Abstract
Introduction
Ingestion of nicotine by smoking, vaping, or other means elicits various effects including reward, antinociception, and aversion due to irritation, bitter taste, and unpleasant side effects such as nausea and dizziness.
Aims and Methods
Here we review the sensory effects of nicotine and the underlying neurobiological processes.
Results and Conclusions
Nicotine elicits oral irritation and pain via the activation of neuronal nicotinic acetylcholine receptors (nAChRs) expressed by trigeminal nociceptors. These nociceptors excite neurons in the trigeminal subnucleus caudalis (Vc) and other brainstem regions in a manner that is significantly reduced by the nAChR antagonist mecamylamine. Vc neurons are excited by lingual application of nicotine and exhibit a progressive decline in firing to subsequent applications, consistent with desensitization of peripheral sensory neurons and progressively declining ratings of oral irritation in human psychophysical experiments. Nicotine also elicits a nAChR-mediated bitter taste via excitation of gustatory afferents. Nicotine solutions are avoided even when sweeteners are added. Studies employing oral self-administration have yielded mixed results: Some studies show avoidance of nicotine while others report increased nicotine intake over time, particularly in adolescents and females. Nicotine is consistently reported to increase human pain threshold and tolerance levels. In animal studies, nicotine is antinociceptive when delivered by inhalation of tobacco smoke or systemic infusion, intrathecally, and by intracranial microinjection in the pedunculopontine tegmentum, ventrolateral periaqueductal gray, and rostral ventromedial medulla. The antinociception is thought to be mediated by descending inhibition of spinal nociceptive transmission. Menthol cross-desensitizes nicotine-evoked oral irritation, reducing harshness that may account for its popularity as a flavor additive to tobacco products.
Implications
Nicotine activates brain systems underlying reward and antinociception, but at the same time elicits aversive sensory effects including oral irritation and pain, bitter taste, and other unpleasant side effects mediated largely by nicotinic acetylcholine receptors (nAChRs). This review discusses the competing aversive and antinociceptive effects of nicotine and exposure to tobacco smoke, and the underlying neurobiology. An improved understanding of the interacting effects of nicotine will hopefully inform novel approaches to mitigate nicotine and tobacco use.
Introduction
Smoking and the consumption of other types of tobacco products continues to be a source of preventable morbidity. In 2015, the WHO estimated that 20.2% of the world population smoked tobacco.1 In 2017, it was estimated that 19.3% of US adults used some type of tobacco product, with 14% smoking cigarettes.2 Although the usage of tobacco products has been declining over the past 10 years, there is increased incidence of vaping especially among adolescents, with 27% of US high schoolers and 7% of middle schoolers reporting current use of tobacco products, and 21% of high schoolers vaping within the past month.3
Tobacco smoke contains nicotine as well as a variety of toxic and carcinogenic substances including particulate matter, nitrosamines, acrolein, formaldehyde and other aldehydes, and flavorants.4 The combusted liquid in electronic cigarettes also contains nicotine as well as numerous toxicants.5
Nicotine is thought to be the main reinforcer in the addictive potential of tobacco products, as evidenced by a lack of widespread use of denicotinized cigarettes compared to those containing nicotine.6 In a double-blind study, never-smokers learned to distinguish between post-ingestional effects of capsules containing nicotine versus placebo, and subsequently 50% chose to receive the nicotine-containing capsule due to its positive affective effects.7 This implies that nicotine can be reinforcing even in tobacco-naïve human subjects.
Often the first encounter with a tobacco product is unpleasant due to the occurrence of dizziness, nausea, and other side effects; yet some people still become addicted. This is generally true of many drugs of abuse including cocaine and opioids that are plant neurotoxins having evolved to deter herbivores, yet having rewarding effects in mammals that lead to drug seeking: the paradox of drug reward.8 The rewarding effect of nicotine is thought to be due to its binding with nicotinic acetylcholine receptors (nAChRs), particularly those containing α− and β-subunits including α 4 β 2, α 3 β 4, and α 7. This leads to increased dopamine release in brain reward circuits including the prefrontal cortex, ventral striatum, and nucleus accumbuns (reviewed in 9).
Nicotine dependence is complex and not only involves the reward circuitry, but also many other sensory and psychological factors. This article will review the sensory properties of nicotine, including its ability to elicit oral pain, irritation and bitter taste, antinociception (pain reduction) and interactions with flavor additives, particularly menthol.
Nicotine Elicits Pain and Oral Irritation
Smoking, vaping, and other types of tobacco consumption can result in coughing, irritation and dryness of the mouth, throat, and eyes; dizziness, headache, shortness of breath, altered taste, nausea, and other symptoms10 as well as ocular inflammation and increased incidence of other ocular diseases.11 Nicotine elicits pain sensation when applied to the human blister base12 or nasal sinus13,14 and nociceptive responses in animals.15 Epilingual application of nicotine elicits irritation that is reduced by mecamylamine,16,17 implicating involvement of nAChRs in the irritant effect.
Sequential epilingual applications of nicotine elicited irritation that declined in intensity across repeated trials at a 1-min interstimulus interval, a phenomenon called desensitization18 (Figure 1A). This is similar to desensitization of irritation elicited by mustard oil,19 menthol,20 and certain other irritants, but different from the increasing irritancy (sensitization) elicited by sequential application of capsaicin18,21 (Figure 1A). Nicotine-evoked irritation is cross-desensitized by menthol,20 capsaicin, and piperine from black pepper.22,23 However, only a high concentration of nicotine (300 mM) reciprocally cross-desensitized capsaicin-evoked oral irritation.23 The magnitude of irritation elicited by 300 mM nicotine was lower when applied within 24 hours but not 48 hours after its initial application.24 These findings indicate that smoking or oral ingestion of a fairly high concentration of nicotine reduces the sensory impact of subsequent nicotine ingestion, at least for 1 day.
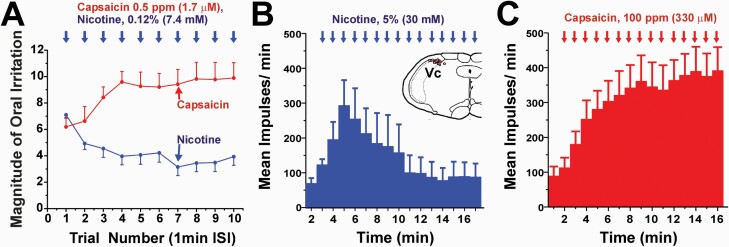
Figure 1.
Oral irritation by nicotine. A. Nicotine or capsaicin was applied to the tongue and subjects rated the intensity of irritation 25 s later, followed by reapplication in the same manner at 1-min intervals. Sequential application of nicotine elicited irritation that decreased across trials (blue) while capsaicin elicited irritation that increased across trials (red). Adapted from reference18, Fig. 3, 1997 with permission from Oxford University Press. B. Application of nicotine to the tongue elicited responses in Vc neurons of anesthetized rats that initially increased, then decreased across applications delivered at 1-min intervals. Inset shows Vc neuronal recording sites. C. Repeated application of capsaicin elicited a progressive increase in Vc neuronal responses. Adapted from reference56, Figs. 5B, 11D, 2000 with permission from the American Physiological Society.
