Abstract
Purpose
Despite the therapeutic advances, disease recurrence remains an ever-present threat to the health and well-being of breast cancer survivors. Assessment of circulating tumor cells (CTCs) and cancer stem cells (CSCs) during and after treatment may be of value in refining treatment.
Methods
Three 5 mL blood samples were taken from each patient: the first, at diagnosis; the second, after completion of neoadjuvant anthracyclin-based chemotherapy; and the third, a month after surgery and completion of adjuvant radiotherapy. The absolute numbers of CTCs were identified as CD45-cytokeratin+ cells. CTCs per 5 mL of blood were determined by recording all events in the whole suspension. CSCs were identified as cytokeratin+CD44+CD24-/CD45- cells. The CSCs were expressed as a percentage of CTCs.
Results
Univariate analysis identified the measurements of baseline CTCs and CSCs, taken after chemotherapy and one month after the cessation of radiotherapy, as prognostic factors for both four-year disease-free survival and four-year overall survival. Multivariable analysis identified the third measurement of CSCs, taken one month after the completion of radiotherapy, as the only independent prognostic factor for the four-year disease-free survival (P < 0.002, hazard ratio [HR] = 1.231, 95% CI 1.077–1.407). The initial CTC measurement was the one factor that reached significance on multivariate analysis (P < 0.03, HR 1.969, 95% CI 1.092–3.551) for the four-year overall survival. Correlation was higher between CTC and CSC counts at diagnosis (r = 0.654, P < 0.001) than after chemotherapy (r = 0.317, P < 0.03), because of the more rapid decrease in the mean CTC count with chemotherapy.
Conclusion
The CTC count could be suitable as one of the measures for monitoring response to chemotherapy, while persistence of CSC after cessation of the treatment of nonmetastatic breast cancer, except hormonal therapy when indicated, may be a reason to consider additional therapy in the future. These findings need confirmation in larger randomized trials.
Keywords: nonmetastatic breast cancer, CTC, CD44+CD24 CSC
Introduction
Breast cancer (BC) is the most common cancer among women. 1 Although the prognosis of nonmetastatic BC is generally good, a significant proportion (20%–40%) of chemotherapy-treated BC patients develop metastatic disease. 2 The identification of BC patients who will relapse is not yet well established. It is becoming increasingly accepted that metastatic disease is initiated by circulating tumor cells (CTCs) that originate from the primary tumor and spread the cancer in the body via the blood circulatory system. 3 The presence of CTCs is commonly associated with an increased risk of metastases.4,5 The detection of CTCs in BC has been performed in patients with diseases ranging from ductal carcinoma in situ to metastatic BC,6–11 and their detection is generally associated with a poor prognosis. Previous studies have shown that the detection of CTCs after the completion of treatment is a prognostic marker.9,12–15 However, it must be noted that considerable variation has been reported in CTC detection rates and correlation with prognosis, 16 and, to date, this limits the routine use of CTC count as a prognostic marker.
CTCs can exist in intermediate states expressing both epithelial and mesenchymal markers to varying degrees.17–21 Subpopulations of these tumor cells at any point may acquire cancer stem cell (CSC) attributes such as quiescence, self-renewal, asymmetric division, drug resistance,19,22 and radiation resistance. 23 After radiation therapy, a greater fraction of CSCs is observed 24 in BC cell lines; thus, it is proposed that hypofractionated irradiation may be more effective for the control of tumors containing these cells. 25 CSC markers have been identified in BC CTC populations by a number of researchers.26–29 It was concluded in a recent review article that further studies assessing the clinical utility of CTCs at different stages of treatment as well as looking at CTC subpopulations, including the presence of CSC populations and their alterations with treatment, are needed. 30 In addition, the link between CTC subsets and the clinical outcome warrants further evaluation. 31 The aim of this study was to assess the clinical impact of the detection of CTCs and CSCs in the peripheral blood of nonmetastatic BC patients measured before and at different stages of treatment.
Methods
This prospective study was conducted from January 2011 to January 2015. Eligible patients were women with nonmetastatic BC stages II to III (T2–T4, N0–N3, and M0) and were aged 18 years or older who have not previously received chemotherapy or hormonal therapy. The patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2. The research was approved by the Institutional Review Board of the South Egypt Cancer Institute and conducted in accordance with the principles of the Declaration of Helsinki. Patients gave their written, informed consent to participate in this research.
