Abstract
Sleep deprived rats undergo a predictable sequence of physiological changes, including changes in skin condition, increased energy expenditure, and altered thermoregulation. Amino-cupric-silver staining was used to identify sleep deprivation related changes in the brain. A significant increase in staining was observed in the supraoptic nucleus (SON) of the hypothalamus of rats with high sleep loss (>45 h) vs. their yoked controls. Follow-up experiments showed that staining was not significantly different in rats sleep deprived for less than 45 h, suggesting that injurious sleep deprivation-related processes occur above a threshold quantity of sleep loss. These anatomical changes suggest that the effects of sleep deprivation may be related to protein metabolism in certain brain regions.
Theme: Neural basis of behavior
Topic: Biological rhythms and sleep
Keywords: Sleep deprivation, Supraoptic nucleus, Neuronal degeneration, Amino cupric silver, Disk-over-water
1. Introduction
Chronic sleep deprivation has produced symptoms ranging from mild behavioral changes to cerebral hemorrhage and disrupted thermoregulation [1–3]. In early studies, deprivation was often not long enough to distinguish between homeostatically regulated increases in sleep tendency and biological deficits. Often such strong stimuli were used to maintain wakefulness that it was difficult to separate the effects of sleep loss from the effects of stress and motor fatigue. Early studies rarely quantified sleep loss.
To achieve long-term, well-controlled sleep deprivation, Rechtschaffen and his colleagues [3] at the University of Chicago designed the disk-over-water method of sleep deprivation. In this method, the rat to be sleep deprived and a disk control animal are placed in neighboring cages whose common floor is a single horizontal disk that is suspended above a pan of shallow water. Whenever the disk rotates, both rats must walk opposite to disk rotation to avoid being carried into the water. The frontal electroencephalogram, EMG, and theta activity from the parietal encephalogram of both rats are monitored, and whenever the sleep deprived rat begins to sleep, a computerized system triggers disk rotation. The control rat is able to sleep as long as the disk is not turning, that is, whenever the sleep deprived rat is spontaneously awake.
This method produces a clear and reproducible syndrome in the sleep deprived rats. They seek higher environmental temperatures, indicating an elevated temperature setpoint [4,5]. Despite significantly increased food consumption, sleep deprived rats lose weight [3,6], indicating increased energy expenditure. Despite the increased energy expenditure and temperature setpoint, temperature eventually decreases, indicating excessive heat loss. The deprived rats develop ulcerative and hyperkeratotic skin lesions on paws and tail, and their fur takes on an unkempt and disheveled appearance during the second half of deprivation. A decrease of 2–3 standard deviations from baseline body temperature heralds a point of no return, past which recovery sleep cannot prevent death [7]. On average, rats die after approximately 2–3 weeks of sleep deprivation [6]. Disk control rats do not experience such extreme effects. In early experiments, no obvious signs of anatomically localized neuronal changes were observed using nissl staining [8]. If localized changes could be found, their sites, timing, and biochemical correlates could provide important clues to the function of sleep.
We used amino-cupric-silver staining to re-examine the question of whether sleep deprivation produces neuro-anatomically localized changes. The amino-cupric-silver method was developed by De Olmos and others [9] to identify damaged cell somas and processes. Staining is thought to result from the precipitation of ionic silver around chemical reducing groups present in damaged subcellular structures [10,11]. Because of their distributions within both somas and processes, cytoskeletal elements represent a likely substrate for amino-cupric-silver reactive staining [10,11]. Onset of reactive staining can occur within minutes of trauma [10] and can persist for days or weeks [9,12] As such, amino-cupric-silver staining can detect degenerative events occurring throughout a much longer time course than stains such as TUNEL, which identify irreversibly damaged cells in the process of apoptotic or necrotic cell death [13]. De Olmos and others have documented amino-cupric-silver reactive staining of cells in response to diverse types of neural trauma, including neurotoxicants, hypoxia, and physical trauma [9,11,12,14]
2. Materials and methods
2.1. Sleep deprivation
All rats were adult Sprague–Dawley males with initial weights of 450–650 g. Sleep deprivation was by the disk-over-water method. Each sleep deprived rat was yoked to a disk control rat and an identically implanted home cage control rat, 14 such triads were formed. The first four yoked triads were implanted with cortical screw electrodes placed over the dura after drilling holes through the cranium. Because the screws slightly depressed the dura, traces of reactive staining were apparent at these cortical sites. Therefore, a different kind of cortical electrode was used for the remaining 10 triads. Small silver plates were held with probes against the cranium at the desired loci while dental cement was placed over the tops of the electrodes and the surrounding cranium. Recording plugs were held to the cranium with dental cement placed over an anchoring screw threaded into the cranium over the cerebellum and over a stainless steel wire net that was threaded across the cranium through the zygomatic arches and small holes drilled through parietal ridges. Cortical electrodes attached in this manner did no damage to underlying tissue, and the only cortical data used were from rats implanted in this fashion.
Sleep deprivation was performed at the Chicago laboratory for 8–10 days using the disk-over-water total sleep deprivation protocol described by Bergmann et al. [15].
However, actual amounts of sleep deprivation vary according to the specific sleep parameters entered into the computer program that controls disk rotation. A low threshold for disk rotation (relatively small increases in EEG amplitude or decreases in EMG amplitude) produces almost complete sleep deprivation in the targeted rat but also significant sleep loss in the control rat. A higher threshold for disk rotation reduces sleep loss in control rats, but also in experimental rats. Usually, lower thresholds increase the difference in sleep deprivation between the pairs [16].