Nicotine Activates C-Fiber Nociceptors
The pain and irritation elicited by exposure to nicotine is due to the activation of C-fibers, including nociceptors, in the skin, ocular and oral mucosa, trachea, and lungs. Cutaneous unmyelinated (C-fiber) sensory nerves were reported to be activated by acetylcholine and other cholinergic agonists25–27 including nicotine which also sensitized C-fiber nociceptors to noxious heat.28 Nicotine activated acetylcholine-sensitive corneal C-fibers that were insensitive to thermal or mechanical stimulation.29,30 Seventeen percent of ethmoid nerve C-fibers in guinea pigs responded to intranasal instillation of nicotine.31 Intranasal vapor-phase nicotine excited ethmoid nerve fibers in a manner that was significantly attenuated by mecamylamine and another nAChR antagonist dihydro- β -erythroidine.32 A subpopulation of lingual nerve C-fibers innervating the oral mucosa responded to nicotine.33 Right-atrial injection of nicotine excited 48% of single pulmonary C-fiber afferents including both rapidly- (RAR) and slowly adapting (SAR) pulmonary stretch receptors.34 Cigarette smoke excited 78.6% and 27.3% of pulmonary SARs and RARs, respectively.35
Nicotine Activates Peripheral Sensory Neurons
A number of studies have used in vitro patch-clamp or calcium imaging techniques to investigate nicotine activation of isolated dorsal root ganglion (DRG), trigeminal ganglion (TG), and nodose/jugular ganglion (NJG) sensory neurons of the vagus nerve. Nicotine (usually 100 μM) excited >50% of rat DRG36–40 and TG cells41 in a manner exhibiting tachyphylaxis. Many nicotine-sensitive neurons also responded to capsaicin. Comparable studies employing calcium imaging have reported that nicotine excites rat DRG42 and NJG neurons,34 many of which also responded to capsaicin. Pharmacological evidence indicates that nicotine acts via α 7*, α 3 β 4*, and α 4 β 2* nAChRs. Evidence for α 7* nAChRs is based on antagonism by choline and MLA, and for α 3 β 4* nAChRs is based on antagonism by mecamylamine and excitation by epibatidine.37–39,42 Evidence also exists for peripheral α 6 β 4* nAChRs.43 In rat DRG cells, nicotine elicited slow- and fast-inactivating currents that were mediated by α 3 β 4* and α 7* nAChRs, respectively.40 However, nicotine was also shown to activate mouse TRPA1 in a mecamylamine-antagonizable manner.44 This may represent a species difference, in that nicotine-evoked responses of rat DRG neurons were blocked by mecamylamine but were not affected by the selective TRPA1 antagonist HC-03003140. A recent study revealed that nicotine activated 85% of human DRG neurons that exhibited only slowly activating and inactivating currents, while mouse DRG cells only exhibited fast-inactivating currents.45 Moreover, human DRG neuronal responses to nicotine were blocked by mecamylamine but not HC-03003145, implying a major role for nAChRs but not TRPA1.
Nicotine Stimulates the Peripheral Release of CGRP
The release of calcitonin gene-related peptide (CGRP) from tracheal or buccal tissue has been investigated as a measure of nicotine activation of peripheral peptidergic nerve fibers. Nicotine induced the release of CGRP from the isolated rat46 and mouse trachea.47,48 A low nicotine concentration (30 μM) elicited CGRP release via nAChRs while a higher concentration (100 μM) also elicited CGRP release in a pH-dependent manner requiring TRPA1 and TRPV147. In the same study, 48% of JNG (but only 14% of DRG) cells were excited by 100 μM nicotine while all cells were excited by a high nicotine concentration (20 mM) at an alkaline pH.47 CGRP release in the mouse buccal mucosa was also elicited by nicotine at alkaline pH, as well as by delivery of cigarette smoke in a manner involving TRPA1 and TRPV1.49
Nicotine Activation of Central Neurons
Nicotine activates peripheral C-fibers, including nociceptors, which convey signals into the spinal and medullary dorsal horn to activate second-order neurons that convey somatosensory information to higher centers. Using c-fos as an immunohistochemical marker of strong neuronal activation, intradermal injection of nicotine in the hindpaw excited neurons in a region of the spinal superficial dorsal horn (laminae I and II) that overlapped with the area of neuronal activation by noxious pinch and injection of other algesic agents including capsaicin, serotonin, histamine, and formalin.50 In functional studies, intradermal injection of nicotine excited superficial dorsal horn neurons in a manner that exhibited tachyphylaxis to repeated injections of high but not low nicotine concentrations, and was antagonized by mecamylamine.51
Application of nicotine to the dorsal anterior tongue elicited c-fos expression in neurons in the dorsomedial trigeminal subnucleus caudalis (Vc) and adjacent paratrigeminal nucleus, nucleus of the solitary tract (NTS), ventrolateral Vc, and area postrema (AP).52,53 The number of neurons in dorsomedial and ventrolateral Vc, NTS and AP was significantly reduced by pretreatment with mecamylamine, as well as a high (1%) but not low (0.1%) dose of atropine53 that may be attributed to a nonspecific local anesthetic effect. Delivery of nicotine to the throat (bypassing the oral mucosa) elicited significant c-fos expression in the same brainstem regions as observed with lingual nicotine application.54 Neurons in the dorsomedial Vc exhibited significant dose-related increases in firing to lingual application of nicotine in the low-to-mid mM range in a manner exhibiting tachyphylaxis.55,56 Nicotine-sensitive Vc neurons also responded to many other irritant chemicals.55 Repeated application of nicotine to the tongue initially excited Vc neurons, followed by a progressive decrease in firing across applications (Figure 1B) consistent with the decline in psychophysical ratings of irritation (Figure 1A). In contrast, repeated application of capsaicin elicited a progressive increase in Vc neuronal firing (Figure 1C) consistent with its sensitizing effect observed psychophysically (Figure 1A).56 Lingual application of nicotine cross-desensitized responses of dorsomedial Vc neurons to the irritant chemical pentanoic acid57 in a manner that was prevented by lingual application of mecamylamine.58
Nicotine excited Vc neurons and elicited an irritant sensation in human subjects when delivered at low (7–30) mM concentrations. The tissue concentration of nicotine at the nociceptive nerve endings is assumed to be 2–3 orders of magnitude lower, consistent with nicotine exciting sensory neurons in vitro in the low μM range.
Nicotine Taste
Nicotine tastes bitter59 and at concentrations above 50 μg/mL elicited aversive behavioral responses in rats,60 hamsters,61 and three strains of mice.62 Rodents generally avoid nicotine in two-bottle preference tests (see below). Conditioned taste aversion (CTA) of mice to nicotine revealed a generalization to quinine (a bitter tastant) as well as the irritant spilanthol and nicotine odor, implying that the orosensory taste, irritant and/or olfactory quality of nicotine is aversive.63 Regarding nicotine’s bitter taste, nAChRs are expressed in TRPM5-positive taste receptor cells64 and nicotine activates both TRPM5-dependent and TRPM5-independent gustatory neurons in the chorda tympani (taste) nerve as well as gustatory cortex in rats and mice.65 Mecamylamine reduced TRPM5-independent gustatory responses and behavioral discrimination of nicotine and quinine, indicating that nAChRs mediate the bitter taste of nicotine. Nicotine activates neurons in the NTS, the first relay in the gustatory pathway.66,67 The excitatory effect of nicotine on NTS neurons was reduced by mecamylamine, and nicotine still excited NTS neurons following trigeminal ganglionectomy, indicating that nicotine directly excited gustatory afferents expressing nAChRs.67 Nicotine also suppressed responses of single NTS neurons to their preferred tastant (sweet, sour, bitter, salty, or umami) in a manner that was reduced by mecamylamine and prevented by trigeminal ganglionectomy, implying that the inhibitory effect was mediated via nAChR-expressing trigeminal afferents.67 Thus, it is clear that nicotine excites the gustatory pathway in addition to its trigeminal chemesthetic effect.
