Abstract
Introduction
Traditional cigarette use influences cost-benefit decision making by promoting impulsive choice. However, the impact of nicotine exposure via electronic nicotine delivery systems on impulsivity remains unclear. Hence, the present study examined the short- and long-term effects of nicotine vapor on impulsive choice.
Methods
Twenty-four adult male rats were trained in the delay discounting task to choose between small immediate food rewards and large delayed food rewards. After 24 days of training in the task rats were exposed to vapor containing either 0, 12, or 24 mg/mL of nicotine for 10 days. To validate inhalation of nicotine vapor serum cotinine levels were analyzed on exposure days 1, 5, and 10 using enzyme-linked immunosorbent assay. Following vapor exposure, rats were retrained in the discounting task until rats displayed stable responding and the effects of nicotine vapor on choice preference were assessed.
Results
Rats exposed to 12 and 24 mg/mL nicotine vapor displayed higher serum cotinine levels than control rats exposed to 0 mg/mL vapor. There were no differences in impulsive choice between any vapor exposure groups when tested 15 days after exposure, across 6 days of stable responding, suggesting that nicotine vapor does not have long lasting effects on impulsive choice. Interestingly, a subsequent nicotine vapor challenge revealed short-term increases in impulsive choice immediately following a single exposure to 24 mg/mL nicotine vapor, relative to choice preference immediately following exposure to 0 mg/mL vapor.
Conclusions
These results suggest that exposure to nicotine vapor causes immediate, short-term increases in impulsive choice.
Implications
E-cigarette use is increasing at an alarming rate, particularly among adolescents and young adults. This is concerning given the lack of research into the effects of nicotine vapor exposure on the brain and behavior. The present study describes a viable rodent model of human e-cigarette use and suggests that exposure to nicotine vapor produces short-term increases in impulsive choice.
Introduction
Nicotine vapor inhalation from electronic nicotine delivery systems, also known as e-cigarettes, is the preferred method for nicotine consumption among adolescents, with e-cigarette use increasing in this population by 19.3% from 2011 to 2018.1 This is concerning as both clinical and preclinical works suggest that repeated exposure to nicotine produces neurobiological adaptations that contribute to impaired cognitive control.2–5 Furthermore, recent studies suggest nicotine vapor inhalation from e-cigarettes may have detrimental effects on human health beyond those from traditional smoking.6,7 E-cigarettes expose users to different concentrations of nicotine, intake patterns, additive chemicals (eg, salt, flavorings), and chemical reaction products (eg, nicotyrine), all of which may enhance nicotine’s addictive properties and promote progression to conventional cigarette use in individuals with no history of smoking.7–12 Preclinical studies investigating the effects of nicotine vapor consumption on the brain and behavior are indeed limited, warranting the need for research into the psychopharmacological, neurobiological, and behavioral effects of nicotine vapor consumption.13,14
Clinical studies have documented both short- and long-term changes in cognitive function resulting from nicotine use in human smokers.15–17 For example, nicotine consumption from traditional cigarette use has been shown to increase impulsive choice.18–20 To examine impulsive choice, preclinical work has relied on the delay discounting task, where rodents can choose between a small immediate reward or a large delayed reward. While most rodent studies have shown nicotine-induced increase in preference for the small immediate reward (increased impulsive choice, like that seen in smokers), some have shown no effects on performance in the delay discounting task following injections of nicotine.21–23 Notably, most of these studies administered nicotine to rodents via injections, which ineffectively models nicotine inhalation in humans.
The lack of preclinical research on the effects of nicotine vapor on the brain and behavior may be due to the lack of validated nicotine vapor delivery systems designed for animal exposure. While efforts to develop novel rodent models of human e-cigarette use are underway, studies examining the effects of nicotine vapor exposure on impulsive choice have yet to be conducted. The current e-cigarette epidemic occurring in adolescents and young adults, along with the well-established increase in risky and impulsive choice observed in smokers, substantiates the need for preclinical research on the effects of nicotine vapor exposure on impulsive choice in these age groups. Here we describe and utilize a relatively novel rodent nicotine vapor delivery system14,24,25 to investigate the effects of nicotine vapor exposure during early adulthood on impulsive decision-making in rats.
Methods
Subjects
Male Sprague-Dawley rats (N = 24) 3 weeks of age were obtained from an outbred stock of animals (Envigo, Inc, Indianapolis, IN). The rats were paired-housed in a humidity- and temperature-controlled (22°C) vivarium on a reverse 12-hour light/dark cycle (lights off at 6:00 am and on at 6:00 pm) with ad libitum access to food and water, except when noted below. Cagemates consisted of outbred non-sibling rats. All animal procedures were performed in accordance with the University of Texas at El Paso animal care and use committee’s regulations.