Flow Cytometric Detection of CTCs and CSCs
The CSC and CTC counts were assessed in three blood samples from each patient. They were detected by fluorescein isothiocyanate-conjugated pan-cytokeratin, phycoerythrin-conjugated CD24, peridinium-chlorophyll-protein-conjugated CD45, and allophycocyanin-conjugated CD44. All monoclonal antibodies were purchased from BD Biosciences. After discarding the first 1 mL of blood to avoid potential contamination with skin epithelial cells, 5 mL of blood were collected. Following lysis of erythrocytes in the 5 mL blood sample, the cell suspension was incubated for 20 minutes in the dark with 10 μL of CD24, CD45, and CD44. After that, the cells were washed with phosphate-buffered saline (PBS), followed by the addition of fixative solution to fix the cells and incubation for 15 minutes. The cells were then washed with PBS, followed by the addition of permeabilizing solution and 10 μL of pan-cytokeratin and incubation for 15 minutes at room temperature. After washing with PBS, the cells were ready for analysis. Flow cytometric analysis was done using the FACSCalibur flow cytometry with CellQuest software (BD Biosciences). Antihuman IgG was used as an isotype-matched negative control for each sample. The absolute numbers of CTCs were identified as CD45-cytokeratin+ cells. CTCs per 5 mL of blood were determined by recording all events in the whole suspension. CSCs were identified as cytokeratin+CD44+CD24–/CD45– cells (Fig. 1). The CSCs were expressed as a percentage of CTCs.
Figure 1.
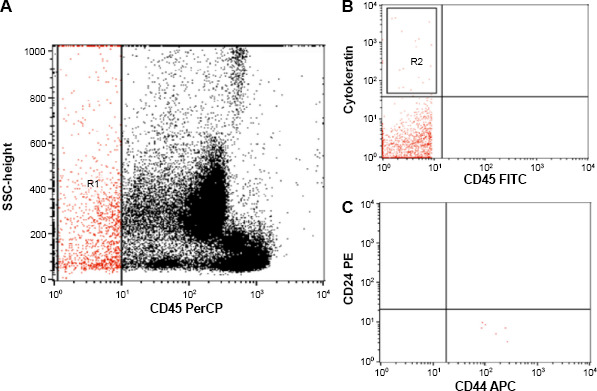
Flow cytometric detection of circulating tumor cells (CTCs) and circulating CSCs. (A) CD45 and side scatter histogram were used to select the CD45– cells (R1). (B) The expression of cytokeratin in CD45– cells (R1) was detected. CTCs were defined as cytokeratin+CD45– (R2). (C) The CTCs were further gated to detect CSCs, which are identified as cytokeratin+CD44+CD24– CD45– cells.
Treatment
Patients received six cycles of neoadjuvant anthracycline-based chemotherapy in one of the following two regimens: FAC (fluorouracil 500 mg/m2, adriamycin 50 mg/m2, and cyclophosphamide 500 mg/m2 every 21 days) and FEC (fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 21 days).
All patients received adjuvant radiotherapy for one or more of the following indications: conservative breast surgery, lymph node positive disease and or tumor 5 cm or more. A hypofractionated accelerated schedule was used, in which the whole breast received 42.5 Gy in 16 fractions of 2.66 Gy each and the lumpectomy site received a 12 Gy electron boost divided in 16 fractions of 0.75 Gy, each given concomitantly over 3.2 weeks. At simulation, all patients underwent computed tomography to generate a three-dimensional plan. The planning target volume (PTV) included the extent of the breast volume as identified on computed tomography, excluding a 0.5-cm skin thickness. The boost PTV was identified using the lumpectomy cavity seroma and/or surgical clips. If the tumor bed seroma was not easily palpated and the surgical clips were not found, a 3- to 4-cm margin was placed parallel to the surgical scar with a 1-cm margin at the ends of the scar to define the boost PTV. The heart and lung were also contoured. Two tangential wedged fields for the whole breast and a matched supraclavicular field was added when indicated. An en face electron field for the boost volume prescribed at the 90% isodose line was given to all patients.
