Abstract
Objective:
This systematic review and meta-analysis aimed to evaluate whether dyslipidemia affects the mortality and severity of COVID-19, we also aimed to evaluate whether other comorbidities influence the association.
Methods:
A systematic literature search using PubMed, Embase, and EuropePMC was performed on 8 October 2020. This study’s main outcome is a poor composite outcome, comprising of mortality and severe COVID-19.
Results:
There were 9 studies with 3663 patients. The prevalence of dyslipidemia in this pooled analysis was 18% (4%-32%). Dyslipidemia was associated with increased composite poor outcome (RR 1.39 [1.02, 1.88], P = .010; I2: 56.7%, P = .018). Subgroup analysis showed that dyslipidemia was associated with severe COVID-19 (RR 1.39 [1.03, 1.87], P = .008; I2: 57.4%, P = .029). Meta-regression showed that the association between dyslipidemia and poor outcome varies by age (coefficient: −0.04, P = .033), male gender (coefficient: −0.03, P = .042), and hypertension (coefficient: −0.02, P = .033), but not diabetes (coefficient: −0.24, P = .135) and cardiovascular diseases (coefficient: −0.01, P = .506). Inverted funnel-plot was relatively symmetrical. Egger’s test indicates that the pooled analysis was not statistically significant for small-study effects (P = .206).
Conclusion:
Dyslipidemia potentially increases mortality and severity of COVID-19. The association was stronger in patients with older age, male, and hypertension.
PROSPERO Registration Number: CRD42020213491
Keywords: coronavirus, COVID-19, dyslipidemia, hyperlipidemia, prognosis
Introduction
Coronavirus disease 2019 (COVID-19) is rapidly increasing and threatens to overwhelm healthcare systems, especially in developing countries. 1 Potential prognostic factors such as comorbidities2-6 and laboratory results7-9 facilitate risk stratification and allocation of high-risk patients to receive more intensive monitoring. This is especially important in developing countries, which may struggle due to a lack of medical resources.
Approximately 30%-60% of the population is affected by dyslipidemia, making it one of the most prevalent conditions in healthy people.10-12 Thus, it is potentially one of the most common comorbidities in patients with COVID-19. The metabolic and lipid profile may even worsen due to less activity and imbalanced diet during the pandemic. However, the evidence on whether dyslipidemia influences the prognosis of COVID-19 remains contradictory.13-16 Previously, a meta-analysis shown that dyslipidemia potentially increases severity. 17 This meta-analysis aimed to evaluate whether dyslipidemia affects the mortality and severity of COVID-19, we also aimed to evaluate whether other comorbidities influence the association using meta-regression analysis.
Material and Methods
Search strategy and study selection
This is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) compliant systematic review. This study is registered in the PROSPERO (CRD42020213491).
Eligibility criteria
The inclusion criteria were studies reporting COVID-19 patients along with the information on dyslipidemia and mortality/severity/progression of COVID-19 disease.
We excluded preprints, letters, conference abstracts, review articles, editorials/opinion, and case reports/series to reduce bias.
Search strategy and study selection
A systematic literature search using PubMed, Embase, and EuropePMC was performed on 8 October 2020. The PubMed search keywords was ([dyslipidemia] OR [hyperlipidemia]) AND ([severity] OR [mortality]) AND ([COVID-19] OR [2019-nCoV] OR [coronavirus] OR [SARS-CoV-2]). After the initial search, we remove duplicates; then, the titles and abstracts were screened and evaluated for their eligibility by 2 independent authors. The literature searchers are experienced in performing systematic reviews.
Data extraction
Two authors independently conducted data extraction with the aid of standardized extraction forms comprised of the first author, year of publication, study design, age, gender, diabetes mellitus, hypertension, cardiovascular disease, smoking, obesity, liver disease, and outcome of interest. The factors chosen were based on the possibility that they may confound the association between dyslipidemia and the main outcome.
