Abstract
Background:
Endovascular repair (EVR) has replaced open surgery as the procedure of choice for patients requiring elective abdominal aortic aneurysm (AAA) repair. Long-term outcomes of the two approaches are similar, making the relative cost of caring for these patients over time an important consideration.
Methods and Results:
We linked Medicare claims to Vascular Quality Initiative registry data for patients undergoing elective EVR or open AAA repair from 2004–2015. The primary outcome was Medicare’s cumulative disease-related spending, adjusted to 2015 dollars. Disease-related spending included the index operation and associated hospitalization, surveillance imaging, reinterventions (AAA-related and abdominal wall procedures) and all-cause admissions within 90 days. We compared the incidence of disease-related events and cumulative spending at 90 days and annually through 7 years of follow-up. The analytic cohort comprised 6,804 EVR patients (median follow-up: 1.85 years; interquartile range (IQR): 0.82–3.22 years) and 1,889 open repair patients (median follow-up: 2.62 years; IQR: 1.13–4.80 years). Spending on index surgery was significantly lower for EVR (median (IQR): $25,924 ($22,280-$32,556) EVR vs. $31,442 ($24,669-$40,419) open; p<0.001), driven by a lower rate of in-hospital complications (6.6% EVR vs. 38.0% open; p<0.001). EVR patients underwent more surveillance imaging (1.8 studies per person-year EVR vs. 0.7 studies per person-year open; p<0.001) and AAA-related reinterventions (4.0 per 100 person-years EVR vs. 2.1 per 100 person-years open; p=0.041). Open repair patients had higher rates of 90-day readmission (12.9% EVR vs. 17.8% open; p<0.001) and abdominal wall procedures (0.6 per 100 person-years EVR vs. 1.5 per 100 person-years open; p<0.001). Overall, EVR patients incurred more disease-related spending in follow-up ($7,355 EVR vs. $2,706 open through 5 years). There was no cumulative difference in disease-related spending between surgical groups by 5 years of follow-up (-$33 EVR; 95% confidence interval: -$1,543 to $1,476).
Conclusions:
We observed no cumulative difference in disease-related spending on EVR and open repair patients 5 years after surgery. Generalized recommendations about which approach to offer elective AAA patients should not be based on relative cost.
The advent and widespread adoption of endovascular repair (EVR) for abdominal aortic aneurysms (AAA) represents a paradigm shift in the armamentarium of vascular surgeons. Between 2000 and 2010 in the United States, the proportion of all repairs performed by an endovascular approach increased from 5% to 74%.1 Endovascular techniques and their related devices are expensive. In the short term, this cost is offset by shorter postoperative hospitalizations and, most importantly, decreased morbidity and mortality for patients exposed to a less invasive procedure.2 In the long-term this trade-off becomes more complex. EVR can be complicated by endoleak requiring reintervention, with reported long-term rates of reintervention as high as 30%.3,4 Society for Vascular Surgery guidelines also recommend annual surveillance imaging for these patients as compared to 5-year interval surveillance for open AAA repair patients, which may drive higher utilization of healthcare resources.5,6
A robust body of literature has evolved to assess the short- and long-term outcomes of these alternative AAA interventions. Missing from most of these analyses, however, is an assessment of the cumulative spending on care for patients with different clinical courses following endovascular or open surgery. An estimated 30,000–40,000 AAA repair procedures incur approximately $1 billion in healthcare expenditure each year in the United States.1 AAA repair patients often require significant healthcare resources following surgery as well. Cost-effectiveness analyses have compared EVR and open AAA repair, but these rely on assumptions about the probability and cost of postoperative events and have often used clinical trial data which may not accurately reflect real-world practice.7–10 A recent characterization of real-world costs of EVR and open repair only considered the index operation.11 Other efforts to measure the downstream costs of open AAA repair and EVR have come from single institutions.12 In a national economic and healthcare reform context that prioritizes managing the rapidly growing costs of care, a better understanding of longitudinal spending on AAA repair patients is needed.