Postmastectomy radiotherapy was similarly given in two tangential fields and a matching supraclavicular field when needed for 42.5 Gy in 16 fractions. The radiation therapy plan was evaluated using a dose–volume histogram. V95 and V107 were defined as the volumes that received 95% and 107% of the prescribed dose, respectively. After the completion of radiotherapy, hormonal therapy was started by either tamoxifen or aromatase inhibitors or a combination for hormonal-positive tumors. Follow-up was every three months for the first year, then every six months for two years, and then yearly thereafter.
Statistical Analysis
Data analysis was performed using the statistical package for social sciences, version 17. The Kaplan-Meier method for the generation of actuarial survival curves was used to assess progression free survival (PFS) and overall survival (OS). The effect of each clinicopathological factor on survival was compared using the log-rank test. Multivariable analysis for PFS and OS included the statistically significant factors by the Kaplan-Meier method and was performed using the Cox regression proportional hazards model. Receiver operating characteristic analysis was used to define the optimum cutoff for each group of CTC and CSC measurements. The two-tailed paired T test was used to compare each CTC and CSC measurement with the subsequent one and correlate them. Pearson's correlation was used to assess the relation between CTC and CSC counts during the course of treatment. The P value was considered as statistically significant if <0.05.
Results
This study included 51 eligible patients. The characteristics of patients and tumors are shown in Table 1. Twenty-one (41.2%) patients were under the age of 50 years, while the rest were 50 years or older. The majority of the patients, 34 (66.7%), had an ECOG PS of 1. Almost half of the patients, 24 (47.1%), had T2 tumors. Grade 2 was the most common tumor grading with 38 patients (74.5%) having it. Thirty-two patients (62.7%) were lymph node negative. Estrogen, progesterone, and Her2-neu receptor status were positive in 29 (56.9%), 19 (37.3%), and 12 patients (23.5%), respectively.
Table 1.
The characteristics of patients and tumors.
| CHARACTERISTIC | NO. (%) |
|---|---|
| Age | |
| <50 | 21 (41.2) |
| ≥50 | 30 (58.8) |
| ECOG PS | |
| 0 | 7 (13.7) |
| 1 | 34 (66.7) |
| 2 | 10 (19.6) |
| T stage | |
| 1 | None |
| 2 | 24 (47.1) |
| 3 | 12 (23.5) |
| 4 | 15 (29.4) |
| Grade | |
| 1 | 2 (3.9) |
| 2 | 38 (74.5) |
| 3 | 11 (21.6) |
| Lymph node metastases | |
| Negative | 32 (62.7) |
| Positive | 19 (37.3) |
| Estrogen receptor | |
| Negative | 22 (43.1) |
| Positive | 29 (56.9) |
| Progesterone receptor | |
| Negative | 32 (62.7) |
| Positive | 19 (37.3) |
| Her2-neu | |
| Negative | 34 (66.7) |
| Positive | 12 (23.5) |
| Unknown | 5 (9.8) |
| Menopausal status | |
| Premenopausal | 21 (41.2) |
| Postmenopausal | 30 (58.8) |
The range, mean, and median of CTC and CSC counts for each sample are shown in Table 2. By receiver operating characteristic analysis, the optimal cutoff for high and low values for the measurements of CTCs in the 5 mL of blood were 4.5, 3.1, and 2.75 for the first, second, and third measurements, respectively, while for the measurements of CSCs, the values were 26.5%, 24%, and 21.75% for the first, second, and third measurements respectively. The four-year PFS for the entire group was 60.7%, while the four-year OS was 75.7%. A univariate analysis of factors possibly affecting the four-year disease-free survival (DFS) and the 4-year OS is shown in Table 3. A statistically significant difference in the four-year DFS was found according to the age (P < 0.004), progesterone receptor positivity (P < 0.02), menopausal status (P < 0.004), baseline CTC count, CTC count after chemotherapy, CTC count after surgery and chemotherapy, baseline CSC count, CSC count after chemotherapy, and CSC count after surgery and chemotherapy (P < 0.001). Factors affecting the four-year OS were progesterone receptor positivity (P < 0.03), baseline CTC count, CTC count after chemotherapy (P < 0.001), CTC count after surgery and radiotherapy (P < 0.02), baseline CSC count, CSC count after chemotherapy, and CSC after surgery and radiotherapy (P < 0.001). By multivariate analysis, however, the third measurement of CSCs, taken one month after the cessation of radiotherapy, was the only independent prognostic factor for DFS (P < 0.002, HR = 1.231, 95% CI 1.077–1.407; Fig. 2). For OS, the one independent prognostic factor was the initial CTC count (P < 0.03, HR 1.969, 95% CI 1.092–3.551; Fig. 3).