Experimental Design
Before the start of the experiment, the rats were handled in the vivarium for 2 consecutive days. The experiment schedule (see Table 1) began with shaping procedures for the delay discounting task, which included magazine training, lever press training for a grain food pellet (left and right lever, counterbalanced), and nose-poke training, in that order, until a criteria of 30 responses in 1 hour was met by all rats for each of the shaping procedures. After shaping, the rats received 24 consecutive days of training in the delay discounting task (Training phase I). After initial training in the discounting task, separate groups of 10-week old rats (an age associated with early adulthood26) were exposed to 1 of 3 nicotine vapor concentrations (0, 12, or 24 mg/mL nicotine) for 10 days. The nicotine vapor concentrations and exposure protocols with rodents were based on established parameters used to model human nicotine consumption rates and serum cotinine levels resulting from daily e-cigarette use.9,27 To verify the delivery of nicotine in rats, blood collection procedures were conducted immediately following vapor exposure on days 1, 5, and 10 and blood serum levels of its metabolite, cotinine, were assessed. Rats resumed daily training in the delay discounting task (Training phase II) the day after nicotine vapor exposure was completed and were trained until criterion for stable responding was achieved across 6 consecutive days (long-term test). To examine the short-term effects of nicotine vapor exposure on impulsive choice, all animals were tested in the delay discounting task immediately following a 0 or 24 mg/mL nicotine vapor challenge (short-term test). One final test of choice preference was conducted 24 hours after the 24 mg/mL nicotine vapor challenge.
Table 1.
Experiment Schedule for Training, Testing, and Vapor Exposure
| Group | Training phase I DD 1–24 | 10 days vapor exposure | Training phase II DD 25–34 | Stability/long-term test DD 35–39 | Vapor challenge/ short-term test DD 41 | Vapor challenge/ short-term test DD 42 | 24 h Post exposure DD 43 |
|---|---|---|---|---|---|---|---|
| Age (PD) | 35–60 | 70–80 | 81–89 | 90–94 | 100 | 101 | 102 |
| 1 | No exposure | 0 mg/mL Nicotine | No exposure | No exposure | 0 mg/mL Nicotine | 24 mg/mL Nicotine | No exposure |
| 2 | No exposure | 12 mg/mL Nicotine | No exposure | No exposure | 0 mg/mL Nicotine | 24 mg/mL Nicotine | No exposure |
| 3 | No exposure | 24 mg/mL Nicotine | No exposure | No exposure | 0 mg/mL Nicotine | 24 mg/mL Nicotine | No exposure |
DD = delay discounting day, PD = postnatal day.
Nicotine Vapor Exposure
To model nicotine vapor exposure from e-cigarettes, rats received passive nicotine vapor exposure 5 days a week (Monday to Friday) using the Four Chamber Benchtop Passive E-Vape Inhalation System (La Jolla Alcohol Research Inc, La Jolla, CA). This system consisted of four sealed chambers (interior dimension of 14.5″ L × 10.5″ W × 9.0″ H), each with two valve ports. One valve port was connected to a small vacuum that controlled the airflow in the chamber at 0.6 L per minute. The vacuum outlet was connected to a Whatman HEPA filter (Millipore Sigma, Darmstadt, Germany) and onto a house exhaust that safely removed the nicotine vapor from the chambers and outside the testing room. The other valve port was connected via PVC tubing to a TFV4 mini-tank (4.9 V, 65.0 W; Smok Inc, Shenzhen, China), where the nicotine e-liquid was heated by a 0.42 Ω atomizer coil.
At 10 weeks of age rats were exposed to either 0, 12, or 24 mg/mL nicotine vapor for 10 consecutive days, with 2 days off between days 5 and 6 of vapor exposure.26 Two rats were exposed per chamber (cagemates) for a total of eight rats per exposure session, with each nicotine vapor concentration group presented in separate sessions each day. Since all four chambers were connected to one minitank, all eight rats in each session were exposed to the same nicotine concentration. To minimize the possibility of higher concentrations contaminating lower concentrations, groups of eight rats were exposed to 0, 12, or 24 mg/mL nicotine concentration in sessions 1, 2, and 3, respectively. Separate PVC tubing and minitanks were used for each concentration of nicotine vapor, and chambers were cleaned after every exposure. The present study used flavorless nicotine e-liquids containing nicotine in its freebase form in 50/50 vegetable glycerin/propylene glycol vehicle. All nicotine e-liquids were purchased from the commercial vendor Vapor Chef (VC Tobacco #13; Bristol, PA).
Exposure parameters were chosen based on pilot studies we conducted suggesting these approximate cotinine levels following electronic cigarettes use in humans, based on a review of the clinical literature.28,29 The rats were exposed to vapor in daily 90-minute sessions consisting of four cycles, with 5-minute inter-cycle intervals. For each cycle nicotine e-liquid was heated to 400°F for a 3-second puff delivery, occurring every 2 minutes and 10 times per cycle, for a total of 40 puff deliveries per day and a cycle duration of 18 minutes and 30 seconds.
Assessment of Cotinine Levels
On exposure days 1, 5, and 10, the rats were briefly anesthetized using an isoflurane/oxygen mixture (1%–3% isoflurane) and received a nick on the end of the tail with a sterile scalpel blade. Blood was collected from the tail in sterile 1 mL Eppendorf tubes and placed on ice. Blood collection from all rats was done at least 15 minutes after removal from vapor. The blood was then centrifuged for 15 minutes at 5000g at 4°C. Serum was analyzed for cotinine using an enzyme-linked immunosorbent assay (catalog no. CO096D-100; Cal Biotech Inc, El Cajon, CA) conducted according to manufacturer instructions. Enzyme-linked immunosorbent assay plates were measured using a standard laboratory plate reader (Spectra Max PLUS 384, Molecular Devices) and the results were analyzed using the Soft Max Pro software (version 5.4).