EEG, EMG, and theta were recorded continuously for each sleep deprived and disk control rat and scored for sleep stages during baseline and experimental periods. Sleep loss was calculated by subtracting average sleep amounts during the experiment from average baseline sleep amounts. Initially, in seven triads of rats, the experimental rats were sleep deprived at a low threshold (high sleep loss, or ‘HSL’ animals), producing 89.1±20.3 h average accumulated sleep loss in the sleep deprived rats and 37.2±22.4 h of sleep loss in the disk controls. Functional sleep loss in HSL rats is probably under-estimated because, as discussed by Everson [17], much of their sleep results from increased low voltage NREM (analogous to human stage 1) which is of dubious functional value. A follow-up experiment, comprised of seven additional triads, examined rats that were moderately sleep deprived (moderate sleep loss, or ‘MSL’ animals). The sleep deprived rats in this group accumulated 41±3.7 h of sleep loss on average, vs. 20±6.3 h in the disk controls.
2.2. Perfusion
At the end of the sleep deprivation period, all three rats in each yoked triad were immediately sacrificed using 0.7 ml of Nembutal administered intraperitoneally. Rats were perfused with 100 ml of a wash solution (9 g/l NaCl and 0.34 g/l Na–Cacodylate) followed by 600 ml of fixative (40 g/l paraformaldehyde and 14.34 g/l Na–Cacodylate). Since manipulation of the brain prior to fixation can cause artifactual amino-cupric-silver staining [9], the brains were left in situ after sacrifice. Small holes were drilled in the skull to allow fix to surround the brain, and the head was left in fixative overnight before the brain was removed. Each brain was assigned an arbitrary code number carrying no information about its experimental condition, and all experimental information was kept in Chicago. Brains were sent to Neuroscience Associates (Knoxville, TN) via the Siegel lab in Los Angeles.
2.3. Analysis of amino-cupric-silver stained tissue
At Neuroscience Associates, the brains of yoked rats were gelatin embedded together in the same block prior to slicing at 40 μm, thereby insuring that the brains of all members of a triad were processed identically. A one-in-eight series (sections 320 μm apart) was stained using the amino cupric silver protocol as described previously [18] and then mounted on slides.
All analyses of the coded brains were performed at the laboratory in Los Angeles. Personnel who stained and evaluated the brains were blind to the experimental condition of each brain. The amino-cupric-silver stained tissue was examined carefully over its entire rostral to caudal extent for any sign of argyrophilic cells or reactive processes by a ‘blind’ evaluator. Once an area containing reactive items was identified, the staining was quantified in all brains by counting all stained objects within that area (profile counts). Any area containing even two or three reactive axons or cells in more than one brain was subjected to counts in all brains and to preliminary statistical testing. Argyrophilic cells were counted individually in serial sections. Virtually the entire supraoptic nucleus (SON) was visible in a single field of view, so all cells were counted. Argyrophilic cells of the SON were observed and counted (left side of the brain only) in all adjacent sections containing the SON (six to 10 sections for each brain in a block). Reactive cells in dorsal cortex were counted over two levels, in areas corresponding to Bregma 3.20 and 1.60 mm and to Bregma −0.40 and −1.80 mm. The average count per section was used for statistical analyses, which were by GLM repeated measures ANOVA followed by post-hoc two-tailed paired t-tests using the SPSS 9.0 statistical package. Reactive cells or processes in the basal forebrain, preoptic area, nucleus accumbens, suprachiasmatic nucleus, paraventricular nucleus of the hypothalamus, diagonal band, lateral hypothalamus, locus coeruleus, and numerous other areas were counted in pilot studies, but no consistent changes linked to experimental condition were seen.
After all the cell counts were performed, codes for the experimental condition of each rat were obtained from the Chicago laboratory.
3. Results
3.1. Supraoptic nucleus of the hypothalamus
The most readily apparent reactive staining appeared in the large somas of the supraoptic nucleus of the hypothalamus (SON). Somas within the SON are easily dis-tinguishable from adjacent cells of ventral lateral preoptic (VLPO) and other areas because of their large size—approximately 30 μm in diameter. Each stained soma contained numerous grains of precipitated silver distributed throughout the cytoplasm (Fig. 1).
Fig. 1.
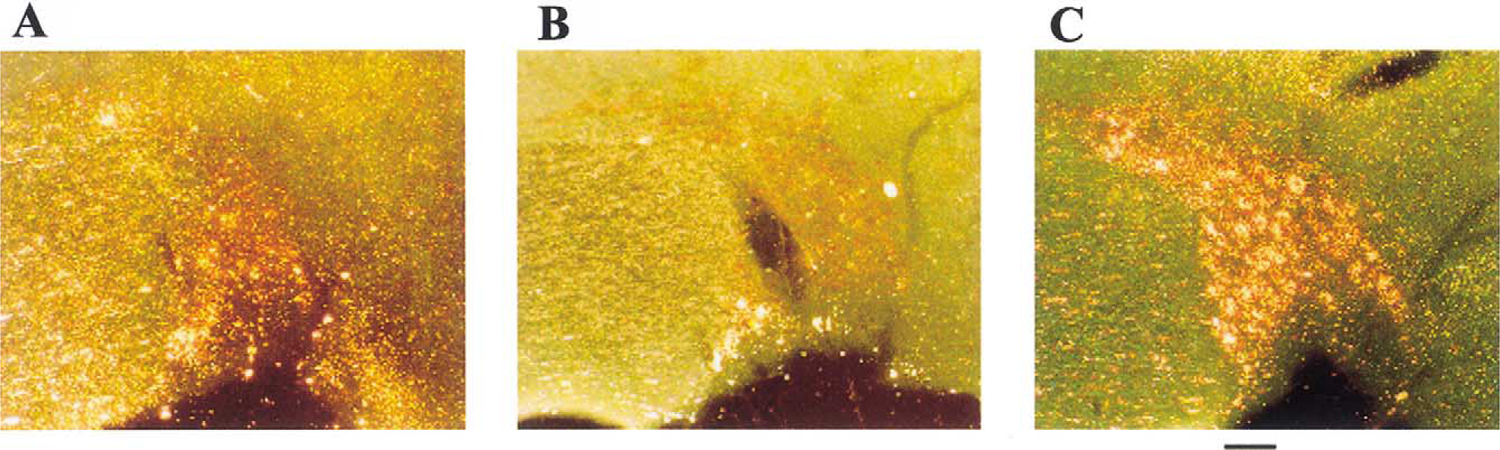
The supraoptic nucleus of the sleep deprived rat shows greater amino-cupric-silver reactive staining than that of controls. (A) In a home caged control rat; (B) in a disk control rat; (C) in a ‘high sleep loss’ sleep deprived rat. Scale bar: approximately 100 μm.
