Abstract
Introduction:
Radiological response assessment to immune checkpoint inhibitor is challenging due to atypical pattern of response and commonly used RECIST 1.1 criteria do not take into account the kinetics of tumor behavior. Our study aimed at evaluating the tumor growth rate (TGR) in addition to RECIST 1.1 criteria to assess the benefit of immune checkpoint inhibitors (ICIs).
Methods:
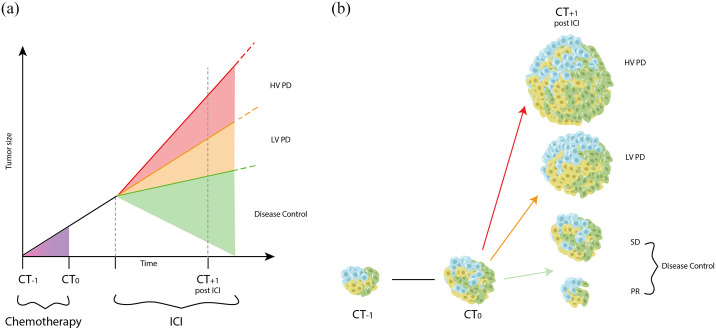
Tumor real volume was calculated with a dedicated computed tomography (CT) software that semi-automatically assess tumor volume. Target lesions were identified according to RECIST 1.1. For each patient, we had 3 measurement of tumor volume. CT-1 was performed 8–12 weeks before ICI start, the CT at baseline for ICI was CT0, while CT + 1 was the first assessment after ICI. We calculated the percentage increase in tumor volume before (TGR1) and after immunotherapy (TGR2). Finally, we compared TGR1 and TGR2. If no progressive disease (PD), the group was disease control (DC). If PD but TGR2 < TGR1, it was called LvPD and if TGR2 ⩾ TGR1, HvPD.
Results:
A total of 61 patients who received ICIs and 33 treated with chemotherapy (ChT) were included. In ICI group, 18 patients were HvPD, 22 LvPD, 21 DC. Median OS was 4.4 months (95% CI: 2.0–6.8, reference) for HvPD, 7.1 months (95% CI 5.4–8.8) for LvPD, p = 0.018, and 20.9 months (95% CI: 12.5–29.3) for DC, p < 0.001. In ChT group, 7 were categorized as HvPD, 17 as LvPD and 9 as DC. No difference in OS was observed in the ChT group (p = 0.786)
Conclusion:
In the presence of PD, a decrease in TGR may result in a clinical benefit in patients treated with ICI but not with chemotherapy. Monitoring TGR changes after ICIs administration can help physician in deciding to treat beyond PD.
Keywords: checkpoint inhibitor, drug resistance, immunotherapy, lung cancer, non-small cell lung cancer (NSCLC)
Introduction
The unprecedented results of immune checkpoint inhibitors (ICIs) have rapidly changed the therapeutic scenario in patients with advanced Non-Small Cell Lung Cancer (NSCLC), becoming standard of care both alone and in combination with chemotherapy in first and further lines of treatment 1 for patients with non-oncogene addicted NSCLC, that represents the majority of lung cancer cases in western populations. 2
A number of randomized studies have demonstrated that ICIs prolong median OS of, as an average, 2–4 months as compared to standard docetaxel-based chemotherapy in patients with advanced pre-treated NSCLC, with a 5-year survival rate of approximately 10%–20%.3–6 However, a significant part of these patients do not seem to really benefit from ICIs treatment as indicated by the low overall RECIST response rate (10%–20%) and the presence of rapid, extensive and, sometimes, highly symptomatic disease progression in a not negligible percentage them.7,8 At present, it is unclear whether achieving an objective response is a prerequisite to attain a survival benefit.
The current standard to evaluate tumor response is the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, originally published in 2000 9 and revised in 2009 as version 1.1. 10 Progression is defined as an increase in tumor size (which is the summation of the longest diameter of five target lesions) of more than 20% compared to the lowest determined tumor size at any time point, the appearance of new lesions, or the un-equivocal progression of non target lesions.
These criteria have been set for anticancer chemotherapy, but the novel mechanism of action of ICIs, with immune and T cell activation, leads to unusual patterns of response on imaging which make assessment of percentage in linear dimensions changes, as in RECIST criteria, insufficient to describe the complex arrays of immunotherapy effects.
Indeed, while a pseudoprogression, defined as an apparent increase of tumor burden due to infiltration by activated T cells, needs a confirmation through a subsequent CT evaluation, the hyperprogression (HPD) pattern, defined as an unexpected acceleration of the tumor kinetics, can only be evaluated via dynamic imaging/ temporal scanning. In addition, some patients, despite being classified as in progressive disease (PD) according to radiological RECIST 1.1, seem to gain a clinical benefit from ICIs, hence justifying the possibility to continue immunotherapy treatment beyond radiological progression (TBP). 11
On the basis of observations of immune-related response patterns, adjustments to the conventional tumor response criteria have been proposed in an attempt to revise and improve their characterization.
