Abstract
The COVID-19 pandemic has profound adverse effects on the population on dialysis. Patients requiring dialysis are at an increased risk of SARS-CoV-2 infection and mortality, and many have experienced psychological distress as well as delayed or suboptimal care. COVID-19 survivors have prolonged viral shedding, but generally develop a robust and long-lasting humoral immune response that correlates with initial disease severity. However, protection against reinfection is incomplete. A growing body of evidence reveals delayed and blunted immune responses to SARS-CoV-2 vaccination. Administration of a third dose within 1 to 2 months of prime-boost vaccination significantly increases antibody levels, in particular in patients with poor initial responses. Patients on dialysis have inferior immune responses to adenoviral vector vaccines than to mRNA vaccines. The immunogenicity of the mRNA-1273 vaccine is markedly better than that of the BNT162b2 vaccine, most likely by virtue of its higher mRNA content. Despite suboptimal immune responses in patients on dialysis, preliminary data suggest that vaccination partially protects against infection and severe disease requiring hospitalization. However, progressive waning of immunity and emergence of SARS-CoV-2 variants with a high potential of immune escape call for a booster dose in all patients on dialysis 4 to 6 months after prime-boost vaccination. Patients with persistent poor vaccine responses may be candidates for primary prophylaxis strategies. In the absence of specific data in patients on dialysis, therapeutic strategies in the event of established COVID-19 must be extrapolated from evidence obtained in the population not on dialysis. Neutralizing monoclonal antibodies may be an attractive option after a high-risk exposure or during the early course of infection.
Keywords: COVID-19, dialysis, hemodialysis, immune response, SARS-CoV-2, treatment, vaccination
Numerous large multicenter studies from across the globe have documented high short-term case fatality rates of coronavirus disease 2019 (COVID-19) in patients on dialysis, ranging between 20% and 30%.1, 2, 3, 4, 5, 6 Population-based studies suggest a 4-fold increased mortality compared to patients not on dialysis even after adjustments for demographic factors and comorbid conditions that are highly prevalent in patients on dialysis and are known to affect disease severity, including hypertension, diabetes, obesity, older age, cardiovascular disease, or poor socioeconomic status.7 , 8 Chronic kidney disease is associated with accelerated immunosenescence, the age-related decline in immune functions, as well as with inflammaging, the low-grade upregulation of certain pro-inflammatory responses that accompanies aging, leading to chronic activation and dysfunction of the innate immune system. Patients on dialysis with fatal outcomes have a strikingly reduced time between symptom onset and death,9, 10, 11 suggesting a lack of adequate infection control in the early phase of the disease.
Asymptomatic infections account for 10% to 50% of cases in the population on dialysis,12, 13, 14, 15, 16 depending on the diagnostic approach. Strikingly, baseline characteristics were not different in patients with asymptomatic and symptomatic infection,13 , 15 highlighting that as yet unidentified factors determine disease course.
Although a comparison of disease severity in peritoneal dialysis and hemodialysis is hampered by differences in baseline patient characteristics, presentation and prognosis appeared similar in small retrospective studies of hospitalized patients.17 , 18 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified in peritoneal effluent fluid in some19 but not all studies.20 Whether peritoneal effluent fluid represents a potential route of viral transmission remains moot.
The emergence of the variants of concern (VOCs) may strongly affect disease course, because they are characterized not only by increased transmissibility but also by the ability to escape innate and acquired immune responses.21 Four main VOCs appeared worldwide in late 2020 to early 2021: B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta). The Delta VOC has globally displaced the other VOCs, is highly transmissible, and has greater pathogenicity.21 The more recently described B.1.1.529 variant (Omicron) displays >30 changes in the spike (S) protein with a high potential of infectivity and immune evasion and has outcompeted Delta to globally predominate. The assessment of the virulence of the VOCs is biased by continuous improvement of preventive and therapeutic strategies during the course of the pandemic. Data on the impact of virus strain on disease severity in patients on dialysis are limited. Patients on hemodialysis infected by a SARS-CoV-2 variant from the B.1.362 lineage had significantly poorer outcomes and higher mortality rates than did those infected by nonvariant SARS-CoV-2, despite a lack of differences in baseline characteristics or treatment.22
The long-term consequences of COVID-19 in patients on dialysis remain poorly described. A long COVID-19 syndrome, defined by the Centers for Disease Control and Prevention as nonresolutive or newly occurring symptoms beyond 4 weeks after initial infection,23 has been identified in patients with very mild to severe COVID-19. The main symptoms include fatigue, dyspnea, cardiac involvement, muscle ache, headache, joint pain, or neuropsychological disorders. Because these symptoms commonly occur in patients on dialysis, the prevalence of long COVID-19 is difficult to assess. A recent study in 183 surviving patients on hemodialysis with 6-month follow-up after the acute infection identified no excess cardiovascular disease or mortality but severe cachexia and extreme muscle weakness in 13% of patients.24
Impact on the population on dialysis
In early 2020, an excess mortality of 15% to 20% (corresponding to 7000–10,000 deaths) was observed in the US population on dialysis.25 , 26 In contrast, other regions reported no overall excess mortality, because COVID-19–related mortality was balanced by lower than anticipated mortality in noninfected patients on dialysis, possibly by a lower incidence of other respiratory infections by virtue of droplet infection prevention measures.1 Although regional disparities were observed, in the United States the number of incident patients and the mean estimated glomerular filtration rate at dialysis initiation were significantly lower in 2020 than in previous years, particularly in elderly patients and non-Hispanic Blacks.27 , 28 Overall, the size of the US population on dialysis shrunk by 1.6% in 2020.29
The rate of hospitalization unrelated to COVID-19 also declined significantly,25 suggesting difficulties in accessing care. In addition, logistical constraints during the first wave resulted in decreased weekly dialysis time in noninfected patients.30 Furthermore, a sharp reduction in kidney transplantation occurred during the first wave in 22 countries worldwide.31 Although data on the benefit of renal transplantation during the pandemic are conflicting,32 , 33 many transplantation programs were interrupted. Finally, patients on dialysis have experienced major psychological distress owing to exposure to a new disease without effective treatment and high case fatality rates.34
Assessment of the immune response to infection and vaccination
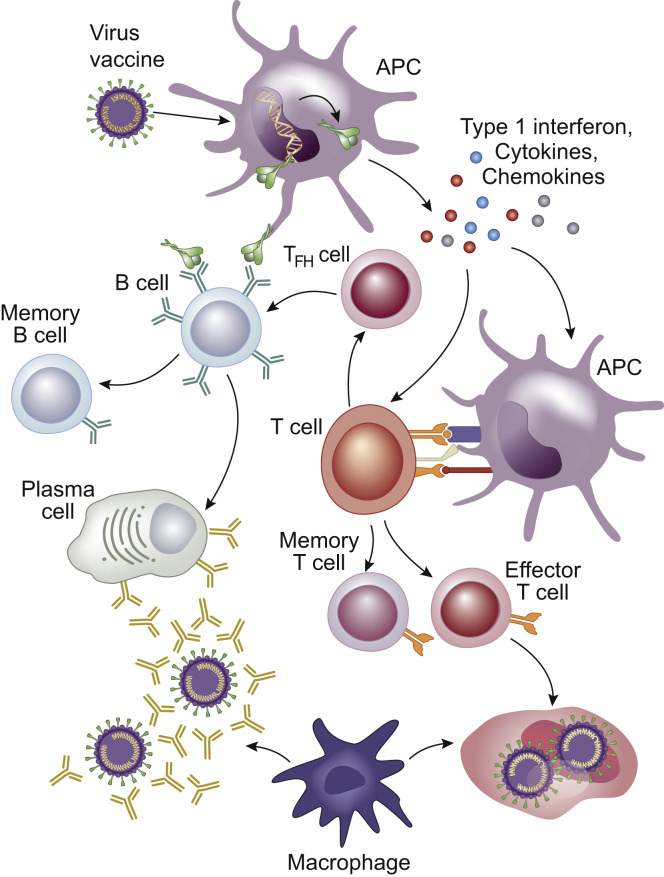
A critical challenge is to identify valid and reproducible biomarkers of the humoral and cellular immune responses to infection or vaccination (Figure 1 ) and link these to clinical outcomes.