The aim of this study was to compare spending by Medicare on care for patients receiving elective EVR or open AAA repair. We used claims data to evaluate per-capita payments made by Medicare for disease-related care from index surgery through last follow-up. We leveraged data from the Vascular Quality Initiative (VQI) encompassing anatomic, procedural and other clinical considerations to calculate adjusted differences in disease-related and total spending by Medicare on AAA repair recipients through seven years of follow-up. This comparison allows for clinically relevant estimates of healthcare utilization and spending on AAA surgical patients that are consequential for health systems, providers, and payors looking to ensure high-value care for their populations.
Methods
Data sources
The VQI was launched by the Society for Vascular Surgery to improve the quality, safety, effectiveness and cost of vascular health care.13 Over 500 centers in the United States and Canada contribute validated data to this registry (https://www.vqi.org/). The VQI dataset was used to identify patients receiving EVR or open AAA surgery between January 1, 2003 and September 30, 2015 and associated information on preoperative risk factors, procedural characteristics and postoperative events.
These patients were linked to Medicare claims using an established social security number to beneficiary identifier crosswalk.14 Use of these claims was pursuant to a Data Use Agreement with the Centers for Medicare and Medicaid Services (CMS) and Institutional Review Board approval was obtained from the Dartmouth College Committee for Protection of Human Subjects with waiver of informed consent. Any data points generated from <11 beneficiaries were suppressed from data presentation per CMS requirements. Because of the sensitive nature of the data collected for this study, requests to access a similar dataset from qualified researchers trained in human subject confidentiality protocols must be made through CMS.
Study population
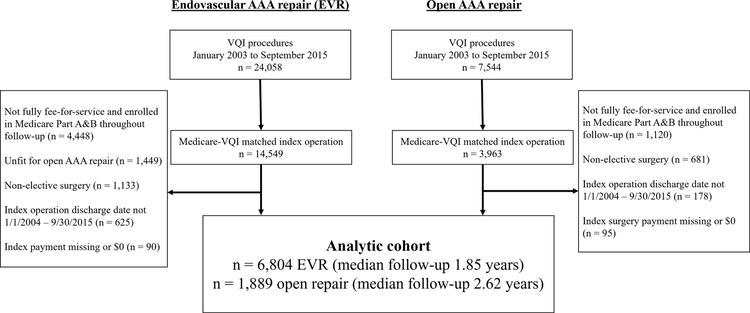
The cohort selection process is outlined in Figure 1. As detailed in prior work, we linked EVR and open AAA repair procedures identified in the VQI to Medicare claims using a 100 percent sample of Medicare beneficiaries, International Classification of Diseases, Ninth Edition (ICD-9) procedure codes, and Medicare Provider Analysis and Review claims for inpatient hospital services.14 Patients were followed from the date of their index operation to the end of the study period or death and were required to be enrolled in Medicare Part A and B and exclusively fee-for-service throughout the study. To facilitate comparison of similar patient populations, we considered only patients having elective surgery as categorized in the VQI. We also excluded EVR patients deemed unfit for open AAA repair by their surgeon. Finally, given the weight of the index operation on cumulative spending, we excluded patients whose index payment was missing or $0.
Figure 1.
Patient selection flowchart
Patient and procedure characteristics at index surgery
Demographic covariates obtained from Medicare claims were age, gender, race/ethnicity and dual-eligible status (defined as those fully eligible for Medicare and Medicaid in the year of surgery). Year of surgery was taken from the VQI. Preoperative clinical characteristics obtained from VQI data were smoking status, body mass index, coronary artery disease, diabetes or chronic obstructive pulmonary disease on therapy, congestive heart failure, and active dialysis. Additional variables obtained from the VQI were the center at which index surgery occurred, urgency of the operation (elective, symptomatic, ruptured), presence of concomitant iliac aneurysm, maximum AAA diameter, postoperative length of stay, and the occurrence of any postoperative complication: access site occlusion (EVR only), surgical site infection, hematoma, stroke, myocardial ischemia or infarction, cardiac dysrhythmia, congestive heart failure, respiratory failure, lower extremity embolization, new dialysis requirement, intestinal ischemia, and return to the operation room.