Table 2.
The CTC and CSC counts.
| CELL COUNT | RANGE | MEAN ± SEM | MEDIAN |
|---|---|---|---|
| CTC baseline | 0.5–10.0 | 3.9157 ± 0.34897 | 3.4 |
| CTC after chemotherapy | 0.2–8.0 | 2.2608 ± 0.29766 | 1.6 |
| CTC after radiotherapy | 0.1–9.40 | 2.0784 ± 0.2985 | 1.0 |
| CSC baseline | 4.5–67.0 | 27.2941 ± 2.4179 | 23.0 |
| CSC after chemotherapy | 4.0–60.0 | 24.0294 ± 2.258 | 19.0 |
| CSC after radiotherapy | 3.9–61.0 | 21.984 ± 2.3285 | 17.0 |
Table 3.
Univariate analysis for the effect of clinicopathological factors on DFS and OS.
| FACTOR | 4-YEAR PFS, % | P VALUE | 4-YEAR OS, % | P VALUE |
|---|---|---|---|---|
| Age | ||||
| <50 | 38.1 | 0.004 | 71.4 | 0.304 |
| ≥50 | 76.7 | 80.0 | ||
| ECOG PS | ||||
| 0 | 80 | 0.708 | 57.1 | 0.137 |
| 1 | 58.3 | 85.3 | ||
| 2 | 60.0 | 60.0 | ||
| T stage | ||||
| 2 | 54.2 | 0.228 | 75.0 | 0.792 |
| 3 | 83.3 | 83.3 | ||
| 4 | 53.3 | 76.5 | ||
| Grade | ||||
| 1 | 100 | 0.372 | 100 | 0.656 |
| 2 | 55.3 | 73.7 | ||
| 3 | 72.7 | 81.8 | ||
| Lymph node metastases | ||||
| Negative | 72.7 | 0.415 | 90.9 | 0.246 |
| Positive | 57.5 | 72.5 | ||
| Estrogen receptor | ||||
| Negative | 54.5 | 0.409 | 63.6 | 0.075 |
| Positive | 65.5 | 86.2 | ||
| Progesterone receptor | ||||
| Negative | 46.9 | 0.014 | 65.6 | 0.023 |
| Positive | 84.2 | 94.7 | ||
| Her2-neu | ||||
| Negative | 67.6 | 0.290 | 85.3 | 0.116 |
| Positive | 50.0 | 58.3 | ||
| Unknown | 40.0 | 60 | ||
| Menopausal status | ||||
| Premenopausal | 38.1 | 0.004 | 71.4 | 0.304 |
| Postmenopausal | 76.7 | 80.0 | ||
| Baseline CTC count | ||||
| Low | 81.8 | 0.001 | 90.9 | <0.001 |
| High | 22.2 | 50.0 | ||
| CTC count after chemotherapy | ||||
| Low | 79.5 | <0.001 | 93.9 | <0.001 |
| High | 0.0 | 44.4 | ||
| CTC count after surgery and radiotherapy | ||||
| Low | 71.4 | <0.001 | 91.9 | <0.02 |
| High | 11.1 | 25.0 | ||
| Baseline CSC count | ||||
| Low | 87.1 | <0.001 | 93.4 | <0.001 |
| High | 20.0 | 47.7 | ||
| CSC count after chemotherapy | ||||
| Low | 80.8 | <0.001 | 94.2 | <0.001 |
| High | 18.8 | 32.8 | ||
| CSC count after surgery and radiotherapy | ||||
| Low | 84.8 | <0.001 | 97.0 | <0.001 |
| High | 16.7 | 31.8 |
Figure 2.
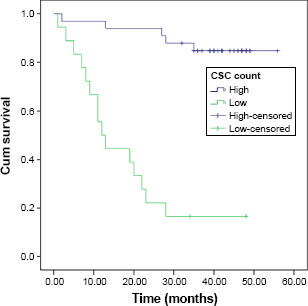
DFS according to the final CSC count.