Figure 1.
Immune response to infection and vaccination. Viral proteins are taken up by antigen presenting cells (APCs) that generate a range of pro-inflammatory cytokines. The antigens are presented to naive T cells that differentiate into different types of cells. T follicular helper (TFH) cells assist B cells to differentiate into plasma cells that produce antigen-specific antibodies to neutralize the virus. A broad range of antibodies are generated against multiple epitopes on the spike protein, but those directed against the highly immunogenic receptor-binding domain appear to have the greatest neutralizing potential because they disrupt the interaction between the spike protein and the angiotensin II converting enzyme 2 receptor. Effector T cells destroy virus-infected cells. Macrophages phagocytose and digest antibody-tagged virus and virus-infected cells. Antigen-specific memory B and T cells develop to prevent future infection. In parallel with the serological response, antigen-specific memory B cells continuously acquire somatic mutations in their variable region genes to improve antigenic affinity. Upon antigenic reexposure, memory B cells drive the recall response by differentiating into high-affinity antibody-secreting plasma cells. Although antibody levels wane, antigen-specific memory B cells progressively become more numerous and mature.
Humoral response
The humoral immune response to SARS-CoV-2 is mediated by antibodies directed to viral proteins, mainly the nucleocapsid protein and the S protein. Anti-nucleocapsid antibodies can be used to diagnose breakthrough infections, because they are specific to infection and not induced by vaccination. The humoral response to vaccination is usually assessed by the measurement of anti-S antibodies. A large number of enzyme-linked immunosorbent assay tests are commercially available, but each has its own cutoff value for positivity and reference range, hampering comparison between assays. The World Health Organization has attempted to introduce standardization by virtue of the World Health Organization International Standard study,35 which provides the mathematical relationship of the individual test units to the World Health Organization binding antibody units (BAUs).
The key question is whether antibody levels can serve as a valid surrogate marker for protection against (re)infection. Data from 7 large vaccination trials and a convalescent cohort revealed a strong relationship between neutralization antibody levels early after vaccination or infection and subsequent protective efficacy,36 suggesting the higher the antibody levels, the better the protection from (re)infection. The estimated neutralizing antibody level required for protection from severe disease appeared to be ∼6-fold lower than the level required for protection from any symptomatic infection.36 A large study in vaccinated health care workers revealed that the risk of a breakthrough infection was associated with the neutralizing antibody titer during the peri-infection period but even stronger with the peak neutralizing antibody titer after vaccination,37 corroborating the value of neutralizing antibody levels as an immune correlate of protective efficacy. A threshold of 264 BAUs/ml, which is associated with an 80% protection against symptomatic COVID-19 caused by the Alpha variant, is currently considered as protective.38
The emergence of VOCs has added a layer of complexity to the assessment of the humoral response. The commercially available anti-S and anti–receptor-binding domain (RBD) enzyme-linked immunosorbent assay tests have been designed on the basis of the S protein sequences of the original virus that were deposited in January 2020. As such, they may not capture all antibodies that are generated against severely mutated S proteins and thus underestimate the strength of the natural humoral response to VOCs. Conversely, levels of antibodies induced by vaccines based on the S protein sequences of the original virus may overestimate the true effectiveness of vaccine-induced humoral immunity against VOCs.
Cellular response
The cellular immune response is likely an important component of the protective adaptive immunity, but its assessment is labor intensive and beyond the abilities of a routine clinical laboratory. Quantification of SARS-CoV-2–specific cellular immunity requires stimulation of whole blood with SARS-CoV-2 peptide pools. Subsequently, proliferation of specific lymphocyte subpopulations, activities of certain signaling pathways, or generation of a number of cytokines (e.g., interferon-γ and interleukin-2) is measured. For none of these tests, the cutoff value that correlates with protection against infection is known.
Response to infection in patients on dialysis
Viral clearance
Patients with chronic kidney disease and in particular those on dialysis characteristically have delayed viral clearance after the resolution of clinical SARS-CoV-2 infection.39, 40, 41 The median time from admission to the first negative polymerase chain reaction test was 18 days in patients with chronic kidney disease versus 11 days in patients without chronic kidney disease, with impaired kidney function as an independent predictor of time to viral clearance.39 More than two-thirds of patients on hemodialysis remained polymerase chain reaction positive 20 days after symptom onset.40 Existing symptom- and time-based strategies to inform the decision to lift quarantine may therefore not be applicable to the population on dialysis. The challenge is to differentiate between the presence of remnant viral RNA and replication-competent (and therefore infectious) virus, because unnecessary prolonging of isolation has adverse logistical and psychological effects. Although no single laboratory method can serve as a reliable predictor of viral infectivity, there is a strong association between quantitative viral load data, polymerase chain reaction cycle threshold values, and the ability to recover SARS-CoV-2 in viral culture.42 A pragmatic approach could be to discontinue quarantine when the viral load is <100,000 copies/ml, corresponding to a cycle threshold value of >28 to 31, depending on the type of analyzer (e.g., cobas 6800 [Roche], RespiTAC [Qiagen], and GeneXpert [Cepheid]) and gene target (nucleocapsid, Open Reading Frame-1 [Orf-1], RNA-dependant RNA Polymerase [RdRP], and E gene; M. Reynders, MD, PhD, oral communication, November 2021).
Humoral and cellular response
After natural SARS-CoV-2 infection, the large majority of patients on hemodialysis develop a robust antibody response.41 , 43, 44, 45, 46, 47, 48, 49, 50, 51 These observations are somewhat counterintuitive in view of the high mortality rates of COVID-19 in the population on hemodialysis and may be partially accounted for by survivor bias, because humoral responses may be better in patients who have recovered from SARS-CoV-2 infection. An alternative explanation may be that patients on hemodialysis develop more severe disease, produce higher levels of pro-inflammatory cytokines, and have prolonged viral shedding, resulting in more intense immune stimulation. Indeed, patients on hemodialysis with a history of severe polymerase chain reaction–confirmed SARS-CoV-2 infection requiring hospitalization had higher antibody levels than did patients who developed only mild or asymptomatic disease, but the decay trajectory of the antibodies was similar in both groups.52 Likewise, symptomatic patients had higher antibody levels than did asymptomatic individuals.45 , 46 Lack of seroconversion has been observed in 5% to 10% of patients on hemodialysis43, 44, 45 and could mainly be attributed to immunosuppressive drugs or chemotherapy.43
Most studies show durable humoral immune responses in patients on hemodialysis with a slow decline over time43 , 44 , 46 , 47 , 49 , 51 , 52 and a longevity that is commensurate with that of the general population. Serological responses have been reported to persist up to >1 year, with a faster decay of anti-nucleocapsid IgG than of anti-S IgG.52
Data on the cellular response after natural infection in patients on dialysis are limited.51 , 53 Interferon-γ secretion upon SARS-CoV-2 antigen exposure in the absence of measurable antibodies was identified in 8 of 11 survivors 6 months after infection.51
Protection against reinfection
Although seropositivity seems to afford protection against reinfection,45 , 51 , 54 this protection is clearly incomplete. An observational study in 2337 patients on hemodialysis revealed that serological evidence of past SARS-CoV-2 infection was associated with a 45% and 79% reduction of the risk of subsequent SARS-CoV-2 infection and clinically manifest COVID-19, respectively.54 For comparison, a large prospective study in health care workers found that antibody positivity conferred a ∼90% reduction of the risk of reinfection.55
The impact of the cellular response on the risk of reinfection in patients on dialysis remains ill-defined.
It should be noted that the reported studies have been conducted when the wild-type virus was the most prevalent strain. The ability of naturally acquired immunity to prevent reinfection with the Delta and Omicron VOC has not been studied in patients on dialysis.