The key exposure in this study was dyslipidemia, defined as dyslipidemia as comorbidity that was diagnosed based on medical history or medical records. Other variables such as diabetes mellitus, hypertension, cardiovascular disease, smoking, obesity, and liver disease were also based on diagnosis from medical history or medical records.
This study’s main outcome is a poor composite outcome, defined as a composite of mortality and severe COVID-19. Mortality was defined as death or non-survivor in the studies. Severe COVID-19 was a composite of severe COVID-19 based on the World Health Organization (WHO)-China joint mission on Coronavirus Disease, need for ICU care or mechanical ventilation, and disease progression respective studies’ definition. The effect measure was risk ratios (RRs). To assess the risk of bias, 2 independent authors use the Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies.
Statistical analysis
STATA 16 was used to perform meta-analyses in this study. Meta-analysis of the proportion was utilized to estimate the pooled prevalence of dyslipidemia in the included studies. The random-effects model calculated using the DerSimonian & Laird method was used to calculate the RR for the main outcome and estimate the heterogeneity. P-values ⩽ .05 were considered as statistically significant. Inter-study heterogeneity was assessed using I-squared (I2) and Cochrane Q test, with a value of >50% or P-value < .10 indicates significant heterogeneity. Subgroup analysis was performed for severe COVID-19. Random-effects meta-regression with restricted-maximum likelihood method was performed using 1 covariate at a time for age, gender, hypertension, diabetes mellitus, cardiovascular disease, smoking, and obesity. Egger’s test was performed to assess small-study effects. Funnel-plot analysis was used to assess the risk of publication bias.
Results
Study selection and baseline characteristics
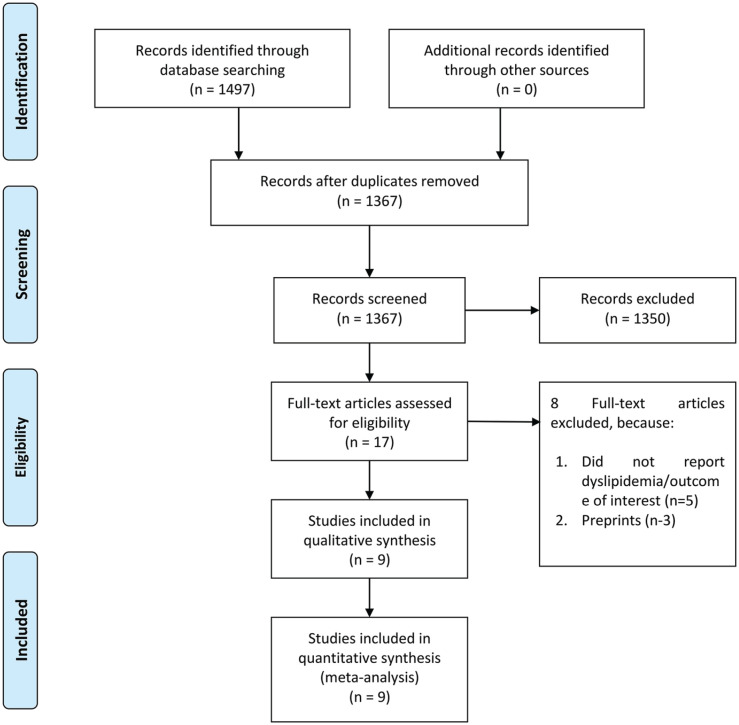
Preliminary several databases search yielded 1497 articles. After subsequent exclusion of duplicates, the remaining articles for the title and abstract screening were 1367 articles. Then, a total of 1350 articles were excluded, with the remaining articles underwent full-text eligibility assessment. Finally, out of 17 studies assessed for full-text eligibility, 9 studies with 3663 patients were included in the meta-analysis.13-16,18-22 (Figure 1, Table 1) The mean NOS of the included studies was 8 ± 0.86, indicating low-to-moderate risk of bias.
Figure 1.
PRISMA flowchart.
Table 1.