Tumor growth rate (TGR) is a parameter developed in different kind of cancers as a tool to estimate the increase in tumor volume over time. It incorporates the time between CT assessments, thus allowing a quantitative and dynamic evaluation of the tumor burden, using each patient as his own control.12–17
In this study, we explored the impact of TGR changes after ICIs administration in advanced NSCLC patients receiving ICIs. Our hypothesis was that ICIs might, in some cases, yield a clinical benefit by slowing down tumor kinetics and that a reduction in TGR could eventually identify a proportion of patients that, despite a RECIST 1.1 PD, might benefit from immunotherapy administration. To discriminate if this finding was typical of ICIs, we also enrolled a control group of patients treated with chemotherapy.
Materials and methods
Patients
Data were retrospectively collected from all consecutive eligible patients with advanced NSCLC treated with ICIs (nivolumab, pembrolizumab, atezolizumab) in second or later lines, from 10 November 2015, to 15 October 2019. We also enrolled a control cohort of patients treated with standard second line chemotherapy (docetaxel, gemcitabine, pemetrexed) between 2 January 2010 and 31 December 2014.
To be eligible, patients had to be 18 years or older, histologically or cytologically confirmed stage III or IV NSCLC and available CT scans scan with one to five measurable target lesion according to RECIST 1.1 criteria for radiological. In the single agent chemotherapy control cohort, patients who received previous or subsequent treatment with ICIs were not eligible. The end of follow-up period was 14 March 2020. The study was approved by local IRB/EC (approval no. 2381/2019) and all patients gave written consent before enrolment in this study.
Tumor volume and tumor growth rate calculation
Patients with at least 3 CT tumor assessments were selected for this study. CT-1 was performed 8–12 weeks before ICI start (while the patient was on the treatment that preceded ICI). The CT performed at baseline for ICI was CT0 (within 6 weeks from ICI start), while CT + 1 was the first assessment after ICI start (8–12 weeks after ICI start). A maximum of 5 target lesions were identified according to RECIST 1.1 criteria at CT-1 (average 1.67 in the chemotherapy group and 2.22 in the immunotherapy group).
Three tumor real volumes, one at each CT assessment (V0, V-1 and V+1) were calculated with a dedicated CT software (Philips IntelliSpace Portal v. 8.0, Philips Medical System, Nederland). In brief, the reader manually selects a small region of interest within a tumor by a mouse click, determining a seed point. The software automatically segments the lesion from the surrounding structures, using a three-dimensional seed-growing algorithm. The reader then visually assessed the automatically segmented tumor contours, and if needed, manually adjusts the contour to generate the final tumor contour. After segmentation and manual correction, tumor volume (measured in cm3) was automatically calculated by the software.
V0 and V-1 were used to calculate TGR1, which corresponds to the daily tumor growth rate before ICI start. TGR2, the daily tumor growth rate after ICI treatment, is calculated using Vi and V + 1, where Vi is the tumor volume just at the time of ICI start. Since baseline CT before ICI start was carried out not exactly the day of treatment start but at a variable number of days before, Vi did not coincide with V0 and, therefore, we could not use V0 to calculate TGR2 but we calculated Vi as an approximation assuming that tumor growth follows an exponential law.
Let t be the time expressed in days at the tumor assessment, the volume at a certain time t, before ICI start can be approximated by the following formula: V(t)=V0 e(TG1 t), where V0 is the volume at time t = 0, corresponding to the day of CT0 and TG1 is the growth constant before ICI start which is given by TG1 = Log(V0/V-1)/T, where T is the time expressed in days between CT-1 and CT0. With this formula Vi is derived, which is given by Vi = V0 e(TG1 ti), where ti is the time, expressed in days, between CT0 and the start of ICI. Using Vi is possible to calculate TG2, which is growth constant after ICI start, as follows: TG2 = Log(V + 1/Vi)/T as previously described. 13
TG1 and TG2 were then used to calculate TGR1 and TGR2 with the following formula: TGR1 = 100 (e^TG1 -1), TGR2 = 100 (e^TG2 -1).
Finally, we compared TGR1 and TGR2 of each patient. As showed in Figure 1, if there was no RECIST 1.1 PD, we called them DC (disease control). If disease progressed (including the appearance of new lesions) but TGR2 was lower than TGR1, we called them LvPD (Lower velocity PD) and if TGR2 was higher than TGR1, HvPD (higher velocity PD).
Figure 1.
Explanation of the three categories according to tumor growth rate.
Statistical analysis
Clinical and pathological information was summarized using summary statistics.