Response to vaccination in patients on dialysis
Humoral response
A recent elegant review summarized 22 studies reporting on early seroconversion rates after COVID-19 mRNA vaccination in patients receiving hemodialysis, 11 of which included a control group generally consisting of health care workers.56 Not included in this review are 2 large multicenter studies57 , 58 as well as a number of more recently published smaller studies.59, 60, 61, 62
The emerging picture is that the development of the serological response in dialysis is substantially delayed. In healthy volunteers, the peak response was achieved at 4 to 5 weeks after the first vaccine dose with stable values thereafter, whereas antibody titers continued to rise in patients on hemodialysis.58
The pooled estimate of the antibody response rate in patients receiving hemodialysis was 45% and 89% after the first and second dose, respectively.56 At first sight, response rates after the second dose do not compare unfavorably with 95% to 100% seroconversion rates in healthy controls. However, seroconversion rates describe only the proportion of patients who cross the detection limit of the antibody test but do not provide information on the size and quality of the humoral response. Indeed, antibody levels are significantly lower in patients on hemodialysis than in healthy volunteers.58 , 63, 64, 65, 66, 67, 68, 69 As an example, 8 weeks after BNT162b2 (Pfizer-BioNTech) vaccination, only 26% of COVID-19–naive patients on hemodialysis but 84% of COVID-19–naive healthy volunteers achieved a titer above 590 BAUs/ml.58
In multivariate analyses, use of immunosuppressive drugs, low serum albumin level, low lymphocyte count, low IgG levels, hepatitis B vaccine nonresponder status, high dialysis vintage, and high i.v. iron dose were identified as independent predictors of a poor serological response.58 , 61 , 70 , 71 Age was retained as an independent predictor in some59 , 61 , 68 , 71 but not all studies,58 , 70 possibly because of the differences in the type and number of parameters included in the multivariate analyses. It is tempting to speculate that markers of immunosenescence may be better predictors of the immune response than chronological age per se. Antibody titers were numerically higher in peritoneal dialysis than in hemodialysis in some72, 73, 74 but not all studies.75 , 76
COVID-19 experience results in a strong vaccine-induced response.56 , 58 , 59 , 61 , 62 Overall, the response in COVID-19–experienced patients on dialysis was in the same range as that in COVID-19–naive healthy volunteers, but a significant correlation was found between the intensity of the vaccine-induced immune response and the severity of the historical SARS-CoV-2 infection.58
A third vaccine dose, generally given 1 to 2 months after the second dose, significantly increased antibody levels in almost all patients on dialysis.77, 78, 79, 80, 81, 82 Patients with poor initial responses appeared to derive the most relative benefit, whereas those with high antibody titers after the second dose featured more modest increases.83 Interestingly, serum from patients with an absent or low response after the second dose who subsequently received a third dose had a greater neutralizing capacity than did serum from patients with a high response to standard prime-boost vaccination.82 The response to the booster dose was similar in patients on hemodialysis and patients on peritoneal dialysis.79
Protection against the SARS-CoV-2 VOCs may require higher antibody levels. Studies conducted in the general population revealed that the vaccine-induced neutralizing activity was only mildly reduced against Alpha, but 5- to 12-fold lower against Beta, 5-fold lower against Gamma, 6-fold lower against Delta, and 10-fold lower against Omicron as compared with the activity against wild-type viruses.84, 85, 86, 87 Data in patients on hemodialysis are limited. The neutralizing activity of serum taken 3 weeks after the second BNT162b2 or mRNA-1273 dose in patients on dialysis was significantly lower against Beta than Alpha88 and against Delta than the wild-type virus.89 Although BNT161b2 vaccination induced comparable neutralizing response against VOCs in patients on dialysis and those not on dialysis, the adenovirus-based vaccine AZD1222 (AstraZeneca) had lower immunogenicity.90 A third vaccine dose induced neutralizing antibodies against the wild-type virus in the large majority of patients, but less than a third developed neutralizing antibodies against Delta.82 In a recent study of hemodialysis patients from the UK, homologous vaccination with 3 BNT161b2 doses resulted in quantifiable neutralizing antibodies against Delta and Omicron in the majority of subjects. In contrast, heterologous vaccination with 2 AZD1222 doses and a BNT161b2 booster dose generated quantifiable neutralizing antibodies against Delta in more than 50% of patients, but the neutralizing antibodies against Omicron were below the quantifiable range in more than 50% of patients.91
Data on the longevity of the humoral response to vaccination in patients on dialysis are rapidly emerging74 , 76 , 92, 93, 94, 95, 96, 97 (Table 1 ) and reveal a gradual waning of antibody levels with a rate of decline similar to that in the general population. Not unsurprisingly, more durable responses were observed when the initial titers were higher, for example, in patients on peritoneal dialysis and in mRNA-1273 vaccine recipients.95
Table 1.
Studies on long-term humoral immune response to SARS-CoV-2 vaccination in patients on dialysis
| Study | Population sample size | Control group | Vaccine | Sampling time | Test type | Findings |
|---|---|---|---|---|---|---|
| De Vriese et al.92 | 492 HD (436 naive) | 75 naive HV | mRNA-1273 (n = 180) BNT162b2 (n = 256) |
24 wk | Anti-S (Abbott) | GMT: HD naive: 702 (mRNA-1273), 226 (BNT162b2) HD exp: 6671 (mRNA-1273), 5220 (BNT162b2) HV naive: 4046 (mRNA-1273), 1521 (BNT162b2) Decline between 8 or 9 and 24 wk: ±80% in HD and HV |
| Angel-Korman et al.93 | 409 naive HD | 148 naive HV | BNT162b2 | 82–89 d | Anti-S (DiaSorin) | GMT: HD: 23.3 HV: 222.7 |
| Anand et al.94 | 2563 D | – | mRNA-1273 (n = 1259) BNT162b2 (n = 1197) Ad26.COV2.S (n = 107) |
4–6 mo | Anti-RBD (Siemens) | No detectable antibody: mRNA-1273: 11% at 5–6 mo BNT162b2: 31% at 5–6 mo Ad26.COV2.S: 57% at 4–5 mo |
| Hsu et al.95 | 1567 naive D | – | mRNA-1273 (n = 779) BNT162b2 (n = 441) Ad26.COV2.S (n = 347) |
1 mo 6 mo |
Anti-RBD (Siemens) | Median: mRNA-1273: from 20 (1 mo) to 6.2 (6 mo) BNT162b2: from 20 (1 mo) to 1.3 (6 mo) Ad26.COV2.S: from <1 (1 mo) to <1 (6 mo) |
| Davidovic et al.96 | 41 HD | – | BNT162b2 | 4 wk 6 mo |
Anti-S (DiaSorin) | Median: from 1110 (4 wk) to 85.6 (6 mo) |
| Goggins et al.97 | 35 HD | – | BNT162b2 | Monthly to 6 mo | Anti-S (Euroimmun) | Mean: 648 (2 mo), 491 (3 mo), 366 (4 mo), 302 (5 mo), 178 (6 mo) |
| Speer et al.74 | 114 naive HD 41 naive PD |
– | BNT162b2 | 3 wk 12 wk |
Anti-S (Siemens) SNA (medac) |
Median: anti-S: HD: from 7 (3 wk) to 3 (12 wk) PD: from 22 (3 wk) to 7 (12 wk) Median: SNA: HD: from 56% (3 wk) to 45% (12 wk) PD: from 77% (3 wk) to 55% (12 wk) |
| Einbinder et al.76 | 118 naive HD 64 naive PD |
– | BNT162b2 | 6 mo | Anti-S (Abbott) | Median/mean: HD: 133/400 PD: 285/384 |
D, dialysis (home hemodialysis, in-center hemodialysis, and peritoneal dialysis); GMT, geometric mean titer; HD, hemodialysis; HV, healthy volunteer; naive, coronavirus disease 2019 naive; PD, peritoneal dialysis; S, spike protein; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SNA, surrogate neutralizing antibody.