Characteristics of patients in the included studies.
| Author | Study design | Sample | Age (mean/median) | Male (%) | Hypertension (%) | Diabetes (%) | Cardiovascular disease (%) | Liver disease (%) | Obesity (%) | Smoking (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al 21 | RC | 211 | 37.55 | 35.1 | 6.2 | 1.9 | 0.9 | — | — | 8 | |
| Chen et al. 14 | RC | 145 | 47.52 | 54.4 | 15.2 | 9.7 | 0.6 | 4.1 | — | 9.9 | 8 |
| Hwang et al. 17 | RC | 77 | 67.62 | 50 | 55 | 34 | 12 | — | — | 8 | |
| Petrilli et al. 16 | PC | 2729 | 63 | 61.3 | 62 | 34.7 | 70.6 | 39.6 | 5.2 | 9 | |
| Santos et al. 18 | RC | 37 | 74.7 | 47.4 | 60.5 | 39.5 | 50 | — | — | 8 | |
| Simonnet et al. 19 | RC | 124 | 60 | 73 | 49 | 23 | — | — | — | 9 | |
| To et al. 20 | PC | 23 | 60.46 | 56.5 | 26 | 17.4 | 8.7 | — | — | 6 | |
| Zhang et al. 13 | RC | 80 | 51.16 | 41.25 | 22.5 | 11.25 | 11.25 | 6.2 | — | — | 8 |
| Zhang et al. 15 | RC | 140 | 57 | 50.7 | 30.0 | 12.1 | — | 5.7 | — | 6.4 | 8 |
Abbreviations: CAD, coronary artery disease; CVD, cardiovascular diseases; RC, retrospective cohort; —, not available/applicable/reported; NOS, Newcastle-Ottawa scale.
Dyslipidemia and composite poor outcome
The prevalence of dyslipidemia in this pooled analysis was 18% (4%-32%). Dyslipidemia was associated with composite poor outcome (RR 1.39 [1.02, 1.88], P = .010; I2: 56.7%, P = .018) (Figure 2). Subgroup analysis showed that dyslipidemia was associated with severe COVID-19 (RR 1.39 [1.03, 1.87], P = .008; I2: 57.4%, P = .029) (Figure 3).
Figure 2.
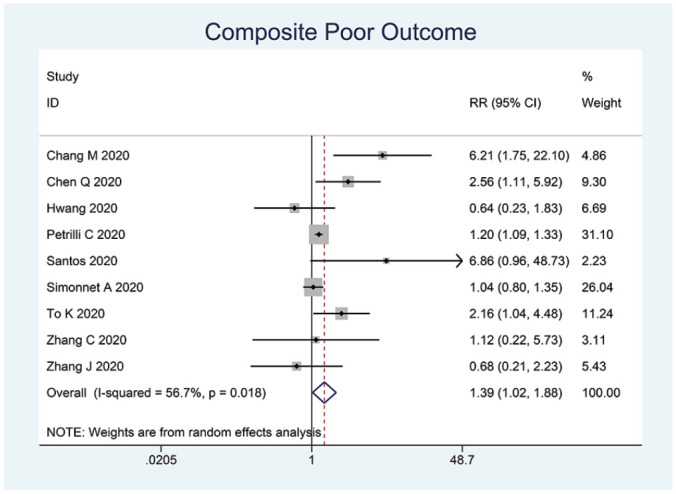
Dyslipidemia and poor outcome.
Figure 3.
