Abstract
Memory, cognition, executive functioning, and spatial cognition loss are prevalent in the normal aging process, but these impairments are observed more extensively in individuals with dementia, specifically Alzheimer’s disease. To improve the impaired functions, serious games targeting the lost functions are commonly developed and used in training programs. In this study, we designed a virtual reality driving simulator (VRDS) as a serious game with different difficulty levels for improving the spatial cognition; we evaluated it on 11 participants with different levels of dementia for two weeks, every day except weekends (10 sessions of practice in total) and 30 min/day. We assessed the participants’ spatial cognition before and after the intervention by an independent assessment (the VR replica of Morris Water test) and also by their performance playing the VRDS during the intervention. We also assessed the participants’ mood by a standard depression scale as well as their plausible experience of simulation sickness. The results showed significant improvement in Morris water test. The participants’ normalized correct trajectory (to find the target) was improved significantly by 44.4% at post-intervention with respect to baseline. Furthermore, on average, the participants progressed to higher (more challenging) levels of the game, and their spatial learning score increased throughout the sessions. Their mood also showed improvement with respect to baseline. Overall, the results hold promise for the designed VRDS as a mood-lifting and enhancing spatial skills serious game for older adults if it is played regularly.
Trial Registry name: Investigating the Effect of Training with a Virtual Reality Driving Simulator
URL: https://clinicaltrials.gov/ct2/show/NCT04074655
Clinical Trials.gov ID: NCT04074655
Keywords: Serious games, virtual reality, driving simulator, spatial cognition, dementia, Alzheimer’s disease
Significance Statement
The application of virtual reality serious games is beneficial in cognitive training and improving brain plasticity.
A custom virtual reality driving simulator is designed and developed to be used as a cognitive training tool in a pilot study for people with different levels of dementia.
Far and near effects of the designed training program was demonstrated to be significantly effective on participants’ spatial cognition.
The results suggest the designed driving simulator can be considered as a mood uplifting, engaging and effective serious game for retraining the spatial cognition of older adults.
Introduction
Dementia, specifically Alzheimer’s disease, is one of the most prevalent health issues of the century. 1 Associated with Alzheimer’s, not only one’s cognition, memory and executive functioning are deteriorated, 2 but also their spatial cognition/processing may become impaired significantly. 3 In comparison to young adults, healthy seniors may show a mild degree of deterioration performing the tasks associated with spatial processing 4 but those affected by Alzheimer’s show significant deterioration.3,5,6
Based on neuroplasticity of the brain, cognitive training can change the brain and generate new brain functional connections. 7 Thus, for slowing the progress of neurodegenerative dementia, cognitive training has been suggested as an approach for improving the impaired brain functions. 8 Virtual reality (VR) has been utilized not only for gaming but also for conducting different neuroscience studies varying from treating phobias and stress9,10 to improve the brain’s cognitive functions.6,11 In comparison to real-world training settings, it is easier to change a virtual environment for training purposes. 10 Moreover, VR trainings are safer, and if designed as serious games, they are more enjoyable for users.9,10,12
VR serious games simulating a naturalistic environment have been used for training or education purposes successfully.6,11,13,14 A virtual driving simulator is a class of VR serious games, which can have various applications such as improving vehicle manufacturing 15 and evaluating one’s hazardous driving habits. 2 For instance, using a semi-immersive driving simulator, researchers have demonstrated that Alzheimer’s patients have a longer reaction time in mental flexibility tasks; that is, they drove with a slower average speed in comparison to their age-matched healthy peers. 2 In another similar study, 16 the relationship between visual attention and driving performance were found to affect one another. It was found training with a commercial semi-immersive driving simulator would positively affect older adults’ visual attention. Furthermore, the effects of simulator training on the participants’ physical mobility were investigated and the results showed no relationship between the two. 16
Another study used a non-immersive driving simulator to compare driving performances of three groups of participants: healthy older adults as the control group, individuals with mild cognitive impairment (MCI) and those with probable Alzheimer’s. 17 The results showed that both MCI and Alzheimer’s groups had a poorer driving performance than the control group. The errors of those two groups were mainly due to attention deficit, false judgment and/or being too cautious.
Using driving simulators, the driving behavior of MCI population has been evaluated.18,19 For instance, various levels of in-vehicle-distractions and their effects on simulator driving performance were evaluated in. 18 Their results showed MCI population were more prone to distractions such as talking on a cellphone than the age-matched healthy controls. Another similar study evaluated the MCI drivers’ estimation of their driving performance and compared it to their actual simulator driving metrics. 19 Their results demonstrated MCI participants mostly overestimated their driving skills.