Cellular response
Prime-boost mRNA vaccination induced CD4+ T cell responses in 60% to 100% of patients on dialysis, a proportion who is similar to or slightly reduced compared with controls.57, 58, 59 , 61 , 66 , 98 , 99 The magnitude of the CD4+ T cell response in patients on hemodialysis paralleled that in controls in some57 , 67 but not all studies.58 S-specific CD4+ T cell responses generally57, 58, 59 , 98 but not always61 correlated with anti-RBD IgG, in line with the cooperation between CD4+ T cells and B cells to generate IgG. Factors independently associated with CD4+ T cell responses included prior SARS-CoV-2 exposure, immunosuppression, nutritional status, lymphopenia, and dialysis vintage.58 CD8+ T cell responses were observed in <50% of patients on dialysis, a proportion similar to that of healthy controls.59 Factors negatively associated with CD8+ T cell responses included immunosuppression and absence of humoral response.59
In a cohort of 23 patients on dialysis, a third vaccine dose led to an increase of CD4+ T cells specific for the wild-type and Delta variants. No effect on CD8+ T cells was observed.82 However, in another study of 75 patients on dialysis, the third dose given within 3 months of the second dose did not significantly affect the CD4+ T cell response, even after stratification for responder status after the second dose.83
A study of the B cell response in 44 patients on dialysis 7 days after a BNT162b2 boost revealed impaired induction of effectors of B cell immunity (e.g., memory B cells and plasmablasts) compared with healthy controls, in line with the defective antibody response.65 In contrast, another study found a similar proportion of RBD-specific memory B cells 1 to 2 months after a BNT162b2 boost in patients on dialysis and controls, despite lower antibody levels in patients on dialysis (Attias, MD, unpublished data, 2022). Although a third dose, given 3.5 months after the second dose, induced a strong RBD-specific memory B cell expansion in SARS-CoV-2–naive patients, this B cell compartment remained unchanged in SARS-CoV-2–experienced patients, suggesting no benefit of an early third dose in these patients.
Vaccine type
In accordance with data in health care workers,100 several studies reported a remarkably better immunogenicity of the mRNA-1273 vaccine (Moderna) compared with the BNT162b2 vaccine in patients on hemodialysis.57 , 58 , 70 , 101 As an example, geometric mean antibody titers were significantly greater and a larger proportion of patients achieved the threshold of 590 BAUs/ml with the mRNA-1273 vaccine (573 BAUs/ml and 53.6%) as compared with the BNT162b2 vaccine (221 BAUs/ml and 31.8%) 8 to 9 weeks after the first dose.58 Similarly, cellular responses were more robust in mRNA-1273 than in BNT162b2 recipients.58 , 61 Both mRNA vaccines consist of mRNA encoding for the SARS-CoV-2 S glycoprotein encapsulated in lipid nanoparticles and have no other content relevant to immunogenicity. However, the mRNA dose of mRNA-1273 (100 μg) is substantially higher than that of BNT162b2 (30 μg). Additional discriminatory elements are the longer interval between priming and boosting for mRNA-1273 (4 weeks) than for BNT162b2 (3 weeks) and a better thermostability and ease of handling of the former. Both mRNA vaccines showed near-maximal clinical efficacy in large randomized trials,102 , 103 but the results were obtained on the short-term in healthy volunteers when the wild-type virus was dominant. Whether the better immunogenicity of the mRNA-1273 vaccine will translate into superior protection of vulnerable populations in the context of waning immunity and more resistant SARS-CoV-2 variants remains to be determined.
Data on the immunogenicity of the other vaccine platforms in the population on hemodialysis are scarcer. The neutralizing ability of serum obtained from 178 COVID-19–naive patients on hemodialysis 33 days after full vaccination with BNT162b2 or AZD1222 was assessed in vitro.90 Compared with BNT162b2 recipients, AZD1222 recipients had a markedly lower capacity to neutralize Alpha (>4-fold reduction), Beta (>3-fold reduction), and Delta (>6-fold reduction).90 Another study found a nonsignificant lower seroconversion rate in AZD1222 recipients (70.6%) than in BNT162b2 recipients (81.8%).104 Finally, in 76 patients on hemodialysis the antibody responses were lower with the Ad26.COV2.S vaccine (Johnson & Johnson) than with mRNA vaccines.62
The durability of the serological response correlates with the robustness of the initial response. Of the 1567 COVID-19–naive patients on dialysis, 67.5% of Ad26.COV2.S, 32.1% of BNT162b2, and 12.3% of mRNA-1273 recipients had undetectable antibodies 4 months after vaccination.95 Likewise, in a cohort of 2563 COVID-19–naive patients on dialysis, anti-RBD antibodies had disappeared in 57% of Ad26.COV2.S recipients 4 to 5 months after vaccination and in 31% and 11% of BNT162b2 and mRNA-1273 recipients 5 to 6 months after vaccination.94
Protection against infection
Because there are currently no universally accepted and validated biomarkers for protection against SARS-CoV-2 infection, disease, and mortality, only data on the incidence and severity of breakthrough infections (i.e., infection >2 weeks after full vaccination) can provide definitive answers on the real-world effectiveness of the COVID-19 vaccines. Reliable data are notoriously difficult to obtain, because the degree of vaccination of the general population and the population on dialysis, the infectiousness and virulence of the prevalent SARS-CoV-2 VOCs, and the local dynamics of the pandemic all have to be taken into account. In a retrospective cohort of >35,000 patients on dialysis from the United States, the hazard ratio for COVID-19 diagnosis was 0.22 and 0.27 after BNT161b2 and mRNA-1273 vaccination, respectively, as compared with propensity score–matched unvaccinated controls.105 Modeling of data from hospitalizations after a SARS-CoV-2 infection in 3620 patients on dialysis and 457,160 people from the general population in France revealed a reduced hospitalization rate in the population on dialysis over time that was independently associated with vaccination coverage of patients on dialysis and their same-age peers from the general population.106 In a cohort of 15,251 patients on maintenance dialysis observed between February 1 and August 26, 2021, fully vaccinated patients were significantly less likely to be diagnosed or hospitalized with COVID-19.107 Nevertheless, 26% of new SARS-CoV-2 infections, 27% of COVID-19–related hospitalizations, and 33% of COVID-19–related deaths occurred in fully vaccinated patients. The vast majority of these had very low or undetectable antibody levels at the time of COVID-19 diagnosis, generally because they had never developed an initial humoral response.107 Breakthrough infections particularly occurred when Delta and Omicron became the dominant strains and were most common in Ad26.COV2.S recipients and least common in mRNA-1273 recipients.107 In another cohort of 2563 vaccinated patients requiring dialysis, 56 breakthrough infections (of which 25 required hospitalization) were identified after a median time from vaccination of 110 days, in particular in patients with low peak and peri-infection anti-RBD antibody levels.94 Taken together, preliminary data reveal a substantial but incomplete clinical effectiveness of the SARS-CoV-2 vaccines in patients on dialysis.
Optimization of the vaccination strategy
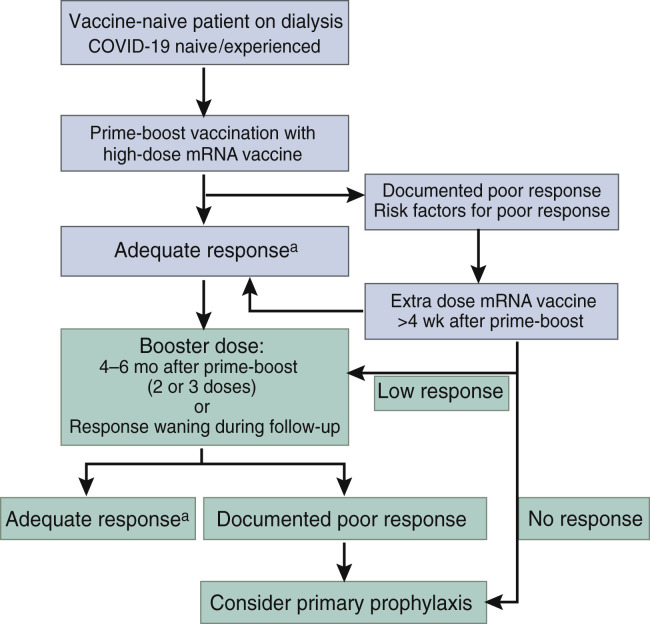
The presence of antibodies does not automatically track with functional humoral or cellular immunity required for long-term protection against SARS-CoV-2. However, they are currently the best surrogate markers to design and validate the optimal vaccination strategy in patients on dialysis (Figure 2 ).38 The extant evidence suggests that not all vaccines are created equal. Hemodialysis and other vulnerable populations with blunted vaccine responses should therefore receive the most immunogenic vaccines, in casu the mRNA vaccines. The observed differences between mRNA-1273 and BNT162b2 suggest a cardinal role for dose and may be conducive to the future development of high-dose vaccines.
Figure 2.