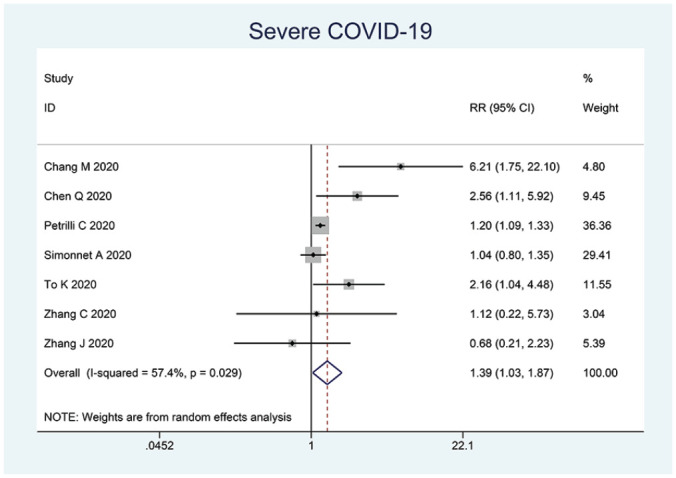
Dyslipidemia and severe COVID-19.
Meta-regression
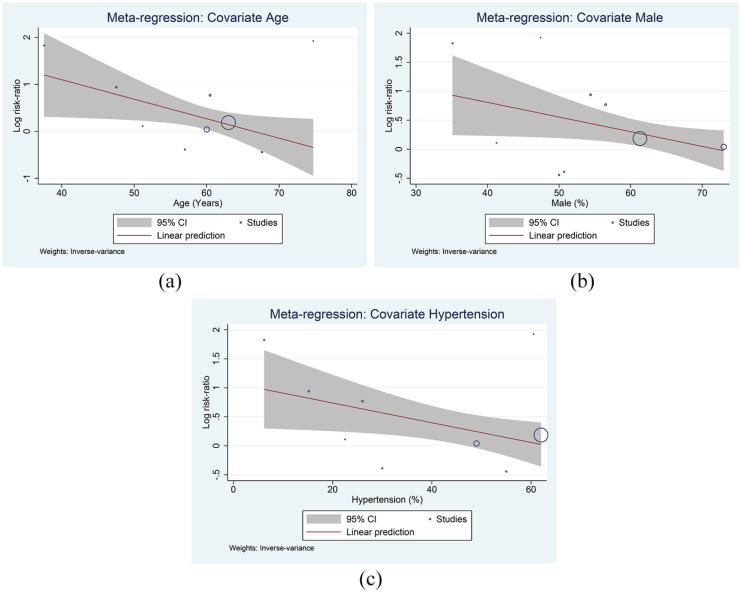
Meta-regression showed that the association between dyslipidemia and poor outcome varies by age (coefficient: −0.04, P = .033) (Figure 4a), gender (male, coefficient: −0.03, P = .042) (Figure 4b), and hypertension (coefficient: −0.02, P = .033) (Figure 4c), but not diabetes (coefficient: −0.24, P = .135) and cardiovascular diseases (coefficient: −0.01, P = .506).
Figure 4.
Meta-regression analysis. Covariates: age (a), male gender (b), and hypertension (c).
Risk of bias assessment
The mean NOS of the included studies was 8 ± 0.86, indicating high-quality studies. Inverted funnel-plot was relatively symmetrical (Figure 5). Egger’s test indicates that the pooled analysis was not statistically significant for small-study effects (P = .206).
Figure 5.
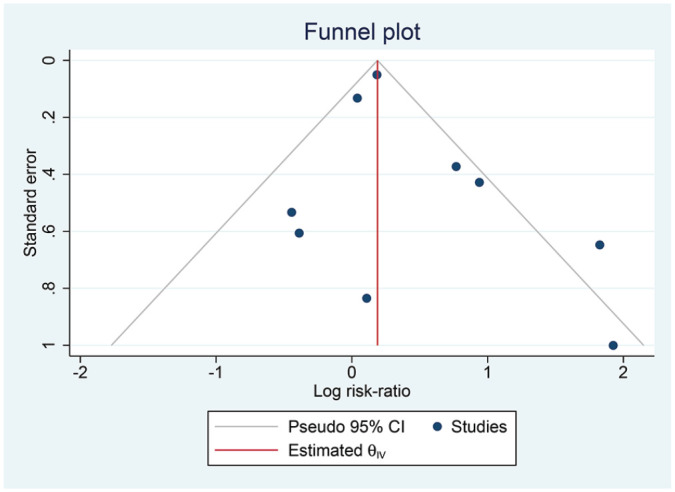
Inverted funnel plot analysis.