Delay Discounting Task
For the delay discounting task, the present study utilized eight standard operant chambers and procedures previously described in our work.30,31 All left and right lever reward assignments and presentations were randomly assigned and counterbalanced during all training and testing sessions. Five days before the delay discounting procedures began, rats were food restricted to 90% of their free-feeding body weight. Subsequent feeding was only allowed for 2 hours per day after behavioral testing was completed for the day. The task began with rats receiving two 45-minute sessions of magazine training consisting of 16 to 22 deliveries of a 45 mg food pellet with an average delivery interval of 2 minutes. This was followed by four consecutive 1 hour sessions of lever press training, where rats were presented with a single lever (2 days with left lever and 2 days with right lever) and allowed to press for a food pellet under a fixed ratio 1 schedule of reinforcement (1 pellet delivery for every lever press). Upon the completion of lever press training (30 presses within the 1 hour sessions), rats were trained to nose poke into an illuminated food trough, after which the 1.12 W trough light was turned off and a single lever (left or right) was presented. When the rats pressed on a lever two food pellets were delivered and the lever was retracted.
Daily delay discounting sessions consisted of five blocks, each with four forced-choice trials (only one lever presented) followed by six free-choice trials (two levers presented at the same time) and lasted 50 minutes. Individual trials began with the presentation of a light cue (food trough light), which was extinguished when the rat entered the food trough, initiating lever extensions. Pressing one of the two levers (left or right of the food cup) resulted in the immediate delivery of a small reward (one grain food pellet), whereas pressing the other lever resulted in the delayed delivery of a large reward (four food pellets) with a determined delay for delivery. A 10-second response window was given for both nose poke and lever press responses. Trials in which rats failed to nose poke or lever press during the 10-second window were scored as omissions and a new trial began. To control for differential effects that have been reported to occur when presenting ascending versus descending delays to the large reward delivery,32 half of the rats in each treatment group had delays to the large reward delivery that increased across session blocks (0, 4, 8, 16, 32 seconds), while the other half of the rats in each group had delays to the large reward delivery that decreased across session blocks (32, 16, 8, 4, 0 seconds, counterbalanced). Both levers were retracted once either lever was pressed or the 10-second response window elapsed. All trials, regardless of the delay or omissions, were set to a fixed duration. Therefore, performance in the task did not affect rate of progress through the trials and the large delayed reward was objectively the “optimal” choice for maximizing food delivery. Performance in this task was not assessed until a stable discounting curve, averaged across all rats, was observed across 6 consecutive days. A stable discounting curve was achieved when a statistically significant effect of delay was obtained (suggesting that rats shifted their choice appropriately based on the delay to the large reward), with no effect of daily training sessions (suggesting that the rate of the shift does not change across training sessions).
Statistical Analysis
Choice preference in delayed reward was compared using a mixed model two-way analysis of variance (ANOVA) with group (0, 12, or 24 mg/mL nicotine) as a between-subject factor and delay or training day as within-subject factors. T tests and Bonferroni corrected alpha levels were used as post hoc analyses. Finally, partial eta squared and Cohen’s d were used to determine effect sizes.
Results
Training in the Delay Discounting Task
Rats were trained in the delay discounting task for 24 days before nicotine vapor exposure. A mixed model ANOVA revealed no main effects of, or interactions with, choice preference across the 5 delay discounting days (DD) before nicotine vapor exposure (training days 20–24; Fs < 1.39, ps > .08). Following vapor exposure, rats continued to train in the delay discounting task for an additional 10 days before 6 days of stable task performance was achieved across all rats. No main effects or interactions with nicotine treatment group were observed across these 10 posttreatment training days (DD 25–34, Fs < 1.12, ps > .24), suggesting that nicotine vapor exposure does not affect learning in the delay discounting task. One-way ANOVAs on the 5 delay discounting training days preceding vapor exposure and on the 10 training days after vapor exposure revealed no differences in discounting task performance between the 10-day nicotine vapor exposure groups (Fs < 2.39, ps > .12). Criteria for stable responding in the delay discounting task was met across DD 35 to 40 and was determined by an observed main effect of delay (F(4,80) = 81.91, p < .001, ηp2 = 0.80), without a main effect of day (F(5,100) = 0.79, p = .56).