Proposal for a vaccination strategy in patients on dialysis.aAn adequate response to vaccination can be defined as antibody levels above a certain antibody threshold 4 weeks after vaccination, for example, 264 binding antibody units (BAUs)/ml.38 A low response can be defined by antibody levels >0 BAU/ml but <264 BAUs/ml. These thresholds need to be redefined for Delta and Omicron. The benefit of primary prophylaxis against variants of concern (VOCs) has not been demonstrated.
An additional dose administered with an interval of at least 4 weeks after the second dose appears especially effective in patients with initially poor or absent responses. As such, patients with a documented poor response or at high risk of a poor response based on their clinical profile may be candidates for an additional vaccine dose. The progressive waning of immunity and appearance of VOCs with a high potential of immune evasion is prompting the administration of a booster dose 4 to 6 months after prime-boost vaccination in all patients on dialysis. A small subgroup (estimated to represent 5%–10% of the population on dialysis) will not mount a protective response following such an approach. As further vaccine doses are unlikely to be effective, these patients may instead benefit from pre-exposure prophylaxis, although monitoring the effectiveness of this strategy against future variants will require further studies. A combination of 2 long-acting monoclonal antibodies (tixagevimab and cilgavimab; AZD7442, AstraZeneca) that target 2 distinct RBD epitopes was associated with an 83% reduction of symptomatic COVID-19 at 6 months in a primary prevention setting (Pre-exposure Prophylaxis of COVID-19 in Adult [PROVENT] trial).108 Nasal administration of the potent anti–SARS-CoV-2 agent niclosamide is currently evaluated as a means to prevent COVID-19 in vulnerable patient populations, including patients on dialysis (PROphylaxis for paTiEnts at Risk of COVID-19 infecTion -V [PROTECT-V]; ClinicalTrials.gov identifier: NCT04870333).
Finally, ring vaccination of household members and other close contacts is mandatory in patients on dialysis, particularly in those with a suboptimal response to vaccination.106
The continuous emergence of VOCs that partially evade the immune response to vaccines based on the original virus strain presents a gargantuan challenge to vaccine development. An updated mRNA-1273 vaccine encoding for the S protein of Beta was highly effective in animal models.109 In the meantime, however, Beta has been completely replaced by the far more contagious Delta and Omicron and is therefore no longer clinically relevant. Several companies have already announced the development of an Omicron-specific vaccine.110, 111, 112 Preliminary evidence fortunately reveals a greatly increased neutralization efficiency after receipt of a third vaccine dose.113 , 114 Ultimately, we may need second-generation coronavirus vaccines that protect against all known and future VOCs. A fascinating study from Singapore found that serum from survivors of the SARS outbreak in 2002 to 2003 who were vaccinated with BNT162b2 had the ability to neutralize SARS-CoV-1, all SARS-CoV-2 variants, as well as several bat and pangolin coronaviruses with potential to cause human infection.115 These findings suggest that it must be feasible to develop a pan-sarbecovirus vaccine through a mechanism of cross-clade boosting. Finally, the potential role of intranasal vaccines has garnered attention by the observation that viral loads are similar in the nose of vaccinated and unvaccinated individuals with SARS-CoV-2 infection.116 Intranasal vaccine delivery may induce mucosal immunity in the respiratory tract, reduce viral shedding in the nose, and thus block viral transmission.
Safety
The short-term adverse effects of vaccines are less common and severe in patients on hemodialysis than in healthy volunteers,58 commensurate with their immunogenicity in these populations. The tolerance of the second and third vaccine doses in patients on hemodialysis appears similar.79 Severe adverse effects are rare in the population on dialysis. In an online survey from the United States, 20% of responders reported vaccine hesitancy, about half of which expressed concerns about adverse effects.117 The odds of vaccine hesitancy were higher in younger, female, Black, Native American, or Pacific Islander patients.117
Treatment
Treatment strategies for COVID-19 are rapidly evolving. As for now, therapy is still largely supportive and focused on the prevention of complications.
Because SARS-CoV-2 S interacts with angiotensin-converting enzyme 2 to enter host cells, the role of renin-angiotensin system (RAS) blockers in SARS-CoV-2 severity has been the subject of intense research. In a French nationwide study of almost 2 million hypertensive people, RAS blockers were associated with a 16% to 26% lower risk of hospitalization for COVID-19 as compared with calcium channel blockers.118 However, these results were not reproduced in other nationwide studies.119 Moreover, losartan introduction did not reduce hospitalization rate in a randomized controlled trial of 117 patients with mild symptomatic COVID-19.120 Data in the population on dialysis are scarce. In a retrospective cohort of 248 patients on dialysis with COVID-19, RAS blockers were associated with a 50% reduced risk of mortality after propensity score matching.121 However, data from the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry obtained in 1052 patients on dialysis with COVID-19 disclosed no modification of fatality risk by RAS blockers after multiple adjustments, despite a trend toward a lower rate of hospitalization.122 To date, no evidence suggests that RAS blockers should be introduced or discontinued after COVID-19 diagnosis in patients on dialysis, unless in case of obvious contraindication.
Thrombotic events are a major cause of COVID-19 morbidity.123 , 124 Patients on dialysis with COVID-19 may experience arteriovenous fistula thrombosis,125 mechanical dysfunction of the catheter, or circuit clotting,126 , 127 although no excess of vascular access thrombosis was observed in another study of 601 patients with arteriovenous fistula/graft.128 Prophylactic anticoagulation is an essential therapeutic strategy for patients on dialysis, in accordance with the recommendations for the population not on dialysis. Anticoagulation protocols are derived from those in the general population129 and adapted to the dialysis modality (intermittent vs. continuous) and ventilation/oxygenation procedures in critically ill patients.124
Since March 2020, numerous therapeutic strategies to control COVID-19 have been or are currently under investigation (Table 2 ). Only those shown to be effective and officially recommended by the Infectious Diseases Society of America or European Respiratory Society will be discussed (Table 3 ).130 Because most prospective therapeutic trials have excluded patients on dialysis for safety reasons131 (including studies of novel antivirals against SARS-CoV-2 with promising results132), therapeutic strategies in patients on dialysis are extrapolated from evidence obtained in the population not on dialysis.
Table 2.
Overview of COVID-19 therapies under investigation
|
Antiviral interventions |
| Monoclonal antibodies |
| Bamlanivimab 700 mg/etesevimab 1400 mg |
| Casirivimab 600 mg/imdevimab 600 mg |
| Sotrovimab 500 mg |
| Small molecule antivirals |
| Remdesivir |
| Molnupiravir |
| Paxlovid |
|
Interventions for the inflammatory response phase |
| Corticosteroids |
| Interleukin-6 receptor antagonists: tocilizumab and sarilumab |
| Janus kinase inhibitors: baricitinib and tofacitinib |
| Interleukin-1 receptor antagonist: anakinra |
| Anti-GM-CSF monoclonal antibodies |
| Interferon beta-1a |
COVID-19, coronavirus disease 2019; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Table 3.
Overview of therapeutic options in COVID-19
| Treatment | Ambulatory care | Hospitalized: mild-to-moderate disease without need for supplemental oxygen | Hospitalized: severe but noncritical disease (Spo2 <94% on room air) | Hospitalized: critical disease (e.g., mechanical ventilation, septic shock, and ECMO) |
|---|---|---|---|---|
| Corticosteroids | Suggest against use | Suggest use; 17% lower mortality | Recommend use; 34% lower mortality | |
| IL-6-RA | Suggest use if CRP level > 75 mg/l; 17% reduced clinical deterioration, trend toward lower mortality | Suggest use if CRP level > 75 mg/l | ||
| JAKi | Suggest use; up to 38% lower mortality; not to be associated with IL-6 inhibitors; more infections when associated with glucocorticoids; no data if eGFR < 30 ml/min per 1.73 m2 or immunodepression | |||
| Remdesivira | Suggest use; trend toward clinical improvement (no benefit on mortality); accumulation in ESKD? | Suggest against use | ||
| Monoclonal antibodies | Suggest use Bamlanivimab/etesevimab: 70% lower hospitalization/mortality. Casirivimab/imdevimab: 73% lower hospitalization. Sotrovimab: 86% lower hospitalization |
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IL-6, interleukin-6; IL-6-RA, interleukin-6 receptor antagonist; JAKi, Janus kinase inhibitor; Spo2, oxygen saturation.