Discussion
Meta-analysis showed that dyslipidemia potentially increases severity and mortality associated with COVID-19. The association was affected by age, male, and hypertension. However, this association is limited by the lack of details regarding comorbidities and medications consumption among the patients. Drugs such as statin has been shown to reduced fatal/severe disease in patients with COVID-19. 23
The findings in our studies strengthen the previous hypothesis, 17 in this study we provide an addition of meta-regression analysis to explore the confounders and source of heterogeneity. Meta-regression analysis showed that older age, male, and hypertension affect the association between dyslipidemia and poor outcome. This may indicate that the association may be due to other conditions accompanying dyslipidemia rather than dyslipidemia itself. Petrilli et al, 16 Simonnet et al, 20 and Chang et al 22 indicates that dyslipidemia was not significant for increased severity in multivariable analysis. Meanwhile, Santos et al 19 reported that dyslipidemia as an independent predictor of mortality. Obesity and chronic liver disease, including fatty liver disease, may affect the association between dyslipidemia and poor outcome; because both obesity and fatty liver disease are associated with poor prognosis.6,24,25 However, meta-regression analysis was not possible because only 3 studies were reporting chronic liver disease, and 1 study reporting obesity.
Plasma lipids and lipoproteins measurement, such as low-density lipoprotein (LDL), very-low-density lipoprotein (VLDL), high-density lipoprotein (HDL), and triglycerides (TG), is a key component in managing the risk of cardiovascular diseases (CVD). Excessive body mass index (BMI) is an independent risk factor for severe COVID-19. A dose-response meta-analysis reported that the risk for composite poor outcome increased by 5% for every 5 kg/mg 2 increase in BMI. 6 Obese individuals are at a higher risk of developing dyslipidemia, insulin resistance, diabetes mellitus, hypertension, CVD, and cerebrovascular disease, which are well-known predictor of poor prognosis in COVID-19 patients.2,3,5,26 Furthermore, the lack of physical inactivity (sedentary lifestyle) in individuals with obesity and dyslipidemia during the pandemic may further undermine their immunity and put them at higher risk of contracting COVID-19.27,28 The presence of coexisting diseases, including cardiac disease, pulmonary disease, chronic kidney disease, liver injury, and orthopedic injuries, are also associated with worse outcomes in patients with COVID-19.29-33
Excessive immune system activation is often found in severe COVID-19 leading to various complications, such as respiratory failure, multi-organ dysfunction, coagulopathy, and ultimately death.8,34 Tissue damage caused by viral invasion promotes the release of pro-inflammatory chemokines and cytokines, including interleukin (IL)-6, macrophage inflammatory protein (MIP), and monocyte chemoattractant protein (MCP)-1, and further attracts defensive host cells, such as neutrophils, macrophages, and T cells. Activation of these cells causes uncontrolled, persistent inflammation, and impaired immunity with further accumulation of eicosanoids, including thromboxane B2 (TXB2), prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and lipoxin A4 (LXA4). 35 The increased eicosanoids and hypercoagulable state in patients with COVID-19 may contribute to the development of life-threatening complications and eventual death.
