Abstract
Objective:
This study aimed to evaluate trends in the use of obesogenic medications among adults.
Methods:
Cross-sectional data on adults aged ≥20 years are from the 1999 to 2018 National Health and Nutrition Examination Survey (n = 52,340). Obesogenic medications were defined according to the 2015 Endocrine Society guidelines on the pharmacological management of obesity. Weight status was categorized according to BMI. Trends in prior 30-day use were evaluated.
Results:
In NHANES 2017–2018, 20.3% of US adults used an obesogenic medication. Beta-blockers (9.8%) and antidiabetics (5.7%) were the most common; antipsychotics (1.0%) were the least common. Most common indications were disorders of glucose metabolism, hypertension, neuralgia or neuritis, heart disease, and musculoskeletal pain and/or inflammation. From 1999 to 2018, the proportional use of obesogenic medications increased for anticonvulsants (34.4% to 55.0%) but decreased for antidepressants (32.1% to 18.8%), antidiabetics (82.9% to 52.5%), and beta-blockers (83.9% to 80.7%). The proportional use of obesogenic medications was not associated with weight status, except for antipsychotics.
Conclusions:
Use of obesogenic medications was common. Differences in the proportional use of obesogenic medication may reflect changing availability of obesogenic versus nonobesogenic medications over time. The decision to prescribe a nonobesogenic alternative, if one exists, is guided by weighing the risks and benefits of available treatments.
INTRODUCTION
The prevalence of obesity among adults in the United States has been increasing since the 1980s (1), and this trend has continued over the past 20 years (2). In the National Health and Nutrition Examination Survey (NHANES) 2017–2018, the prevalence of obesity (BMI ≥ 30 kg/m2) among adults aged 20 years and older was 42.4%, and the prevalence of class 3 obesity (BMI ≥ 40 kg/m2) was 9.2% (2). The high prevalence of obesity can be attributed to increased calorie intake and decreased energy expenditure, which are influenced by the availability of low-cost, energy-dense, highly palatable foods and an increasingly sedentary environment (3); however, other environmental factors such as medication use may also play a role. Many prescription medications have weight gain as an adverse and unintentional effect (“obesogenic” medications) (4,5), and the use of these medications has been recognized as a preventable cause of obesity (6). Although “obesogenic” as a term may be limited because weight gain does not always result in BMI ≥ 30 kg/m2, it is widely used to describe medication-induced weight gain. The use of obesogenic medications diminishes weight loss achieved as part of weight loss programs (4,7) and after laparoscopic sleeve gastrectomy (5). In NHANES 2011–2012, more than one half (51%) of US adults used a prescription medication, and 15% used five or more prescription medications (8); however, the prevalence of the use of obesogenic prescription medications is unknown.
The use of any prescription medication and the use of multiple medications are more common among adults with obesity. In NHANES 2005–2008, 61.5% of US adults with obesity used at least one prescription medication compared with 52.7% of adults with normal weight, and 20.2% of adults with obesity used five or more prescription medications compared with 9.4% of adults with normal weight (9). Obesity is a known risk factor for several health conditions, such as hypertension, heart disease, and type 2 diabetes, that are often treated using obesogenic medications, which could, in turn, lead to increased weight gain or difficulty in losing weight.
The objectives of this study were (1) to evaluate trends in the use of obesogenic prescription medications by therapeutic class among US adults from 1999 to 2018, (2) to examine the relationship between weight status and use of obesogenic prescription medications by therapeutic class, and (3) to determine the reasons for use of obesogenic medications.
Methods
Data source
This analysis used data from NHANES, a cross-sectional survey representing the US community-dwelling population (10). Participation in NHANES included an in-home interview as well as physical examinations and laboratory investigations conducted at mobile examinations centers. NHANES data have been released in 2-year cycles since 1999. This study used data from NHANES 1999–2000 to 2017–2018, the most recent years for which data are available, to examine trends over time. The interviewed response rates for each cycle from 1999–2000 to 2017–2018 were 76.2%, 78.3%, 72.9%, 74.1%, 73.4%, 74.1%, 67.4%, 65.7%, 57.2%, and 47.9%. Because NHANES uses sample weights to represent the US population, the (weighted) demographics in this study match the distribution found in the adult US population during the specified periods.
Obesogenic and nonobesogenic medications
During the in-home interview, survey participants were asked whether they have used a prescription medication in the prior 30 days. If the answer was “yes,” the interviewer asked participants to provide all prescription medication containers and recorded the names, durations of use, and reasons for use. If the container was unavailable, the participant verbally reported the name of the medication. Medication names were matched to the standard drug name in the Multum Lexicon Plus database (11). Medications that were only available over the counter during the 2-year cycle were excluded, except over-the-counter insulin. Beginning in NHANES 2013–2014, participants’ reported reasons for use were matched to a prespecified list of three usual and possible off-label indications (12).
Prescription medications reported by NHANES participants were categorized based on the Multum Lexicon Plus therapeutic classification system (11). Medications were classified as obesogenic according to the 2015 Endocrine Society guidelines on the pharmacological management of obesity (6), which was based on a systematic review commissioned by the Endocrine Society (13) as well as existing systematic reviews, randomized trials, and observational studies. The Endocrine Society guidelines identified certain antipsychotics, antidepressants, anticonvulsants, anti-inflammatories (corticosteroids), beta-blockers, and antidiabetics as obesogenic, and all other medications in these therapeutic classes were considered nonobesogenic. Supporting Information Table S1 shows the categorization of all obesogenic and nonobesogenic medications for those therapeutic classes that contain obesogenic medications, reported by participants in NHANES 1999–2018, as well as published estimates of weight gain associated with each obesogenic medication (6,13–18). Weight gain for individual medications ranged from 0.3 to 15.3 kg. Antihistamines, antiretrovirals, and protease inhibitors were also identified as obesogenic but they did not have prescription nonobesogenic alternatives for comparison and therefore were not included in this analysis. Use of an obesogenic medication was defined as the use of at least one obesogenic medication in the prior 30 days, with or without concurrent use of any nonobesogenic medications. Use of a nonobesogenic medication was defined as the use of at least one nonobesogenic medication in the prior 30 days without concurrent use of any obesogenic medication.
Weight status
Use of medications by weight status was also examined. Survey participants’ height and weight were measured according to a standardized protocol (19), and BMI was calculated as weight in kilograms divided by height in meters squared and rounded to the nearest tenth. Weight status was categorized as normal weight (BMI < 25), overweight (BMI 25–29.9), class 1 and 2 obesity (BMI 30–39.9), and class 3 obesity (BMI ≥ 40) (20).
Statistical analysis
Participants who had missing data for medication use were excluded from all analyses. Participants who were pregnant or who had missing height or weight were excluded from analyses that included weight status. Medications with a missing reason for use were excluded from that part of the analysis.