Associations between DC, LvPD or HvPD and categorical or continuous variables were evaluated using the Fisher exact test and the t test, respectively. OS was estimated using the Kaplan-Meier method. The median follow-up was calculated with reverse Kaplan Meyer method. The hazard ratio (HR) was estimated using the univariate Cox proportional hazards regression model. All p values were 2 sided, and values less than 0.05 were considered statistically significant. A test for interaction between TGR categorization and treatment administered was performed and retained if p value for interaction was less than 0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) program version 25.0 (IBM, Armonk, NY).
Results
In all, 61 patients were retrospectively enrolled in ICIs group. Baseline characteristics are summarized in Table 1. The median follow-up for ICIs group was 23.4 months (95% CI: 14.2–32.6). Median OS was 10.8 months (95% CI: 6.0–15.6). Mean age was 71.0 years (SD = 17.8), 80% were men, 62% were current or former smoker, 16% and 25% had liver and bone metastasis respectively, 12% had an ECOG PS of 2 or more, 26% had derived neutrophile to lymphocyte ratio (dNL)⩾3% and 72% did not receive any subsequent line of treatment after progression to ICI. A total of 6 patients had a partial response as best response to ICI (9.8%), 15 stable disease (24.6%). Of the 40 patients who had RECIST 1.1 disease progression at first CT scan (65.6%), 22 continued ICIs after radiological disease progression. Of them, 1 was subsequently confirmed as pseudo progression (2.5%). PD-L1 expression was available for 38 patients.
Table 1.
Clinic-pathological characteristics of the HvPD, LvPD and DC group in patients treated with immunotherapy.
| HvPD (n = 18) | LvPD (n = 22) | DC (n = 21) | All patients (n = 61) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean, SD) | 69.3 (19.8) | 73.1 (10.0) | 70.3 (13.8) | 71.0 (17.8) | 0.778 | |||||
| Sex | Male | 13 | 72.2% | 20 | 90.9% | 16 | 76.2% | 49 | 80.3% | 0.282 |
| Female | 5 | 27.8% | 2 | 9.1% | 5 | 23.8% | 12 | 19.7% | ||
| Smoking | Current | 2 | 11.1% | 3 | 13.6% | 3 | 14.3% | 8 | 13.1% | 0.321 |
| Former | 9 | 50.0% | 13 | 59.1% | 14 | 66.7% | 36 | 59.0% | ||
| Never | 5 | 27.8% | 2 | 9.1% | 1 | 4.8% | 8 | 13.1% | ||
| N/A | 2 | 11.1% | 4 | 18.2% | 3 | 14.3% | 9 | 14.8% | ||
| Liver metastasis | Yes | 3 | 16.7% | 4 | 18.2% | 3 | 14.3% | 10 | 16.4% | 0.972 |
| No | 15 | 83.3% | 18 | 81.8% | 18 | 85.7% | 51 | 83.6% | ||
| Bone metastasis | Yes | 9 | 50.0% | 4 | 18.2% | 2 | 9.5% | 15 | 24.6% | 0.009 |
| No | 9 | 50.0% | 18 | 81.8% | 19 | 90.5% | 46 | 75.4% | ||
| Ecog PS | 0–1 | 13 | 72.2% | 21 | 95.5% | 20 | 95.2% | 54 | 88.5% | 0.035 |
| 2 | 5 | 27.8% | 1 | 4.5% | 1 | 4.8% | 7 | 11.5% | ||
| dNLR | ⩾ 3 | 6 | 33.3% | 7 | 31.8% | 3 | 14.3% | 16 | 26.2% | 0.282 |
| < 3 | 12 | 66.7% | 15 | 68.2% | 18 | 85.7% | 45 | 73.8% | ||
| Subsequent therapy | Yes | 4 | 22.2% | 4 | 18.2% | 3 | 14.3% | 11 | 18.0% | 0.908 |
| No | 14 | 77.8% | 15 | 68.2% | 13 | 61.9% | 44 | 72.1% | ||
| Treatment Ongoing | 0 | 0.0% | 3 | 13.6% | 3 | 14.3% | 6 | 9.8% | ||
| Lost at f.u. during ICI | 0 | 0 | 2 | 9.5% | ||||||
| Treatment Beyond Radiological Progression at first CT-scan | Yes | 11 | 61.1% | 11 | 50.0% | 0.521 | ||||
| No | 7 | 38.9% | 10 | 45.5% | ||||||
| PD-L1 Expression | Mean % (SD) | 9.7 (21.9) | 21.8 (29.1) | 24.2 (29.7) | 0.413 | |||||
| N/A | 6 | 33.3% | 7 | 31.8% | 10 | 47.6% | 23 | 37.7% | ||
| Local Ablative treatment within 6 months after ICIs start | 0.205 | |||||||||
| Yes | 4 | 22.2% | 2 | 9.1% | 1 | 4.8% | 7 | 11.5% | ||
| No | 14 | 77.8% | 20 | 90.9% | 20 | 95.2% | 54 | 88.5% | ||
DC, disease control; dNLR, derived neutrophil/lymphocyte ratio; ECOG PS, ECOG performance status; HvPD, higher velocity PD; ICI, immune checkpoint inhibitors; LvPD, lower velocity PD; SD, standard deviation.