Figure 3.
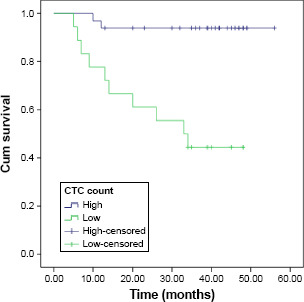
OS according to the initial CTC count.
Correlation was done to assess the relations between every two consecutive measurements of CTCs and CSCs (Table 4). The first and second measurements of CTCs were significantly correlated (r = 0.730, P < 0.001); however, they were less closely correlated than the second and third measurements (r = 0.927, P < 0.001), indicating that the greatest change in CTC count occurred after chemotherapy, more than that which occurred after radiotherapy and surgery. Serial CSC counts were also highly correlated with r being 0.986 for the first and second measurements and 0.978 for the second and third measurements (P < 0.001), indicating less of a change between measurements. When correlation was done to determine how closely related the CTC and CSC counts were, it was found that they were most closely related initially (r = 0.654, P < 0.001; Fig. 4), more than in the sample after chemotherapy (r = 0.317, P < 0.03; Fig. 5), or after surgery and radiotherapy (r = 0.368, P < 0.008), which may be due to that the CTC count decreased more noticeably than the CSC count with treatment, especially after chemotherapy.
Table 4.
Correlation between serial measurements of CTCs and CSC and between measurements of CTC and CSC.
| R VALUE | P VALUE | |
|---|---|---|
| Correlation for CTC counts | ||
| Baseline and second measurements | 0.730 | <0.001 |
| Second and third measurements | 0.927 | <0.001 |
| Correlation for CSC counts | ||
| Baseline and second measurements | 0.986 | <0.001 |
| Second and third measurements | 0.978 | <0.001 |
| Correlation for CTC and CSC counts | ||
| Baseline measurements | 0.654 | <0.001 |
| Second measurements | 0.317 | <0.03 |
| Third measurements | 0.368 | <0.008 |
Figure 4.
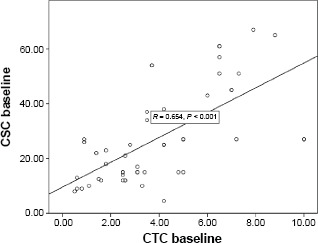
Correlation between CTC and CSC counts at diagnosis.
Figure 5.
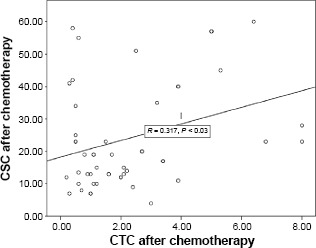
Correlation between CTC and CSC counts after chemotherapy.
Discussion
Conventional therapeutic approaches (chemotherapy and radiotherapy) as well as most of the current targeted therapies are based on the intention to target tumor cells using maximum tolerated doses. The failure of these treatments to eradicate the disease, in a substantial number of patients, has renewed the interest in CSCs. 30 By definition, recurrence originates from residual treatment-resistant cells, which regenerate the BC phenotype. The existence of radiation-resistant subpopulations of tumor cells has been long proposed by radiobiologists. 22 How important understanding the tumor cell origin will be in improving BC outcome is still debatable, so further studies of these cells at different stages of treatment and their correlation with the outcome may aid in clarifying how they could possibly be incorporated in BC treatment and follow-up. In this study, we measured CTCs and CSCs before, during, and after the completion of treatment, except continuing hormonal treatment.
The prognostic value, observed in this study, of the CTC count at diagnosis being an independent prognostic factor for OS in nonmetastatic BC has been reported in numerous trials, and many of them were included in a meta-analysis by Zhang et al. 5 The pooled HR, for patients considered positive for CTCs, was reported to be 2.78 (95% CI 2.22–3.48, P = 0.00). In this study, the CTC count at diagnosis was a significant prognostic factor for DFS as well. This was similarly found in the meta-analysis in which the pooled HR, in 19 studies, for patients considered to have high CTCs, was 2.86 (95% CI, 2.19–3.75, P = 0.00). Therefore, the prognostic significance of the CTC count before treatment has been proven repeatedly; however, fewer trials have assessed its value during and after treatment.