Data were aggregated over NHANES 2015–2018 for examining patterns in medication use by weight status and reasons for use of medications because it increased the reliability and precision of estimates. Examination sample weights that account for nonresponse, noncoverage, and unequal probabilities of selection were used. Estimates were weighted to be nationally representative, unless otherwise stated. Standard errors were estimated using Taylor series linearization, and 95% CIs were constructed using Korn and Graubard’s method (21). Data management was performed using R version 3.5.2 (R Foundation for Statistical Computing), and the R “survey” package (22) was used for statistical analyses to account for the complex survey design. Statistical reliability of proportions was evaluated according the National Center for Health Statistics Data Presentation Standards (23).
Analyses of trends over time were conducted according to the National Center for Health Statistics Guidelines (24). Linearity was evaluated using orthogonal polynomial logistic regression that included the 2-year survey cycle as a continuous variable. Trends with a significant quadratic term were then evaluated with National Cancer Institute’s Joinpoint Regression Program version 4.8.0.1 (25), allowing a maximum of one Joinpoint over the ten 2-year cycles. Any significant (p < 0.05) Joinpoint was then evaluated using piecewise regression and reported if the difference between the slopes before and after the Joinpoint was statistically significant. Linearity of trends in overweight categories was evaluated using orthogonal polynomial logistic regression. Trends with a significant quadratic term were evaluated using two-sided pairwise t tests.
NHANES was approved by the National Center for Health Statistics research ethics review board, and adult participants provided written consent.
RESULTS
Of the 52,398 adults aged 20 or over in the NHANES 1999–2018 examination sample, 58 (0.1%) who had missing data on prescription medication use were excluded, leaving 52,340 participants (Figure 1). Analyses using 2015–2018 data stratified by weight status excluded 282 (282/10,726 = 2.6%) participants who were pregnant or who had missing height or weight data. The median duration of use for individual obesogenic medications ranged from 12 months for anti-inflammatories to 61 months for beta-blockers (Supporting Information Table S2).
FIGURE 1.
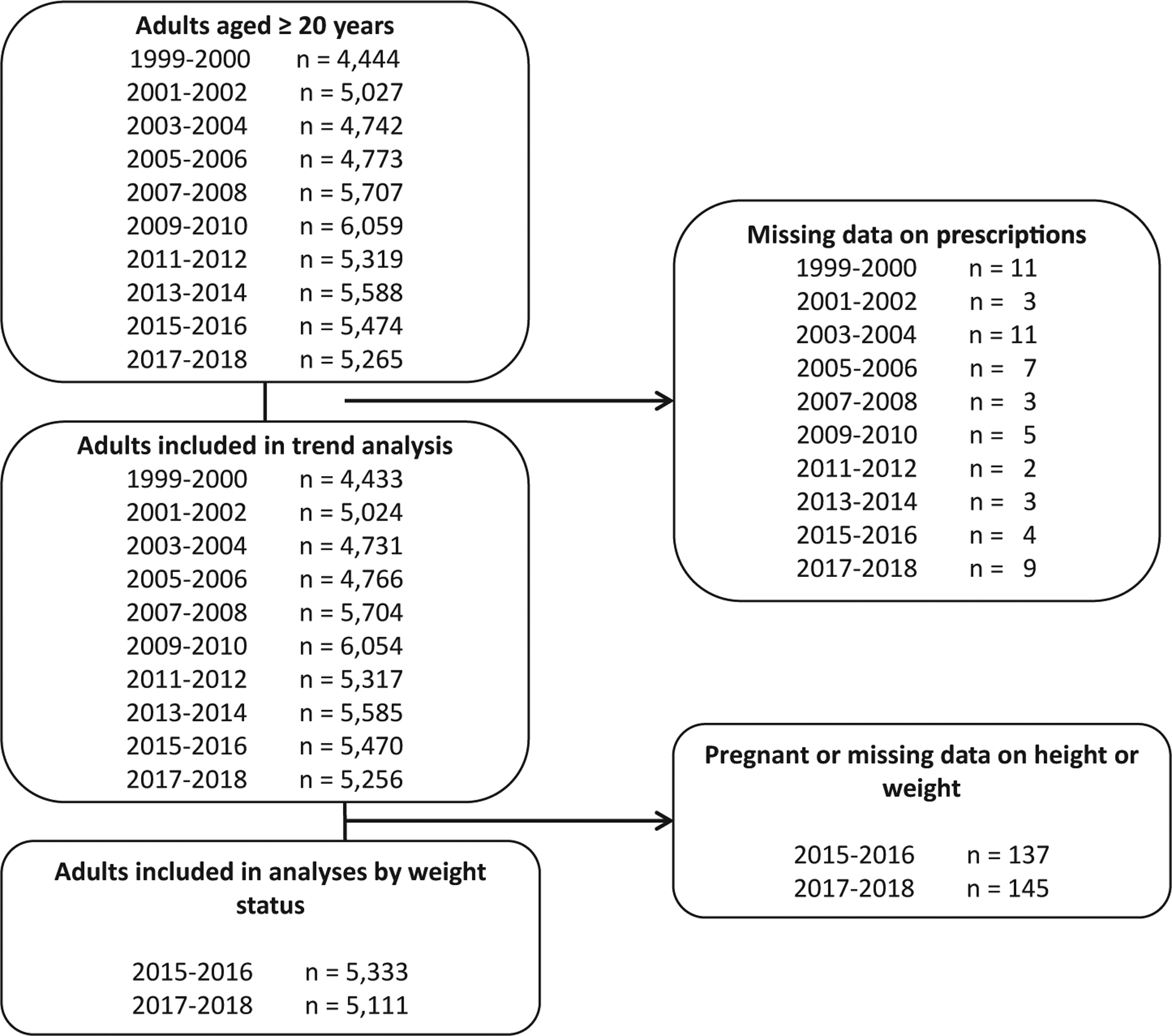
Flow diagram of included survey participants by survey year: National Health and Nutrition Examination Survey, 1999–2018
In 2017–2018, 20.3% (95% CI: 18.5%−22.2%) of adults took at least one obesogenic medication (Table 1). Use of obesogenic beta-blockers was highest (9.8%), followed by obesogenic antidiabetics (5.7%), anticonvulsants (4.5%), antidepressants (2.7%), anti-inflammatories (1.8%), and antipsychotics (1.0%); use of obesogenic medications as a proportion of medication use within the class was highest for beta-blockers (80.7%), followed by antipsychotics (61.0%), anticonvulsants (55.0%), antidiabetics (52.5%), anti-inflammatories (20.0%), and antidepressants (18.8%). Overall, the use of obesogenic medications constituted 55.8% of medication use within these classes in 2017–2018.
TABLE 1.