Modified from the Infectious Diseases Society of America and European Respiratory Society treatment guidelines, accessed December 19, 2021.130
No longer recommended in the European Respiratory Society guidelines.
Glucocorticoids are now standard treatment in patients with severe to critical disease by virtue of their benefit in the hyperinflammatory phase of COVID-19.133 Interleukin-6 receptor antagonists (tocilizumab and sarilumab) are proposed in hospitalized patients with severe or critical illness and elevated inflammation markers, with the intention to limit the hyperinflammatory syndrome.134 The Janus kinase inhibitors baricitinib and tofacitinib are proposed in patients with severe noncritical disease.
Remdesivir, a nucleotide analogue that inhibits SARS-CoV-2 RNA transcription, has shown limited benefit in hospitalized patients needing oxygen supplementation, but not in patients on invasive ventilation or extracorporeal membrane oxygenation.135 , 136 Remdesivir and its active metabolites are predominantly eliminated by the kidneys. Remdesivir was well tolerated in an observational study of 48 patients on dialysis with SARS-CoV-2 infection.137
Neutralizing monoclonal antibodies targeting the RBD of the S protein to inhibit virus entry in host cells are a therapeutic option in ambulatory mild to moderate COVID-19 at high risk of progression,138, 139, 140, 141 but may be less effective in already hospitalized patients.142 The efficacy of neutralizing antibodies against Delta and Omicron are the subject of intense research.143 Neutralizing antibodies are well tolerated, and no evidence suggests specific adverse effects in patients on dialysis. Concerns have been raised regarding the selection of resistant SARS-CoV-2 variants in immunocompromised patients, including patients on dialysis.144 Although no studies have been performed specifically in the population on dialysis, they represent an attractive option after a high-risk exposure or during the early course of infection.
Conclusion
The dramatic impact of the COVID-19 pandemic on the population on dialysis has stimulated collective efforts to unravel the pathophysiology of COVID-19 and develop effective preventive and therapeutic measures. An optimized vaccination strategy and actions to improve vaccine acceptance clearly have the best chances at success, along with continued attention to droplet infection prevention measures. Patients with inadequate responses to vaccination may be candidates for a primary prevention strategy. The prevalence of long-lasting physical and neuropsychological consequences of past infection, the protective efficacy of natural and vaccine-induced immunity against new virus variants, and the long-term consequences of the pandemic on a population scale are among the many subjects that require further research. The fight against this pandemic is far from over.
Disclosure
All the authors declared no competing interests.
References
- 1.De Meester J., De Bacquer D., Naesens M., et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol. 2021;32:385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couchoud C., Bayer F., Ayav C., et al. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng J.H., Hirsch J.S., Wanchoo R., et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberici F., Delbarba E., Manenti C., et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilbrands L.B., Duivenvoorden R., Vart P., et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35:1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenzato L., Botton J., Drouin J., et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158. doi: 10.1016/j.lanepe.2021.100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goicoechea M., Sánchez Cámara L.A., Macías N., et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lano G., Braconnier A., Bataille S., et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J. 2020;13:878–888. doi: 10.1093/ckj/sfaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller N., Chantrel F., Krummel T., et al. Impact of first-wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: the observational COVIDIAL study. Nephrol Dial Transplant. 2020;35:1338–1411. doi: 10.1093/ndt/gfaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke C., Prendecki M., Dhutia A., et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969–1975. doi: 10.1681/ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rincón A., Moreso F., López-Herradón A., et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J. 2020;13:542–549. doi: 10.1093/ckj/sfaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creput C., Fumeron C., Toledano D., et al. COVID-19 in patients undergoing hemodialysis: prevalence and asymptomatic screening during a period of high community prevalence in a large Paris center. Kidney Med. 2020;2:716–723.e1. doi: 10.1016/j.xkme.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H., Tian J.B., Dong J.W., et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis. 2020;76:490–499.e1. doi: 10.1053/j.ajkd.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau K., Muller M.P., Lin M., et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76:690–695.e1. doi: 10.1053/j.ajkd.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachdeva M., Uppal N.N., Hirsch J.S., et al. COVID-19 in hospitalized patients on chronic peritoneal dialysis: a case series. Am J Nephrol. 2020;51:669–674. doi: 10.1159/000510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H.J., Tang H., Xiong F., et al. COVID-19 in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2020;16:121–123. doi: 10.2215/CJN.07200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vischini G., D’Alonzo S., Grandaliano G., D’Ascenzo F.M. SARS-CoV-2 in the peritoneal waste in a patient treated with peritoneal dialysis. Kidney Int. 2020;98:237–238. doi: 10.1016/j.kint.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Shamy O., Vassalotti J.A., Sharma S., et al. Coronavirus disease 2019 (COVID-19) hospitalized patients with acute kidney injury treated with acute peritoneal dialysis do not have infectious peritoneal dialysis effluent. Kidney Int. 2020;98:782. doi: 10.1016/j.kint.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao K., Tzou P.L., Nouhin J., et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021;22:757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wand O., Mor O., Zuckerman N., et al. Outcomes from infections with variant strains of SARS-CoV-2 among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2021;78:617–619. doi: 10.1053/j.ajkd.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crook H., Raza S., Nowell J., et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 24.Chawki S., Buchard A., Sakhi H., et al. Long-term impact of COVID-19 among maintenance haemodialysis patients. Clin Kidney J. 2021;15:262–268. doi: 10.1093/ckj/sfab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinhandl E.D., Wetmore J.B., Peng Y., et al. Initial effects of COVID-19 on patients with ESKD. J Am Soc Nephrol. 2021;32:1444–1453. doi: 10.1681/ASN.2021010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziemba R., Campbell K.N., Yang T.H., et al. Excess death estimates in patients with end-stage renal disease—United States, February-August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:825–829. doi: 10.15585/mmwr.mm7022e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen K.H., Thorsness R., Hayes S., et al. Evaluation of racial, ethnic, and socioeconomic disparities in initiation of kidney failure treatment during the first 4 months of the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wetmore J.B., Johansen K.L., Liu J., et al. Changes in treatment of patients with incident ESKD during the novel coronavirus disease 2019 pandemic. J Am Soc Nephrol. 2021;32:2948–2957. doi: 10.1681/ASN.2021040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinhandl E.D., Gilbertson D.T., Wetmore J.B., Johansen K.L. COVID-19-associated decline in the size of the end-stage kidney disease population in the United States. Kidney Int Rep. 2021;6:2698–2701. doi: 10.1016/j.ekir.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chazot C., Weis L., Hebibi H., et al. Impact of first wave COVID-19 crisis on dialysis parameters of COVID-free hemodialysis patients: a NephoCare France Longitudinal Retrospective Cohort Study. Blood Purif. https://doi.org/10.1159/000517493 Published online August 26, 2021. [DOI] [PMC free article] [PubMed]
- 31.Aubert O., Yoo D., Zielinski D., et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. 2021;6:e709–e719. doi: 10.1016/S2468-2667(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goffin E., Candellier A., Vart P., et al. COVID-19 related mortality in kidney transplant and hemodialysis patients: a comparative, prospective registry based study. Nephrol Dial Transplant. 2021;36:2094–2105. doi: 10.1093/ndt/gfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinson A.J., Kiberd B.A., Tennankore K.K. Panic in the pandemic: when should kidney transplant programs close? Kidney Int Rep. 2021;6:1232–1241. doi: 10.1016/j.ekir.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z.H., Pan X.T., Chen Y., et al. Psychological profiles of Chinese patients with hemodialysis during the panic of coronavirus disease 2019. Front Psychiatry. 2021;12:616016. doi: 10.3389/fpsyt.2021.616016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO/BS.2020.2403 establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody. https://www.who.int/publications/m/item/WHO-BS-2020.2403