In the HSL experiment, there were significant differences in SON amino-cupric-silver staining among HSL rats, disk control rats, and home caged control rats (repeated measures GLM ANOVA, F2,5=13.9, P<0.01). Sleep deprived rats had significantly higher levels of reactive staining than their yoked disk controls (post-hoc paired t-test, two-tailed, t6=−3.008, P<0.02). These differences were also significantly different between home caged controls and disk controls (post-hoc paired t-test, two-tailed, t6=−3.323, P<0.02) and between home caged controls and sleep deprived rats (post-hoc paired t-test, two-tailed, t6=−5.772, P<0.001). In the MSL rats, there was no significant difference between SON counts of yoked disk controls and sleep deprived rats (Fig. 2).
Fig. 2.
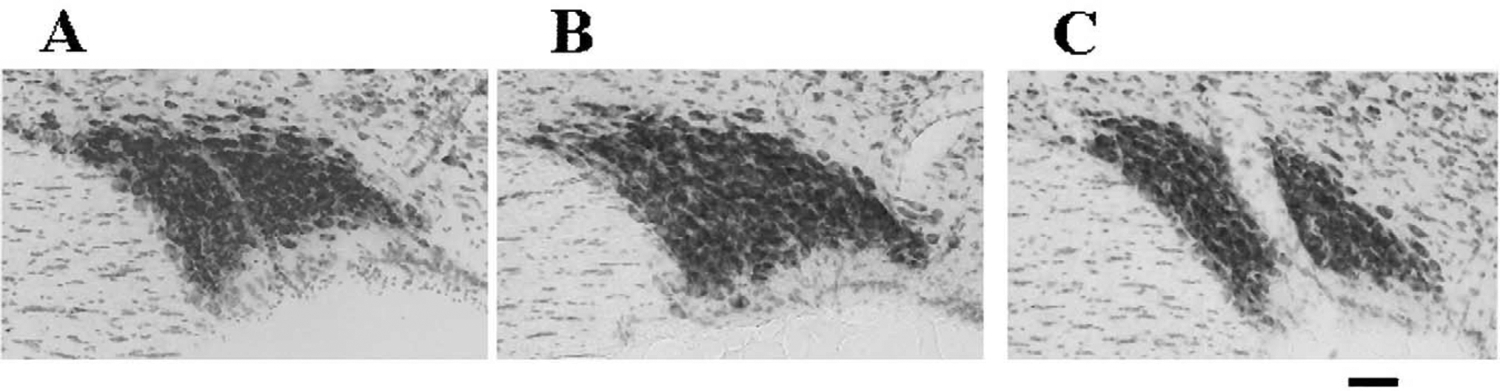
The supraoptic nucleus of the sleep deprived rat does not appear appreciably different from its controls using a nissl stain. (A) In a home caged control rat; (B) in a disk control rat; (C) in a ‘high sleep loss’ sleep deprived rat. Scale bar: approximately 100 μm.
3.2. Dorsal cortex
Because drilling through the skull could have produced cortical damage, results for the cortex were considered only for the 10 triads of rats implanted without penetration through the skull. In seven out of 30 rats implanted using this technique, a diffuse, dust-like reactive staining and reactive somas appeared in a wide area of dorsal cortex including motor, somatosensory, and visual areas as defined by Paxinos and Watson [19]. The affected area, crescent-shaped in coronal views, covered approximately 12 mm in its rostral-to-caudal extent (4.2 to −8.3 mm Bregma and 1–4 Interaural in Paxinos and Watson). This type of staining appeared most prominently in the three sleep deprived rats in the HSL group and weakly, or not at all, in their yoked controls. There was virtually no staining of this type in the MSL rats. Therefore, the difference between disk control and sleep deprived rats was not significant, perhaps due to the low incidence of cortical staining.
4. Discussion
4.1. The nature of the deleterious effects on the brain
The results show that sleep loss produces amino-cupric-silver staining of the supraoptic nucleus of the rat hypothalamus. Other observations of changes in the brain after sleep deprivation have not controlled for the strength of the stimulus [1,7]. Though the present study involved a complete rostral-to-caudal analysis of the entire brain, we have reported only those areas where reactive staining differed significantly between sleep deprived and disk control rats. Surprisingly, areas closely associated with sleep mechanisms—including but not limited to the SCN, preoptic area, locus coeruleus, and raphe—showed little or no amino cupric silver reactivity under high sleep loss conditions. Though these areas may have undergone changes that remained undetected by our sampling technique, the SON is the only affected area we were able to identify.