Trends in obesogenic and total prescription medication usea among adults, by therapeutic classb, United States, 1999–2018
| Survey year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999–2000, n = 4,433 |
2001–2002, n = 5,024 |
2003–2004, n = 4,731 |
2005–2006, n = 4,766 |
2007–2008, n = 5,704 |
2009–2010, n = 6,054 |
2011–2012, n = 5,317 |
2013–2014, n = 5,585 |
2015–2016, n = 5,470 |
2017–2018, n = 5,256 |
Trendc | |
| Anticonvulsants, % (95% CI) | |||||||||||
| Obesogenic | 0.8 (0.4–1.3) | 1.5 (1.1–1.9) | 1.9 (1.4–2.5) | 1.7 (1.2–2.3) | 2.5 (1.9–3.3) | 2.6 (2.2–3.0) | 2.4 (1.9–3.0) | 3.5 (2.7–4.4) | 4.2 (3.5–4.9) | 4.5 (3.4–5.8) | Increasing |
| Total | 2.3 (1.7–2.9) | 3.4 (2.5–4.4) | 4.7 (3.7–5.8) | 4.3 (3.6–5.1) | 5.2 (4.3–6.2) | 5.3 (4.8–5.9) | 5.4 (4.4–6.4) | 7.1 (6.3–8.0) | 7.4 (6.3–8.7) | 8.2 (6.9–9.7) | Increasing |
| Proportion obesogenic | 34.4 (20.2–50.9) | 43.9 (37.6–50.4) | 41.4 (34.9–48.2) | 39.2 (29.7–49.4) | 48.7 (42.2–55.2) | 49.0 (42.9–55.1) | 45.4 (36.4–54.5) | 49.4 (38.2–60.6) | 56.0 (48.2–63.6) | 55.0 (46.9–63.0) | Increasing |
| Antidepressants, % (95% CI) | |||||||||||
| Obesogenic | 2.3 (1.9–2.9) | 3.1 (2.4–4.0) | 3.2 (2.7–3.7) | 2.2 (1.8–2.6) | 2.2 (1.9–2.6) | 1.9 (1.3–2.7) | 2.4 (1.9–3.0) | 2.4 (1.9–3.1) | 1.8 (1.2–2.5) | 2.7 (2.0–3.7) | Nonsignificant |
| Total | 7.3 (6.2–8.5) | 9.3 (8.1–10.6) | 11.2 (9.9–12.7) | 11.4 (10.3–12.6) | 12 (10.4–13.7) | 10.8 (9.3–12.6) | 12.9 (10.4–15.6) | 15.2 (13.3–17.1) | 13 (11–15.2) | 14.5 (12.3–16.9) | Increasing |
| Proportion obesogenic | 32.1 (26.2–38.5) | 33.4 (25.9–41.6) | 28.2 (23.8–32.9) | 19.0 (15.6–22.6) | 18.4 (15.9–21.3) | 17.2 (12.1–23.3) | 18.5 (14.2–23.3) | 16.1 (12.5–20.1) | 13.8 (10–18.4) | 18.8 (14–24.5) | Decreasing |
| Antidiabetics, % (95% CI) | |||||||||||
| Obesogenic | 4.0 (3.3–4.9) | 4.3 (3.6–5.0) | 5.4 (4.5–6.5) | 4.9 (4.1–5.8) | 5.8 (4.6–7.2) | 5.3 (4.5–6.1) | 5.4 (4.6–6.3) | 5.2 (4.5–6.0) | 5.2 (4.3–6.2) | 5.7 (5.1–6.4) | Increasing |
| Total | 4.8 (3.9–5.9) | 5.3 (4.4–6.2) | 6.4 (5.4–7.5) | 6.4 (5.5–7.4) | 7.7 (6.2–9.3) | 7.8 (6.8–8.8) | 8.3 (7.2–9.5) | 9.5 (8.4–10.7) | 10.3 (8.9–11.8) | 10.9 (10–11.8) | Increasing |
| Proportion obesogenic | 82.9 (77.5–87.5) | 80.8 (72.9–87.3) | 84.7 (77.5–90.3) | 76.9 (71.7–81.5) | 75.6 (70–80.5) | 67.9 (62.1–73.4) | 64.7 (61–68.3) | 54.7 (51.2–58.2) | 50.3 (45–55.7) | 52.5 (46.9–58.0) | Decreasing |
| Anti-inflammatories, % (95% CI) | |||||||||||
| Obesogenic | 2.1 (1.7–2.7) | 1.8 (1.3–2.5) | 1.5 (1.1–2.0) | 1.6 (1.1–2.3) | 1.3 (1.0–1.6) | 1.2 (0.8–1.6) | 1.5 (1.1–1.9) | 1.5 (1.1–2.0) | 1.5 (1.0–2.0) | 1.8 (1.4–2.2) | Decreased from 1999–2000 to 2009–2010 and then increased from 2009–2010 to 2017–2018 |
| Total | 10.0 (8.4–11.9) | 9.9 (8.6–11.4) | 12.9 (11.4–14.6) | 8.4 (7.4–9.5) | 8.0 (6.5–9.7) | 8.1 (7.0–9.2) | 7.2 (5.7–9.0) | 8.6 (7.6–9.7) | 7.7 (6.4–9.2) | 8.9 (7.7–10.2) | Decreasing |
| Proportion obesogenic | 21.3 (18.4–24.4) | 18.4 (14.0–23.6) | 11.6 (8.4–15.6) | 19.3 (13.1–26.8) | 16.0 (11.7–21.0) | 14.6 (10.5–19.6) | 20.8 (17.1–24.9) | 17.2 (12.5–22.8) | 18.8 (14.1–24.2) | 20.0 (15.3–25.5) | Nonsignificant |
| Antipsychotics, % (95% CI) | |||||||||||
| Obesogenic | 0.6 (0.2–1.3) | 0.8 (0.4–1.4) | 0.7 (0.3–1.1) | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) | 0.8 (0.5–1.2) | 1.0 (0.6–1.4) | 1.0 (0.7–1.3) | 1.0 (0.7–1.4) | 1.0 (0.7–1.3) | Nonsignificant |
| Total | 1.1 (0.6–1.9) | 1.1 (0.6–1.7) | 1 (0.5–1.8) | 1.4 (1–1.8) | 1.4 (0.9–2.0) | 1.2 (0.9–1.6) | 1.6 (1.1–2.1) | 1.6 (1.2–2.1) | 1.8 (1.2–2.5) | 1.6 (1.3–2.0) | Increasing |
| Proportion obesogenic | 58.8 (33.3–81.2) | 75.2 (58.2–87.9) | 66.2 (39.9–86.8) | 69.4 (54.6–81.8) | 64.3 (51.1–76.1) | 66.0 (51.1–78.9) | 62.5 (46.3–76.9) | 59.7 (46.1–72.2) | 54.7 (39.2–69.6) | 61.0 (49.6–71.5) | Nonsignificant |
| Beta-blockers, % (95%CI) | |||||||||||
| Obesogenic | 5.1 (4.2–6.2) | 5.6 (4.5–6.9) | 8.1 (7.2–9.1) | 9.4 (7.6–11.5) | 8.7 (7.7–9.6) | 8.9 (7.7–10.2) | 8.3 (6.5–10.4) | 8.0 (7.0–9.1) | 7.8 (6.7–9.1) | 9.8 (8.7–11.1) | Increased from 1999–2000 to 2003–2004 and was then stable from 2003–2004 to 2017–2018 |
| Total | 6.1 (5.1–7.2) | 6.8 (5.5–8.3) | 9.5 (8.5–10.6) | 10.9 (9.1–12.9) | 10.1 (9.1–11.3) | 11.4 (9.8–13.2) | 10.6 (8.6–12.9) | 11.2 (10.2–12.3) | 10.2 (8.8–11.8) | 12.2 (11.2–13.3) | Increased from 1999–2000 to 2003–2004 and then increased at a lower rate from 2003–2004 to 2017–2018 |