The ongoing inflammatory processes result in HDL-related apolipoproteins’ modulation, causing increased serum amyloid protein A and decreased apolipoprotein (Apo) A-I, ApoM, and ApoE, which negatively impacts the antioxidant, anti-inflammatory, and immunomodulatory roles of HDL.35,36 It is known that HDL acts to stimulate reverse-cholesterol transport from the peripheral parts to the liver, while also enhance immune system modulation and help to control infectious diseases. Apart from their antioxidant, antithrombotic, and immunomodulatory roles, HDL have a high binding affinity and may neutralize pathogen-associated lipids that mediate an exaggerated immune response in sepsis. 35 Antioxidant and anti-inflammatory properties of HDL are markedly reduced during infections such as in influenza and human immunodeficiency virus infection.37,38 In the context COVID-19, low total cholesterol, LDL-C, and HDL-C increases COVID-19 severity and its associated mortality, while plasma TG is often elevated during infection and inflammation.39-41
Inflammation alters the composition of HDL apolipoprotein, but the exact mechanism for the phenomenon is currently unknown. The imbalances in the antioxidant mechanism results in generation of oxidized HDL (oxHDL) which binds to and activates the pro-inflammatory patent recognition scavenger receptor lectin-like oxLDL (LOX-1). Exaggerated inflammatory response and oxidative stress, coupled with inactivation of enzyme paraoxonase 1 (PON1) in HDL, further stimulates lipid oxidation, which further compromise HDL function. 35 Low PON1 activity was shown to be associated with poor prognosis in patients with cardiovascular diseases and was reported to be reduced in various inflammatory and infectious diseases. 42
The excessive accumulation of oxHDL and oxLDL, which are potent LOX-1 activators, stimulates further inflammatory processes that exacerbate tissue injury. This cycle leads to changes in the transport of lipoprotein and disruption of the RCT pathway characterized by inadequate interaction between ApoA-I and adenosine triphosphate-binding cassette transporter A1 or ABCG1 on macrophages and reduced cholesterol esterification by lecithin cholesterol acyltransferase (LCAT). These processes reduce cholesteryl esters return to the liver directly after interaction with the hepatic scavenger receptor B1 receptors or indirectly after transfer to LDL by cholesteryl ester transfer protein and uptake by hepatic LDL receptors. Low ApoE and ApoC-III levels on HDL lead to reduced activity of the lipoprotein lipase (LPL), which results in the accumulation of VLDL and TG. 35
Hypercholesterolemia causes cholesterol to accumulate in macrophages and other cells of the immune system, which stimulates inflammatory responses, including enhanced Toll-like receptor (TLR) signaling. LDL is the primary transporter of cholesterol and phospholipids in the circulatory system. During acute inflammation, LDL and apoB are oxidized (oxLDL),43,44 the accumulation of LDL promotes the formation of cholesterol crystals in macrophages and stimulates the activation of the inflammasome, which induces the release of inflammatory cytokines, such as IL-1B and Il-18, and ultimately exacerbates the inflammation in damaged tissue. High levels of LDL and TG also causes endothelial dysfunction, which predisposes the patient to CVD-related complications which increase mortality in COVID-19. 35 Additionally, cardiovascular risk factors such as dyslipidemia and more specifically oxLDL induce “immune training” in myeloid cells predisposing them to exaggerated inflammatory responses following an innate immunity challenge such as SARS-CoV2 infection. 45
A sustained inflammatory response driven by a viral-induced cytokine storm is regarded as 1 plausible explanation for dyslipidemia and complications in patients with COVID-19. The hyperinflammatory state cause immune-mediated inflammatory dyslipoproteinemia, resulting in the low levels of HDL-C, LDL-C, and ApoE, high level of TG, increased lipoprotein oxidation, and impaired inflammation resolution due to decreased specialized pro-resolving mediators (SPM) biosynthesis, such as omega-3 polyunsaturated fatty acids. Modification of the abnormal values of plasma lipid through the use of pharmacological agents may prove to be useful for patients with COVID-19. For example, improving HDL level may restore lipid transport function and improve antioxidant, anti-inflammatory, and immunomodulatory properties. 35 The use of statins can be considered, given its immunomodulatory properties and their interactions with TLR on host cell membrane. Statin use was shown to reduce the risk of adverse outcomes in COVID-19 patients. 46
Exercise should be encouraged during the pandemic, a worsening metabolic and lipid profile not only increases the risk for severe COVID-19, but may also increase the risk of acute cardiovascular events. These events may lead to epidemic of cardio-cerebrovascular diseases post-pandemic.26,47,48
Limitations
The majority of the studies in this meta-analysis were retrospective in nature. There was lack of data in the drugs used in these study population. Most of the studies did not report the TG, LDL, and HDL; the extent of dyslipidemia may affect the prognosis. Most of the studies did not report obesity, which is important in patients with dyslipidemia. Additionally, the included studies were not geared toward evaluating dyslipidemia’s effect on COVID-19; thus, the characteristics were not grouped by patients with dyslipidemia versus no-dyslipidemia, but rather poor outcome versus non-poor outcome. This also means that the details of lipid measurement are lacking and their cut-off points. There was no information on years since diagnosis and use of drugs such as statin.