In this study, the CTC count measured after chemotherapy was of prognostic significance for DFS and OS. Among the most important studies that reported the CTC count after chemotherapy, as well as before chemotherapy, and its relation with survival, is the Success trial 11 in which 2026 early BC patients participated. This trial proved that the persistence of CTCs after chemotherapy showed a negative influence on DFS (HR = 1.12, 95% CI 1.02–1.25, P = 0.02) and on OS (HR = 1.16, 95% CI 0.99–1.37) as well. The multivariable analysis conducted in the Success trial also proved the CTC count before chemotherapy to be an independent prognostic factor for both DFS (P < 0.0001) and OS (P < 0.002), similar to the present study.
Studies evaluating the effect of CD44+/CD24– CSCs on clinical outcome are less common, especially those assessing them in the peripheral blood of the patients. These cells have been linked to worse BC-specific survival when studied in BC tissue in 1342 patients (P = 0.001). 32 The presence of CSC in the bone marrow was found to be an independent prognostic factor for DFS (HR = 15.8, P = 0.017) in a study conducted in MD Anderson. 33 In this study, three peripheral blood samples were taken from each patient and the presence of high CD44+/CD24– CSC was associated with a decreased DFS and OS every time. The third sample taken in this study (after surgery and radiotherapy) was the factor that reached statistical significance on multivariate analysis for DFS. So the CSCs, which persisted after treatment, were the strongest factor linked to recurrence. Assessment of CSC after the completion of all treatments of BC, except for hormonal, may be one of the methods of identifying patients in need of further treatment.
The measured counts of CTCs and CSCs were the most highly correlated initially in this study, more than after chemotherapy or after surgery and radiotherapy. This could be due to that, in these patients, the mean change in the CTC count with treatment was greater than the mean decrease in the CSC count, most noticeable in the sample after chemotherapy, so CTC count may be more useful for response assessment, during and after chemotherapy because it decreased more rapidly than the CSC count. Consideration of switching to a different chemotherapy may be a future direction upon lack of response to a chemotherapy regimen assessed during treatment. This strategy was tested in the SWOG0500 trial, 34 but for patients with metastatic BC, and although it showed no OS advantage for the patients who switched to a different cytotoxic regimen upon lack of decrease in the CTC count, this may not be true for nonmetastatic tumors and it may also not be true for all alternative chemotherapy regimens, so it should be tested during neoadjuvant chemotherapy for early-to-advanced BC with different cytotoxic regimens.
Conclusion
In the early- to high-risk nonmetastatic BC patients, involved in this study, the only independent prognostic factor for OS was the initial CTC count, and it was concluded to be more appropriate for monitoring response to therapy than the CSC count due to its more rapid decrease with chemotherapy. Persistent CSCs, however, were the independent prognostic factor for DFS, so their assessment may be used for the consideration of additional treatment in the future. These results need to be confirmed in larger trials.
Author Contributions
Conceived and designed the experiments: AMZ and MS. Analyzed the data: MS. Wrote the first draft of the article: MS. Contributed to the writing of the article: AMZ. Agreed with the article results and conclusions: MS, AMZ, MSFH, and DOM. Jointly developed the structure and arguments for the article: MS and AMZ. Made critical revisions and approved the final version: MS and AMZ. All authors reviewed and approved the final article.