| Proportion obesogenic | 83.9 (76.5–89.7) | 82.1 (74.9–88.0) | 85.5 (80.3–89.7) | 86.5 (80.8–91.0) | 85.3 (82–88.3) | 77.7 (73.6–81.5) | 78.7 (72.3–84.2) | 71.7 (66.3–76.6) | 76.6 (70.9–81.6) | 80.7 (73.1–86.8) | Decreasing |
| Any medication, % (95% CI)b | |||||||||||
| Obesogenic | 13.2 (11.3–15.3) | 14.3 (12.4–16.3) | 17.3 (15.8–18.9) | 17.5 (15.1–20.0) | 17.6 (15.7–19.7) | 16.9 (15.3–18.5) | 17.2 (15.1–19.4) | 17.7 (16.0–19.6) | 17.0 (15.4–18.8) | 20.3 (18.5–22.2) | Increasing |
| Total | 22.6 (20.2–25.1) | 24.4 (22.4–26.6) | 30.8 (28.4–33.3) | 29.4 (26.9–32.0) | 29.7 (27.0–32.5) | 29.7 (27.3–32.2) | 31.0 (27.5–34.8) | 33.5 (31.5–35.4) | 32.1 (29.7–34.6) | 34.8 (32.3–37.3) | Increasing |
| Proportion obesogenic | 52.2 (48.5–55.9) | 53.3 (49.3–57.2) | 53.7 (51.3–56.2) | 57.0 (52.9–61.1) | 56.8 (53.5–60.1) | 54.8 (51.1–58.4) | 52.4 (48.7–56.2) | 50.2 (46.2–54.2) | 51.2 (47.4–54.9) | 55.8 (50.7–60.9) | Nonsignificant |
Note: For each therapeutic class, the prevalence estimates for obesogenic medications include adults who take at least one obesogenic medication and may also take nonobesogenic medications in the same therapeutic class.
Obesogenic and total percentages indicate prevalence of use among the US adult population. Proportion obesogenic indicates the relative proportion within each class.
Only selected therapeutic classes that contain obesogenic medications.
Linear trends are significant at p < 0.05. Linearity was evaluated using orthogonal polynomial logistic regression that included the 2-year survey cycle as a continuous variable. Nonlinear trends were then evaluated with Joinpoint software, and any significant Joinpoint result was confirmed using piecewise regression.
Use of any obesogenic medication among US adults increased from 13.2% in 1999–2000 to 20.3% in 2017–2018 (Table 1). Use of obesogenic anticonvulsants increased from 0.8% to 4.5%, and use of obesogenic antidiabetics increased from 4.0% to 5.7% over this period, whereas the use of obesogenic beta-blockers increased from 5.1% in 1999–2000 to 8.1% in 2003–2004 and it was stable thereafter (Table 1, Figure 2). There was no overall significant increase or decrease in the use of obesogenic antidepressants, anti-inflammatories, or antipsychotics. Obesogenic medication use as a proportion of total use within the therapeutic class from 1999–2000 to 2017–2018 decreased for antidepressants (32.1% to 18.8%), antidiabetics (82.9% to 52.5%), and beta-blockers (83.9% to 80.7%), whereas proportional use of obesogenic medications increased for anticonvulsants (34.4% to 55.0%) and there was no significant trend for anti-inflammatories or antipsychotics.
FIGURE 2.
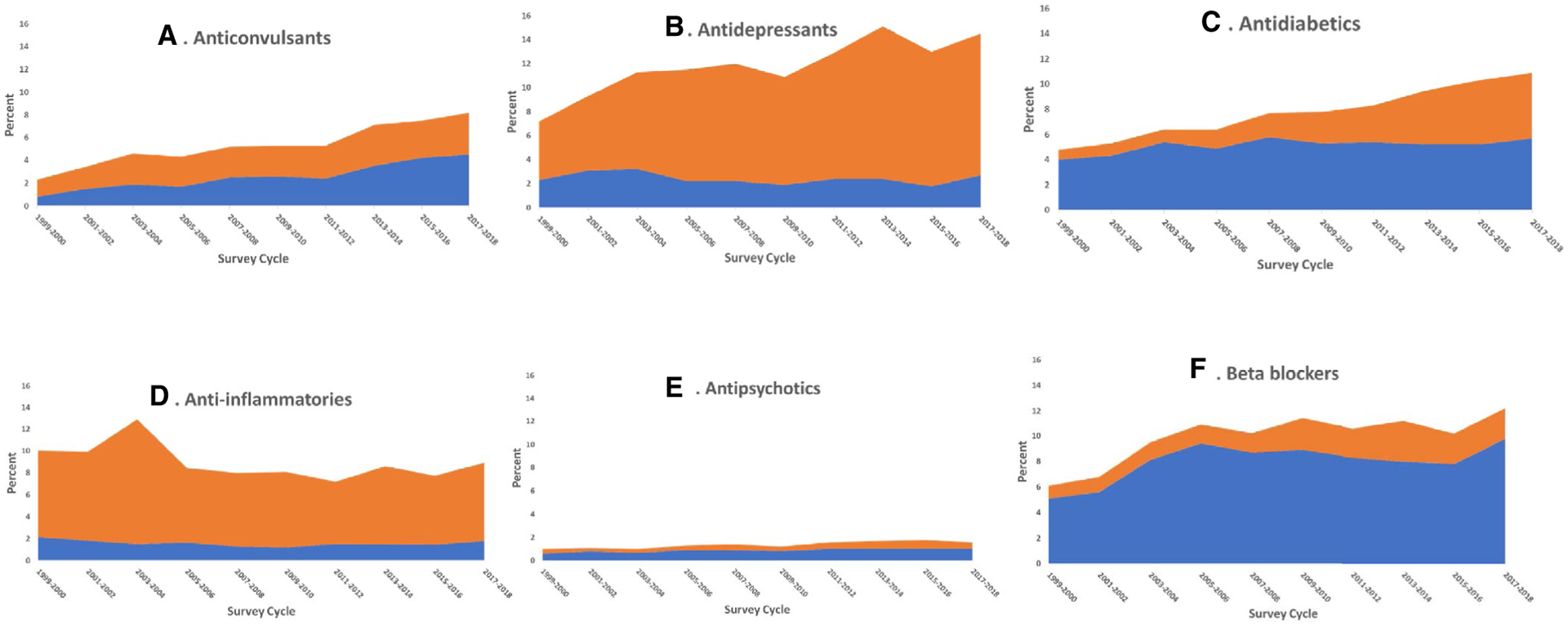
Trends in obesogenic and nonobesogenic prescription medication use among adults, United States, 1999–2018. Percentages indicate prevalence of use among the US adult population. Prevalence estimates for obesogenic medications include adults who may also take nonobesogenic medications in the same therapeutic class. The estimates for nonobesogenic medications exclude adults who take obesogenic medications in the same therapeutic class but do not exclude adults taking an obesogenic medication in another therapeutic class
In 2015–2018, not only obesogenic but also total medication use in select therapeutic classes was positively associated with weight status, with 11.5% obesogenic medication use among adults with normal weight to 25.6% among adults with class 3 obesity (BMI ≥ 40) and 22.4% total medication use among adults with normal weight to 47.5% among adults with class 3 obesity (BMI ≥ 40) (Table 2). Overall, obesogenic medication use as a proportion of use within the therapeutic class was not associated with weight status. Similar patterns were observed for anticonvulsants, antidiabetics, anti-inflammatories, and beta-blockers (Table 2, Figure 3). Only for antipsychotics was the proportion of obesogenic medication use relative to total antipsychotic use positively associated with weight status, from 52.9% among those with normal weight to 80.9% among those with class 3 obesity (BMI > 40).