Conclusion
Dyslipidemia potentially increases mortality and severity of COVID-19. The association was stronger in patients with older age, male, and hypertension.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ISA and MAL contributes to conceptualization and design. JH and MAL performed data curation. EY and RV performed data extraction. RP contributes statistical analysis. EY, RV, and RP contributes to data interpretation and analysis. ISA, MAL, EY, JH wrote the manuscript draft. BR, RV, and RP reviewed and provide critical revision to the manuscript.
Abbreviations: ACE2: angiotensin converting enzyme 2
BMI: body mass index
CVD: cardiovascular diseases
COVID-19: coronavirus disease 2019
HDL: high-density lipoprotein
LDL: low-density lipoprotein
VLDL: very-low-density lipoprotein
TG: triglycerides
ORCID iDs: Michael Anthonius Lim  https://orcid.org/0000-0001-7631-6835
https://orcid.org/0000-0001-7631-6835
Raymond Pranata  https://orcid.org/0000-0003-3998-6551
https://orcid.org/0000-0003-3998-6551
Data Availability: Data is available on reasonable request
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article. The corresponding author (ISA) can be contacted for more information.
References
- 1. World Health Organization. Weekly Epidemiological Update: Coronavirus Disease 2019 (COVID-19) 10 November 2020. World Health Organization; 2020. [Google Scholar]
- 2. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. 2020;14:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pranata R, Permana H, Huang I, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14:983-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21:147032032092689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pranata R, Lim MA, Yonas E, et al. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. Published online July 29, 2020. doi: 10.1016/j.diabet.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim MA, Pranata R, Huang I, et al. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Heal Dis. 2020; 7: 205435812093857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pranata R, Huang I, Lukito AA, et al. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. Published online May 20, 2020. doi: 10.1136/postgradmedj-2020-137884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcez MR, Pereira JL, Fontanelli M, Marchioni DM, Fisberg RM. Prevalence of dyslipidemia according to the nutritional status in a representative sample of São Paulo. Arq Bras Cardiol. 2014;103:476-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goff DC, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647-656. [DOI] [PubMed] [Google Scholar]
- 12. Opoku S, Gan Y, Fu W, et al. Prevalence and risk factors for dyslipidemia among adults in rural and urban China: findings from the China National Stroke Screening and prevention project (CNSSPP). BMC Public Health. 2019;19:1500. doi: 10.1186/s12889-019-7827-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang C, Qin L, Li K, et al. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. Published online June 5, 2020. doi: 10.3389/fcimb.2020.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020;75:1730-1741. [DOI] [PubMed] [Google Scholar]
- 16. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi GJ, Kim HM, Kang H. The potential role of dyslipidemia in COVID-19 severity: an umbrella review of systematic reviews. J Lipid Atheroscler. 2020;9:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwang JM, Kim JH, Park JS, et al. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci. 2020;41:2317-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santos CS, Morales CM, Álvarez ED, et al. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. To KKW, Tsang OTY, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang MC, Park YK, Kim BO, et al. Risk factors for disease progression in COVID-19 patients. BMC Infect Dis. 2020;20:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen VL, Hawa F, Berinstein JA, et al. Hepatic steatosis is associated with increased disease severity and liver injury in coronavirus disease-19. Dig Dis Sci. Published online September 27, 2020. doi: 10.1007/s10620-020-06618-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim T, Roslin M, et al. Body mass index as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity. Published