. [DOI] [PubMed] [Google Scholar]
- 24. Mikkelsen MK, Nielsen DL, Vinther Aet al. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment - A qualitative interview study. Eur J Oncol Nurs 2019;41:16-23. [DOI] [PubMed] [Google Scholar]
- 25. Schulz KF, Altman DG, Moher D.. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikkelsen MK, Lund CM, Vinther Aet al. Engaging the older cancer patient; patient activation through counseling, exercise and mobilization - pancreatic, biliary tract and lung cancer (PACE-Mobil-PBL) - study protocol of a randomized controlled trial. BMC Cancer 2018;18:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Thielke Ret al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rödjer L, Jonsdottir IH, Rosengren Aet al. Self-reported leisure time physical activity: A useful assessment tool in everyday health care. BMC Public Health 2012;12:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rikli RE, Jones CJ.. Senior Fitness Test Manual. 2nd ed. Human Kinetics; 2001. [Google Scholar]
- 30. Mathiowetz V, Weber K, Volland Get al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222-226. [DOI] [PubMed] [Google Scholar]
- 31. Cleeland CS, Mendoza TR, Wang XSet al. Assessing symptom distress in cancer patients: The M.D. Anderson symptom inventory. Cancer 2000;89:1634-1646. [DOI] [PubMed] [Google Scholar]
- 32. Aaronson NK, Ahmedzai S, Bergman Bet al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 33. Zigmond AS, Snaith RP.. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 34. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: National Cancer Institute, 2019. [Google Scholar]
- 35. Wright AA, Cook CE, Baxter GDet al. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 2011;41:319-327. [DOI] [PubMed] [Google Scholar]
- 36. Litterini AJ, Fieler VK, Cavanaugh JTet al. Differential effects of cardiovascular and resistance exercise on functional mobility in individuals with advanced cancer: A randomized trial. Arch Phys Med Rehabil 2013;94:2329-2335. [DOI] [PubMed] [Google Scholar]
- 37. Oldervoll LM, Loge JH, Lydersen Set al. Physical exercise for cancer patients with advanced disease: A randomized controlled trial. The Oncologist 2011;16:1649-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from https://www.R-project.org/. [Google Scholar]
- 39. Pergolotti M, Deal AM, Williams GRet al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer. J Geriatr Oncol 2017;8:249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cocks K, King MT, Velikova Get al. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29:89-96. [DOI] [PubMed] [Google Scholar]
- 41. Forbes CC, Swan F, Greenley SLet al. Physical activity and nutrition interventions for older adults with cancer: A systematic review. J Cancer Surviv 2020;14:689-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mikkelsen MK, Juhl CB, Lund CMet al. The effect of exercise-based interventions on health-related quality of life and physical function in older patients with cancer receiving medical antineoplastic treatments: A systematic review. Eur Rev Aging Phys Act 2020;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Srdic D, Plestina S, Sverko-Peternac Aet al. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer 2016;24:4495-4502. [DOI] [PubMed] [Google Scholar]
- 44. Pin F, Couch ME, Bonetto A.. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 2018;12:420-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cruz-Jentoft AJ, Bahat G, Bauer Jet al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peddle-McIntyre CJ, Singh F, Thomas Ret al. Exercise training for advanced lung cancer. Cochrane Database Syst Rev 2019;2:CD012685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wiskemann J, Clauss D, Tjaden Cet al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: A randomized controlled trial. Pancreas 2019;48:257-266. [DOI] [PubMed] [Google Scholar]
- 48. Steindorf K, Clauss D, Tjaden Cet al. Quality of life, fatigue, and sleep problems in pancreatic cancer patients—a randomized trial on the effects of exercise. Dtsch Arztebl Int 2019;116:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McTiernan A, Friedenreich CM, Katzmarzyk PTet al. Physical activity in cancer prevention and survival: A systematic review. Med Sci Sports Exerc 2019;51:1252-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loughney L, West MA, Kemp GJet al. Exercise intervention in people with cancer undergoing adjuvant cancer treatment following surgery: A systematic review. Eur J Surg Oncol 2015;41:1590-1602. [DOI] [PubMed] [Google Scholar]
- 51. Palma S, Hasenoehrl T, Jordakieva Get al. High-intensity interval training in the prehabilitation of cancer patients - A systematic review and meta-analysis. Support Care Cancer 2020;29:1781-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGettigan M, Cardwell CR, Cantwell MMet al. Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst Rev 2020;5:CD012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morishita S, Hamaue Y, Fukushima Tet al. Effect of exercise on mortality and recurrence in patients with cancer: A systematic review and meta-analysis. Integr Cancer Ther 2020;19:1534735420917462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bland KA, Zadravec K, Landry Tet al. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol 2019;136:79-85. [DOI] [PubMed] [Google Scholar]
- 55. Caan BJ, Meyerhardt JA, Brown JCet al. Recruitment strategies and design considerations in a trial of resistance training to prevent dose-limiting toxicities in colon cancer patients undergoing chemotherapy. Contemp Clin Trials 2021;101:106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinmeyer Z, Gérard S, Filleron Tet al. Low lean mass and chemotherapy toxicity risk in the elderly: The Fraction study protocol. BMC Cancer 2019;19:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.