TABLE 2.
Obesogenic and total prescription medication usea among adults by weight status and therapeutic category, United States, 2015–2018
| Weight statusb | Trend | ||||
|---|---|---|---|---|---|
| Normal weight (BMI < 25), n = 2,792 |
Overweight (BMI 25.0–29.9 kg), n = 3,357 |
Class 1 or 2 obesity (BMI 30.0–39.9), n = 3,402 |
Class 3 obesity (BMI ≥ 40.0), n = 893 |
||
| Anticonvulsants, % (95% CI) | |||||
| Obesogenic | 2.6 (1.9–3.5) | 3.7 (3.0–4.5) | 5.5 (4.4–6.8) | 6.5 (5.0–8.4) | Increasingc |
| Total | 6.1 (4.9–7.5) | 6.6 (5.2–8.2) | 9.1 (7.7–10.5) | 11.4 (8.7–14.6) | Increasingc |
| Proportion obesogenic | 43.0 (32.9–53.5) | 55.6 (48.4–62.6) | 60.9 (52.4–69.0) | 57.4 (39.9–73.6) | Nonsignificant |
| Antidepressants, % (95% CI) | |||||
| Obesogenic | 2.0 (1.2–3.2) | 2.1 (1.3–3.1) | 2.4 (1.7–3.5) | 3.5 (2.1–5.5) | Stable |
| Total | 9.9 (8.4–11.6) | 12.5 (10.8–14.4) | 16.5 (14.1–19.2) | 20.9 (16.4–26.0) | Increasingc |
| Proportion obesogenic | 20.3 (12.6–30.1) | 16.5 (10.8–23.6) | 14.8 (10.1–20.6) | 16.7 (9.7–25.8) | Nonsignificant |
| Antidiabetics, % (95% CI) | |||||
| Obesogenic | 1.8 (1.4–2.4) | 4.8 (3.7–6.1) | 7.8 (6.7–9.1) | 9.6 (7.8–11.8) | Increasingc |
| Total | 3.7 (2.7–4.8) | 8.9 (7.5–10.5)d | 15.0 (13.6–16.6)d,e | 21.0 (17.1–25.5)d,e,f | Increasing |
| Proportion obesogenic | 50.5 (39.8–61.1) | 53.4 (46.6–60.1) | 52.2 (45.5–59.0) | 45.7 (35.5–56.2) | Nonsignificant |
| Anti-inflammatories, % (95% CI) | |||||
| Obesogenic | 1.8 (1.2–2.6) | 1.0 (0.7–1.4) | 1.9 (1.3–2.8) | 1.9 (1.0–3.4) | Nonsignificant |
| Total | 6.0 (4.8–7.4) | 7.4 (6.0–8.9) | 9.9 (8.5–11.4) | 13.6 (9.2–19.2) | Increasingc |
| Proportion obesogenic | 30.6 (20.6–42.0) | 13.9 (9.9–18.7) | 19.5 (13.7–26.4) | 14.3 (6.6–25.7) | Nonsignificant |
| Antipsychotics, % (95% CI) | |||||
| Obesogenic | 0.7 (0.4–1.2) | 0.4 (0.2–0.8) | 1.4 (1.0–1.9) | 2.0 (0.9–3.9) | Increasingc |
| Total | 1.4 (0.9–1.9) | 1.3 (0.9–1.9) | 2.1 (1.5–2.8) | 2.5 (1.3–4.3) | Increasingc |
| Proportion obesogenic | 52.9 (34.1–71.1) | 31.2 (13.9–53.3) | 67.6 (49.4–82.5) | 80.9 (42.9–98.2) | Increasingc |
| Beta-blockers, % (95% CI) | |||||
| Obesogenic | 4.8 (3.7–6.1) | 9.4 (8.3–10.7)d | 11.3 (9.5–13.3)d | 11.1 (8.6–14.0)d | Increasing |
| Total | 6.2 (4.9–7.6) | 11.0 (9.7–12.3)d | 15.0 (13.2–16.9)d,e | 14.4 (11.3–18.1)d,e | Increasing |
| Proportion obesogenic | 77.6 (70.3–83.8) | 86 (80.9–90.1) | 75.4 (68.5–81.4) | 76.8 (65.5–85.9) | Nonsignificant |
| Any medication, % (95% CI)g | |||||
| Obesogenic | 11.5 (9.8–13.4) | 17.6 (15.5–19.8) | 23.9 (21.6–26.3) | 25.6 (21.7–29.8) | Increasing |
| Total | 22.4 (20.4–24.4) | 30.9 (28.0–34.0)d | 41.6 (38.9–44.4)d,e | 47.5 (41.3–53.7)d,e | Increasingc |
| Proportion obesogenic | 47.5 (41.0–54.2) | 54.9 (50.7–59.0) | 55.7 (51.0–60.4) | 51.7 (42.2–61.1) | Nonsignificant |
Note: For each therapeutic class, the prevalence estimates for obesogenic medications include adults who take at least one obesogenic medication and may also take nonobesogenic medications in the same therapeutic class.