Driving simulators have also been used to investigate the biomechanical aspects of driving in healthy adults and seniors. 20 In that study, an immersive driving simulator was exploited to evaluate the neck and the whole upper body rotation and their effects on participants’ performance for blind spot checking. Their results showed that in blind spot-checking task, the upper body rotation is significantly greater than the neck rotation. Furthermore, seniors had a poorer performance in the driving task in comparison to younger adults. 20
Other than previous studies, our team used an immersive simple driving simulator for a participant at the onset of Alzheimer’s as a cognitive training program. 14 The results of that case-study showed the participant did improve in simulated driving task and enjoyed the practice sessions immensely. In this current pilot study, we designed a VR driving simulator and evaluated it in a cognitive training program for older adults with various degrees of dementia. Our program’s aim was to improve users’ navigational abilities and spatial cognition as well as providing an enjoyable and uplifting experience for individuals with dementia.
Materials and methods
We designed a driving simulator, named VRDS, as a serious game with three different difficulty levels and a naturalistic rural road with several traffic elements and checkpoints. The VRDS was designed using Unity 3D game engine and Integrated Development Environment version 5.1.3. along with realistic 3D models and assets as the main simulator tool 21 and a head mounted display (HMD) (Oculus Rift DK2) as the main immersion tool. The VRDS is rendered using NVIDIA GTX980M G-SYNC GPU and is run on a personal laptop. Wingman formula force GP, including steering wheel and pedals, is used as the main input device and interaction paradigm, to communicate commands and act as the interface between users and the simulator. It is also possible to control the driving simulator using keyboards’ arrow keys.
Based upon the game’s level, there are a number of intersections in the rural road with stop signals and traffic lights. The initial state of the traffic light is always green. When the virtual vehicle gets close enough to the intersection, that is, 30 Virtual Units (VUs), the state of the traffic light changes to red. Afterwards with a 0.1 frequency, the state of the traffic light alternates between green and red. There are also upcoming cars at different points of the road. At the beginning of each level and 90 VUs from the start line, a deer is hidden inside of bushes. When the distance between the virtual vehicle and the deer is less than 12 VUs, the deer starts running towards the other side of the road. If users brake right upon observing the deer, the vehicle stops after 1 second and 5.55 VUs from the deer. Otherwise, the animal will be hit.
To evaluate the VRDS’s participants’ performance, using an interactive input field, the game generates a .CSV file that is unique for each user and each trial a participant uses the game. We placed several invisible checkpoints at various points of the road. The indices of the passed checkpoints, the speed of the vehicle while passing the checkpoints, the vehicle’s trajectory in the Cartesian system and with respect to Unity axes, the time of passing each checkpoint, the stops at all traffic lights and signs (if they did stop) and the number of crashes between each two consecutive checkpoints (if any) are logged in the generated .CSV file of each user at each training trial.
When playing a VR game in the immersive mode, it is more probable for users to experience simulation sickness syndrome.22,23 Hence, the VRDS has been designed to operate in both immersive or non-immersive VR modes using either Oculus Rift-DK2 or a regular laptop screen. In the case of using the VRDS with the HMD, the design has been ensured to have a smooth graphical transition between the data frames of the headset. In our study’s intervention phase, we started the training with the VRDS in the immersive mode for all of the participants. A trained research assistant was present during the entire time of the game’s usage to monitor for any plausible simulation sickness symptoms and change the setup of the viewing mode to non-immersive if necessary, that is, when a participant experienced symptoms of simulation sickness.
The designed VRDS consists of two phases: (1) Demo, in which users observe a virtual vehicle being driven automatically on a path; they are instructed by the game’s audio message to memorize the observed path, specifically the correct turns at intersections. (2) Training phase, in which users are instructed by an audio message to recall the demo path to drive it themselves. If users turn into a wrong direction at an intersection, an audio message warns them to go back to the correct route. Furthermore, when users finish a level, a reward message accompanying audio and fireworks are displayed for them.
Study participants
We evaluated our VRDS training program on 11 participants (3 males, 77 ± 10.1 years). The level of cognitive status of the participants was assessed by Montreal Cognitive Assessment (MoCA) 24 at baseline. Out of the 11 participants, 3 of them were healthy with MoCA>25, 4 had 21≤ MoCA≤25 and were considered as MCI, and 4 participants had MoCA<21 and were diagnosed with early to moderate stage of Alzheimer’s. In addition, we enrolled one participant (77 years) with advanced Alzheimer’s and a MoCA score of 2 as an especial case. All participants (and also their legal guardian in case of the Alzheimer’s participants) signed a written informed consent form approved by the Biomedical Research Ethics Board of the University of Manitoba (approval number: HS23059 (B2019:073)) prior to being enrolled into the study. Table 1 demonstrates the demographic information of the participants.
Table 1.