Nicotine Self-administration
Animal models have been developed in an attempt to mimic the reinforcing property of nicotine and conditions associated with nicotine addiction. That nicotine is reinforcing or rewarding to rodents and nonhuman primates is based on studies using intravenous or oral nicotine self-administration, conditioned place preference, and intracranial self-stimulation.68–72 Although there are many conflicting findings, the consensus is that nicotine is rewarding but with large inter-individual differences,73–76 and animals will self-administer nicotine by oral or intravenous routes with effects more pronounced in adolescent male and adult female rodents.
Nicotine also has central aversive effects thought to be mediated via α 5* and α 3 β 4* nAChRs.77 Intravenous self-administration of nicotine was enhanced in α 5* nAChR knockout mice and “rescued” to wildtype levels by reintroduction of α 5* nAChRs into neurons of the habenula-interpeduncular tract.78 Verenicline (Chantix®), which blocks rewarding effects of nicotine, at a higher dose induced conditioned place aversion that was reduced in α 5* nAChR knockout mice (Pfizer, New York, NY).79
The intravenous route rapidly delivers nicotine to the brain, similar to inhalation of cigarette smoke. However, this method requires instrumentation and operant training, as opposed to the two-bottle paradigm of oral self-administration that is easier to implement. Oral self-administration is thought to better reflect smokers’ behavior, despite the fact that the nicotine is metabolized during the first pass through the liver so that a lower concentration reaches the brain more slowly. Thus there are numerous studies investigating oral self-administration of nicotine, but also many associated problems with this approach.6
One potential problem is that nicotine tastes bitter and is an irritant, and thus may be avoided by rodents. Indeed, intraoral delivery of nicotine elicited behavioral signs of aversion in rats60 presumably due to its bitter and/or chemesthetic quality. Moreover, numerous studies have failed to demonstrate a preference for nicotine in two-bottle tests.60,80–82 However, comparison of three common inbred mouse strains (C57BL/6J [B6], DBA/2J, and A/J) revealed that each strain exhibited equivalent reductions in licking in a brief access test, yet the B6 strain (especially females) exhibited greater consumption of nicotine (75 μg/mL) in a two-bottle test, suggesting that inter-strain variations in nicotine intake are not due to differences in taste or chemesthetic sensitivity,62 but rather genetic factors (CHRNA5).
Moreover, the addition of sweeteners to mask the bitterness of nicotine either increased74,83 or had no effect73 on nicotine intake.6 Studies from our laboratory suggest that sweeteners do not mask nicotine bitterness/irritancy, and that rats can use flavor cues to develop a learned avoidance of nicotine. In a two-bottle preference test, male Sprague–Dawley rats did not show a preference for 10% Kool Aid (which is 94% sugars) with either grape or cherry flavor (Figure 2A). However, when nicotine was added to one flavor, rats consistently avoided that flavor (Figure 2B); when the nicotine was removed, the learned avoidance extinguished over the ensuing 2 weeks.
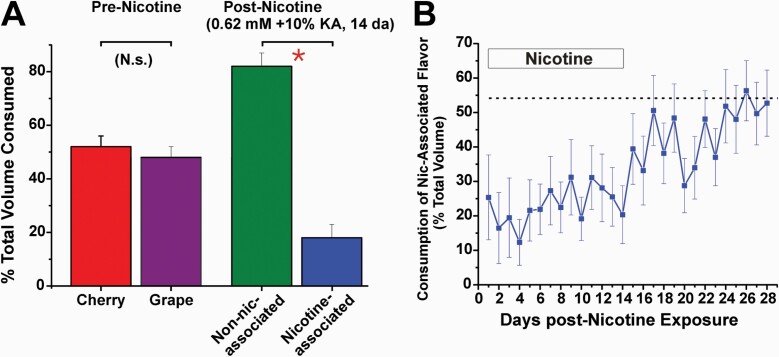
Figure 2.
Learned avoidance of nicotine. A. Graph plots mean consumption of the two different Kool-Aid flavors in a two-bottle paradigm, before and after addition of nicotine to one of the bottles. Prior to nicotine, rats consumed equal amounts of cherry and grape Kool-Aid (10%) more than 4 days (left-hand bars, p > 0.05; n = 10). Nicotine (0.62 mM) was then added to one flavor (either grape or cherry, counterbalanced across rats), with the other flavor untainted. Rats had free access to the two solutions for 14 days, with bottle positions swapped daily. They developed a learned avoidance of nicotine, with significantly less consumption of the nicotine-treated solution compared to the untreated Kool-Aid flavor (right-hand bars; p < 0.001; t-test). B. Extinction of learned avoidance. Nicotine was paired with one flavor of Kool-Aid for 14 days, after which it was removed and rats had free access to the either of the Kool-Aid flavors for another 14 days. The graph plots consumption of the Kool-Aid flavor paired with nicotine (as a percentage of total volume consumed per day) versus time. Rats exhibited a significant avoidance of the flavor paired with nicotine over the first 14 days (p < 0.05, ANOVA), with a gradual trend toward greater consumption that reached 50% (extinction of learned avoidance; no preference) during weeks 3–4 when nicotine was no longer paired with the flavor. K. Zanotto, M. Iodi Carstens, E. Carstens, unpublished observations.
In contrast to the studies described above, other investigators report that rats and mice develop a preference for nicotine in two-bottle preference tests.76,84–87 The most likely explanation is that the post-ingestional rewarding effect of nicotine intake eventually overrides the aversive taste and/or irritancy of nicotine to result in a preference for nicotine. The rewarding effect of nicotine appears to involve α 4* and α 6* nAChR subunits in the ventral tegmental area.88
There are numerous other drawbacks of the two-bottle preference test. First, animals exhibit a side preference so that the position of the bottle containing nicotine must be changed regularly. For individual testing rodents must be singly housed, which induces social isolation stress. Another problem is that the two bottles are often fairly close together making it more difficult for animals to remember which side contains nicotine. Female B6 mice exhibited a progressive increase in nicotine consumption (up to a mean of 5 mg/kg/day), while A/J males exhibited a significant decrease (to <1 mg/kg/day) over a 42-day period when the bottles were separated by 19 cm,85 suggesting that preference for or avoidance of nicotine was improved by the bottle separation. Individuals within and across strains also exhibited a large amount of variability in nicotine consumption.73,74
Nicotine Antinociception
Tobacco was reported to relieve pain as early as the 16th century.89 It is now recognized that smoking or other forms of nicotine intake has an antinociceptive effect, and abstinence from smoking is often associated with increased pain that contributes to relapse in smokers trying to quit.90 The antinociceptive effect of nicotine no doubt contributes to the reciprocal relationship between chronic pain that can motivate the use of tobacco, and chronic tobacco usage that can lead to the development of chronic pain.91,92 A role for nAChRs in the antinociceptive effect of nicotine was bolstered by the discovery of the nAChR agonist epibatidine from the skin of Ecuadoran poison frogs, which has a potent antinociceptive effect exceeding and independent of that of morphine.93
Antinociception From Tobacco Smoke
A recent meta-analysis of 13 human studies reported that groups receiving nicotine consistently exhibited small to moderate increases in pain threshold and pain tolerance.94 In the selected studies, most comparisons were made between smoker and nonsmoker groups, but also included comparisons between groups receiving nicotine by patch or snuff. There was no apparent relationship between nicotine delivery method and the degree of pain reduction.