References
- 1.Forman D., Bray F., Brewster D.H. et al. Cancer Incidence in Five Continents (CI5). Lyon: IARC Scientific Publications; 2014: 164. [Google Scholar]
- 2.Albain K., Anderson S., Arriagada R. et al. Comparisons between different poly-chemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomized trials. Lancet. 2012; 379(9814): 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tjensvoll K., Oltedal S., Heikkila R. et al. Persistent tumor cells in bone marrow of non-metastatic breast cancer patients after primary surgery are associated with inferior outcome. BMC Cancer. 2012; 12: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janni W., Vogl F.D., Wiedswang G. et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse— a European pooled analysis. Clin Cancer Res. 2011; 17(9): 2967–2976. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L., Riethdorf S., Wu G. et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012; 18(20): 5701–5710. [DOI] [PubMed] [Google Scholar]
- 6.Ignatiadis M., Rothe F., Chaboteaux C. et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011; 6(1): e15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanger N., Effenberger K.E., Riethdorf S. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011; 129(10): 2522–2526. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M., Budd G.T., Ellis M.J. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004; 351(8): 781–791. [DOI] [PubMed] [Google Scholar]
- 9.Xenidis N., Ignatiadis M., Apostolaki S. et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol. 2009; 27(13): 2177–2184. [DOI] [PubMed] [Google Scholar]
- 10.Diel I.J., Kaufmann M., Costa S.D. et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst. 1996; 88(22): 1652–1658. [DOI] [PubMed] [Google Scholar]
- 11.Rack B., Schindlbeck C., Juckstock J. et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014; 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes D.F., Cristofanilli M., Budd G.T. et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006; 12(14): 4218–4224. [DOI] [PubMed] [Google Scholar]
- 13.Mathiesen R.R., Borgen E., Renolen A. et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res. 2012; 14(4): 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slade M.J., Payne R., Riethdorf S. et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br J Cancer. 2009; 100(1): 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidard F.C., Mathiot C., Delaloge S. et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010; 21(4): 729–733. [DOI] [PubMed] [Google Scholar]
- 16.Van der Auwera I., Peeters D., Benoy I.H. et al. Circulating tumour cell detection: a direct comparison between the CellSearch System, the AdnaTest and CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer. Br J Cancer. 2010; 102(2): 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M., Bardia A., Wittner B.S. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013; 339(6119): 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnomet A., Syne L., Brysse A. et al. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012; 31(33): 3741–3753. [DOI] [PubMed] [Google Scholar]
- 19.Raimondi C., Gradilone A., Naso G. et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011; 130(2): 449–455. [DOI] [PubMed] [Google Scholar]
- 20.Paterlini-Brechot P., Benali N.L. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007; 253(2): 180–204. [DOI] [PubMed] [Google Scholar]
- 21.Lecharpentier A., Vielh P., Perez-Moreno P., Planchard D., Soria J.C., Farace F. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer. 2011; 105(9): 1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel A.P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008; 3(8): e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich J.N. Cancer stem cells in radiation resistance. Cancer Res. 2007; 67(19): 8980–8984. [DOI] [PubMed] [Google Scholar]
- 24.Philips T.M., McBride W.H., Pajonk F. The response of CD24(–/low)/CD44+ breast cancer initiating cells to radiaition. J Natl Cancer Inst. 2006; 98(24): 1777–1785. [DOI] [PubMed] [Google Scholar]
- 25.Lin S.C., Xu Y.C., Gan Z.H. et al. Monitoring cancer stem cells: insights into clinical oncology. Onco Targets Ther. 2016; 9: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L., Ridgway L.D., Wetzel M.D. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl Med. 2013; 5(180): 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theodoropoulos P.A., Polioudaki H., Agelaki S. et al. Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett. 2010; 288(1): 99–106. [DOI] [PubMed] [Google Scholar]
- 28.Wang N., Shi L., Li H. et al. Detection of circulating tumor cells and tumor stem cells in patients with breast cancer by using flow cytometry: a valuable tool for diagnosis and prognosis evaluation. Tumour Biol. 2012; 33(2): 561–569. [DOI] [PubMed] [Google Scholar]
- 29.Aktas B., Tewes M., Fehm T., Hauch S., Kimmig R., Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009; 11(4): R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McInnes L.M., Jacobson N., Redfern A., Dowling A., Thompson E.W., Saunders C.M. Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol. 2015; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao H., Burke P.A., Chen X. et al. Analysis and characterization of subpopulation of circulating tumor cells in patients with breast cancer. J Clin Oncol. 2011; 29(15). [Google Scholar]
- 32.Liu C., Luo Y., Liu X., Lu P., Zhao Z. Clinical implications of CD44+/CD24– tumor cell ratio in breast cancer. Cancer Biother Radiopharm. 2012; 6: 324–328. [DOI] [PubMed] [Google Scholar]
- 33.Giordano A., Gao H., Cohen E.N. et al. Clinical relevance of cancer stem cells in bone marrow of early breast cancer patients. Ann Oncol. 2013; 24(10): 2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smerage J.B., Barlow W.E., Hortobagyi G.N. et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOGS050 0. J Clin Oncol. 2014; 32: 3483–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]