Participants’ Demographic Information; numbers show mean ± stdev.
| Group | MoCA score | Age (years) | Sex |
|---|---|---|---|
| Healthy (n = 3) | 26.7 ± 1.2 | 85 ± 6 | 1 Male |
| MCI (n = 4) | 22.8 ± 1.5 | 79 ± 10.5 | 1 Male |
| Alzheimer’s (n = 4) | 15.3 ± 5.6 | 69 ± 7.1 | 1 Male |
| All (n = 11) | 21.1 ± 5.9 | 77 ± 10.1 | 3 Males |
| Advanced Alzheimer’s participant | 2 | 77 | Male |
Study protocol
A previously conducted study has demonstrated the effectiveness of cognitive training programs for improving spatial cognition in seniors with cognitive declines, if it is practiced for at least 30 minutes per day and for minimum of 10 sessions of practice. 6 As a pilot study and also that our driving simulator was a relatively simple game to learn, we considered the duration of our cognitive driving simulator program to be 10 sessions of practice and for half-an-hour per day. As our primary outcome measure (VR Morris water test) has a 2-week washout period, we scheduled the baseline and post-intervention assessments to be at least 2 weeks apart.
We used a non-immersive VR replica of the standard spatial Morris water test 25 as an independent assessment to evaluate the participants’ spatial cognition; this test was also used in one of our team’s previous studies. 14 Furthermore, we used Montgomery Asberg Depression Scale (MADRS) questionnaire for rating the participants’ mood and depression level, and Simulator Sickness Questionnaire (SSQ) for quantifying their plausible simulation sickness degree. We assessed the participants’ spatial cognition and depression at baseline and post-intervention. SSQ assessment was run after the first and last sessions of training with the driving simulator. The especial case of the study was not assessed with the independent assessment (VR Morris water test) as was not capable of understanding the instructions of the test due to his advanced Alzheimr’s.
The VR Morris water test assesses the spatial cognition and spatial learning capability. In this test, participants are instructed to locate a fixed-position hidden target within a circular arena of diameter 5 VUs, using distal cues present in the environment, such as different trees, that are learnt and practiced during training trials. This test has four training and one test trials. In training trials, participants have 45 seconds to locate the target. If they are not successful in finding the target before 45 seconds, the target becomes visible to participants; thus, they can learn and remember the target’s location. The target is always located in the middle of the Northwest or the Southwest quadrant of the maze; its location is counter-balanced between the participants and baseline and post-intervention assessment sessions. On the other hand, the initial location of the user changes in each trial. In the test trial, participants have to locate the target within 45 seconds, before the trial ends automatically. The target is not shown to the participants at the end.
To evaluate the performance of participants in the VR Morris water test, the traversed trajectory of each participant in the test trial was plotted, and the total traversed path and the correct trajectory were extracted. The correct trajectory is defined as the part of the trajectory that occurs in the quadrant of the target. We normalized the correct trajectory with respect to the total trajectory and defined it as the performance metric for the testing trials of VR Morris water test. Moreover, for each training trial of this test, the total time spent for finding the target was also calculated as another evaluation metric.
In addition, we evaluated the participants’ progress while training with the VRDS. Using the logged information of the game, it is possible to decide whether users have braked for red signals and stop signs and/or whether they have turned to the correct directions at the intersections. Using this information, we defined a (spatial) learning score as follows:
| (1) |
| (2) |
| (3) |
In equation (1), the direction error is when participants turn to a wrong direction at an intersection, or a direction other than what had been demonstrated in the demo of the game. Traffic light error and stop sign error indicate participants did not stop when they should have.
The maximum error in equation (2) represents a situation, in which a participant turns to the wrong direction at all intersections and passes every red signal and every stop sign without stopping properly. The overall spatial Learning Score, defined in equation (3), considers the difference in difficulty of the levels, and assigns more weight to the learning at more challenging levels.
Hypotheses and Statistical Analysis
We hypothesized: (1) Repeated practice with the driving simulator would improve participants’ spatial cognition measured by the spatial learning score and Morris water test outcomes, (2) The designed cognitive program would have a positive effect on users’ mood measured by MADRS assessment score, and (3) Repeated exposure to the VRDS would not significantly impact the severity of the simulation sickness measured by SSQ questionnaire score.
The statistical analysis was conducted in R. 26 To investigate the first hypothesis, we used repeated measure multivariate analysis of variance (MANOVA) using R’s MANOVA.RM package, 27 followed by post-hoc analysis for each of the dependent variables of the Morris water test (time and normalized correct trajectory). Furthermore, for investigating the other hypotheses, we used paired t-test. In case, the paired t-test assumptions were not met, we used the equivalent nonparametric and distribution-free Wilcoxon-sign ranked test. In all instances, a P-value ≤.05 was considered significant; in post-hoc analysis, the Bonferroni-correction was applied to consider a P-value ≤ .025 as significant (2 dependent variables and 2 time points).