Both our studies and others’ related studies suggest that the reactive SON and cortical cells we observed were most likely not dead or dying, but that they may have been undergoing a degenerative process with the potential for recovery. SON and cortical cells were not visibly shrunken in nissl sections (Fig. 3) (Ref. [20]; our own observations). Electron microscopic studies by Feng et al. found no differences in microtubule density in mitochondria, Golgi apparatus, ribosomes, nucleoli, or lysosomes in cortex, preoptic anterior hypothalamus, or dorsal-lateral pons [21] or in neuronal ultrastructure [20]. However, the maximum sleep debt in these rats was just 72 h (recalculated from abstract data in Feng et al. [20,21], whereas the effects we noted in cortex occurred only after 70 h. Feng et al. [20,21] did not examine the SON. Some treatments, such as administration of protein synthesis inhibitors, can cause very similar Golgi-like argyrophilic impregnation of nuclei in the hypothalamus including the PVN [22]. Cirelli et al. [23] found no significant TUNEL staining for apoptosis in rats deprived by the disk-over-water method. However, TUNEL identifies only cells which are dying and is effective only during the DNA degradation process. These factors restrict TUNEL staining to a time window on the order of minutes or hours, not days. Amino-cupric-silver labels damaged neurons over a much longer period and includes both cells in which damage may still be reversible and cells that will later die [12]. Increases in FluoroJade staining have also not been found in SON or cortex of sleep deprived rats [23]. However, Poirier et al. [14] found that FluoroJade and silver staining appear to highlight separate aspects of neural damage. In their study, silver staining identified hippocampal neurons damaged by status epilepticus within 2 h of the insult, whereas FluoroJade staining was maximal after 24 h. Maximal FluoroJade staining coincided with neuron loss and onset of spontaneous recurrent seizures, but amino-cupric-silver staining had already disappeared at this point. The authors concluded that the silver stain identified neurons that were responding to the insult and had an increased probability of dying, whereas the FluoroJade labeled cells were irreversibly damaged. In addition, sleep deprivation-related changes in gene expression can occur in the absence of significant staining by TUNEL or FluoroJade, as shown by Cirelli and Tononi [23] in their studies of the effects of short-term sleep deprivation in cortex. Changes in gene expression in SON have not been examined.
Fig. 3.
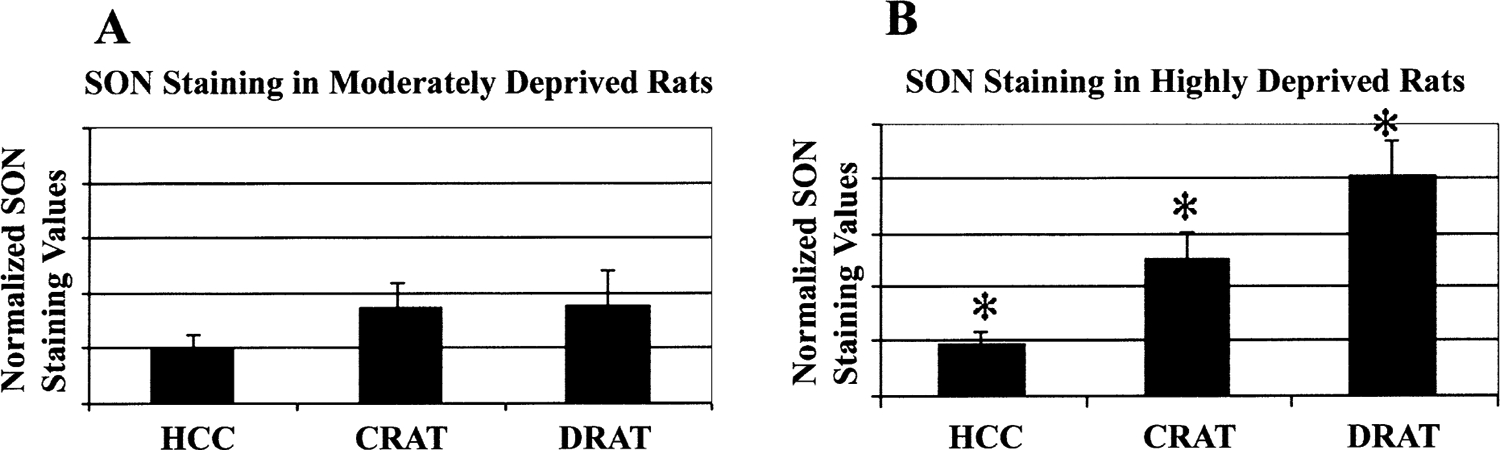
Only high sleep loss produces SON changes that are significantly different from disk controls. (A) SON changes in moderate sleep loss (MSL) rats. (B) SON changes in high sleep loss (HSL) rats. All HSL rat groups show significant differences from each other (see text), which is represented by *. Bars represent mean±S.E.M. Abbreviations: HCC, home caged controls; CRAT, disk controls; DRAT, sleep deprived rats.
4.2. SON changes
The SON is part of the hypothalamic–pituitary axis and is located in the anterior hypothalamus, an area known to be sleep-related since the studies of von Economo and confirmed by numerous subsequent studies [24,25]. The SON is situated among a number of hypothalamic nuclei which participate in aspects of sleep–wake control, such as the suprachiasmatic nucleus, the paraventricular nucleus, and the preoptic area. The SON (or the zone immediately dorsal to it) receives projections from several of these, including the median preoptic nucleus, preoptic area, PVN, SCN, and lateral hypothalamus, as well as the tuberomammillary nucleus of the hypothalamus (reviewed in Ref. [26]; [27–29]). It also receives projections from sleep-related areas outside the hypothalamus, including the ipsilateral locus coeruleus, raphe, and diagonal band of Broca [27,28].