Only a few studies have investigated the antinociceptive effect of tobacco smoke in animals. Daily exposure of rats to cigarette smoke for 10 min resulted in antinociception in the tail flick test on the first day of exposure, followed by the rapid development of tolerance on subsequent days.95 Similar results were obtained with systemic nicotine treatment (1 mg/kg sc). Our group exposed rats in an environmental chamber to tobacco smoke in weekly 5-day blocks (6 h/day) over 4 weeks, with a mean plasma nicotine concentration of 95.4 ng/mL comparable to that of heavy smokers.96 Smoke exposure resulted in significant antinociception in the tail flick test (Figure 3A). There was recovery between blocks and a reduction in the magnitude of the antinociceptive effect across the four blocks of smoke exposure, indicating tolerance.97 The antinociceptive effect of smoke exposure on the first day was prevented in rats receiving mecamylamine via osmotic minipumps (Figure 3B), but was not significantly affected by the μ -opioid antagonist naloxone.98
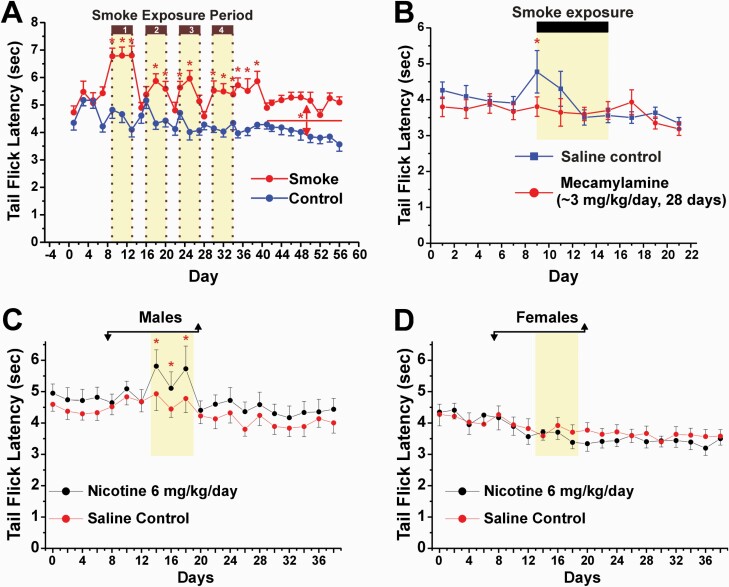
Figure 3.
Analgesia elicited by exposure to tobacco smoke or nicotine. A. Adult male rats were placed in an environmental chamber and exposed to tobacco smoke (approximately 90 mg/m3 suspended particulate matter; 4–5 mg/mm3 nicotine) for 6 h/day for blocks of 5 days, repeated 4 times (yellow shading). Smoke exposure resulted in mean plasma nicotine levels of 95.4 ng/mL. Tail flick testing was done immediately after each daily period of smoke exposure. Control animals were similarly housed and exposed to room air. *Significant difference between smoke-exposed and control groups (p < 0.05, ANOVA). Adapted from reference97, Fig. 2B, 2004 with permission from Elsevier. B. Analgesia from tobacco smoke exposure is prevented by mecamylamine. Mecamylamine or saline was delivered by osmotic minipump for 28 days. Rats were exposed to tobacco smoke (as in A) for 5 days, 6 h/day (yellow shading). *Significant difference between smoke-exposed and control groups (p < 0.05, ANOVA). Adapted from reference98, Fig. 2A, 2005 with permission from Elsevier. C. Analgesic effect of systemic nicotine in male rats. Nicotine or saline was delivered by osmotic minipump for 2 weeks (bar with arrows). *Significant difference between smoke-exposed and control groups (p < 0.05, ANOVA). D. Lack of analgesic effect of nicotine in female rats (format as in C). C and D Adapted from reference99, Figs. 2A, 2B, 2001 with permission from Springer/Nature.
Antinociceptive Effect of Nicotine
Many animal studies have shown antinociceptive effects of nicotine or nAChR agonists delivered systemically (see99 and references therein), intrathecally100–102 and by intracranial injection.103–107
Antinociception From Systemic Nicotine Administration: Tolerance and Sex Differences
Our group delivered nicotine to rats via osmotic minipump, which induced antinociception in male (Figure 3C) but not female (Figure 3D) rats.99 The antinociceptive effect in males confirms a previous study.108 Many other studies have demonstrated antinociception elicited by systemic nicotine, which exhibits the rapid development of tolerance, differences by pain test, and antagonism by mecamylamine and other nAChR antagonists.
The exact mechanism underlying tolerance to the antinociceptive effect of nicotine is uncertain, but likely involves desensitization of nAChRs, which can lead to increased expression of nAChRs in the brain.109 Tolerance to nicotine antinociception was prevented by pretreatment with mecamylamine110 and buproprion111 potentially by antagonizing nAChRs (mainly α 4 β 2*). Tolerance to the antinociceptive effect of nicotine was also reduced by interference with downstream calcium signaling,112 suggesting that tolerance also depends partly on post-receptor events.
A sex difference in nicotine antinociception as in Figure 3C,D has been previously reported, with a majority of studies showing greater antinociception in males than females but a minority showing the reverse or no difference.113 Moreover, a human study reported that nicotine patch treatment reduced pain to electrocutaneous shock in male but not female subjects, and also that male but not female smokers had higher pain threshold and tolerance levels.114 However, subsequent work indicates that both male and female smokers had higher pain threshold and tolerance levels compared to non-smokers.115
Spinal (Intrathecal) Administration
Intrathecal administration of nicotine elicited antinociception,100 with the (−) enantiomer eliciting stronger antinociception in a mecamylamine-sensitive manner.101 Intrathecal administration of epibatidine elicited nocifensive behavioral responses as well as a short-lasting antinociception that was blocked by mecamylamine.102 In a spinal cord slice preparation nAChR agonists facilitated spinal inhibition via α 4 β 2* but not α 7* nAChRs.116,117
Supraspinal Administration
Microinjection of nicotine into the pedunculopontine tegmentum and rostral ventromedial medulla (RVM) elicited antinociception104,105 possibly via afferent inputs to the RVM, an area giving rise to descending pain-modulatory pathways.118 Nicotine antinociception depends largely on α 4 and β 2 nAChR subunits based on genetic knockout studies.119 Microinjection of the α 4 β 2 nAChR agonist epibatidine into the RVM elicited antinociception dependent mainly on α 4 β 2 but to a lesser extent also on α 7 subunits.120 α 7 agonists injected intracerebroventricularly121–123 or into the ventrolateral periaqueductal gray (PAG)124 also elicited antinociception (Figure 4).