Serum Cotinine
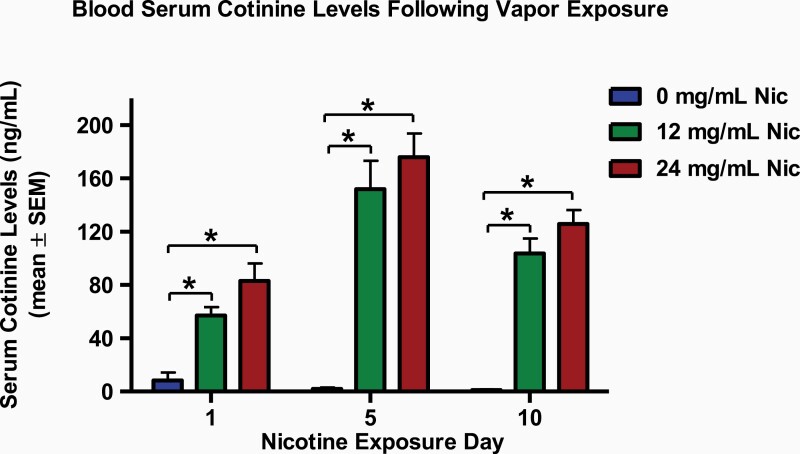
Blood serum cotinine levels on vapor exposure days 1, 5, and 10 were analyzed in a mixed model ANOVA with nicotine vapor group as a between-subject factor and blood collection day as a within-subject factor (Figure 1). Analysis revealed a main effect of nicotine vapor group (F(2,21) = 81.10, p < .001, ηp2 = 0.89) and blood collection day (F(2,42) = 21.35, p < .001, ηp2 = 0.51), as well as an interaction of nicotine vapor group and day (F(4,42) = 6.51, p < .01, ηp2 = 0.38). Bonferroni post hoc analyses were conducted and revealed a difference between the 0 mg/mL nicotine control groups and both the 12 and 24 mg/mL nicotine groups, across all 3 timepoints (ps< .001, Cohen’s d > 3.81). No differences between the 12 mg/mL and 24 mg/mL nicotine treatment groups were observed for the cotinine analysis timepoints (p = .09). Together, these findings suggest that passive exposure to 12 and 24 mg/mL nicotine vapor similarly increase serum cotinine levels, relative to passive exposure to 0 mg/mL nicotine vapor.
Figure 1.
Blood serum cotinine levels of rats exposed to nicotine or vehicle vapors on exposure days 1, 5, and 10. The rats that were exposed to the 12 mg/mL nicotine vapor (12 mg/mL Nic, green bars) and 24 mg/mL nicotine vapor (24 mg/mL Nic, red bars) displayed an increase in serum cotinine levels when compared with the control rats that were exposed to 0 mg/mL nicotine vapor (0 mg/mL Nic, blue bars). No significant differences were observed between the 12 and 24 mg/mL nicotine treatment groups. Data are presented as mean ± SEM. Asterisk (*) indicates a significant difference between the 0 and 12 or 24 mg/mL nicotine vapor treatment groups. Critical p value is .05.
Long-Term Effects of Nicotine Vapor Exposure on Impulsive Choice
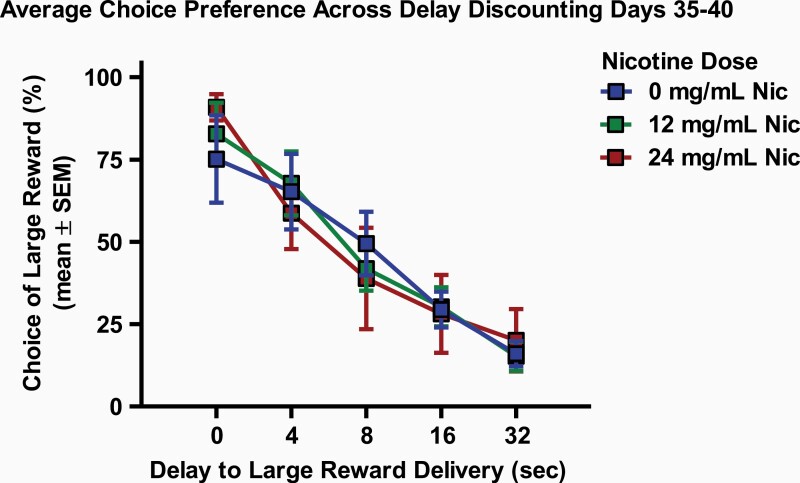
To assess the long-term effects of repeated nicotine vapor exposure on choice preference in the delay discounting task, performance on the delay discounting task was compared between the 10-day vapor treatment groups on each of the 6 training days in which rats showed stable responding (DD 35–40, beginning 15 days after exposure), as well as the average performance across these 6 days (Figure 2). A main effect of delay was observed on the averaged discounting curve from days 35 to 40 (F(4,84) = 79.96, p < .001, ηp2 = 0.88); however, no interaction of delay and nicotine group (F(8,84) = 1.17, p = .33) or main effect of nicotine group (F(2,21) = 0.001, p = .99) were observed. A repeated-measures ANOVA revealed a marginal interaction between 10-day treatment group and delay to large reward delivery on training day 37 (F(8,84) = 2.02, p = .05, ηp2 = 0.16). No other interactions or main effects of nicotine vapor exposure were observed on any of these 6 individual DD or the average of these 6 days (Fs < 1.58, ps > .14). Post hoc analysis revealed no differences between nicotine vapor groups on any of these test days (ps > .37).
Figure 2.
Long-term effects of nicotine vapor exposure on average choice preference across 6 days of stable responding. Rats showed no significant long-term effects on impulsive choice 15 days after a 10-day exposure to 0 mg/mL nicotine vapor (0 mg/mL Nic, blue squares), 12 mg/mL nicotine vapor (12 mg/mL Nic, green squares), or 24 mg/mL nicotine vapor (24 mg/mL Nic, red squares). Impulsive choice was averaged across 6 days of stable responding in the delay discounting task (delay discounting days 35–40) and data are presented as mean ± SEM.