Results
We had to exclude data of one of the healthy participants (MoCA = 28) due to her eyes’ sensitivity that she could not focus on the screen and finish the assessments. The results presented here are the average outcomes of the 10 remaining participants. We also reported the progress of the especial advanced Alzheimer’s participant in using the VRDS.
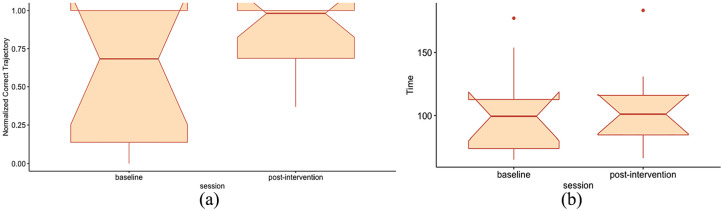
The main intendent spatial assessment for this study was the virtual replica of the Morris water test. None of the participants were able to pass the training trials of the VR Morris water test fully (finding the target before 45 seconds) neither at baseline nor at post-intervention. The repeated measure MANOVA on the time variable of the training trials and the normalized correct trajectory of the test trials showed significant difference at post-intervention with respect to baseline (P < .01). Both the total time and the normalized correct trajectory metrics increased from baseline to post-intervention: the total time from 103.5 ± 11.6 seconds to 105.2 ± 11.0 seconds and the normalized correct trajectory from 0.58 ± 0.14 to 0.84 ± 0.07 as a significant improvement in the pos-hoc analysis (P < .025). The results are shown in Figure 1 and Table 2.
Figure 1.
VR Morris water test, performance metrics: (a) normalized correct trajectory, testing trials and (b) time, training trials.
Table 2.
Descriptive statistics (mean ± SE) of the evaluated variables.
| MWT, training trials (n = 10) | MWT, testing trials (n = 10) | SSQ (n = 10) | ||||
|---|---|---|---|---|---|---|
| Descriptive statistics | Total time | Normalized correct trajectory | Nausea | Oculomotor | Disorientation | Total |
| Baseline | 103.5 ± 11.6 | 0.58 ± 0.14 | 7.6 ± 4 | 7.6 ± 3.2 | 4.2 ± 3 | 7.9 ± 3.1 |
| Post-intervention | 105.2 ± 11.0 | 0.84 ± 0.07* | 8.6 ± 3.3 | 4.7 ± 1.7 | 9.7 ± 5.5 | 8.1 ± 3.1 |
Abbreviation: MWT, Morris water test.
Significant difference (P << .025).
Although the number of participants in this pilot study were small, since their cognitive status differed significantly, we also calculated the above metrics in three subgroups of healthy, MCI and Alzheimer’s. Figure 2 shows the difference in the averaged normalized correct trajectory for the participants’ subgroups from baseline to post-intervention. As can be seen, the participants’ normalized correct trajectory improved at post-intervention noticeably with greater difference for the subgroups with higher MoCA score. We did not run statistical analysis on the subgroups’ data as the sample size in each subgroup was too small for a meaningful statistical analysis.
Figure 2.
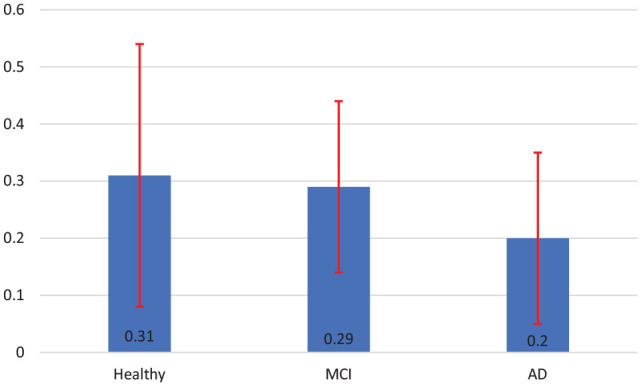
Difference in the normalized correct trajectory from baseline to post-intervention, testing trials of the Morris water test (mean ± SE), Healthy participants (n = 2), MCI participants (n = 4) and Alzheimer’s participants (n = 4).