online November 11, 2020. doi: 10.1002/oby.23076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim MA, Huang I, Yonas E, et al. A wave of non-communicable diseases following the COVID-19 pandemic. Diabetes Metab Syndr Clin Res Rev. 2020;14:979-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim MA, Pranata R. Sports activities during any pandemic lockdown. Irish J Med Sci (1971 -). Published online July 4, 2020. doi: 10.1007/s11845-020-02300-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim MA. Exercise addiction and COVID-19-associated restrictions. J Ment Heal. Published online August 5, 2020. doi: 10.1080/09638237.2020.1803234 [DOI] [PubMed] [Google Scholar]
- 29. Yonas E, Alwi I, Pranata R, et al. Effect of heart failure on the outcome of COVID-19 — A meta analysis and systematic review. Am J Emerg Med. Published online July 9, 2020. doi: 10.1016/j.ajem.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pranata R, Soeroto AY, Ian H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24:838-843. doi: 10.5588/ijtld.20.0278 [DOI] [PubMed] [Google Scholar]
- 31. Lim MA, Pranata R. The importance of COVID-19 prevention and containment in hemodialysis unit. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420939256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pranata R, Supriyadi R, Huang I, et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. Published online September 2020;14. doi: 10.1177/1179548420959165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim MA, Pranata R. Coronavirus disease 2019 (COVID-19) markedly increased mortality in patients with hip fracture – a systematic review and meta-analysis. J Clin Orthop Trauma. Published online September 17, 2020. doi: 10.1016/j.jcot.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang I, Pranata R, Lim MA, et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:175346662093717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorokin AV, Karathanasis SK, Yang Z-H, et al. COVID-19-Associated dyslipidemia: implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020;34:9843-9853. doi: 10.1096/fj.202001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764-772. [DOI] [PubMed] [Google Scholar]
- 37. Feingold KR, Krauss RM, Pang M, et al. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low density lipoprotein subclass pattern B. J Clin Endocrinol Metab. 1993;76:1423-1427. [DOI] [PubMed] [Google Scholar]
- 38. Van Lenten BJ, Wagner AC, Nayak DP, et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283-2288. [DOI] [PubMed] [Google Scholar]
- 39. Hu X, Chen D, Wu L, et al. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou, China. China. 2020. [Google Scholar]
- 40. Wei X, Zeng W, Su J, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khovidhunkit W, Kim M-S, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169-1196. [DOI] [PubMed] [Google Scholar]
- 42. Farid AS, Horii Y. Modulation of paraoxonases during infectious diseases and its potential impact on atherosclerosis. Lipids Health Dis. 2012;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryoo S, Bhunia A, Chang F, et al. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stancel N, Chen C-C, Ke L-Y, et al. Interplay between CRP, Atherogenic LDL, and LOX-1 and its potential role in the pathogenesis of atherosclerosis. Clin Chem. 2016;62:320-327. [DOI] [PubMed] [Google Scholar]
- 45. Erol A. Role of oxidized LDL-induced “trained macrophages” in the pathogenesis of COVID-19 and benefits of pioglitazone: a hypothesis. Diabetes Metab Syndr Clin Res Rev. 2020;14:713-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daniels LB, Sitapati AM, Zhang J, et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149-155. doi: 10.1016/j.amjcard.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pranata R, Lim MA, Yonas E, et al. Out-of-hospital cardiac arrest prognosis during the COVID-19 pandemic. Intern Emerg Med. Published online November 14, 2020;15. doi: 10.1007/s11739-020-02428-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. July J, Pranata R. Impact of the coronavirus disease pandemic on the number of strokes and mechanical thrombectomies: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29:105185. doi: 10.1016/j.jstrokecerebrovasdis.2020.105185 [DOI] [PMC free article] [PubMed] [Google Scholar]