Short-Term Effects of Nicotine Vapor Exposure on Impulsive Choice
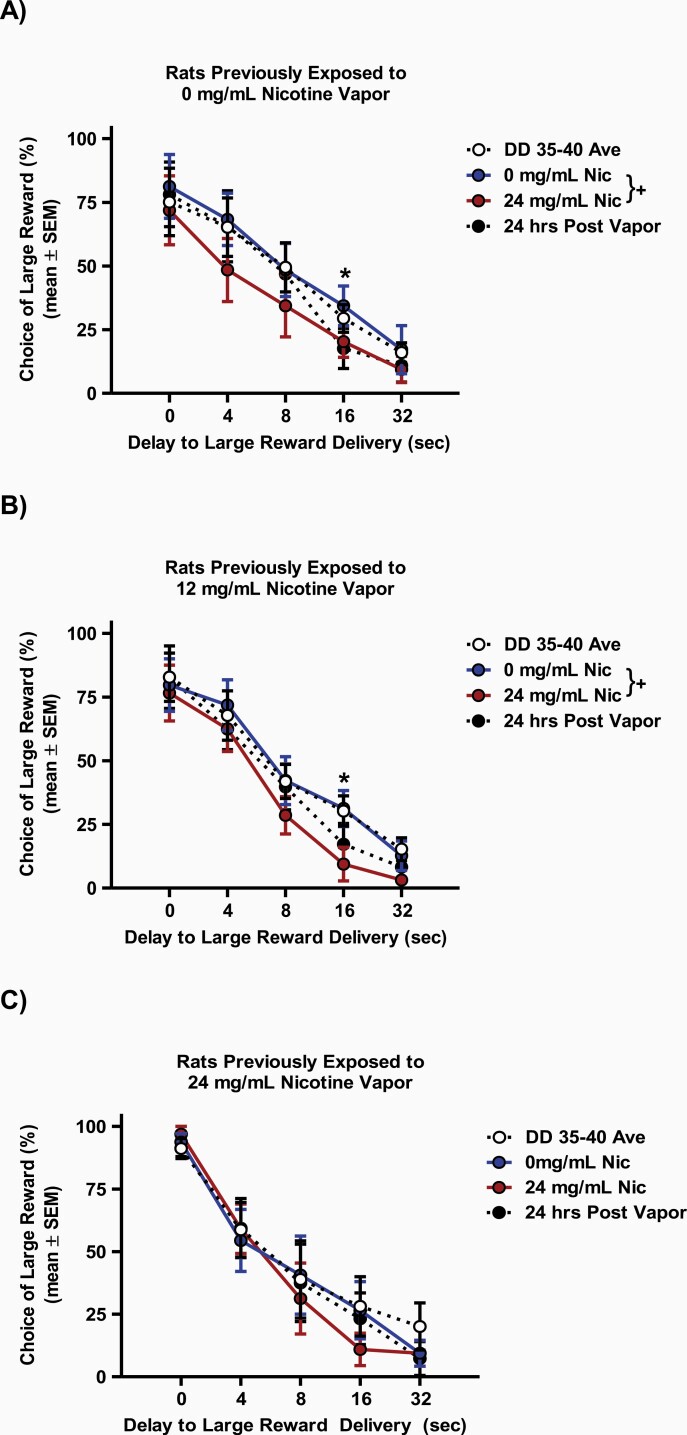
Following analysis of the long-term effects of nicotine vapor exposure, the short-term effects of a passive nicotine vapor exposure were assessed separately in each 10-day vapor treatment group, immediately following a 0 mg/mL (DD 41) and 24 mg/mL (DD 42) nicotine vapor challenge. No differences were observed in any 10-day treatment groups when using repeated-measures ANOVA to compare choice preference on the last day of stable responding (DD 40) to choice preference immediately following the 0 mg/mL nicotine vapor challenge (Fs < 0.38, ps > .56). However, a repeated-measures ANOVA comparing choice preference immediately following the 0 mg/mL nicotine vapor challenge to choice preference immediately following the 24 mg/mL nicotine vapor challenge revealed a main effect of treatment day (F(1,7) = 6.57, p < .05, ηp2 = 0.48) and delay (F(4,28) = 22.63, p = .001, ηp2 = 0.76) in the group that previously received 0 mg/mL nicotine vapor for 10 days (Figure 3A). No interaction of day and delay to large reward delivery (F(4,28) = 0.31, p = .87) was observed in this group. This finding suggests that a single exposure to 24 mg/mL nicotine vapor can cause immediate increase in impulsive choice. Post hoc analysis further revealed that significant increases in impulsive choice in this group, following the 24 mg/mL nicotine vapor challenge, occurred during the 16 seconds delay to delivery of the large reward (t(7) = 3.21, p = .01, Cohen’s d = 0.71).
Figure 3.
Short-term effects of nicotine vapor challenge on choice preference in rats previously treated with 10 days of (A) 0, (B) 12, or (C) 24 mg/mL of nicotine vapor. Rats previously exposed to 0 and 12 mg/mL nicotine vapor demonstrated increased impulsive choice (decreased choice of the large delayed reward). Data shown include choice preference averaged across delay discounting training days 35–40 (DD 35–40 Ave, white circles), immediately after 0 mg/mL nicotine vapor challenge (0 mg/mL Nic, blue circles), immediately after 24 mg/mL nicotine vapor challenge (24 mg/mL Nic, red circles), and 24 hours after 24 mg/mL nicotine vapor challenge (24 hours post vapor, black circles). Data are presented as mean ± SEM. Plus sign (+) indicates a significant difference in delay discounting between the 0 and 24 mg/mL nicotine vapor challenge days, while asterisk (*) indicates significant difference on delay discounting between the 0 and 24 mg/mL nicotine vapor on identified delay block. Critical p value is .05.