The SON integrates these inputs in order to homeostatically regulate a number of physiological functions related to osmotic balance and vasoconstriction [30], to facilitate the pulsatile hormone release required for lactation, parturition, and ejaculation [26,31], and to influence various species-specific behaviors such as maternal care of the young [32]. The primary output used to achieve these effects is the release of neuropeptides oxytocin and vasopressin via the pituitary [33].
Vasopressin has long been of interest to sleep researchers because of its release in the SCN, the circadian pacemaker. Vasopressin is known to be involved in setting the amplitude of the circadian rhythm, with higher relative concentrations correlating with higher amplitude of the circadian rhythm [34–38]. As such, cerebrospinal fluid (CSF) levels of vasopressin appear to contribute directly to regulation of amounts of sleep and wakefulness. In addition to synaptic release, AVP may be released directly into both CSF and blood, indirectly into CSF from blood plasma, or indirectly from neuronal release into CSF [39–41].
AVP is also involved in the central regulation of blood pressure [40,42–46], hypertension [47,48], vascular resistance [42,45,46,49,50]; and body temperature [40,51]. Many aspects of these systemic effects on cardiac function, skin arterioles, and kidney function appear to be interrelated, primarily via VP’s dual role in osmoregulation and vasoconstriction. Though the majority of the central effects of AVP may be exerted on the capillary beds of the kidney [45], the VP producing cells of the hypothalamus (including the SON) also receive afferent fibers from the kidney which appear to create feedback loops affecting cardiovascular control (as reviewed in Refs. [46,52]). Blood pressure can also, in turn, influence wakefulness [53]. Rechtschaffen’s original studies of sleep deprived rats showed significant increases in heart rate, which were interpreted as a natural correlate of their greatly increased energy expenditure [17] but which also appears consistent with the aforementioned cardiovascular factors. SON function is, in turn, clearly affected by circuits normally involved in sleep–wake control. For instance, the SON (or the zone immediately dorsal to it) receives projections from the median preoptic nucleus, ipsilateral locus coeruleus, raphe, preoptic area, and diagonal band of Broca [27,28].
The SON receives direct connections from and is profoundly influenced by the tuberomammillary nucleus, a posterior hypothalamic area involved in arousal [33]. The projections of the tuberomammillary nucleus have reciprocal effects on the vasopressin and oxytocin containing cells—exciting and inhibiting them, respectively [29,33,54,55].
The locus coeruleus and diagonal band constitute a circuit that affects blood pressure via the SON [47,56–58], but is also a major player in sleep control [59,60]. Both the diagonal band [61,62] and the medial preoptic/preoptic area [59,61,63–65] are known to contain temperature-sensitive neurons that are involved in sleep regulation. The latter area also influences the activity of the SON [64]. The SON may be particularly involved in thermoregulatory control under fever conditions or during dysregulation of the thermoregulatory system [51]. The mechanisms of thermoregulatory control by the SON and their relationship to several sleep-related areas have been addressed directly by several groups [64,66–68].
The relationship between SON function, thermoregulatory control, and sleep–wake state are consistent with the finding that one major consequence of long-term sleep deprivation in the rat is a severe disruption of thermoregulation [3]. Since thermoregulatory challenges provide the strongest source of daily variability in sleep architecture yet found [62], the involvement of the SON has immediate relevance to sleep control. This information also implies that sleep deprivation may influence cardiac and blood pressure regulation.
Possibly because of its level of peptide production, the SON has the highest level of protein synthesis in the brain in waking or in sleep [69]. Glushchenko et al. [70] found that after only 24 h of REM sleep deprivation the total protein content of the SON decreased by 17–22%. As mentioned previously, protein synthesis inhibitors are known to cause Golgi-like silver staining of some hypothalamic cells [22]. These findings invite the speculation that chronic sleep deprivation in the present study might have severely limited protein synthesis in the SON, which in turn might have affected the amino-cupric staining of SON cells. Alternate explanations for decreased protein content include decrease in peptide content due to increased release, enhanced vesicular transport, or other factors. This alternate explanation would be consistent with a hyperactivation of the SON.
Impaired SON function could contribute to physiological changes induced by sleep deprivation. If sleep deprivation alters synthesis or release of neurohormones in the SON, it could also disrupt the autonomic functions associated with them. Indeed, Zenko et al. [16] have noted a smaller increase in vascular resistance in sleep deprived rats than in disk controls, which would contribute to reduced ability to retain heat and a consequent elevation of energy expenditure. The relationship between sleepiness and vasodilation is echoed by findings in humans that the level of vasodilation in the extremities is a strong predictor of the quality of ensuing sleep [71]. Decreased release of vasopressin, which affects both alertness and vasoconstriction, could explain these findings. Impairment of vasopressin release by sleep deprivation could correlate with sleepiness in sleep-deprived rats. Arnauld et al. [36] found that vasopressin promotes wakefulness at the expense of slow wave sleep without any subsequent rebound, implying that vasopressin is directly involved in homeostatic regulation of the sleep–wake cycle. Short-term sleep deprivation can impair other functions of the SON, such as secretion of oxytocin and milk ejection in female rats [72].
In conclusion, amino-cupric-silver reactive staining of the SON is strongly increased by chronic sleep deprivation in the rat. In light of its vulnerability to sleep deprivation, its homeostatic and behavioral functions, and its connections with nuclei important to sleep–wake regulation, the SON appears to have important unexplored relevance to sleep function. Nonsignificant trends also suggest an effect of chronic sleep deprivation on amino-cupric-silver staining of cortical cells.