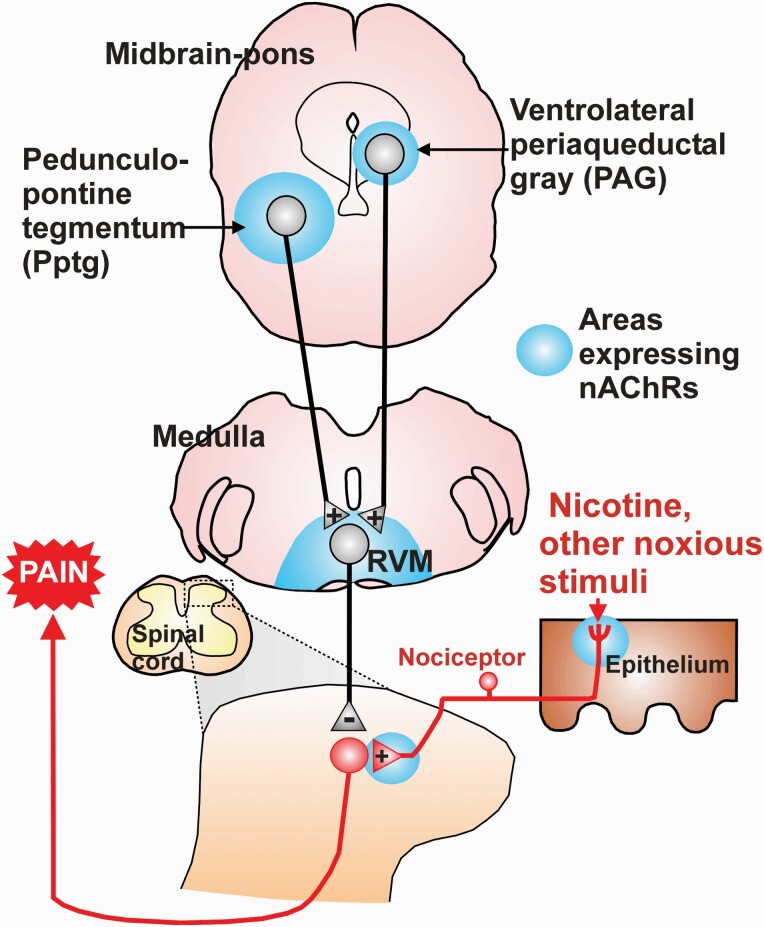
Figure 4.
Schematic showing brain regions with elevated levels of nAChRs, within which microinjection of nicotine elicited analgesia. The ventrolateral PAG and Pptg project to RVM, which gives rise to bidirectional modulation of spinal nociceptive transmission. Nicotine acting at supraspinal sites is postulated to engage RVM-spinal descending pathways that exert a predominantly inhibitory effect on spinal nociceptive transmission. Blue shading: areas with neurons expressing nAChRS. Abbreviations: RVM, rostral ventromedial medulla. +, excitatory synapse; −, inhibitory synapse.
The neurotransmitter serotonin (5-HT) has been implicated in nicotine antinociception. Serotonergic neurons in RVM express the α 2 nAChR subunit.125 Antinociception elicited by systemic nicotine was significantly reduced by pretreatment with 5-HT1a antagonists 8-OH-DPAT and buspirone126 and by pretreatment with the 5-HT synthesis inhibitor para-chlorophenylalanine.127 In contrast, there is less evidence that opioidergic mechanisms contribute to nicotine antinociception, since μ-opioid antagonists such as naloxone have mixed and often no effect (discussed in 91). For example, we found that systemic infusion of naloxone by osmotic minipump had no effect on the antinociception observed following exposure to tobacco smoke.98 Evidence thus indicates that the central antinociceptive action of nicotine is mediated in part by activation of α 4 β 2* and α7* nAChRs to activate serotonergic RVM neurons with descending antinociceptive effects on spinal pain transmission. This may work in combination with nicotine enhancement of spinal inhibition of nociceptive transmission, as noted above.
Flavor Additives and Interaction With Nicotine
Menthol is by far the most common flavor additive to cigarettes, and since 2009 the only one allowed in the United States. The rate of consumption of mentholated cigarettes is highest among youth (52.5%) and African Americans (86.5%).128 Although cigarette consumption has declined in the United States by 46% between 2000 and 2018, a large majority of the total decline (85%, and 91% since 2009) is in non-menthol cigarettes. Menthol contributes to the addictiveness of cigarettes by altering the expression of nAChRs, increasing nicotine bioavailability, reducing the sensory impact of smoke, and serving as a conditioned cue.129
Menthol is a cooling agent that acts via TRPM8,130,131 a cold-sensitive ion channel expressed by sensory nerve fibers.132–134 Menthol at sufficiently high concentration is irritating.135,136 We showed that oral irritation elicited by repeated application of menthol at 60-sec intervals significantly decreased across trials (desensitization) and cross-desensitized oral irritation elicited by nicotine even after the cooling effect of the menthol had dissipated.20 Menthol delivered by chewing gum transiently reduced irritation elicited by nicotine gum but the effect was no longer significant after 5 minutes.137 In an animal study using a two-bottle paradigm, mice exhibited aversion to menthol at concentrations above 100 μg/mL consistent with menthol’s irritant effect.138 However, wildtype mice preferred solutions containing nicotine plus menthol compared with nicotine (200 μg/mL) alone, while TRPM8 knockout mice preferred nicotine alone over a mixture of nicotine and menthol. These results suggest that menthol acting via TRPM8 counteracts nicotine irritation, but adds to nicotine irritation in mice lacking TRPM8.138 This is supported by the observation that menthol dose-dependently increased oral nicotine consumption in mice in a manner dependent on sex, age, and α 7* nAChRs.139 The aversion elicited by high menthol concentrations was abolished in TRPA1-deficient mice,140 implicating TRPA1 in menthol-induced aversion and respiratory irritation (see below).
In mice, braking (cessation of inspiration) was used as a readout of respiratory irritation elicited by cigarette smoke and constituent tobacco irritants including acrolein, cyclohexanone, and acetic acid.141,142 Co-inhalation of menthol reduced or prevented respiratory irritation elicited by these irritants. The suppression of acrolein-evoked respiratory irritation by menthol and eucalyptol, another TRPM8 agonist, was blocked by a TRPM8 antagonist.141 TRPM8-mediated inhibition of irritation might speculatively be due to menthol activation of peripheral cold fibers that excite spinal inhibitory interneurons to suppress pain transmission.143, 144 Since acrolein acts at TRPA1145 and nicotine acts at nAChRs and TRPA1 (at least in mice; see above), the ability of mM concentrations of menthol to inhibit TRPA1146, 147 may also contribute to reduced respiratory irritation as well as aversion to high oral concentrations of menthol.140 However, this may not apply in humans since menthol only acts as an agonist but not as an antagonist at human TRPA1.148 These results imply that menthol acting at TRPM8 (and/or inhibiting TRPA1) reduces respiratory irritation, thus allowing increased volume of inhaled tobacco smoke or nicotine vapor.
Systemic149 or oral150, 151 administration of menthol to rats increased intravenous nicotine self-administration (but see also152), suggesting that menthol promotes nicotine dependence.
The incidence of vaping and other means of electronic nicotine delivery is increasing especially among adolescents.153 Popular flavorants include tobacco, menthol, cherry, coffee, and chocolate/sweet.154 A higher menthol concentration (3.5%) reduced the irritation elicited by a high concentration of nicotine (24 mg/mL) and slightly increased the liking of e-cigarettes.155 Thus, part of menthol’s appeal as a flavorant may be its ability to reduce the harshness of inhaled nicotine.