When comparing immediate effects of 0 and 24 mg/mL nicotine vapor in rats with prior 10-day exposure to 12 (Figure 3B) and 24 (Figure 3C) mg/mL nicotine vapor, repeated-measures ANOVA revealed a main effect of delay (Fs > 32.79, p < .001, ηp2 = 0.82), but no interaction of treatment day and delay (Fs< 1.24, ps> .32) in either group. Interestingly, there was a main effect of nicotine vapor challenge day in the group with previous exposure to 10 days of 12 mg/mL nicotine vapor (F(1,7) = 8.33, p < .05, ηp2 = 0.54, Figure 3B), but not in the group with previous exposure to 10 days of 24 mg/mL nicotine vapor (F(1,7) = 0.32, p < .59, Figure 3C). Post hoc analyses were conducted to characterize the main effect of challenge day observed in the 12 mg/mL nicotine group and revealed that the 24 mg/mL nicotine vapor challenge caused immediate increase in choice for the small immediate reward during the 16 seconds delay (t(7) = 3.33, p = .01, Cohen’s d = 1.13).
To assess the persistence of the observed increase in impulsive choice following a single nicotine vapor exposure, a separate repeated-measures ANOVA was run comparing choice preference immediately following the 0 mg/mL nicotine vapor challenge to choice preference 24 hours after the 24 mg/mL nicotine vapor challenge (DD 41). No significant effects of day were observed in any of the 10-day treatment groups 24 hours after the 24 mg/mL nicotine vapor challenge (Fs< 2.42, ps> 0.16), suggesting that the observed immediate increases in impulsive choice following exposure to 24 mg/mL nicotine vapor are not long-lasting.
Discussion
The present study used a relatively novel rodent e-cigarette delivery system27 to assess the effects of passive nicotine vapor exposure on serum cotinine levels and impulsive choice in rats. Our findings indicate that passive exposure to nicotine vapor using our rodent vapor exposure system significantly increases serum cotinine levels. Furthermore, our analysis demonstrates that passive nicotine vapor exposure can cause short-term increases in impulsive choice. Together, this work describes a viable rodent model of human e-cigarette use and suggests that exposure to nicotine vapor may affect cost-benefit decision making immediately after nicotine vapor exposure, similar to the effects observed following traditional cigarette use.
Nicotine vapor exposure increased blood serum cotinine levels in rats exposed to 12 and 24 mg/mL nicotine vapor, relative to 0 mg/mL vapor controls, on all exposure days assessed. In the present study, we observed higher cotinine levels when compared with recently published studies investigating cotinine levels in rodents exposed to nicotine vapor. For example, a recent study from the Gilpin laboratory25 observed cotinine levels in the range of 10–20 ng/mL using the 20 ng/mL dose. Similarly, Javadi-Paydar et al.14 found cotinine levels below 50 ng/mL following exposure to vapor from an e-liquid containing a 30 ng/mL nicotine concentration. These discrepancies in cotinine levels may be explained by the different experimental conditions under which nicotine vapor was generated. For example, the present study adopted a lower vacuum flow rate (0.6 L per minute) and atomizer resistance (0.24 Ω), higher wattage (4.9 V), and more nicotine puffs per session (40). Together these differences can affect the vaporization efficiency of nicotine e-liquid, resulting in a slower rate of nicotine clearance from the chamber, while more nicotine vapor is being generated. Interestingly, cotinine levels in our study appeared to increase in the 12 and 24 mg/mL nicotine vapor groups between exposure days 1 and 5 and decrease between exposure days 5 and 10. This pattern of changes in cotinine is consistent with those observed in another report that examined cotinine levels 7, 10, or 14 days after nicotine osmotic pump implantation in rats.25,33 We suggest that this effect may be because of nicotine exposure decreasing enzymes that metabolize nicotine. Indeed, previous work has shown that nicotine can decrease hepatic CYP2A6.34 Future work is needed to understand the effects of nicotine vapor and its constituents on nicotine metabolism.