Acknowledgements
This work was funded by the Veterans Administration Medical Research Service, NIH/NHLBI 59594, NS14610, and the UCLA Sleep Multisite Program. The authors wish to thank Kristie Turner for her administrative support and Kari Bauer for her technical assistance in this work.
References
- [1].Kleitman N, Deprivation of Sleep. On Sleep and Wakefulness, University of Chicago Press, Chicago, 1963, pp. 215–229. [Google Scholar]
- [2].de Manaceine M, Quelques observations experimentales sur l’influence de l’insomnie absolue, Arch. Ital. Biol 21 (1894) 322–325. [Google Scholar]
- [3].Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB, Physiological correlates of prolonged sleep deprivation in rats, Science 221 (1983) 182–184. [DOI] [PubMed] [Google Scholar]
- [4].Prete FR, Bergmann BM, Holtzman P, Obermeyer W, Rechtschaffen A, Sleep deprivation in the rat: XII. Effect on ambient temperature choice, Sleep 14 (1991) 109–115. [DOI] [PubMed] [Google Scholar]
- [5].Shaw PJ, Bergmann BM, Rechtschaffen A, Operant control of ambient temperature during sleep deprivation, Am. J. Physiol 272 (1997) R682–R690. [DOI] [PubMed] [Google Scholar]
- [6].Rechtschaffen A, Bergmann BM, Sleep deprivation in the rat by the disk-over-water method, Behav. Brain Res 69 (1995) 55–63. [DOI] [PubMed] [Google Scholar]
- [7].Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA, Sleep deprivation in the rat: I. Conceptual issues, Sleep 12 (1989) 1–4. [DOI] [PubMed] [Google Scholar]
- [8].Gilliland MA, Wold L, Wollman R, Eschenbach K, Rechtschaffen A, Pathology in sleep deprived rats is not reflected in histologic abnormalities, Sleep Res. 13 (1984) 190. [Google Scholar]
- [9].de Olmos JS, Beltramino CA, de Olmos DL, Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma, Neurotoxicol. Teratol 16 (1994) 545–561. [DOI] [PubMed] [Google Scholar]
- [10].Beltramino CA, Silver Staining as a Tool for Neurotoxic Assessment, [136], 1993. Rockville, MD, NIH. Assessing Neurotoxicity of Drugs of Abuse, NIDA Research Monograph. [DOI] [PubMed] [Google Scholar]
- [11].Switzer RC III, Application of silver degeneration stains for neurotoxicity testing, Toxicol. Pathol 28 (2000) 70–83. [DOI] [PubMed] [Google Scholar]
- [12].Jensen KF, Neuroanatomical techniques for labeling neurons and their utility in neurotoxicology, in: Chang LW, Slikker W Jr. (Eds.), Neurotoxicology: Approaches and Methods, Academic Press, San Diego, 1995, pp. 27–66. [Google Scholar]
- [13].Charriaut-Marlangue C, Ben Ari Y, A cautionary note on the use of the TUNEL stain to determine apoptosis, Neuroreport 7 (1995) 61–64. [PubMed] [Google Scholar]
- [14].Poirier JL, Capek R, De Koninck Y, Differential progression of Dark Neuron and Fluoro-Jade labelling in the rat hippocampus following pilocarpine-induced status epilepticus, Neuroscience 97 (2000) 59–68. [DOI] [PubMed] [Google Scholar]
- [15].Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A, Sleep deprivation in the rat: II. Methodology, Sleep 12 (1989) 5–12. [DOI] [PubMed] [Google Scholar]
- [16].Zenko CE, Bergmann BM, Rechtschaffen A, Vascular resistance in the rat during baseline, chronic total sleep deprivation, and recovery from total sleep deprivation, Sleep 23 (2000) 341–346. [PubMed] [Google Scholar]
- [17].Everson CA, Bergmann BM, Rechtschaffen A, Sleep deprivation in the rat: III. Total sleep deprivation, Sleep 12 (1989) 13–21. [DOI] [PubMed] [Google Scholar]
- [18].Siegel JM, Nienhuis R, Gulyani S, Ouyang S, Wu MF, Mignot E, Switzer RC, McMurry G, Cornford M, Neuronal degeneration in canine narcolepsy, J. Neurosci 19 (1999) 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paxinos G, Watson C, The Rat Brain in Stereotaxic Coordinates, Compact, Academic Press, San Diego, 1997. [Google Scholar]
- [20].Feng P-F, Bergmann BM, Rechtschaffen A, Effect of total sleep deprivation on neuronal ultrastructure in the rat, Sleep Res. Abstr 25 (1996) 466. [Google Scholar]
- [21].Feng P-F, Bergmann BM, Rechtschaffen A, Effect of total sleep deprivation on microtubule density in the rat brain, Sleep Res. Abstr 24 (1995) 443. [Google Scholar]
- [22].Warach S, Armstrong WE, Hatton GI, Puromycin-induced argyrophilia as an alternative to Golgi methods: reliable impregnations of hypothalamic cell groups following lateral ventricular injections in rats, Neurosci. Lett 23 (1981) 223–228. [DOI] [PubMed] [Google Scholar]
- [23].Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G, No evidence of brain cell degeneration after long-term sleep deprivation in rats, Brain Res. 840 (1999) 184–193. [DOI] [PubMed] [Google Scholar]
- [24].Kleitman N, On Sleep and Wakefulness, University of Chicago Press, Chicago, 1963. [Google Scholar]
- [25].vonEconomo C, N. J. Med 71 (1930) 249–259. [Google Scholar]