Obesogenic and total percentages indicate prevalence of use among the US adult population. Proportion obesogenic indicates the relative proportion within each class.
BMI calculated as weight in kilograms divided by height in meters squared.
Significant linear increase across weight status categories.
Significantly different compared with normal-weight category.
Significantly different compared with overweight category.
Significantly different compared with class 1 or 2 obesity.
Only selected therapeutic classes that contain obesogenic medications.
FIGURE-3.
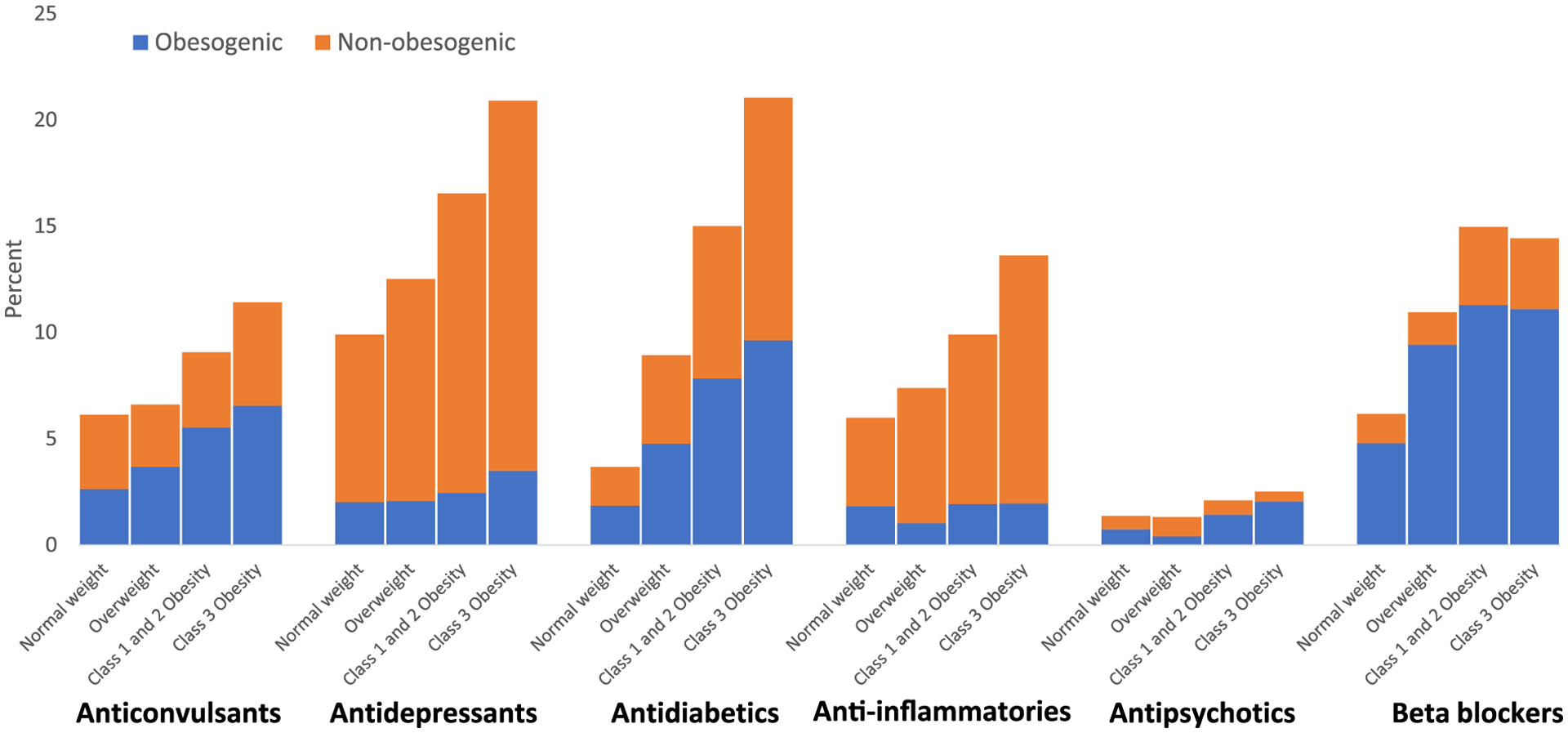
Obesogenic and nonobesogenic medication use among adults, by weight status: United States, 2015–2018. Percentages indicate prevalence of use among the US adult population. For each therapeutic class, the prevalence estimates for obesogenic medications include adults who may also take nonobesogenic medications in the same therapeutic class. The estimates for nonobesogenic medications exclude adults who take obesogenic medications in the same therapeutic class but do not exclude adults taking an obesogenic medication in another therapeutic class
Among adults who took at least one obesogenic anticonvulsant, the most common reasons for use were neuralgia or neuritis, musculoskeletal pain and/or inflammation, mood disorder, and seizure disorder (Table 3). The most common reasons for the use of obesogenic antidepressants were mood disorder, nervousness or anxiety, insomnia or sleep disorder, other neurological disorder, and neuralgia or neuritis, and the most common reasons for the use of antipsychotics were mood disorder, insomnia or sleep disorder, schizophrenia or psychosis, and nervousness or anxiety. The most common reasons for use of obesogenic anti-inflammatories were chronic lung disease, allergy, and musculoskeletal pain and/or inflammation.
TABLE 3.