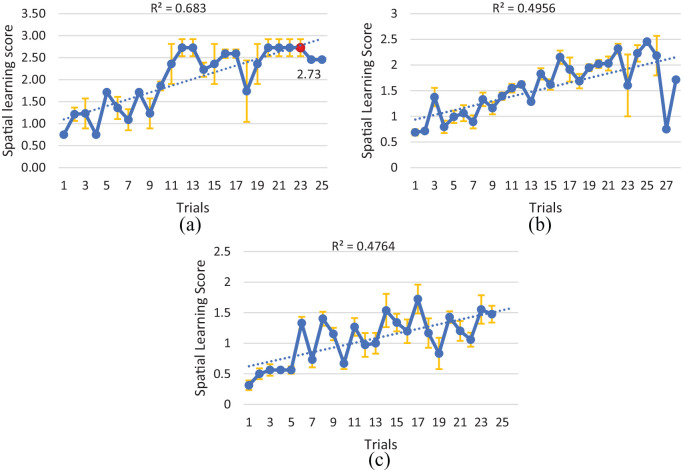
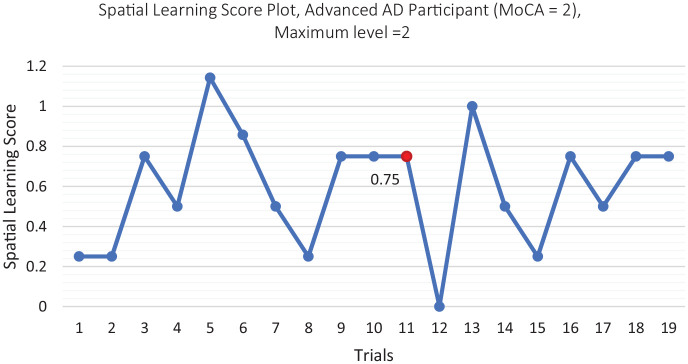
Table 3 demonstrates the details of how many trials and at what difficulty level each participant played the VRDS over the two weeks period of the study. Figure 3 shows the spatial learning score plots of the three cognitive subgroups over the two weeks period of the study. As can be seen, the spatial learning scores of all subgroups show an increasing trend (improvement) as expected. Figure 4 shows the spatial learning score of the advance Alzheimer’s participant, who was enrolled in the study as an especial case. His spatial learning scores also have improved throughout the training sessions.
Table 3.
Participants’ descriptive information in using the VRDS.
| Participant | Condition | Total number of trials | Number of trials-Level 1 | Number of trials-Level 2 | Number of trials-Level 3 |
|---|---|---|---|---|---|
| 1 | Healthy | 26 | 6 | 8 | 12 |
| 2 | Healthy | 23 | 4 | 7 | 12 |
| 3 | MCI | 20 | 4 | 9 | 7 |
| 4 | MCI | 21 | 9 | 8 | 4 |
| 5 | MCI | 26 | 5 | 13 | 8 |
| 6 | MCI | 43 | 12 | 21 | 10 |
| 7 | AD | 27 | 14 | 11 | 2 |
| 8 | AD | 26 | 12 | 14 | 0 |
| 9 | AD | 17 | 8 | 7 | 2 |
| 10 | AD | 24 | 12 | 12 | 0 |
| Mean ± stdev. | 25.3 ± 7.0 | 8.6 ± 3.7 | 11 ± 4.3 | 5.7 ± 4.7 | |
| 11 | Advanced AD | 19 | 17 | 2 | 0 |
Abbreviations: MCI, Mild Cognitive Impairment; AD, Alzheimer’s disease.
Figure 3.
Spatial learning score plot (mean ± SE): (a) healthy participants (n = 2), optimum learning: red, (b) MCI participants (n = 4), and (c) Alzheimer’s participants (n = 4).
Figure 4.
Spatial learning score plot, especial case of the study, the red dot refers to the marked improvement.
The participants’ MADRS score comparison analysis at post-intervention with respect to baseline, showed 14.3% improvement (from 2.1 ± 0.6 at baseline to 1.8 ± 0.4 at post-intervention); this difference was not statistically significant. However, if we exclude participants with the MADRS score equal to zero at baseline, the improvement becomes 23.8% (from 2.6 ± 0.6 at baseline to 2.0 ± 0.5 at post-intervention).
From the participants of our study, only three of them (two healthy participants and one participant with Alzheimer’s) were able to use the immersive mode of the VRDS fully. Other participants were experiencing dizziness, i.e. disorientation, as the main symptom of simulation sickness. Due to the vulnerability of our participants we did not insist on using the immersive mode and upon the slightest discomfort or the slightest symptoms of simulation sickness the training session was continued in the non-immersive mode using a regular laptop screen.
We analyzed the SSQ assessment and its three subcategories, oculomotor, nausea and disorientation symptoms, at baseline and post-intervention. All the sub-categories of the SSQ assessment and also its total mark showed no significant difference between baseline and post-intervention (Table 2).
Discussion
It has been demonstrated that computer based cognitive training programs can be beneficial for the population with MCI and dementia. These programs have had significant positive effects on the MCI population’s general cognition, verbal learning, verbal memory and working memory. Visuospatial memory and executive functioning have been reported to show improvement although not extensively by some cognitive training. 28 The overall effect of conducting computer training programs for dementia participants has been shown to be positive, with significantly positive outcomes on their spatial cognition. 28 In a case study, outcomes of a VR spatial training program were evaluated for a participant at the onset of Alzheimer’s. 6 The study demonstrated the training program’s promising effects on the patients’ VR navigation and real-world navigation. The participant was not only able to complete the navigational tasks in the VR environment with zero error after 8 weeks of training, but also, he was able to start driving independently. 6
Evaluation of a cognitive training serious game, such as our VRDS in this study, can be done from two different but related perspectives: near and far effects of the program’s repeated usage. Near effects are the improvements (if any) achieved on the trained tasks during a cognitive training program; that is the trend of the spatial learning score plot in our study. Far effects are the plausible improvements measured by an independent assessment (but conceptually similar to the training task) on what the training is focused on; that is the results of the Morris water test in our study.