Of the patients included, 18 were categorized as HvPD, 22 as LvPD and 21 as DC. Figure 2 shows an example of HvPD and one of LvPD.
Figure 2.
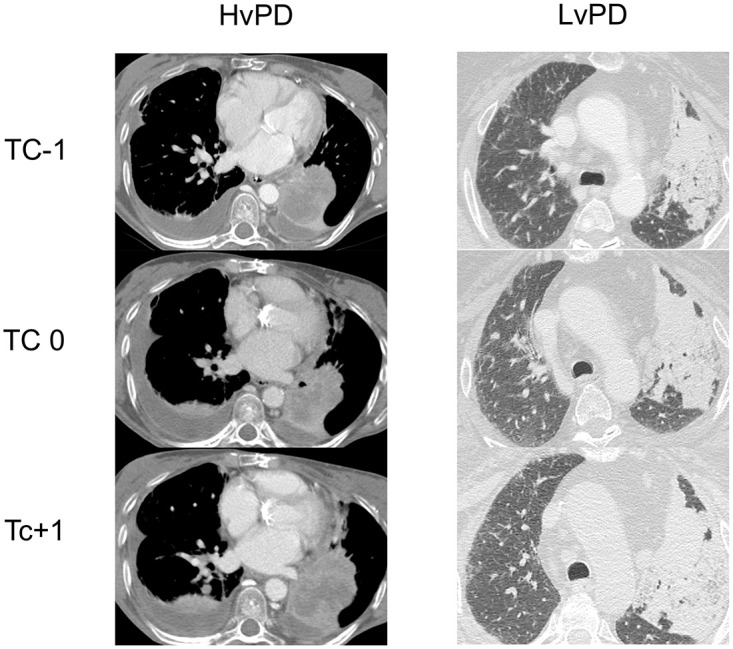
Example of a high velocity PD and a low velocity PD (LvPD); CT-1 was performed 8–12 weeks before ICI start (while the patient was on the treatment that preceded ICI) The CT performed at baseline for ICI was CT0 (within 6 weeks from ICI start), while CT + 1 was the first assessment after ICI start (8–12 weeks after ICI start).
Baseline characteristics were well balanced among the 3 categories, apart from ECOG PS 2 and the presence of bone metastasis, that were more common in HvPD group (p = 0.009 and p = 0.035, respectively), Table 1. In particular, Local Ablative Treatments were administered in 4/18 patients in HvPD group, in 2/22 in LvPD group and in 1/21 in DC group (p = 0.205).
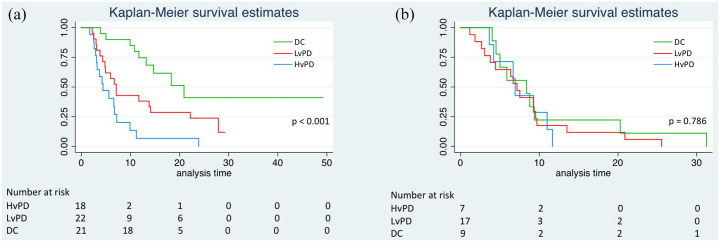
Median overall survival (mOS) was 4.4 months (95% CI: 2.0–6.8, reference) for HvPD, 7.1 months (95% CI: 5.4–8.8) for LvPD, HR 0.43 (95% CI: 0.21–0.87), p = 0.018, and 20.9 months (95% CI: 12.5–29.3) for DC group, HR 0.17 (95% CI: 0.07–0.39), p < 0.001. The difference between LvPD and DC group was also significant, HR 2.55 (95% CI: 1.13–5.74), p = 0.024 (Figure 3(a)).
Figure 3.
Kaplan Meyer curves of overall survival according to the HvPD, LvPD and DC categories in immunotherapy (a) and chemotherapy (b) groups.
Moreover, we analyzed the impact of treatment beyond progression both in HvPD and LvPD, finding no difference (HvPD, p = 0.207; LvPD p = 0.131). A control group of 33 patients who received second line standard chemotherapy and did never receive ICI was also studied. Of those, 21 received docetaxel, 9 pemetrexed and 3 gemcitabine. Of the patients included, 7 were categorized as HvPD, 17 as LvPD and 9 as DC.
Mean age was 61.1 years (SD = 7.4), 66% were men, 39% were current or former smoker, 30% and 39% had liver and bone metastasis respectively, 3% had an ECOG PS of 2 or more, 26% had dNLR ⩾ 3. Median baseline tumor size (BTS) was 65.4 cm3.