- [26].Hatton GI, Emerging concepts of structure–function dynamics in adult brain: the hypothalamo–neurohypophysial system, Prog. Neurobiol 34 (1990) 437–504. [DOI] [PubMed] [Google Scholar]
- [27].Senatorov VV, Renaud LP, Projections of medullary and pontine noradrenergic neurons to the horizontal limb of the nucleus of diagonal band in the rat, Neuroscience 88 (1999) 939–947. [DOI] [PubMed] [Google Scholar]
- [28].Anderson WA, Bruni JE, Kaufmann A, Afferent connections of the rat’s supraoptic nucleus, Brain Res. Bull 24 (1990) 191–200. [DOI] [PubMed] [Google Scholar]
- [29].Weiss ML, Yang QZ, Hatton GI, Magnocellular tuberomammillary nucleus input to the supraoptic nucleus in the rat: anatomical and in vitro electrophysiological investigations, Neuroscience 31 (1989) 299–311. [DOI] [PubMed] [Google Scholar]
- [30].Crosby EC, Humphrey T, Lauer EW (Eds.), Correlative Anatomy of the Nervous System, Hypothalamic Functions, Macmillan Company, New York, 1962, pp. 323–342. [Google Scholar]
- [31].Swanson LW, Sawchenko PE, Hypothalamic integration: organization of the paraventricular and supraoptic nuclei, Annu. Rev. Neurosci 6 (1983) 269–324. [DOI] [PubMed] [Google Scholar]
- [32].Young LJ, Wang Z, Insel TR, Neuroendocrine bases of mono-gamy, Trends Neurosci. 21 (1998) 71–75. [DOI] [PubMed] [Google Scholar]
- [33].Hatton GI, Li ZH, Neurophysiology of magnocellular neuroendocrine cells: recent advances, Prog. Brain Res 119 (1998) 77–99. [DOI] [PubMed] [Google Scholar]
- [34].Ibata Y, Okamura H, Tanaka M, Tamada Y, Hayashi S, Iijima N, Matsuda T, Munekawa K, Takamatsu T, Hisa Y, Shigeyoshi Y, Amaya F, Functional morphology of the suprachiasmatic nucleus, Front. Neuroendocrinol 20 (1999) 241–268. [DOI] [PubMed] [Google Scholar]
- [35].Brown MH, Nunez AA,Vasopressin-deficient rats show a reduced amplitude of the circadian sleep rhythm, Physiol Behav. 46 (1989) 759–762. [DOI] [PubMed] [Google Scholar]
- [36].Arnauld E, Bibene V, Meynard J, Rodriguez F, Vincent JD, Effects of chronic icv infusion of vasopressin on sleep–waking cycle of rats, Am. J. Physiol 256 (1989) R674–R684. [DOI] [PubMed] [Google Scholar]
- [37].Forsling ML, Diurnal rhythms in neurohypophysial function, Exp. Physiol 85 (2000) 179S–186S. [DOI] [PubMed] [Google Scholar]
- [38].Kruisbrink J, Mirmiran M, Van der Woude TP, Boer GJ, Effects of enhanced cerebrospinal fluid levels of vasopressin, vasopressin antagonist or vasoactive intestinal polypeptide on circadian sleep–wake rhythm in the rat, Brain Res. 419 (1987) 76–86. [DOI] [PubMed] [Google Scholar]
- [39].Robinson ICAF, Coombes JE, Neurohypophysial peptides in cerebrospinal fluid: an update, Ann. N.Y. Acad. Sci 689 (1993) 269–284. [DOI] [PubMed] [Google Scholar]
- [40].Doris PA, Vasopressin and central integrative processes, Neuroendocrinology 38 (1984) 75–85. [DOI] [PubMed] [Google Scholar]
- [41].Coculescu M, Serbanescu A, Temeli E, Influence of arginine vasotocin administration on nocturnal sleep of human subjects, Waking Sleeping 3 (1979) 273–277. [PubMed] [Google Scholar]
- [42].Bosch J, Lebrec D, Jenkins SA, Development of analogues: successes and failures, Scand. J. Gastroenterol. Suppl 226 (1998) 3–13. [DOI] [PubMed] [Google Scholar]
- [43].Portaluppi F, Vergnani L, Manfredini R, Fersini C, Endocrine mechanisms of blood pressure rhythms, Ann. N.Y. Acad. Sci 783 (1996) 113–131. [DOI] [PubMed] [Google Scholar]
- [44].Akins VF, Bealer SL, Hypothalamic histamine release, neuroendocrine and cardiovascular responses during tuberomammillary nucleus stimulation in the conscious rat, Neuroendocrinology 57 (1993) 849–855. [DOI] [PubMed] [Google Scholar]
- [45].Jover B, Dupont M, Mimran A, Woods R, McGrath B, Vasoconstrictor role for vasopressin in conscious, sodium-depleted rats, Am. J. Physiol 253 (1987) H763–H769. [DOI] [PubMed] [Google Scholar]
- [46].Pang CC, Vasopressin and angiotensin in the control of arterial pressure and regional blood flow in anaesthetized, surgically stressed rats, Can. J. Physiol Pharmacol 61 (1983) 1494–1500. [DOI] [PubMed] [Google Scholar]
- [47].Veerasingham SJ, Vahid-Ansari F, Leenen FH, Neuronal Fos-like immunoreactivity in ouabain-induced hypertension, Brain Res. 876 (2000) 17–21. [DOI] [PubMed] [Google Scholar]
- [48].Johnston CI, Vasopressin in circulatory control and hypertension, J. Hypertens 3 (1985) 557–569. [DOI] [PubMed] [Google Scholar]
- [49].Ishikawa S, Goldberg JP, Schrier DM, Aisenbrey GA, Schrier RW, Interrelationship between subpressor effects of vasopressin and other vasoactive hormones in the rat, Miner. Electrolyte Metab 10 (1984) 184–189. [PubMed] [Google Scholar]