Reason for use of obesogenic medications among adults, by therapeutic class, 2015–2018
| Therapeutic class | Reason for use of obesogenic medication | No. of participants | % (95% CI) |
|---|---|---|---|
| Anticonvulsants (n = 534) | Neuralgia or neuritis | 280 | 54.1 (47.0–61.0) |
| Musculoskeletal pain and/or inflammation | 123 | 24.2 (19.4–29.6) | |
| Mood disorder | 39 | 9.4 (5.3–15.2) | |
| Seizure disorder | 30 | 7.1 (4.6–10.4) | |
| Antidepressants (n = 224) | Mood disorder | 87 | 36.7 (27.2–47.0) |
| Nervousness or anxiety | 56 | 30.1 (21.4–40.0) | |
| Insomnia/sleep disorder | 49 | 19.0 (13.3–25.9) | |
| Other neurological disorder | 14 | 10.3 (3.8–21.3)a | |
| Neuralgia or neuritis | 24 | 8.7 (3.8–16.5)a | |
| Gastrointestinal disorder | 5 | 5.0 (0.9–14.6)a | |
| Antidiabetics (n = 840) | Diabetes mellitus or elevated glucose or hemoglobin A1c | 816 | 98.0 (96.7–98.9) |
| Anti-inflammatories (n = 182) | Chronic lung disease | 29 | 13.5 (8.3–20.4) |
| Allergy | 22 | 12.3 (6.3–20.9) | |
| Musculoskeletal pain and/or inflammation | 30 | 11.5 (6.8–17.9) | |
| Immune disorder | 13 | 10.9 (6.5–16.9) | |
| Other neurological disorder | 7 | 8.7 (1.9–22.7)a | |
| Connective tissue disorder | 12 | 6.4 (2.5–13.2)a | |
| Other pain | 6 | 5.5 (1.2–14.6)a | |
| Antipsychotics (n = 117) | Mood disorder | 62 | 56.2 (41.7–70.0) |
| Insomnia/sleep disorder | 27 | 23.3 (13.9–35.1) | |
| Schizophrenia or psychosis | 22 | 17.7 (8.2–31.2)a | |
| Nervousness or anxiety | 15 | 14.0 (5.6–27.2)a | |
| Developmental disorder | 11 | 10.5 (5.0–18.8)a | |
| Beta-blockers (n = 1, 046) | Hypertension | 701 | 61.2 (55.5–66.8) |
| Tachycardia, atrial fibrillation, or other cardiac arrhythmia | 134 | 17.9 (13.6–22.9) | |
| Other heart disease | 113 | 10.9 (7.6–15.1) |
Among 3,421 medications (unweighted) reported in 2015–2018, 3,279 (96% unweighted) had at least one reason for use reported; among these, 238 (7% unweighted) had two reasons for use reported and 30 (1% unweighted) had three reasons reported. Reasons for use are presented if the frequency was ≥5%.
Estimate does not meet National Center for Health Statistics standards of reliability.
DISCUSSION
Just over one in five adults in the United States used an obesogenic prescription medication in NHANES 2017–2018. Beta-blockers and antidiabetics were the most common obesogenic medications, whereas antipsychotics were the least common. From 1999–2000 to 2017–2018, different trends occurred in the use of obesogenic medications as a proportion of total use within each class. Proportional use of obesogenic medications increased for anticonvulsants but decreased for antidepressants, antidiabetics, and beta-blockers. While the use of all medications in selected classes was higher in higher weight status categories, obesogenic medication use as a proportion of use within the therapeutic class was not associated with weight status for all classes except antipsychotics; proportional use of obesogenic antipsychotics was positively associated with weight status.
Commonly reported reasons for using obesogenic medications include several reasons not implied by the name of the therapeutic class, such as “neuralgia or neuritis” or “musculoskeletal pain and/or inflammation” for anticonvulsants and antidepressants, “mood disorders” for anticonvulsants and antipsychotics, and “nervousness or anxiety” for antidepressants and antipsychotics. Obesogenic anticonvulsants were more commonly used for neuralgia or neuritis and musculoskeletal pain and/or inflammation than for seizure disorder.
No previous nationally representative study has compared trends in the prevalence of obesogenic medication use. A previous study using NHANES data showed that the use of at least one prescription medication, three or more medications, and five or more medications increased with increasing weight status (9). This study also showed that use of antihypertension medications, lipid-lowering medications, analgesics, antidepressants, proton pump inhibitors, thyroid medications, antidiabetics, and bronchodilators increased with increasing weight status.
Obesogenic medications may promote weight gain through stimulation of hypothalamic appetite regulation centers, a reduction of energy expenditure, or other metabolic disturbances (26). The pattern and magnitude of weight gain varies significantly by medication. Weight gain may be limited to the initial period of medication use or may continue throughout use (26). This study showed that the median duration of use for a given obesogenic medication ranged from 2 to 5 years by therapeutic class.
Obesity is a well-known risk factor for several health conditions that are commonly treated with obesogenic medications. Examples of these health conditions include hypertension, heart disease, and atrial fibrillation (beta-blockers) (27); type 2 diabetes (antidiabetics) and diabetic neuropathy (certain antidepressants and anticonvulsants) (27,28); and mood disorders (antidepressants, anticonvulsants, and certain antipsychotics) (29). Because NHANES is a cross-sectional study, directionality of causation cannot be determined, and associations between obesity and use of obesogenic medications are almost certainly bidirectional. Taking an obesogenic medication or even a nonobesogenic alternative in the same therapeutic class may be an indicator that the person has an obesity-related chronic illness. Indeed, this study showed that the use of both obesogenic and total medications increased with increasing weight status within most therapeutic classes.
Antipsychotics was the only therapeutic classes in which obesogenic medication use as a proportion of total use within the class was positively associated with higher weight status. Although obesity has been shown to be associated with certain psychiatric illnesses such as major depression, bipolar disorder, and anxiety disorders (29,30), the association between obesity and other psychotic disorders is less clear (31).
The Endocrine Society and the strategies to overcome and prevent (STOP) Obesity Alliance recommend that the evaluation of adults for obesity include reviewing current use of potentially obesogenic prescription medications and considering switching to medications that are weight neutral or that may even assist with weight loss (6,32). For an individual patient, the selection of an obesogenic versus a nonobesogenic medication within a therapeutic class may be influenced by health care provider and patient awareness of the obesogenic properties of medications, availability of nonobesogenic options, potential impact of weight gain on the patient’s health, relative efficacy and side effects of obesogenic and nonobesogenic options, and medication cost (6,13,33–36). Certain nonobesogenic alternatives, such as the antidepressant bupropion (in combination with naltrexone) (37), the anticonvulsant topiramate (in combination with phentermine) (38), and the antidiabetics liraglutide and semaglutide (39), are approved by the Food and Drug Administration for weight loss.
This study had several limitations. First, there is no consensus list of obesogenic medications. Some obesogenic medications may be misclassified as nonobesogenic in this study because of lack of evidence, leading to underestimation of the use of obesogenic medications. Variation in the type and quality of studies that do exist have led to variations in the medications identified as obesogenic by different systematic reviews (13,26,36,40). In the interest of consistency with current recommendations, this study relied on only one source, namely, the 2015 Endocrine Society guidelines, although some interpretation of the text was still required for classification of individual obesogenic medications. Second, NHANES participants were asked about their prescription medication use in the prior 30 days only. Use of prescription medications before this period and its association with weight status are unknown. Data on use of over-the-counter medications were not collected. Third, because associations with medication use were evaluated by weight category, associations with smaller increments of weight gain may not be captured. Fourth, because NHANES is a cross-sectional survey, the direction of causality between obesity and medication use could not be determined. Fifth, NHANES response rates have declined; however, an investigation of 2017–2018 data showed that nonresponse errors were minimized with weighting adjustments (41). Sixth, the reason for taking the medication was self-reported. However, the potential for recall bias was minimized by matching the reported reason for use to up to three medication-specific usual and possible off-label indications.