- 36.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 37.Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S., Phillips D.J., White T., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Sullivan E.D., Lees J.S., Howie K.L., et al. Prolonged SARS-CoV-2 viral shedding in patients with chronic kidney disease. Nephrol (Carlton) 2021;26:328–332. doi: 10.1111/nep.13844. [DOI] [PubMed] [Google Scholar]
- 40.Shaikh A., Zeldis E., Campbell K.N., Chan L. Prolonged SARS-CoV-2 viral RNA shedding and IgG antibody response to SARS-CoV-2 in patients on hemodialysis. Clin J Am Soc Nephrol. 2021;16:290–292. doi: 10.2215/CJN.11120720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vriese A.S., Reynders M. IgG antibody response to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis. 2020;76:440–441. doi: 10.1053/j.ajkd.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binnicker M.J. Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00469-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakhi H., Dahmane D., Attias P., et al. Kinetics of anti-SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection. J Am Soc Nephrol. 2021;32:1033–1036. doi: 10.1681/ASN.2020111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbes S., Davari M., Gnanasampanthan S., et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol Dial Transplant. 2021;36:1292–1297. doi: 10.1093/ndt/gfab066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banham G.D., Godlee A., Faustini S.E., et al. Hemodialysis patients make long-lived antibodies against SARS-CoV-2 that may be associated with reduced reinfection. J Am Soc Nephrol. 2021;32:2140–2142. doi: 10.1681/ASN.2021020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muir L., Jaffer A., Rees-Spear C., et al. Neutralizing antibody responses after SARS-CoV-2 infection in end-stage kidney disease and protection against reinfection. Kidney Int Rep. 2021;6:1799–1809. doi: 10.1016/j.ekir.2021.03.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudreuilh C., Roper T., Breen C., et al. IgG SARS-CoV-2 antibodies persist at least for 10 months in patients on hemodialysis. Kidney Int Rep. 2021;6:1961–1964. doi: 10.1016/j.ekir.2021.03.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anft M., Blazquez-Navarro A., Paniskaki K., et al. SARS-CoV-2-reactive cellular and humoral immunity in hemodialysis population. Kidney Int. 2021;99:1489–1490. doi: 10.1016/j.kint.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anand S., Montez-Rath M.E., Han J., et al. Serial SARS-CoV-2 receptor-binding domain antibody responses in patients receiving dialysis. Ann Intern Med. 2021;174:1073–1080. doi: 10.7326/M21-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labriola L., Scohy A., Seghers F., et al. A longitudinal, 3-month serologic assessment of SARS-CoV-2 infections in a Belgian hemodialysis facility. Clin J Am Soc Nephrol. 2021;16:613–614. doi: 10.2215/CJN.12490720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke C.L., Prendecki M., Dhutia A., et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99:1470–1477. doi: 10.1016/j.kint.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Vriese A.S., Van Praet J., Reynders M., et al. Longevity and correlation with disease severity of the humoral and cellular response to SARS-CoV-2 infection in haemodialysis patients. Clin Kidney J. 2021;14:2446–2448. doi: 10.1093/ckj/sfab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Candon S., Guerrot D., Drouot L., et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21:854–863. doi: 10.1111/ajt.16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen D.E., Sibbel S., Marlowe G., et al. Antibody status, disease history, and incidence of SARS-CoV-2 infection among patients on chronic dialysis. J Am Soc Nephrol. 2021;32:1880–1886. doi: 10.1681/ASN.2021030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lumley S.F., O’Donnell D., Stoesser N.E., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr E.J., Kronbichler A., Graham-Brown M., et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Praet J., Reynders M., De Bacquer D., et al. Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: a multicenter observational study. J Am Soc Nephrol. 2021;32:3208–3220. doi: 10.1681/ASN.2021070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Espi M., Charmetant X., Barba T., et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100:928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lacson E., Argyropoulos C.P., Manley H.J., et al. Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol. 2021;32:2735–2742. doi: 10.1681/ASN.2021040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broseta J.J., Rodríguez-Espinosa D., Rodríguez N., et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78:571–581. doi: 10.1053/j.ajkd.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulhern J.G., Fadia A., Patel R., et al. Humoral response to mRNA versus an adenovirus vector-based SARS-CoV-2 vaccine in dialysis patients. Clin J Am Soc Nephrol. 2021;16:1720–1722. doi: 10.2215/CJN.06450521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grupper A., Sharon N., Finn T., et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16:1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yanay N.B., Freiman S., Shapira M., et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99:1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rincon-Arevalo H., Choi M., Stefanski A.L., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 66.Schrezenmeier E., Bergfeld L., Hillus D., et al. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front Immunol. 2021;12:690698. doi: 10.3389/fimmu.2021.690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sattler A., Schrezenmeier E., Weber U.A., et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simon B., Rubey H., Treipl A., et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36:1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goupil R., Benlarbi M., Beaubien-Souligny W., et al. Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ. 2021;193:E793–E800. doi: 10.1503/cmaj.210673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anand S., Montez-Rath M.E., Han J., et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agur T., Ben-Dor N., Goldman S., et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—a prospective cohort study. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab155 Published online April 11, 2021. [DOI] [PMC free article] [PubMed]
- 72.Duarte R., Roldão M., Figueiredo C., et al. Humoral response to BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis patients: a comparative study. Ther Apher Dial. Published online November 27, 2021. [DOI] [PMC free article] [PubMed]
- 73.Quiroga B., Soler M.J., Ortiz A., et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab313 Published online November 12, 2021. [DOI] [PMC free article] [PubMed]
- 74.Speer C., Schaier M., Nusshag C., et al. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines. 2021;9:1130. doi: 10.3390/vaccines9101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patecki M., Merscher S., Dumann H., et al. Similar humoral immune responses in peritoneal dialysis and haemodialysis patients after two doses of the SARS-CoV-2 vaccine BNT162b2. Perit Dial Int J. 2022;42:100–101. doi: 10.1177/08968608211055631. [DOI] [PubMed] [Google Scholar]
- 76.Nacasch N., Cohen-Hagai K., Benchetrit S., et al. Comparison of long-term antibody response to mRNA SARS-CoV-2 vaccine among peritoneal dialysis and hemodialysis patients. Nephrol Dial Transplant. 2022;37:602–604. doi: 10.1093/ndt/gfab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ducloux D., Colladant M., Chabannes M., et al. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100:702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frantzen L., Thibeaut S., Moussi-Frances J., et al. COVID-19 vaccination in haemodialysis patients: good things come in threes…. Nephrol Dial Transplant. 2021;36:1947–1949. doi: 10.1093/ndt/gfab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bensouna I., Caudwell V., Kubab S., et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis. 2022;79:185–192.e1. doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longlune N., Nogier M.B., Miedougé M., et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant. 2021;36:1704–1709. doi: 10.1093/ndt/gfab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dekervel M., Henry N., Torreggiani M., et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin Kidney J. 2021;14:2349–2355. doi: 10.1093/ckj/sfab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stervbo U., Blazquez-Navarro A., Blanco E.V., et al. Improved cellular and humoral immunity upon a second BNT162b2 and mRNA-1273 boost in prime-boost vaccination no/low responders with end-stage renal disease. Kidney Int. 2021;100:1335–1337. doi: 10.1016/j.kint.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Espi M., Charmetant X., Barba T., et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2022;101:390–402. doi: 10.1016/j.kint.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P., Nair M.S., Liu L., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 85.Chen X., Chen Z., Azman A.S., et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled meta-analysis. Clin Infect Dis. 2022;74:734–742. doi: 10.1093/cid/ciab646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wall E.C., Wu M., Harvey R., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khoury D.S., Steain M., Triccas J., et al. A meta-analysis of early results to predict vaccine efficacy against Omicron. medRxiv. https://doi.org/10.1101/2021.12.13.21267748 Published online December 17, 2021.