Cotinine levels did not differ between 12 and 24 mg/mL nicotine vapor groups; however, when compared to the 12 mg/mL, the 24 mg/mL nicotine group did show a trend for higher serum cotinine levels. Surprisingly, the 0 mg/mL nicotine group displayed trace amounts of cotinine. We suggest that contamination or third-hand nicotine vapor exposure may have occurred, despite our efforts to avoid this. Indeed, other groups have also reported trace cotinine levels (under 10 ng/mL) in rodents exposed to commercially available 0 mg/mL nicotine solutions,13 and third-hand nicotine exposure has been reported in humans when exposed to environments where nicotine vapor had been consumed.35 However, it is also possible that trace amounts of cotinine in control samples are overestimated by technical factors related to detecting near zero values using enzyme-linked immunosorbent assay procedures, including limitations associated with detection levels, plate reader, and/or timing of procedures. Future studies employing nicotine vapor procedures should aim to assess the presence of nicotine in commercially available “0 mg/mL” nicotine liquids or develop their own nicotine liquids in the laboratory. Additionally, to avoid possible third-hand exposure, animal models of nicotine vapor should utilize separate vapor exposure chambers when exposing control groups to vehicles. Finally, the validation of a response-contingent vapor administration system will be an important development for rodent models of human e-cigarette use, as research has shown that passive and self-administration of drugs of abuse can result in different biological and behavioral outcomes.36,37
In the present study, we sought to test for long-lasting effects of nicotine vapor exposure on impulsive choice. Thus, we investigated the effects of nicotine vapor on impulsive choice 15 days after repeated exposure. The results revealed that chronic nicotine vapor exposure did not produce long-term effects on impulsive choice. This finding is in line with previous studies showing that nicotine injections do not produce long-term increases in impulsive choice in rats.21,22 Research with humans has also suggested that immediate shifts in choice preference following nicotine use may not be long-lasting.18,38 While the present study investigated the long-term effects of repeated nicotine vapor exposure on impulsive choice, future research will need to investigate the choice preference effects of repeated nicotine vapor exposure within the first 15 days after repeated exposure.
Analysis of impulsive choice immediately following a single exposure to 24 mg/mL nicotine vapor revealed a shift in choice preference towards the small immediate reward (increase in impulsive choice) in rats with no history of nicotine vapor exposure (ie, rats previously exposed to 10 days of 0 mg/mL nicotine vapor). Interestingly, a similar immediate effect of acute nicotine vapor exposure on impulsive choice was seen in rats exposed to 10 days of 12, but not 24, mg/mL nicotine vapor. It is important to note that significant effects of nicotine vapor on impulsive choice were limited to the center of the discounting curve. This is consistent with previous work from our lab showing that the effects of drugs of abuse on discounting curves are often maximized at the center of the curve, where the optimal choice can be relatively less evident.23,30 A similar preference was observed immediately following exposure to 0 or 24 mg/mL nicotine vapor when there was no delay in the delivery of the larger four pellet reward (0 seconds delay block), suggesting that nicotine vapor exposure does not affect the ability to perceive differences in reward magnitude. The immediate effects of exposure to 0 or 24 mg/mL nicotine vapor on choice were also comparable during the longest delay to large reward delivery (32 seconds delay block), indicating that nicotine vapor exposure does not affect the ability to bridge temporal gaps for action-outcome contingencies. Finally, no significant effects on choice preference were seen 24 hours after a single exposure to 24 mg/mL nicotine vapor, suggesting that immediate effects of nicotine vapor on impulsive choice are transient. Together, our findings suggest that while significant increases in impulsive choice can occur immediately following e-cigarette use, these effects may be limited to the hours following nicotine vapor exposure.
The present study only included male subjects. However, it should be noted that sex differences in the behavioral effects of nicotine are prevalent in the literature. For example, females display greater intravenous nicotine self-administration as well as anxiety-like behavior during nicotine withdrawal.39–41 Females also accumulate greater levels of nicotine in the brain faster42 while metabolizing nicotine slower than males.43 Also, recent work examining sex differences in nicotine-induced impulsive choice suggests that females display increase in impulsive choice at lower doses of nicotine as compared with males.44 Future studies are needed to elucidate sex differences in the behavioral effects of nicotine vapor exposure. Lastly, notable strain differences to the effects of nicotine have been documented, warranting the study of strain differences in the behavioral effects of nicotine vapor (for a review see,45–47).
Summary and Conclusions
Previous work in rodent models using nicotine liquid injections or minipumps have reported short- and long-term increases in impulsive choice.22,48 Our study vertically extends prior work by utilizing novel nicotine vapor inhalation in rodents that models e-cigarette use in humans. The present study suggests that exposure to nicotine vapor produces immediate changes in impulsive choice in rats and that these effects are not long lasting. This suggests that the direct effects of nicotine have the potential to alter impulsive choices during e-cigarette use. The data also suggests that there are no long-term residual effects on impulsive choice following e-cigarette use. These findings are particularly relevant for adolescents and young adults who display increase in impulsivity, relative to older adults.49 Overall, the present study contributes the viability of the described vaping system as a valid rodent model of human e-cigarette use and highlights a need for future studies to further characterize the effects of nicotine vapor exposure on the brain and behavior.
Supplementary Material
Acknowledgments
We would like to thank Michelle Martinez, Tania Miramontes, Melissa Ibarra, and Gabriel Frietze for their assistance and technical support on this project.
Declaration of Interests
All authors have contributed to the manuscript and approve the final submission. The authors report no conflicts of interest.
Funding
This work was supported by the National Institute on Drug Abuse, grant numbers R01-DA021274 and SC2-DA052119, as well as by the University of Texas at El Paso School of Pharmacy.