Baseline characteristics were well balanced among the categories (Table 2).
Table 2.
Clinic-pathological characteristics of the HvPD, LvPD and DC group in patients treated with chemotherapy.
| Age (mean, SD) | HvPD (n = 7) | LvPD (n = 17) | DC (n = 9) | All patients (n = 33) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 5 | 71.4% | 11 | 64.7% | 6 | 66.7% | 22 | 67% | 0.147 |
| Female | 2 | 28.6% | 6 | 35.3% | 3 | 33.3% | 11 | 33% | ||
| Smoking | Current | 0 | 0.0% | 1 | 5.9% | 1 | 11.1% | 2 | 6% | 0.69 |
| Former | 4 | 57.1% | 5 | 29.4% | 2 | 22.2% | 11 | 33% | ||
| Never | 1 | 14.3% | 4 | 23.5% | 2 | 22.2% | 7 | 21% | ||
| N/A | 2 | 28.6% | 7 | 41.2% | 4 | 44.4% | 13 | 40% | ||
| Liver metastasis | Yes | 2 | 28.6% | 3 | 17.6% | 3 | 33.3% | 8 | 24% | 0.22 |
| No | 5 | 71.4% | 14 | 82.4% | 6 | 66.7% | 25 | 76% | ||
| Bone metastasis | Yes | 3 | 42.9% | 6 | 35.3% | 4 | 44.4% | 13 | 39% | 0.535 |
| No | 5 | 71.4% | 11 | 64.7% | 5 | 55.6% | 21 | 61% | ||
| Ecog PS | 0–1 | 6 | 85.7% | 10 | 58.8% | 5 | 55.6% | 21 | 64% | 0.592 |
| 2 | 0 | 0.0% | 1 | 5.9% | 0 | 0.0% | 1 | 3% | ||
| NA | 1 | 14.3% | 6 | 35.3% | 4 | 44.4% | 11 | 33% | ||
DC, disease control; ECOG PS, ECOG performance status; HvPD, higher velocity PD; LvPD, lower velocity PD; SD, standard deviation.
All patients were deceased at the time of the analysis, therefore no median follow-up time was calculated. Median OS for the whole population in the control group was 6.90 months (95% CI: 5.72–8.08). Median OS was 6.90 months for HvPD (95% CI: 6.47–7.33), 6.70 months for LvPD (95% CI: 5.13–8.27), and 8.40 months for DC group (95% CI: 1.10–15.70). The difference among categories was non-significant (p = 0.786; Figure 3(b))
An interaction test for the differential effect of our model and chemotherapy vs immunotherapy was performed and resulted statistically significant (HR: 0.46; 95% CI: 0.25–0.86; p = 0.016) therefore supporting the specificity of our finding for immunotherapy.
To address the secondary aim of our study, we analyzed the correlation between TGR1 and OS, with the aim to explore the effect of the of the disease aggressiveness before ICI and the outcome of treatment. We found that there was no association between TGR1 as continuous variable and OS (HR 0.91; 95% CI: 0.62–1.33; p = 0.613). There was no difference also using median TGR1 to dichotomize (p = 0.668). Similarly, no difference was seen in chemotherapy control group (continuous variable: HR: 0.86; 95% CI: 0.57–1.29; p = 0.470).
Discussion
It is common experience that some patients under ICIs treatment seem to benefit from a prolonged disease stabilization or even a limited and slow disease progression. Consistently with this concept, it has become common practice to continue immunotherapy despite RECIST 1.1PD in cases where PD does not appear to be clinically relevant. Indeed, a number of papers evaluated the benefit of ICI treatment beyond progression (TBP), finding a better outcome for those patients who continued the treatment respect to those who discontinued.11,18 These data come from retrospective analysis and the decision on treating beyond progression was based on physician decision supported by iRECIST 1.1 criteria. In most clinical trial, TBP treatment could continue beyond confirmed PD where the patient display a clinical benefit and met the protocol-defined criteria for continuation, such as: absence of symptoms indicating unequivocal PD, no decline in performance status due to PD and absence of tumor progression at critical anatomical sites (e.g. leptomeningeal disease). 19
Despite these reports, the decision on whether continuing the treatment beyond the first CT scan in NSCLC patients treated with ICI is often challenging, in case of progression, due to the absence of a reliable and reproducible method to identify patients who could benefit from ICI continuation despite radiological PD.