- 88.Speer C., Benning L., Töllner M., et al. Neutralizing antibody response against variants of concern after vaccination of dialysis patients with BNT162b2. Kidney Int. 2021;100:700–702. doi: 10.1016/j.kint.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bassi J., Giannini O., Silacci-Fregni C., et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carr E.J., Wu M., Harvey R., et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet. 2021;398:1038–1041. doi: 10.1016/S0140-6736(21)01854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carr E.J., Wu M., Harvey R., et al. Omicron neutralising antibodies after COVID-19 vaccination in haemodialysis patients. Lancet. 2022;399:800–802. doi: 10.1016/S0140-6736(22)00104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Vriese AS, Van Praet J, Reynders M, et al., Longevity and clinical effectiveness of the humoral and cellular response to SARS-CoV-2 vaccination in hemodialysis patients. Kidney Int Rep. Published online February 22, 2022. https://doi.org/10.1016/j.ekir.2022.02.007 [DOI] [PMC free article] [PubMed]
- 93.Angel-Korman A., Peres E., Bryk G., et al. Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel: the case for a third vaccine dose. Clin Kidney J. 2021;15:226–234. doi: 10.1093/ckj/sfab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anand S., Montez-Rath M.E., Han J., et al. SARS-CoV-2 vaccine antibody response and breakthrough infection in dialysis. Ann Intern Med. https://doi.org/10.7326/M21-4176 Published online December 14, 2021. [DOI] [PMC free article] [PubMed]
- 95.Hsu C.M., Weiner D.E., Manley H.J., et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients over six months. medRxiv. https://doi.org/10.1101/2021.09.13.21263535 Published online September 17, 2021.
- 96.Davidovic T., Schimpf J., Abbassi-Nik A., et al. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021;100:1334–1335. doi: 10.1016/j.kint.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goggins E., Sharma B., Ma J.Z., et al. Long term humoral immunity decline in hemodialysis patients following SARS-CoV-2 vaccination. medRxiv. https://doi.org/10.1101/2021.12.01.21265957 Published online December 17, 2021.
- 98.Strengert M., Becker M., Ramos G.M., et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis. EBioMedicine. 2021;70:103524. doi: 10.1016/j.ebiom.2021.103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bertrand D., Hamzaoui M., Lemée V., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32:2147–2152. doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steensels D., Pierlet N., Penders J., et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaiser R.A., Haller M.C., Apfalter P., et al. Comparison of BNT162b2 (Pfizer-BioNtech) and mRNA-1273 (Moderna) SARS-CoV-2 mRNA vaccine immunogenicity in dialysis patients. Kidney Int. 2021;100:697–698. doi: 10.1016/j.kint.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Billany R.E., Selvaskandan H., Adenwalla S.F., et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int. 2021;99:1492–1494. doi: 10.1016/j.kint.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sibbel S., McKeon K., Luo J., et al. Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in patients on hemodialysis. J Am Soc Nephrol. 2022;33:49–57. doi: 10.1681/ASN.2021060778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El Karoui K., Hourmant M., Ayav C., et al. Vaccination and COVID-19 dynamics in hemodialysis patients: a population-based study in France. medRxiv. https://doi.org/10.1101/2021.07.06.21259955 Published online July 7, 2021.
- 107.Manley H.J., Aweh G.N., Hsu C.M., et al. SARS-CoV-2 vaccine effectiveness and breakthrough infections in maintenance dialysis patients. medRxiv. https://doi.org/10.1101/2021.09.24.21264081 Published online September 29, 2021.
- 108.New analyses of two AZD7442 COVID-19 phase III trials in high-risk populations confirm robust efficacy and long-term prevention. https://www.astrazeneca.com/media-centre/press-releases/2021/new-analyses-of-two-azd7442-covid-19-phase-iii-trials-in-high-risk-populations-confirm-robust-efficacy-and-long-term-prevention.html
- 109.Wu K., Choi A., Koch M., et al. Variant SARS-CoV-2 mRNA vaccines confer broad neutralization as primary or booster series in mice. Vaccine. 2021;39:7394–7400. doi: 10.1016/j.vaccine.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pfizer and BioNTech provide update on omicron variant. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant
- 111.Johnson & Johnson to evaluate its COVID-19 vaccine against new Omicron COVID-19 variant. https://www.jnj.com/johnson-johnson-to-evaluate-its-covid-19-vaccine-against-new-omicron-covid-19-variant
- 112.Moderna announces preliminary booster data and updates strategy to address Omicron variant. https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx
- 113.Nemet I., Kliker L., Lustig Y., et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doria-Rose N.A., Shen X., Schmidt S.D., et al. Booster of mRNA-1273 vaccine reduces SARS-CoV-2 Omicron escape from neutralizing antibodies. medRxiv. https://doi.org/10.1101/2021.12.15.21267805 Published online December 15, 2021.
- 115.Tan C.W., Chia W.N., Young B.E., et al. Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N Engl J Med. 2021;385:1401–1406. doi: 10.1056/NEJMoa2108453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubin R. Trying to block SARS-CoV-2 transmission with intranasal vaccines. JAMA. 2021;326:1661–1663. doi: 10.1001/jama.2021.18143. [DOI] [PubMed] [Google Scholar]
- 117.Garcia P., Montez-Rath M.E., Moore H., et al. SARS-CoV-2 vaccine acceptability in patients on hemodialysis: a nationwide survey. J Am Soc Nephrol. 2021;32:1575–1581. doi: 10.1681/ASN.2021010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Semenzato L., Botton J., Drouin J., et al. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loader J., Lampa E., Gustafsson S., et al. Renin-angiotensin aldosterone system inhibitors in primary prevention and COVID-19. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Puskarich M.A., Cummins N.W., Ingraham N.E., et al. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37:100957. doi: 10.1016/j.eclinm.2021.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chawki S., Buchard A., Sakhi H., et al. Treatment impact on COVID-19 evolution in hemodialysis patients. Kidney Int. 2020;98:1053–1054. doi: 10.1016/j.kint.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Soler M.J., Noordzij M., Abramowicz D., et al. Renin-angiotensin system blockers and the risk of COVID-19-related mortality in patients with kidney failure. Clin J Am Soc Nephrol. 2021;16:1061–1072. doi: 10.2215/CJN.18961220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gerber G.F., Chaturvedi S. How to recognize and manage COVID-19-associated coagulopathy. Hematology Am Soc Hematol Educ Program. 2021;2021:614–620. doi: 10.1182/hematology.2021000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Desbuissons G., Michon A., Attias P., et al. Arteriovenous fistulas thrombosis in hemodialysis patients with COVID-19. J Vasc Access. https://doi.org/10.1177/1129729821996091 Published online February 24, 2021. [DOI] [PubMed]
- 126.Khoo B.Z.E., Lim R.S., See Y.P., Yeo S.C. Dialysis circuit clotting in critically ill patients with COVID-19 infection. BMC Nephrol. 2021;22:141. doi: 10.1186/s12882-021-02357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grenon E., Canet E. High incidence of circuit clotting in critically ill COVID-19 patients treated with renal replacement therapy. J Am Soc Nephrol. 2021;32:1823–1824. doi: 10.1681/ASN.2021040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seet C., Lindsey B., Sivaprakasam R., et al. The management of dialysis access thrombosis during the COVID-19 pandemic. J Vasc Access. https://doi.org/10.1177/11297298211045578 Published online September 18, 2021. [DOI] [PubMed]
- 129.ATTACC Investigators. ACTIV-4a Investigators. REMAP-CAP Investigators. Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bhimraj A., Morgan R.L., Shumaker A.H., et al. IDSA guidelines on the treatment and management of patients with COVID-19. COVID-19 guideline, Part 1: treatment and management. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- 131.Major R., Selvaskandan H., Makkeyah Y.M., et al. The exclusion of patients with CKD in prospectively registered interventional trials for COVID-19—a rapid review of international registry data. J Am Soc Nephrol. 2020;31:2250–2252. doi: 10.1681/ASN.2020060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sidebottom D.B., Smith D.D., Gill D. Safety and efficacy of antivirals against SARS-CoV-2. BMJ. 2021;375:n2611. doi: 10.1136/bmj.n2611. [DOI] [PubMed] [Google Scholar]
- 133.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., Al-Beidh F., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aiswarya D., Arumugam V., Dineshkumar T., et al. Use of remdesivir in patients with COVID-19 on hemodialysis: a study of safety and tolerance. Kidney Int Rep. 2021;6:586–593. doi: 10.1016/j.ekir.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Brien M.P., Forleo-Neto E., Musser B.J., et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 142.ACTIV-3/TICO LY-CoV555 Study Group. Lundgren J.D., Grund B., Barkauskas C.E., et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hastie K.M., Li H., Bedinger D., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vellas C., Del Bello A., Debard A., et al. Influence of treatment with neutralizing monoclonal antibodies on the SARS-CoV-2 nasopharyngeal load and quasispecies. Clin Microbiol Infect. 2022;28:139.e5–139.e8. doi: 10.1016/j.cmi.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]