CONCLUSION
In this nationally representative survey of adults in the United States, just over 20% of adults used an obesogenic medication in NHANES 2017–2018. The most common indications for obesogenic medications use were disorders of glucose metabolism, hypertension, neuralgia or neuritis, heart disease, and musculoskeletal pain and/or inflammation. The proportion of obesogenic medication use to total use within the therapeutic class differed by class, and this may reflect factors such as changing availability of obesogenic versus nonobesogenic medications within the class over time. Obesity is a risk factor for several conditions treated with obesogenic medications, which, in turn, may lead to further weight gain. The decision to prescribe a nonobesogenic alternative, if one exists, is guided by weighing the risks and benefits of available treatments.
Supplementary Material
Study Importance.
What is already known?
The use of prescription medications that cause weight gain (obesogenic medications) has been recognized as a preventable cause of obesity. However, the prevalence of obesogenic medication use is unknown.
What does this study add?
One in five US adults used an obesogenic medication in NHANES 2017–2018; beta-blockers and antidiabetics were the most common. The proportional use of obesogenic medication over time differed by therapeutic class and may reflect changing availability of obesogenic versus nonobesogenic medications.
How might these results change the focus of clinical practice?
Obesity is a risk factor for several conditions that are treated with obesogenic medications, which, in turn, may lead to further weight gain. The decision to prescribe a nonobesogenic alternative, if one exists, is guided by weighing the risks and benefits of available treatments.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Center for Health Statistics, the Centers for Disease Control and Prevention, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, or the United States Department of Health and Human Services.
DISCLOSURE
The authors declared no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and Over: United States, 1960–1962 through 2015–2016. Health E-Stats. National Center for Health Statistics; 2018. [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no. 360. National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Adult obesity causes & consequences. Updated March 22, 2021. Accessed April 12, 2021. https://www.cdc.gov/obesity/adult/causes.html
- 4.Desalermos A, Russell B, Leggett C, et al. Effect of obesogenic medications on weight-loss outcomes in a behavioral weight-management program. Obesity (Silver Spring). 2019;27(5):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leggett CB, Desalermos A, Brown SD, et al. The effects of provider-prescribed obesogenic drugs on post-laparoscopic sleeve gastrectomy outcomes: a retrospective cohort study. Int J Obes (Lond). 2019;43(6):1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. [DOI] [PubMed] [Google Scholar]
- 7.Shukla AP, Mandel LS, Tchang BG, et al. Medical weight-loss outcomes in patients receiving concomitant psychotropic medication: a retrospective cohort study. Obesity (Silver Spring). 2020;28(9):1671–1677. [DOI] [PubMed] [Google Scholar]
- 8.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kit BK, Ogden CL, Flegal KM. Prescription medication use among normal weight, overweight, and obese adults, United States, 2005–2008. Ann Epidemiol. 2012;22(2):112–119. [DOI] [PubMed] [Google Scholar]
- 10.Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National Health and Nutrition Examination Survey, 2015–2018: Sample design and estimation procedures: data evaluation and methods research. Vital and Health Statistics, series 2, no. 184. National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1988–2018 data documentation, codebook, and frequencies: prescription medications - drug information (RXQ_DRUG). Published August 2007. Updated September 2021. Accessed April 12, 2021. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm
- 12.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2017–2018 data documentation, codebook, and frequencies: prescription medications (RXQ_RX_J). Published March 2020. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/RXQ_RX_J.htm
- 13.Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(2):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Menachem E Weight issues for people with epilepsy– a review. Epilepsia. 2007;48:42–45. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res. 2003;37(3):193–220. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh B, Cameron C, Singh SR, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5(1):e35–e48. [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry. 2000;157(6):975–981. [DOI] [PubMed] [Google Scholar]
- 18.Sharma AM, Pischon T, Hardt S, Kunz I, Luft FC. Hypothesis: beta-adrenergic receptor blockers and weight gain: a systematic analysis. Hypertension. 2001;37(2):250–254. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. NHANES questionnaires, datasets, and related documentation. Accessed April 12, 2021. https://wwwn.cdc.gov/nchs/nhanes
- 20.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24:193–201. [Google Scholar]
- 22.Lumley T Survey: Analysis of Complex Survey Samples. Accessed March 22, 2021. https://CRAN.R-project.org/package=survey
- 23.Parker JD, Talih M, Malec DJ, et al. National Center for Health Statistics data presentation standards for proportions: data evaluation and methods research. Vital and Health Statistics, series 2, no. 175. National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 24.Ingram DD, Malec DJ, Makuc DM, et al. National Center for Health Statistics guidelines for analysis of trends: data evaluation and methods research. Vital and Health Statistics, series 2, no. 179. National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 25.National Cancer Institute. Joinpoint Trend Analysis Software. Accessed November 23, 2021. https://surveillance.cancer.gov/joinpoint/
- 26.Verhaegen AA, Van Gaal LF. Drug-induced obesity and its metabolic consequences: a review with a focus on mechanisms and possible therapeutic options. J Endocrinol Invest. 2017;40(11):1165–1174. [DOI] [PubMed] [Google Scholar]
- 27.Saydah S, Bullard KM, Cheng Y, et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring). 2014;22(8):1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. [DOI] [PubMed] [Google Scholar]
- 30.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215–220. [DOI] [PubMed] [Google Scholar]
- 32.Dietz WH, Gallagher C. A proposed standard of obesity care for all providers and payers. Obesity (Silver Spring). 2019;27(7):1059–1062. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S90–S102. [DOI] [PubMed] [Google Scholar]
- 34.Manrique C, Whaley-Connell A, Sowers JR. Nebivolol in obese and non-obese hypertensive patients. J Clin Hypertension (Greenwich). 2009;11(6):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders KH, Igel LI, Shukla AP, Aronne LJ. Drug-induced weight gain: rethinking our choices. J Fam Pract. 2016;65(11):780–788. [PubMed] [Google Scholar]
- 36.Wharton S, Raiber L, Serodio KJ, Lee J, Christensen RA. Medications that cause weight gain and alternatives in Canada: a narrative review. Diabetes Metab Syndr Obes. 2018;11:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313(12):1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfaris N, Minnick AM, Hopkins CM, Berkowitz RI, Wadden TA. Combination phentermine and topiramate extended release in the management of obesity. Expert Opin Pharmacother. 2015;16(8):1263–1274. [DOI] [PubMed] [Google Scholar]
- 39.Yanovski SZ, Yanovski JA. Progress in pharmacotherapy for obesity. JAMA. 2021;326(2):129–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs: a systematic review. QJM. 2007;100(7):395–404. [DOI] [PubMed] [Google Scholar]
- 41.Fakhouri THI, Martin CB, Chen TC, et al. An investigation of nonresponse bias and survey location variability in the 2017–2018 National Health and Nutrition Examination Survey: data evaluation and methods research. Vital and Health Statistics, series 2, no. 185. National Center for Health Statistics; 2020. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