Cinnamaldehyde is an irritant selective for TRPA1.156 When delivered independently by chewing gum, both cinnamaldehyde and nicotine elicited oral irritation with that of nicotine being greater; there was little effect on the level of nicotine-evoked irritation when nicotine and cinnamaldehyde gum were chewed simultaneously.157
Conclusions
In conclusion, nicotine activates centrally mediated reward systems but at the same time has aversive sensory properties. Nicotine elicits irritation and pain via activation of nAChRs and possibly TRPA1 (at least in mice) expressed by peripheral sensory neurons. This activates trigeminal pain pathways to excite neurons in Vc and other brainstem areas in a pattern of neuronal firing that is consistent with the psychophysical desensitizing effect of nicotine and the sensitizing effect of capsaicin. Tobacco smoke and systemic administration of nicotine also induces antinociception by activating brainstem neurons expressing nAChRs, giving rise to descending inhibition of the spinal transmission of pain signals. The rewarding and antinociceptive effects of nicotine are seemingly at odds with its aversive properties (see “paradox of drug reward” above), and interact in a complex manner that leads to addiction in some individuals.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
References 101–157 are available as Supplementary Material.
Declaration of Interests
None declared.
Funding
The authors gratefully acknowledge support from the National Institutes of Health and the California Tobacco-Related Disease Research Program for their work presented in this review.
References
- 1. World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025, Second Edition. Geneva: World Health Organization; 2018. www.who.int/tobacco/publications/surveillance/trends-tobacco-smoking-second-edition/en/. [Google Scholar]
- 2. Wang TW, Asman K, Gentzke AS, et al. . Tobacco product use among adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. doi: 10.15585/mmwr.mm6744a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavallo DA, Krishnan-Sarin S. Nicotine use disorders in adolescents. Pediatr Clin North Am. 2019;66(6):1053–1062. [DOI] [PubMed] [Google Scholar]
- 4. U.S. Food & Drug Administration. Harmful and potentially harmful constituents in tobacco products and tobacco smoke: Established list. 2012. www.fda.gov. Accessed July, 2020.
- 5. Clapp PW, Jaspers I. Electronic cigarettes: Their constituents and potential links to asthma. Curr Allergy Asthma Rep.. 2017;17(11):79. doi: 10.1007/s11882-017-0747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins AC, Pogun S, Nesil T, Kanit L. Oral nicotine self-administration in rodents. J Addict Res Ther.. 2012;(suppl 2):004. doi: 10.4172/2155-6105.S2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duke AN, Johnson MW, Reissig CJ, Griffiths RR. Nicotine reinforcement in never-smokers. Psychopharmacology (Berl). 2015;232(23):4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hagen EH, Sullivan RJ, Schmidt R, Morris G, Kempter R, Hammerstein P. Ecology and neurobiology of toxin avoidance and the paradox of drug reward. Neuroscience. 2009;160(1):69–84. [DOI] [PubMed] [Google Scholar]
- 9. Smith LC, George O. Advances in smoking cessation pharmacotherapy: Non-nicotinic approaches in animal models. Neuropharmacology. 2020;178:108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King JL, Reboussin BA, Wiseman KD, et al. . Adverse symptoms users attribute to e-cigarettes: Results from a national survey of US adults. Drug Alcohol Depend. 2019;196:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011;22(6):477–482. [DOI] [PubMed] [Google Scholar]
- 12. Keele CA, Armstrong D.. Substances Producing Pain and Itch. London: Edward Arnold; 1964. [Google Scholar]
- 13. Hummel T, Livermore A, Hummel C, Kobal G. Chemosensory event-related potentials in man: Relation to olfactory and painful sensations elicited by nicotine. Electroencephalogr Clin Neurophysiol. 1992;84(2):192–195. [DOI] [PubMed] [Google Scholar]
- 14. Greiff L, Wollmer P, Erjefält I, Andersson M, Pipkorn U, Persson CG. Effects of nicotine on the human nasal mucosa. Thorax. 1993;48(6):651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansco N, Jansco-Gabor A, Takats I. Pain and inflammation induced by nicotine, acetylcholine and structurally related compounds and their prevention by desensitizing agents. Acta Physiol Acad Sci Hung. 1961;19:113–132. PMID: 13789367. [PubMed] [Google Scholar]
- 16. Jarvik ME, Assil KM. Mecamylamine blocks the burning sensation of nicotine on the tongue. Chem Senses.. 1988;13(2):213–217. [Google Scholar]
- 17. Dessirier JM, O’Mahony M, Sieffermann JM, Carstens E. Mecamylamine inhibits nicotine but not capsaicin irritation on the tongue: Psychophysical evidence that nicotine and capsaicin activate separate molecular receptors. Neurosci Lett. 1998;240(2):65–68. doi: 10.1016/s0304-3940(97)00930-0. [DOI] [PubMed] [Google Scholar]
- 18. Dessirier JM, O’Mahony M, Carstens E. Oral irritant effects of nicotine: psychophysical evidence for decreased sensation following repeated application and lack of cross-desensitization to capsaicin. Chem Senses. 1997;22(5):483–492. doi: 10.1093/chemse/22.5.483. [DOI] [PubMed] [Google Scholar]
- 19. Simons CT, Carstens MI, Carstens E. Oral irritation by mustard oil: Self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28(6):459–465. doi: 10.1093/chemse/28.6.459. [DOI] [PubMed] [Google Scholar]
- 20. Dessirier JM, O’Mahony M, Carstens E. Oral irritant properties of menthol: Sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav.. 2001;73(1–2):25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- 21. Green BG. Capsaicin sensitization and desensitization on the tongue produced by brief exposures to a low concentration. Neurosci Lett.. 1989;107(1–3):173–178. doi: 10.1016/0304-3940(89)90812-4. [DOI] [PubMed] [Google Scholar]
- 22. Dessirier JM, Nguyen N, Sieffermann JM, Carstens E, O’Mahony M. Oral irritant properties of piperine and nicotine: psychophysical evidence for asymmetrical desensitization effects. Chem Senses. 1999;24(4):405–413. doi: 10.1093/chemse/24.4.405. [DOI] [PubMed] [Google Scholar]
- 23. Dessirier JM, Chang HK, O’Mahony M, Carstens E. Cross-desensitization of capsaicin-evoked oral irritation by high but not low concentrations of nicotine in human subjects. Neurosci Lett. 2000;290(2):133–136. doi: 10.1016/s0304-3940(00)01339-2. [DOI] [PubMed] [Google Scholar]
- 24. Carstens E, Albin KC, Simons CT, Carstens MI. Time course of self-desensitization of oral irritation by nicotine and capsaicin. Chem Senses. 2007;32(9):811–816. doi: 10.1093/chemse/bjm048. [DOI] [PubMed] [Google Scholar]
- 25. Douglas WW, Ritchie JM. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol. 1960;150(3):501–514. doi: 10.1113/jphysiol.1960.sp006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fjallbrant N, Iggo A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steen KH, Reeh PW. Actions of cholinergic agonists and antagonists on sensory nerve endings in rat skin, in vitro. J Neurophysiol. 1993;70(1):397–405. doi: 10.1152/jn.1993.70.1.397. [DOI] [PubMed] [Google Scholar]