The standard method to evaluate treatment efficacy is based on RECIST 1.1 criteria, which does not consider tumor growth characteristics and, notably, its pre-treatment component. Thus, the categorization of tumor response according to RECIST 1.1 criteria may not reflect the ability of an anticancer treatment to modify tumor growth by inducing some degree of tumor regression or by slowing down tumor growth. Furthermore, the velocity of tumor progression might be quite heterogeneous not only across tumor types, but also across patients suffering from the same malignancy, owing to different tumor biological characteristics.12,20,21
Previous studies reported the importance of adopting different response assessment methods beyond RECIST 1.1, especially for those malignancies where dimensional radiological criteria are not able to catch the real efficacy at the beginning of an antineoplastic treatment.22,23 TGR is a quantitative measure able to better reflect dynamic changes in cancer cells proliferation, capturing the ability of anticancer drug to affect tumor regression by slowing down tumor growth. It provides a dynamic and quantitative evaluation of tumor kinetics and, in addition, it allows standardizing inter-patients’ variability, each patient being its own control. Recent studies have addressed the issue of tumor kinetics evaluation to predict the clinical benefit from anti PD-1/anti PD-L1 agents. 21 In this context, Tumor Growth Rate (TGR) has been retrospectively exploited to demonstrate possible tumor growth acceleration after anti PD-L1/PD-1 onset 13 in some patients with so-called ‘Hyperprogressive disease’.
We sought to demonstrate that ICIs may slow down TGR, thus prolonging survival, even in the presence of a formal PD according to RECIST 1.1 categorization. In our model we hypothesized that cases of Progressive Disease at first follow-up CT scan could be divided in two groups, according to the slope of TGR curve. One group showing a deceleration of the TGR respect to the last chemotherapy treatment, and a second group with no deceleration. Finally, we used a chemotherapy-tread control group, to verify whether the possible survival benefit throughout a TGR slow-down was specific to immunotherapy or rather a more generalized phenomena emerging with any anticancer treatment. In addition, we worked on the hypothesis that pre-treatment TGR could predict the outcome of ICIs treatment, as it is common belief that rapidly growing and aggressive tumors may not respond well to immunotherapy
The results of our study, while refuting the prognostic role of pre-treatment tumor growth velocity, demonstrate that ICIs may yield a slow-down of TGR in patients with RECIST 1,1 PD which, in turns, leads to a survival improvement, thus supporting the hypothesis that a slope in growth rate could predict a better outcome over ICIs even in the case of progressive disease according to RECIST 1.1. Indeed, patients progressing over immunotherapy regimen according to RECIST 1.1 criteria, belonged to two groups that differ significantly in terms of prognosis. The LvPD group, included patients with slower disease progression from the last chemotherapy, while in the other group, named HvPD, the tumor growth rate did not slow down upon immunotherapy. The difference between LvPD group and HvPD suggests that a slow-down of the tumor growth curve could be itself a sign of activity, even in presence of a progression of disease according to RECIST 1.1 criteria. The effect was not due to oligo-progression with subsequent LAT and treatment continuation, as shown by the absence of difference in LAT in favor of LvPD (4/18 in HvPD, 2/22 in LvPD).
Moreover, this effect seems to be ICIs specific, as suggested by the absence of difference in the outcome of the three control group categories receiving single-agent chemotherapy.
An noteworthy and novel feature of our study is the adoption of a semi-automated method of volume assessment, that could help to capture the real variation is size, compared to unidimensional measurement, particularly at the first radiological assessement.24,25 Moreover, other papers showed how a semi-automatic volumetric analysis grants higher interobserver reproducibility,26,27 which allows for more accurate measurements, thus balancing the relatively low number of patients enrolled.
Another strength of our approach lies in the mathematical calculation of theoretical tumor volume right at the moment of treatment initiation. As our patients were treated within clinical practice, there was a difference in time between CT0 and treatment initiation and during this time the tumor is still growing.
Our results suggest that TGR measurement is also influential beside radiological assessment of response especially for those patients experiencing a progressive disease over ICIs administration.
This is of great value considering the importance of avoiding the discontinuation of a potentially useful treatment in the absence of a prompt response at first radiological assessment (treatment beyond progression). Not only but it may be helpful in avoiding the detrimental administration of a useless treatment that could also result in an acceleration of tumor growth, related to a poor ICI benefit in HPD specific pattern.
Finally, the absence of impact of TGR1 on OS suggests that the outcome of ICI administration is independent from the aggressiveness of the disease, for example, that a higher velocity of tumor progression after chemotherapy did not result in a worse survival.
Among the limitations of this work, we recognize that the retrospective nature of the study and the relatively low number of patients assessed could impair the strength of our findings. In addition, in our study, PD-L1 expression was not available for a high proportion of the patients (23/61). Moreover, TGR based on RECIST 1.1 criteria does not take into account the appearance of new lesions and, of course, patients died before having their first evaluation were not included as they did not have a CT + 1 (albeit this is a common limitation of all studies on radiological assessment criteria). Despite these limitations, we believe that our sample size and the strict methodology used are sufficient to support the hypothesis that ICIs may yield to a survival benefit in pre-treated advanced NSCLC, in some cases also by slowing down GR regardless of the type of RECIST 1.1 response.