References
- 1. Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: Tobacco product use among middle and high school students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2019;68(6):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aşçioglu M, Dolu N, Gölgeli A, Süer C, Ozesmi C. Effects of cigarette smoking on cognitive processing. Int J Neurosci. 2004;114(3):381–390. [DOI] [PubMed] [Google Scholar]
- 3. Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–1470. [DOI] [PubMed] [Google Scholar]
- 4. Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53(4):606–617. [DOI] [PubMed] [Google Scholar]
- 5. Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callahan-Lyon P. Electronic cigarettes: Human health effects. Tob Control. 2014;23(Suppl 2):ii36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L193–L206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall BJ, Wells C, Allenby C, et al. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacol Biochem Behav. 2014;120:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapor inhalation escalates nicotine self-administration. Addict Biol. 2014;19(4):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among US adolescents and young adults. JAMA Pediatr. 2015;169(11):1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS One. 2018;13(1):e0189015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith D, Aherrera A, Lopez A, et al. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One. 2015;10(9):e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, Taffe MA. Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug Alcohol Depend. 2019;198:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geist CR, Herrmann SM. A comparison of the psychological characteristics of smokers, ex-smokers, and nonsmokers. J Clin Psychol. 1990;46(1):102–105. [DOI] [PubMed] [Google Scholar]
- 16. Havermans RC, Debaere S, Smulders FT, Wiers RW, Jansen AT. Effect of cue exposure, urge to smoke, and nicotine deprivation on cognitive performance in smokers. Psychol Addict Behav. 2003;17(4):336–339. [DOI] [PubMed] [Google Scholar]
- 17. Sakurai Y, Kanazawa I. Acute effects of cigarettes in non-deprived smokers on memory, calculation and executive functions. Hum Psychopharmacol. 2002;17(7):369–373. [DOI] [PubMed] [Google Scholar]
- 18. Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. [DOI] [PubMed] [Google Scholar]
- 19. Chivers LL, Hand DJ, Priest JS, Higgins ST. E-cigarette use among women of reproductive age: Impulsivity, cigarette smoking status, and other risk factors. Prev Med. 2016;92:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3(4):261–275. [DOI] [PubMed] [Google Scholar]
- 21. Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010;21(8):754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16(1):15–23. [DOI] [PubMed] [Google Scholar]
- 23. Mendez IA, Gilbert RJ, Bizon JL, Setlow B. Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology. 2012;224(4):489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miliano C, Scott ER, Murdaugh LB, et al. Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems. J Neurosci Methods. 2020;330:108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Montanari C, Kelley LK, Kerr TM, Cole M, Gilpin NW. Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharmacology (Berl). 2020;237(3):613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sengupta P. The laboratory rat: Relating its age with human’s. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 27. Qasim H, Karim ZA, Silva-Espinoza JC, et al. Short-term e-cigarette exposure increases the risk of thrombogenesis and enhances platelet function in mice. J Am Heart Assoc. 2018;7(15):e009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee WH, Ong SG, Zhou Y, et al. Modeling cardiovascular risks of e-cigarettes with human-induced pluripotent stem cell-derived endothelial cells. J Am Coll Cardiol. 2019;73(21):2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marsot A, Simon N. Nicotine and cotinine levels with electronic cigarette: A review. Int J Toxicol. 2016;35(2):179–185. [DOI] [PubMed] [Google Scholar]
- 30. Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121(3):543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mendez IA, Simon NW, Hart N, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: Interactions with order of delay presentation. Psychopharmacology. 2014;231(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Torres OV, Gentil LG, Natividad LA, Carcoba LM, O’Dell LE. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front Psychiatry. 2013;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferguson CS, Miksys S, Palmour RM, Tyndale RF. Differential effects of nicotine treatment and ethanol self-administration on CYP2A6, CYP2B6 and nicotine pharmacokinetics in African green monkeys. J Pharmacol Exp Ther. 2012;343(3):628–637. [DOI] [PubMed] [Google Scholar]
- 35. Bush D, Goniewicz ML. A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int J Drug Policy. 2015;26(6):609–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24(11):566–573. [DOI] [PubMed] [Google Scholar]
- 37. Metaxas A, Bailey A, Barbano MF, Galeote L, Maldonado R, Kitchen I. Differential region-specific regulation of α4β2* nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice. Addict Biol. 2010;15(4):464–479. [DOI] [PubMed] [Google Scholar]
- 38. Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146(4):455–464. [DOI] [PubMed] [Google Scholar]
- 39. Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. [DOI] [PubMed] [Google Scholar]
- 40. Flores RJ, Uribe KP, Swalve N, O’Dell LE. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol Behav. 2019;203:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flores RJ, Cruz B, Uribe KP, et al. Estradiol promotes and progesterone reduces anxiety-like behavior produced by nicotine withdrawal in female rats. Psychoneuroendocrinology. 2020;119:104694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11(6):863–870. [DOI] [PubMed] [Google Scholar]
- 43. Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES. Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos. 1988;16(6):823–828. [PubMed] [Google Scholar]
- 44. Íbias J, Nazarian A. Sex differences in nicotine-induced impulsivity and its reversal with bupropion in rats. J Psychopharmacol. 2020;34(12):1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collins AC, Pogun S, Nesil T, Kanit L. Oral nicotine self-administration in rodents. J Addict Res Ther. 2012;S2:004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Overstreet DH. Differential effects of nicotine in inbred and selectively bred rodents. Behav Genet. 1995;25(2):179–185. [DOI] [PubMed] [Google Scholar]
- 47. Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129(1):35–43. [DOI] [PubMed] [Google Scholar]
- 48. Kayir H, Semenova S, Markou A. Baseline impulsive choice predicts the effects of nicotine and nicotine withdrawal on impulsivity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:6–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behav Neurosci. 2012;126(5):735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.