The results of our independent assessment to investigate the far effect of our training program (VR Morris water test) show significant improvement from baseline to post-intervention for the normalized correct trajectory (Figure 2). On the other hand, none of the participants were able to find the target within the cut-off duration of the assessment’s training trials (45 seconds). This may imply that, although the Morris water test is a challenging assessment for older cognitively impaired participants, they are still able to demonstrate improved performance.
In previous studies, it was shown healthy (control) participants were able to exploit distal cues more efficiently than participants with dementia, to travel a direct path towards the target in the Morris water test.29,30 In another study, younger adults in comparison to healthy seniors had more cross overs with the target and spent most of their traversed trajectory in the quadrant of the target (higher normalized correct trajectory). 31 Congruent with those studies, the participants of our study spent a larger portion of their traversed trajectory in close approximation of the target after the two weeks training with the VRDS.
The near effect of our training program can be observed by the upward trend of spatial learning scores during the intervention sessions. As expected and congruent with previous research, 32 the healthy group learned better than MCI group, and also the MCI group learned better than Alzheimer’s group. To investigate the learning effect further, we define an optimum learning for each level, and investigate which participants’ subgroup reached that optimum learning. We say a cognitive subgroup has achieved the optimum learning when the subgroup could achieve a learning score of at least, 80% of the level’s maximum score, and maintain that learning score for at least three consecutive trials of the game at the same level. In the spatial learning score plots, the optimum learning points (if it was reached) are demonstrated by a red mark on Figure 3. As observed on Figure 3, only healthy participants achieved optimum learning (Figure 3a). This implies the MCI and Alzheimer’s participants might have needed a longer training period to reach a plateau in their learning score plots. On the other hand, the especial case of the study (the advance stage Alzheimer’s participant, Figure 4) showed a plateau equal to 75%. Although he did not achieve the optimum learning, he had an improvement roughly equal to the optimum learning. This implies even an advanced Alzheimer’s participant can improve by training but perhaps needs much longer training period. Thus, the two weeks duration of our training program is probably enough for healthy older adults to reach a marked improvement, but not long enough for MCI and Alzheimer’s. In previous training programs for individuals with some memory problems, the duration of the study was 8 weeks for 3 days/week. 33
Comparing the results of the SSQ assessment between baseline and post-intervention did not demonstrate any significant increase or decrease in the severity of the simulation sickness, which verifies our hypothesis. This was more and less the same for those three participants who used the VRDS in immersive and the rest who used it in non-immersive mode. Previously it was shown that repeated exposure to a VR environment would decrease the severity of simulation sickness, 34 but repeated practice with the VRDS did not cause VR adaptation, nor it increased its symptoms. A previous study 35 compared the effect of the input devices on simulation sickness when the VR task involves navigation. That study found that the best input paradigm to reduce the simulation sickness of the immersive mode of the operation is when users experience VR movement while moving in the real world; that was achieved by a custom designed wheelchair. 12 However, such an input device needs a large space for movement which was not affordable in our study. Thus, we considered several other approaches to reduce the plausible simulation sickness. While designing the VRDS, we tested it many times on a few older adult volunteers of our team. Furthermore, we designed the simulator to have smooth frame transition in the immersive mode. We also manipulated the steering wheel to have a low range of rotation for preventing the participants to experience simulation sickness, while turning in intersections. Moreover, we reduced the maximum speed of the virtual vehicle to be as low as 20 .
A previous study, 22 quantified the simulation sickness experienced by participants due to using various types of non/immersive displays. In that study, after watching a panoramic video on a VR platform, participants were asked to fill the SSQ assessment. The reported result for each display was averaged between the participants. Their results demonstrated the lowest SSQ scores for the non-immersive VR display (a TV screen) in comparison to other types of immersive headsets. 22 Comparing our SSQ assessment results with the result of that study for the non- immersive TV screen, our participants who only used the VRDS’s non-immersive mode of operation, experienced more or less the same severity of simulation sickness with the users of the TV screen.
One of our main goals in this study was to design a driving simulator training program that is engaging. The decreasing trend of depression scores of the participants from baseline to post-intervention implies the program was uplifting, even though the scores did not reach the statistical significance level; that could be due to small number of participants and also that there were a few participants with zero depression scores (power of the test less than 0.1). Nevertheless, the feedbacks from all participants were very positive.