Medicare uses diagnosis-related groups (DRGs) as the basis for reimbursing most inpatient care, and we extracted the DRG assigned at index surgery from Medicare claims. These designations changed in 2008 for both EVR and open AAA repair, from 110 and 111 (Major vessel operation except heart, with and without major complication or comorbidity, respectively) to 237 and 238 (Major cardiovascular procedures, with and without major complication or comorbidity, respectively).
Outcomes
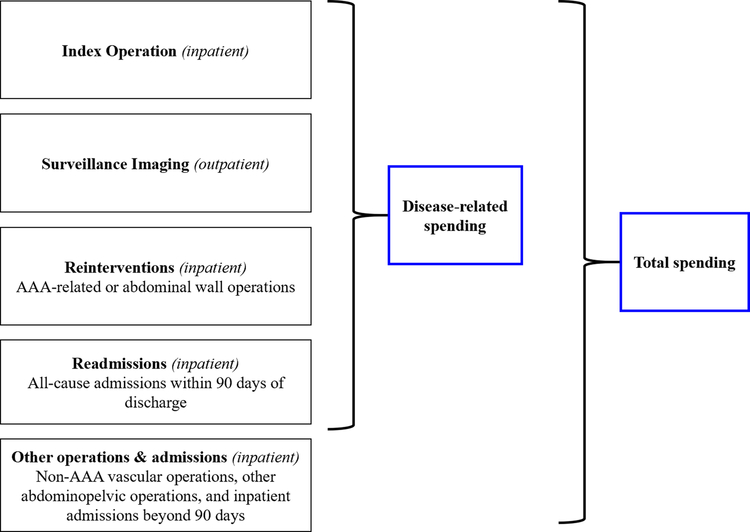
Spending on index surgery was calculated by summing Medicare’s total hospital payment, professional service payment and pass-through payment (temporary add-on fees for innovative technologies). Reintervention events were flagged using ICD-9-CM procedure codes; we categorized reinterventions as disease-related or other interventions. Disease-related reinterventions were comprised of AAA-related procedures and abdominal wall procedures, which included procedures for incisional hernia repair and adhesiolysis. Other interventions were categorized as vascular or non-vascular. Reintervention payments were determined from the related inpatient hospital and professional services payment amounts for the reintervention claim record. Post-procedure imaging was defined by Current Procedural Terminology (CPT) codes using outpatient and Part B/carrier line item files, and we considered only the line payment associated with the image claim. Other inpatient spending was measured using all claims that were not otherwise flagged as disease-related or other vascular / non-vascular procedures. All-cause readmissions occurring within 90 days of discharge were considered disease-related events.
The disease-related and total spending outcomes are summarized in Figure 2 and code lists used for event detection are provided in Supplemental Tables A–G. Final code lists were the result of blinded categorization of appropriate codes by two clinicians based on lists used in prior work, and discrepancies were adjudicated by the senior author.15 All payment amounts were adjusted to 2015 US dollars using the Bureau of Labor Statistics Consumer Price Index for Medical Care Services.16
Figure 2.
Conceptual model of Medicare spending outcomes
Statistical analyses
We compared preoperative clinical and procedural characteristics of patients undergoing EVR versus open AAA repair using appropriate univariate tests. We included total inpatient spending in the 12 months prior to index surgery as a baseline spending variable. The rate of disease-related events was compared using unadjusted Poisson regression. We performed crude comparison of median differences in spending for index surgery, individual follow-up events, and cumulative spending at different follow-up intervals (90 days post discharge then annually through 7 years) using quantile regression. Finally, as described in other work examining longitudinal healthcare costs, we calculated the adjusted difference in cumulative AAA-related and total spending at these intervals using multivariable quantile regression, incorporating variables that were different (p<0.10) at index surgery.17 The final model included clustering at the level of VQI center where the index operation occurred. A sensitivity analysis incorporating only patients who had surgery from 2011–2015 was also performed. All statistical analysis was performed using Stata/MP 15.1 (StataCorp LLC, College Station, TX).