In perspective, considering that ICIs is now an established frontline treatment of advanced NSCLC, we propose to now test our findings in a focused study on first line therapy. In particular, a TGR analysis could be of interest in a PD-L1 positive population (TPS ⩾ 50%), where it could allow to selectively start immunotherapy, and possibly add chemotherapy, only in patients experiencing HvPD, sparing unnecessary added toxicity in those with LvPD or DC. This could be challenging as patients treated in first line could not have pre-baseline CT scan, however novel techniques could be implemented in the study. For example, blood based serum tumor markers or ctDNA assessment are more accessible in ambulatory care and accumulating evidences are supporting their endorsement as valid surrogate markers of tumor burden. In addition, their fluctuation over time could be a useful parameter in treatment monitoring.28,29 Their assessment at diagnosis, treatment start and at the first evaluation could overcome the difficulties in TGR analysis in 1st line setting in the near future. Future analysis could also include PET scan derived parameters, such as metabolic tumor volume, that may have a role in evaluating response to ICI as well as they have in prediting response30,31 thanks to its ability of taking into account the whole tumor burden instead of the RECIST based target lesions.
Conclusion
In the presence of a radiological disease progression, the evaluation of tumor growth rate changes over ICI treatment could help physicians to establish which patients are gaining benefit from immunotherapy and should not, therefore, have a treatment withdraw. Our results highlight and support the monitoring of TGR as a tool for treatment response assessment and as a valuable aid to difficult clinical decisions in patients treated with ICIs. Prospective studies to assess whether TGR evaluation could be transferable in front line ICIs therapy of metastatic NSCLC are warranted, albeit the difficulties in assessing TGR1 in untreated patients.
Acknowledgments
We thank Giulia Manferrari for the editing work.
Footnotes
Author contributions: Filippo G. Dall’Olio: Conceptualization; Formal analysis; Methodology; Writing-original draft
Claudia Parisi: Data curation; Investigation; Writing-original draft
Laura Marcolin: Data curation; Investigation; Software
Stefano Brocchi: Conceptualization; Data curation; Methodology; Supervision; Writing-original draft
Caroline Caramella: Methodology; Supervision; Writing-review & editing
Nicole Conci: Data curation; Investigation
Giulia Carpani: Data curation; Investigation; Software
Francesco Gelsomino: Investigation; Writingreview & editing
Stefano Ardizzoni: Formal analysis; Methodology; Software
Paola Valeria Marchese: Data curation; Investigation
Alexandro Paccapelo: Formal analysis; Methodology
Giada Grilli: Data curation; Formal analysis
Rita Golfieri: Supervision; Writing-review & editing
Benjamin Besse: Supervision; Writing-review & editing
Andrea Ardizzoni: Conceptualization; Funding acquisition; Methodology; Supervision; Writingreview & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Caroline Caramella: has acted as a consultant of AstraZeneca, BMS, MSD, and Roche Benjamin Besse: has conducted research funded by Abbvie, Amgen, AstraZeneca, Biogen, Blueprint Medicines, BMS, Celgene, Eli Lilly, GSK, Ignyta, Ipsen, Merck, MSD, Nektar, Onxeo, Pfizer, Pharma Mar, Sanofi, Spectrum Pharmaceuticals, Takeda and Tiziana Pharm 4D Pharma, Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Inivata, Janssen, Onxeo, OSE immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, Tolero Pharmaceuticals. Andrea Ardizzoni: Grants from BMS and Celgene; Personal Fees from BMS, MSD, Eli Lilly, Boehringer, Pfizer and Celgene. The other authors declare no potential conflicts of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Filippo G. Dall’Olio  https://orcid.org/0000-0003-0903-6109
https://orcid.org/0000-0003-0903-6109
Francesco Gelsomino  https://orcid.org/0000-0002-9204-1728
https://orcid.org/0000-0002-9204-1728
Contributor Information
Filippo G. Dall’Olio, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Via Albertoni 15, 40138 Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, Policlinico di Sant’Orsola University Hospital, Bologna, Italy; Cancer Medicine Department, Gustave Roussy, Villejuif, France.
Claudia Parisi, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, Policlinico di Sant’Orsola University Hospital, Bologna, Italy; Cancer Medicine Department, Gustave Roussy, Villejuif, France.
Laura Marcolin, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Stefano Brocchi, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Caroline Caramella, Department of Radiology, Hôpital Marie Lannelongue, Le Plessis-Robinson, France.
Nicole Conci, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, Policlinico di Sant’Orsola University Hospital, Bologna, Italy.
Giulia Carpani, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Francesco Gelsomino, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Stefano Ardizzoni, Department of Engineering and Architecture, University of Parma, Parma, Italy.