The decreasing trend of the participants’ depression scores from baseline to post-intervention is in accordance with previous studies. A number of studies have demonstrated the effectiveness of cognitive serious gamification for improving mental health.28,36 These studies have demonstrated a moderate effect of computerized cognitive training programs for treating neuropsychiatric and depression symptoms in MCI population and participants with dementia with long lasting effects beyond follow up assessment sessions. 28 We observed improvement in our participants’ mood after the intervention; however, since we only assessed them at baseline and post-intervention, we are not sure how long that positive mood impact might have lasted. In a future study, we should conduct follow-up assessment sessions, for instance one month and 28 weeks after the end of intervention, to evaluate the temporal pattern of mood enhancement.
Conclusion
The main limitation of this research was its small sample size; thus, the power of our statistical tests was low (0.55). However, without a pilot study such as this one, it is usually not possible to estimate the required sample size to investigate a hypothesis with statistical rigor. Using the outcomes of this study and the suggested formula for sample size estimation, 37 a minimum of 34 participants is needed to achieve statistical tests with a moderate effect size of 0.5 and statistical power of at least 0.8 with P-value < .05.
Overall, the results of this pilot study show that the repeated practice with the designed VRDS, other than being an uplifting experience, has positive effects on older adults’ spatial cognition, even on those with different degrees of dementia. We plan to use the VRDS as another serious game in our current large study investigating the effect of brain exercises in individuals with dementia.
Acknowledgments
We acknowledge Dr. Kazushige Kimura for his comments and recommendations during the design and development stage of the driving simulator. Moreover, we acknowledge the volunteers who participated in the conducted pilot study to help us evaluate the cognitive effects of our designed serious game.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Mitacs accelerate program (Application Ref. IT15620) in partnership with Riverview Health Centre Foundation, Winnipeg, MB, Canada. The funding sources had no involvement in the collection, analysis and interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Note: Sogol Masoumzadeh is now affiliated with Electrical and Computer Engineering, McGill University, Montreal, Canada.
Author Contributions: Sogol Masoumzadeh: Methodology, Software, Verification, Formal Analysis, Investigation, Writing- Original Draft, Writing, Review and Editing, Project administration. Zahra Moussavi: Conceptualization, Methodology, Verification, Resources, Data Curation, Writing- Review and Editing, Supervision, Funding acquisition.
ORCID iDs: Sogol Masoumzadeh  https://orcid.org/0000-0003-3639-194X
https://orcid.org/0000-0003-3639-194X
Zahra Moussavi  https://orcid.org/0000-0001-9202-949X
https://orcid.org/0000-0001-9202-949X
References
- 1. Román GC. Stroke, cognitive decline and vascular dementia: the silent epidemic of the 21st century. Neuroepidemiology. 2003;22:161-164. [DOI] [PubMed] [Google Scholar]
- 2. Etienne V, Marin-Lamellet C, Laurent B. Mental flexibility impairment in drivers with early Alzheimer’s disease: a simulator-based study. IATSS Res. 2013;37:16-20. [Google Scholar]
- 3. Zen D, Byagowi A, Garcia M, Kelly D, Lithgow B, Moussavi Z. The perceived orientation in people with and without Alzheimer’s. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER); November 6-8, 2013; San Diego, CA. [Google Scholar]
- 4. Samadani A-A, Moussavi Z. The effect of aging on human brain spatial processing performance. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2012; San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 5. Pouya OR, Byagowi A, Kelly DM, Moussavi Z. Introducing a new age-and-cognition-sensitive measurement for assessing spatial orientation using a landmark-less virtual reality navigational task. Q J Exp Psychol. 2017;70:1406-1419. [DOI] [PubMed] [Google Scholar]
- 6. White PJ, Moussavi Z. Neurocognitive treatment for a patient with Alzheimer’s disease using a virtual reality navigational environment. J Exp Neurosci. 2016;10:JEN.S40827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20:265-276. [PMC free article] [PubMed] [Google Scholar]
- 8. Park DC, Bischof GN. The aging mind: neuroplasticity in response to cognitive training. Dialogues Clin Neurosci. 2013;15:109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strickland D, Hodges L, North M, Weghorst S. Overcoming phobias by virtual exposure. Commun ACM. 1997;40:34-39. [Google Scholar]