- 28. Bernardini N, Sauer SK, Haberberger R, Fischer MJ, Reeh PW. Excitatory nicotinic and desensitizing muscarinic (M2) effects on C-nociceptors in isolated rat skin. J Neurosci. 2001;21(9):3295–3302. doi: 10.1523/JNEUROSCI.21-09-03295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanelian DL. Cholinergic activation of a population of corneal afferent nerves. Exp Brain Res. 1991;86(2):414–420. doi: 10.1007/BF00228966. [DOI] [PubMed] [Google Scholar]
- 30. MacIver MB, Tanelian DL. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993;13(10):4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sekizawa SI, Tsubone H. Nasal receptors responding to noxious chemical irritants. Respir Physiol. 1994;96(1):37–48. doi: 10.1016/0034-5687(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 32. Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25(1):61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J Gen Physiol. 1993;101(6):843–866. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu J, Yang W, Zhang G, Gu Q, Lee LY. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L54–L61. doi: 10.1152/ajplung.00182.2006. [DOI] [PubMed] [Google Scholar]
- 35. Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127(2–3):113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 36. Sucher NJ, Cheng TP, Lipton SA. Neural nicotinic acetylcholine responses in sensory neurons from postnatal rat. Brain Res. 1990;533(2):248–254. doi: 10.1016/0006-8993(90)91346-i. [DOI] [PubMed] [Google Scholar]
- 37. Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86(4):1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 38. Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci. 2004;113(1-2):32–42. doi: 10.1016/j.autneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 39. Rau KK, Johnson RD, Cooper BY. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J Neurophysiol. 2005;93(3):1358–1371. doi: 10.1152/jn.00591.2004. [DOI] [PubMed] [Google Scholar]
- 40. Zhang XL, Albers KM, Gold MS. Inflammation-induced increase in nicotinic acetylcholine receptor current in cutaneous nociceptive DRG neurons from the adult rat. Neuroscience. 2015;284:483–499. doi: 10.1016/j.neuroscience.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu L, Simon SA. Capsaicin and nicotine both activate a subset of rat trigeminal ganglion neurons. Am J Physiol.. 1996;270(6 Pt 1):C1807–C1814. doi: 10.1152/ajpcell.1996.270.6.C1807. [DOI] [PubMed] [Google Scholar]
- 42. Fucile S, Sucapane A, Eusebi F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol. 2005;565(Pt 1):219–228. doi: 10.1113/jphysiol.2005.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hone AJ, Meyer EL, McIntyre M, McIntosh JM. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype. FASEB J. 2012;26(2):917–926. doi: 10.1096/fj.11-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Talavera K, Gees M, Karashima Y, et al. . Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12(10):1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 45. Zhang X, Hartung JE, Friedman RL, Koerber HR, Belfer I, Gold MS. Nicotine evoked currents in human primary sensory neurons. J Pain. 2019;20(7):810–818. doi: 10.1016/j.jpain.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jinno S, Hua XY, Yaksh TL. Nicotine and acetylcholine induce release of calcitonin gene-related peptide from rat trachea. J Appl Physiol (1985). 1994;76(4):1651–1656. doi: 10.1152/jappl.1994.76.4.1651. [DOI] [PubMed] [Google Scholar]
- 47. Kichko TI, Lennerz J, Eberhardt M, et al. . Bimodal concentration-response of nicotine involves the nicotinic acetylcholine receptor, transient receptor potential vanilloid type 1, and transient receptor potential ankyrin 1 channels in mouse trachea and sensory neurons. J Pharmacol Exp Ther. 2013;347(2):529–539. doi: 10.1124/jpet.113.205971.E. [DOI] [PubMed] [Google Scholar]
- 48. Kichko TI, Kobal G, Reeh PW. Cigarette smoke has sensory effects through nicotinic and TRPA1 but not TRPV1 receptors on the isolated mouse trachea and larynx. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L812–L820. doi: 10.1152/ajplung.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kichko TI, Neuhuber W, Kobal G, Reeh PW. The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur J Neurosci. 2018;47(3):201–210. doi: 10.1111/ejn.13799. [DOI] [PubMed] [Google Scholar]
- 50. Jinks SL, Simons CT, Dessirier JM, Carstens MI, Antognini JF, Carstens E. C-fos induction in rat superficial dorsal horn following cutaneous application of noxious chemical or mechanical stimuli. Exp Brain Res. 2002;145(2):261–269. doi: 10.1007/s00221-002-1128-3. [DOI] [PubMed] [Google Scholar]
- 51. Jinks SL, Carstens E. Activation of spinal wide dynamic range neurons by intracutaneous microinjection of nicotine. J Neurophysiol. 1999;82(6):3046–3055. doi: 10.1152/jn.1999.82.6.3046. [DOI] [PubMed] [Google Scholar]
- 52. Carstens E, Saxe I, Ralph R. Brainstem neurons expressing c-Fos immunoreactivity following irritant chemical stimulation of the rat’s tongue. Neuroscience. 1995;69(3):939–953. doi: 10.1016/0306-4522(95)00297-v. [DOI] [PubMed] [Google Scholar]
- 53. Carstens E, Simons CT, Dessirier JM, Carstens MI, Jinks SL. Role of neuronal nicotinic-acetylcholine receptors in the activation of neurons in trigeminal subnucleus caudalis by nicotine delivered to the oral mucosa. Exp Brain Res. 2000;132(3):375–383. doi: 10.1007/s002210000351. [DOI] [PubMed] [Google Scholar]
- 54. Boucher Y, Simons CT, Cuellar JM, Jung SW, Carstens MI, Carstens E. Activation of brain stem neurons by irritant chemical stimulation of the throat assessed by c-fos immunohistochemistry. Exp Brain Res. 2003;148(2):211–218. doi: 10.1007/s00221-002-1308-1. [DOI] [PubMed] [Google Scholar]
- 55. Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80(2):465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 56. Dessirier JM, Simons CT, Sudo M, Sudo S, Carstens E. Sensitization, desensitization and stimulus-induced recovery of trigeminal neuronal responses to oral capsaicin and nicotine. J Neurophysiol. 2000;84(4):1851–1862. doi: 10.1152/jn.2000.84.4.1851. [DOI] [PubMed] [Google Scholar]
- 57. Sudo S, Sudo M, Simons CT, Dessirier JM, Carstens E. Sensitization of trigeminal caudalis neuronal responses to intraoral acid and salt stimuli and desensitization by nicotine. Pain. 2002;98(3):277–286. doi: 10.1016/s0304-3959(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 58. Simons CT, Sudo S, Sudo M, Carstens E. Mecamylamine reduces nicotine cross-desensitization of trigeminal caudalis neuronal responses to oral chemical irritation. Brain Res.. 2003;991(1–2):249–253. doi: 10.1016/s0006-8993(03)03539-x. [DOI] [PubMed] [Google Scholar]
- 59. Pfaffmann C. The sense of taste. In: J Field, HW Magoun, VE Hall, eds. Handbook of Physiology: Neurophysiology. Vol 1. Washington, DC: American Physiological Society; 1959:507–533. [Google Scholar]
- 60. Flynn FW, Webster M, Ksir C. Chronic voluntary nicotine drinking enhances nicotine palatability in rats. Behav Neurosci. 1989;103(2):356–364. doi: 10.1037//0735-7044.103.2.356. [DOI] [PubMed] [Google Scholar]
- 61. Brining SK, Belecky TL, Smith DV. Taste reactivity in the hamster. Physiol Behav. 1991;49(6):1265–1272. doi: 10.1016/0031-9384(91)90361-q. [DOI] [PubMed] [Google Scholar]