Paola Valeria Marchese, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, Policlinico di Sant’Orsola University Hospital, Bologna, Italy.
Alexandro Paccapelo, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Giada Grilli, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Rita Golfieri, Department of Radiology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Benjamin Besse, Cancer Medicine Department, Gustave Roussy, Villejuif, France.
Andrea Ardizzoni, Division of Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine, Policlinico di Sant’Orsola University Hospital, Bologna, Italy.
References
- 1. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol; 2018; 29(Suppl. 4): iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 2. Dall’Olio FG, Conci N, Rossi G, et al. Comparison of sequential testing and next generation sequencing in advanced lung adenocarcinoma patients – a single centre experience. Lung Cancer 2020; 149: 5–9. [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herbst RS, Baas P, Kim D-WW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 7. Russo GL, Facchinetti F, Tiseo M, et al. Hyperprogressive disease upon immune checkpoint blockade: focus on non–small cell lung cancer. Curr Oncol Rep; 2020; 22: 41. [DOI] [PubMed] [Google Scholar]
- 8. Guaitoli G, Baldessari C, Bertolini F, et al. Are we ready to describe response or progression to immunotherapy in lung cancer? Crit Rev Oncol Hematol 2019; 138: 112–119. [DOI] [PubMed] [Google Scholar]
- 9. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216, http://www.ncbi.nlm.nih.gov/pubmed/10655437 (accessed 27 May 2017). [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline; (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 11. Won SE, Park HJ, Byun S, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology 2020; 9: 1776058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomez-Roca C, Koscielny S, Ribrag V, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer 2011; 47: 2512–2516. [DOI] [PubMed] [Google Scholar]
- 13. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 14. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res; 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017; 28: 1605–1611. [DOI] [PubMed] [Google Scholar]
- 16. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 2018; 4: 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim CG, Kim KH, Pyo K-H, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019; 30: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 18. Stinchcombe TE, Miksad RA, Gossai A, et al. Real-world outcomes for advanced non-small cell lung cancer patients treated with a PD-L1 inhibitor beyond progression. Clin Lung Cancer 2020; 21: 389–394.e3. [DOI] [PubMed] [Google Scholar]
- 19. Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol; 2018; 13: 1906–1918. [DOI] [PubMed] [Google Scholar]
- 20. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer; 2018; 88: 38–47. [DOI] [PubMed] [Google Scholar]
- 21. Ten Berge DMHJ, Hurkmans DP, den Besten I, et al. Tumour growth rate as a tool for response evaluation during PD-1 treatment for non-small cell lung cancer: a retrospective analysis. ERJ Open Res; 2019; 5: 00179-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schiavon G, Ruggiero A, Schöffski P, et al. Tumor volume as an alternative response measurement for imatinib treated GIST patients. PLoS ONE 2012; 7: e48372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishino M, Dahlberg SE, Cardarella S, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy. Cancer 2013; 119: 3761–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayes SA, Pietanza MC, O’Driscoll D, et al. Comparison of CT volumetric measurement with RECIST response in patients with lung cancer. Eur J Radiol 2016; 85: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Force J, Rajan A, Dombi E, et al. Assessment of objective responses using volumetric evaluation in advanced thymic malignancies and metastatic non-small cell lung cancer. J Thorac Oncol 2011; 6: 1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodman LR, Gulsun M, Washington L, et al. Inherent variability of CT lung nodule measurements in vivo using semiautomated volumetric measurements. Am J Roentgenol 2006; 186: 989–994. [DOI] [PubMed] [Google Scholar]
- 27. Dinkel J, Khalilzadeh O, Hintze C, et al. Inter-observer reproducibility of semi-automatic tumor diameter measurement and volumetric analysis in patients with lung cancer. Lung Cancer 2013; 82: 76–82. [DOI] [PubMed] [Google Scholar]
- 28. Dall’Olio FG, Abbati F, Facchinetti F, et al. CEA and CYFRA 21-1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther Adv Med Oncol 2020; 12: 1758835920952994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg SB, Narayan A, Kole AJ, et al. Assessing response to immunotherapy in patients with non-small cell lung cancer using circulating tumor DNA. Ann Oncol 2017; 28: v478. [Google Scholar]
- 30. Castello A, Rossi S, Mazziotti E, et al. Hyperprogressive disease in patients with non-small cell lung cancer treated with checkpoint inhibitors: the role of 18F-FDG PET/CT. J Nucl Med; 2020; 61: 821–826. [DOI] [PubMed] [Google Scholar]
- 31. Dall’Olio FG, Calabrò D, Conci N, et al. Baseline total metabolic tumour volume on 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non–small cell lung cancer treated with first-line pembrolizumab. Eur J Cancer 2021; 150: 99–107. [DOI] [PubMed] [Google Scholar]