- 10. Maples-Keller JL, Bunnell BE, Kim S-J, Rothbaum BO. The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harv Rev Psychiatry. 2017;25:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aldaba C. Investigation of virtual reality locomotion technology effects on simulator sickness and application for neuro-cognitive training for participants with memory problems. 2018. https://search.lib.umanitoba.ca/discovery/search?query=any,contains,Investigation%20of%20virtual%20reality%20locomotion%20technology%20effects%20on%20simulator%20sickness%20and%20application%20for%20neuro-cognitive%20training%20for%20participants%20with%20memory%20problems&tab=Everything&search_scope=MyInst_and_CI&vid=01UMB_INST:UMB&offset=0
- 12. Byagowi A, Mohaddes D, Moussavi Z. Design and application of a novel virtual reality navigational technology (VRNChair). J Exp Neurosci. 2014;8:JEN.S13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García-Betances RI, Waldmeyer MTA, Fico G, Cabrera-Umpiérrez MF. A succinct overview of virtual reality technology use in Alzheimer’s disease. Front Aging Neurosci. 2015;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White P. An immersive virtual reality navigational tool for diagnosing and treating neurodegeneration. Libertas. 2016. [Google Scholar]
- 15. Lawson G, Salanitri D, Waterfield B. VR processes in the automotive industry. In: Kurosu M, eds. Human-Computer Interaction: Users and Contexts. HCI 2015. Lecture Notes in Computer Science, Cham: Springer; 2015; vol. 9171. [Google Scholar]
- 16. Haeger M, Bock O, Memmert D, Hüttermann S. Can driving-simulator training enhance visual attention, cognition, and physical functioning in older adults? J Aging Res. 2018; 2018:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein AC, Dubinsky RM. Driving simulator performance in patients with possible and probable Alzheimer’s disease. Ann Adv Automot Med. 2011; 55: 325-334. [PMC free article] [PubMed] [Google Scholar]
- 18. Beratis IN, Pavlou D, Papadimitriou E, et al. Mild cognitive impairment and driving: does in-vehicle distraction affect driving performance? Accid Anal Prev. 2017;103:148-155. [DOI] [PubMed] [Google Scholar]
- 19. Fragkiadaki S, Kontaxopoulou D, Beratis IN, et al. Self-evaluation of driving ability through a driving simulator experiment: differences between patients with Mild Cognitive Impairment (MCI) and healthy elderly drivers. Alzheimers Dement. 2017;13:724-724. [Google Scholar]
- 20. Chen KB, Xu X, Lin J-H, Radwin RG. Evaluation of older driver head functional range of motion using portable immersive virtual reality. Exp Gerontol. 2015; 70:150-156. [DOI] [PubMed] [Google Scholar]
- 21. Unity Technologies. Unity. https://unity.com. 2019.
- 22. Somrak A, Humar I, Hossain MS, Alhamid MF, Hossain MA, Guna J. Estimating VR sickness and user experience using different HMD technologies: an evaluation study. Future Gener Comput Syst. 2019; 94:302-316. [Google Scholar]
- 23. Brooks JO, Goodenough RR, Crisler MC, et al. Simulator sickness during driving simulation studies. Accid Anal Prev. 2010;42:788-796. [DOI] [PubMed] [Google Scholar]
- 24. Nasreddine ZS, Phillips NA, Bédirian VR, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [DOI] [PubMed] [Google Scholar]
- 25. Morris RG. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239-260. [Google Scholar]
- 26. R Core Team. R: a language and environment for statistical computing, 2017. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/ [Google Scholar]
- 27. Friedrich S, Konietschke F, Pauly M. MANOVA.RM: resampling-based analysis of multivariate data and repeated measures designs, 2019 R package version 0.3.4. https://CRAN.R-project.org/package=MANOVA.RM
- 28. Hill NT, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174:329-340. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton DA, Driscoll I, Sutherland RJ. Human place learning in a virtual Morris water task: some important constraints on the flexibility of place navigation. Behav Brain Res. 2002;129:159-170. [DOI] [PubMed] [Google Scholar]
- 30. Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77-84. [DOI] [PubMed] [Google Scholar]
- 31. Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851-859. [DOI] [PubMed] [Google Scholar]
- 32. Cherrier MM, Mendez M, Perryman K. Route learning performance in Alzheimer disease patients. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:159-168. [PubMed] [Google Scholar]
- 33. Garcia-Campuzano MT, Virues-Ortega J, Smith S, Moussavi Z. Effect of cognitive training targeting associative memory in the elderly: a small randomized trial and a longitudinal evaluation. J Am Geriatr Soc. 2013;61:2252-2254. [DOI] [PubMed] [Google Scholar]
- 34. Kawano N, Iwamoto K, Ebe K, et al. Slower adaptation to driving simulator and simulator sickness in older adults aging clinical and experimental research. Aging Clin Exp Res. 2012;24:285-289. [DOI] [PubMed] [Google Scholar]
- 35. Aldaba CN, Moussavi Z. Effects of virtual reality technology locomotive multi-sensory motion stimuli on a user simulator sickness and controller intuitiveness during a navigation task. Med Biol Eng Comput. 2020;58:143-154. [DOI] [PubMed] [Google Scholar]
- 36. Fleming TM, Bavin L, Stasiak K, et al. Serious games and gamification for mental health: current status and promising directions. Front Psychiatry. 2017;7:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suresh KP, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012; 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]