- 62. Glatt AR, Denton K, Boughter JD Jr. Variation in nicotine consumption in inbred mice is not linked to orosensory ability. Chem Senses. 2009;34(1):27–35. doi: 10.1093/chemse/bjn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gyekis JP, Dingman MA, Revitsky AR, et al. . Gustatory, trigeminal, and olfactory aspects of nicotine intake in three mouse strains. Behav Genet. 2012;42(5):820–829. doi: 10.1007/s10519-012-9546-x. [DOI] [PubMed] [Google Scholar]
- 64. Qian J, Mummalaneni S, Grider JR, Damaj MI, Lyall V. Nicotinic acetylcholine receptors (nAChRs) are expressed in Trpm5 positive taste receptor cells (TRCs). PLoS One.. 2018;13(1):e0190465. doi: 10.1371/journal.pone.0190465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, et al. . Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci U S A. 2009;106(5):1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94(6):3719–3729. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- 67. Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006;96(4):1877–1886. doi: 10.1152/jn.00345.2006. [DOI] [PubMed] [Google Scholar]
- 68. Pogun S, Yararbas G, Nesil T, Kanit L. Sex differences in nicotine preference. J Neurosci Res. 2017;95(1-2):148–162. doi: 10.1002/jnr.23858. [DOI] [PubMed] [Google Scholar]
- 69. O’Dell LE, Khroyan TV. Rodent models of nicotine reward: What do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91(4):481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Y, Le Foll B, Liu Y, Wang X, Lu L. Conditioned place preference induced by licit drugs: Establishment, extinction, and reinstatement. ScientificWorldJournal. 2008;8:1228–1245. doi: 10.1100/tsw.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: A review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res. 2015;17(11):1297–1310. doi: 10.1093/ntr/ntv002. [DOI] [PubMed] [Google Scholar]
- 72. Flores RJ, Uribe KP, Swalve N, O’Dell LE. Sex differences in nicotine intravenous self-administration: A meta-analytic review. Physiol Behav. 2019;203:42–50. doi: 10.1016/j.physbeh.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl). 1996;124(4):332–339. [DOI] [PubMed] [Google Scholar]
- 74. Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61(1-2):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nesil T, Kanit L, Li MD, Pogun S. Nine generations of selection for high and low nicotine intake in outbred Sprague-Dawley rats. Behav Genet. 2013;43(5):436–444. doi: 10.1007/s10519-013-9605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nesil T, Kanit L, Ugur M, Pogun S. Nicotine withdrawal in selectively bred high and low nicotine preferring rat lines. Pharmacol Biochem Behav. 2015;131:91–97. doi: 10.1016/j.pbb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 77. Stoker AK, Markou A. Unraveling the neurobiology of nicotine dependence using genetically engineered mice. Curr Opin Neurobiol. 2013;23(4):493–499. doi: 10.1016/j.conb.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bagdas D, Alkhlaif Y, Jackson A, Carroll FI, Ditre JW, Damaj MI. New insights on the effects of varenicline on nicotine reward, withdrawal and hyperalgesia in mice. Neuropharmacology. 2018;138:72–79. doi: 10.1016/j.neuropharm.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Le Houezec J, Martin C, Cohen C, Molimard R. Failure of behavioral dependence induction and oral nicotine bioavailability in rats. Physiol Behav. 1989;45(1):103–108. [DOI] [PubMed] [Google Scholar]
- 81. Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50(4):619–626. [DOI] [PubMed] [Google Scholar]
- 82. Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: A dose-response experiment. Pharmacol Biochem Behav. 2004;78(1):13–25. [DOI] [PubMed] [Google Scholar]
- 83. Smith A, Roberts DC. Oral self-administration of sweetened nicotine solutions by rats. Psychopharmacology (Berl). 1995;120(3):341–346. doi: 10.1016/j.neuropharm.2020.108225. [DOI] [PubMed] [Google Scholar]
- 84. Adriani W, Macrì S, Pacifici R, Laviola G. Restricted daily access to water and voluntary nicotine oral consumption in mice: Methodological issues and individual differences. Behav Brain Res. 2002;134(1-2):21–30. doi: 10.1016/S0166-4328(01)00448-X. [DOI] [PubMed] [Google Scholar]
- 85. Locklear LL, McDonald CG, Smith RF, Fryxell KJ. Adult mice voluntarily progress to nicotine dependence in an oral self-selection assay. Neuropharmacology. 2012;63(4):582–592. [DOI] [PubMed] [Google Scholar]
- 86. Peng C, Engle SE, Yan Y, et al. . Altered nicotine reward-associated behavior following α4 nAChR subunit deletion in ventral midbrain. PLoS One. 2017;12(7):e0182142. doi: 10.1371/journal.pone.0182142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bagdas D, Diester CM, Riley J, et al. . Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology. 2019;157:107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Exley R, Maubourguet N, David V, et al. . Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108(18):7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Umana IC, Danidle CA, McGehee DS. Neuronal nicotinic receptors as analgesic targets: It’s a winding road. Biochem Pharmacol.. 2013;86:1208–1214. doi: 10.1016/j.bcp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ditre JW, Heckman BW, LaRowe LR, Powers JM. Pain status as a predictor of smoking cessation initiation, lapse, and relapse. Nicotine Tob Res.. 2021;23(1):186–194. doi: 10.1093/ntr/ntaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113(4):977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- 92. LaRowe LR, Ditre JW. Pain, nicotine, and tobacco smoking: Current state of the science. Pain. 2020;161(8):1688–1693. doi: 10.1097/j.pain.0000000000001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Spande TF, Garraffo HM, Yeh HJ, Pu QL, Pannell LK, Daly JW. A new class of alkaloids from a dendrobatid poison frog: A structure for alkaloid 251F. J Nat Prod. 1992;55(6):707–722. doi: 10.1021/np50084a002. [DOI] [PubMed] [Google Scholar]
- 94. Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA. Acute analgesic effects of nicotine and tobacco in humans: A meta-analysis. Pain. 2016;157(7):1373–1381. doi: 10.1097/j.pain.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mousa SA, Aloyo VJ, Van Loon GR. Tolerance to tobacco smoke- and nicotine-induced analgesia in rats. Pharmacol Biochem Behav. 1988;31(2):265–268. doi: 10.1016/0091-3057(88)90344-9. [DOI] [PubMed] [Google Scholar]
- 96. Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol.. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. Neurosci Lett. 2004;366(1):86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 98. Simons CT, Cuellar JM, Moore JA, et al. . Nicotinic receptor involvement in antinociception induced by exposure to cigarette smoke. Neurosci Lett. 2005;389(2):71–76. doi: 10.1016/j.neulet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 99. Carstens E, Anderson KA, Simons CT, Carstens MI, Jinks SL. Analgesia induced by chronic nicotine infusion in rats: Differences by gender and pain test. Psychopharmacology (Berl). 2001;157(1):40–45. doi: 10.1007/s002130100770. [DOI] [PubMed] [Google Scholar]
- 100. Aceto MD, Bagley RS, Dewey WL, Fu TC, Martin BR. The spinal cord as a major site for the antinociceptive action of nicotine in the rat. Neuropharmacology. 1986;25(9):1031–1036. doi: 10.1016/0028-3908(86)90198-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.