- [50].Arnolda L, McGrath BP, Johnston CI, Vasopressin and angiotensin II contribute equally to the increased afterload in rabbits with heart failure, Cardiovasc. Res 25 (1991) 68–72. [DOI] [PubMed] [Google Scholar]
- [51].Pittman QJ, Poulin R, Wilkinson MF, Role of neurohypophysial hormones in temperature regulation, Ann. N.Y. Acad. Sci 689 (1993) 375–381. [DOI] [PubMed] [Google Scholar]
- [52].Stella A, The kidney as a sensor: functional evidence, J. Hypertens. Suppl 10 (1992) S113–S119. [PubMed] [Google Scholar]
- [53].Baust W, Heinemann H, The role of the baroreceptors and of blood pressure in the regulation of sleep and wakefulness, Exp. Brain Res 3 (1967) 12–24. [DOI] [PubMed] [Google Scholar]
- [54].Hatton GI, Yang QZ, Ionotropic histamine receptors and H2 receptors modulate supraoptic oxytocin neuronal excitability and dye coupling, J. Neurosci 21 (2001) 2974–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yang QZ, Hatton GI, Histamine mediates fast synaptic inhibition of rat supraoptic oxytocin neurons via chloride conductance activation, Neuroscience 61 (1994) 955–964. [DOI] [PubMed] [Google Scholar]
- [56].Renaud LP, Cunningham JT, Nissen R, Yang CR, Electrophysiology of central pathways controlling release of neurohypophysial hormones. Focus on the lamina terminalis and diagonal band inputs to the supraoptic nucleus, Ann. N.Y. Acad. Sci 689 (1993) 122–132. [DOI] [PubMed] [Google Scholar]
- [57].Cunningham JT, Nissen R, Renaud LP, Catecholamine depletion of the diagonal band reduces baroreflex inhibition of supraoptic neurons, Am. J. Physiol 263 (1992) R363–R367. [DOI] [PubMed] [Google Scholar]
- [58].Cunningham JT, Nissen R, Renaud LP, Norepinephrine injections in diagonal band of Broca selectively reduced the activity of vasopressin supraoptic neurons in the rat, Brain Res 610 (1993) 152–155. [DOI] [PubMed] [Google Scholar]
- [59].Glotzbach SF, Heller HC, Changes in the thermal characteristics of hypothalamic neurons during sleep and wakefulness, Brain Res. 309 (1984) 17–26. [DOI] [PubMed] [Google Scholar]
- [60].Berridge CW, Foote SL, Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain, J. Neurosci 16 (1996) 6999–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Alam MN, McGinty D, Szymusiak R, Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep, Brain Res. 752 (1997) 81–89. [DOI] [PubMed] [Google Scholar]
- [62].McGinty D, Alam MN, Szymusiak R, Nakao M, Yamamoto M, Hypothalamic sleep-promoting mechanisms: coupling to thermoregulation, Arch. Ital. Biol 139 (2001) 63–75. [PubMed] [Google Scholar]
- [63].Ogawa Y, Kawamura H, Increase of multiple unit activity during slow wave sleep in the cat preoptic area, Brain Res. Bull 20 (1988) 897–902. [DOI] [PubMed] [Google Scholar]
- [64].Matsumura K, Nakayama T, Tamaki Y, Effects of preoptic and hypothalamic thermal stimulation on electrical activity of neurosecretory cells in the supraoptic nucleus, Brain Res. 346 (1985) 327–332. [DOI] [PubMed] [Google Scholar]
- [65].Datta S, Mohan K, Chhina GS, Singh B, Tonic activity of medial preoptic norepinephrine mechanism for body temperature maintenance in sleeping and awake rats, Brain Res. Bull 15 (1985) 447–451. [DOI] [PubMed] [Google Scholar]
- [66].Amir S, De Blasio E, Activation of brown adipose tissue thermogenesis by chemical stimulation of the hypothalamic supraoptic nucleus, Brain Res. 563 (1991) 349–352. [DOI] [PubMed] [Google Scholar]
- [67].Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y, Opposite regulation of body temperature by cholinergic input to the paraventricular nucleus and supraoptic nucleus in rats, Brain Res. 909 (2001) 102–111. [DOI] [PubMed] [Google Scholar]
- [68].Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y, Cholinergic input to the supraoptic nucleus increases Fos expression and body temperature in rats, Pflugers Arch. 442 (2001) 451–458. [DOI] [PubMed] [Google Scholar]
- [69].Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson W, Mishkin M, Kennedy C, Gillin JC, Smith CB, Sokoloff L, Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta, Eur. J. Neurosci 9 (1997) 271–279. [DOI] [PubMed] [Google Scholar]
- [70].Glushchenko TS, Malinauskaite LO,Klenikova VA, Demin NN, Quantitative changes in the total proteins of the cells of the hypothalamic supraoptic nucleus after extreme exposures: acute immobilization and deprivation of the paradoxical sleep phase, Zh. Evol. Biokhim. Fiziol 26 (1990) 715–719. [PubMed] [Google Scholar]
- [71].Krauchi K, Cajochen C, Werth E, Wirz-Justice A, Warm feet promote the rapid onset of sleep, Nature 401 (1999) 36–37. [DOI] [PubMed] [Google Scholar]
- [72].Voloschin LM, Gallardo MG, Tramezzani JH, Suckling-induced serum prolactin levels are modified by interference with milk ejection in lactating rats, Biol. Reprod 59 (1998) 182–189. [DOI] [PubMed] [Google Scholar]