Results
Cohort and index surgery characteristics
The analytic cohort was comprised of 6,804 EVR patients (median follow-up: 1.85 years; interquartile range (IQR): 0.82–3.22 years) and 1,889 open repair patients (median follow-up: 2.62 years; IQR: 1.13–4.80 years). A total of 1,021 EVR patients and 618 open repair patients completed at least 5 years of follow-up.
The preoperative and procedural characteristics of patients at index AAA repair is summarized in Table 1. EVR patients were older (75.4 vs. 72.9 years; p<0.001) and had more comorbidities such as diabetes (14.9% EVR vs. 12.0% open; p<0.001), mild to severe congestive heart failure (4.1% EVR vs 3.0% open; p<0.001), and dialysis dependence (1.1% EVR vs. 0.6% open; p<0.001). There was no difference in inpatient spending in the 12 months prior to surgery between cohorts (mean ± standard deviation (SD): $3,997 ± $11,498 EVR vs. $3,773 ± $10,436 open).
Table 1.
Characteristics of EVR and open AAA surgery patients at index surgery
| Variable | EVR, % (N=6,804) | Open Repair, % (N=2,616) | P value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age, mean years (SD) | 75.4 (7.1) | 72.9 (6.5) | <0.001 |
| Male Gender, % | 81.5 | 68.3 | <0.001 |
| Race, % | 0.227 | ||
| Non-Hispanic White | 93.3 | 94.6 | |
| Non-Hispanic Black | 3.2 | 2.6 | |
| Hispanic | 1.7 | 1.3 | |
| Other | 1.9 | 1.5 | |
| Dual-Eligible for Medicaid, % | 6.3 | 7.5 | 0.113 |
| Inpatient spending prior 12 months, mean $ (SD) | $3,997 ($11,498) | $3,773 ($10,436) | 0.445 |
| Year of surgery | <0.001 | ||
| 2004–2007 | 4.4 | 21.8 | |
| 2008–2011 | 18.8 | 22.3 | |
| 2012–2015 | 76.8 | 55.9 | |
| CLINICAL FACTORS | |||
| Smoking, % | <0.001 | ||
| Never | 15.5 | 9.0 | |
| Prior | 58.1 | 54.3 | |
| Current | 26.4 | 36.7 | |
| BMI, mean kg/m2 (SD) | 27.8 (5.3) | 26.9 (5.1) | <0.001 |
| Diabetes on therapy, % | 14.9 | 12.0 | 0.002 |
| COPD on therapy, % | 19.7 | 21.8 | 0.050 |
| Coronary artery disease, % | 0.352 | ||
| No MI history | 70.9 | 70.9 | |
| MI history, asymptomatic | 22.0 | 22.9 | |
| Angina (stable or unstable) | 7.1 | 6.2 | |
| Congestive heart failure, % | 0.001 | ||
| No | 89.4 | 92.2 | |
| Asymptomatic history | 6.5 | 4.8 | |
| Mild to severe | 4.1 | 3.0 | |
| Active dialysis, % | 1.1 | 0.60 | 0.060 |
| AAA max diameter, mean cm (SD) | 5.5 (1.5) | 6.0 (1.3) | <0.001 |
| Iliac aneurysm present, % | 23.9 | 25.0 | 0.312 |
| POSTOPERATIVE FACTORS | |||
| Length of stay, mean days (SD) | |||
| ICU | 0.6 (1.7) | 4.1 (6.7) | <0.001 |
| Total | 3.0 (5.3) | 8.9 (9.4) | <0.001 |
| Postoperative complication*, % | 6.6 | 38.0 | <0.001 |
| Index DRG Code, % | <0.001 | ||
| Without MCC (DRG 111/238) | 86.3 | 49.8 | |
| With MCC (DRG 110/237) | 8.4 | 46.1 | |
| Other | 5.3 | 4.1 | |
| In-hospital mortality, % | 0.37 | 3.49 | <0.001 |
Access site occlusion (EVR only), surgical site infection, hematoma, stroke, myocardial ischemia or infarction, cardiac dysrhythmia, congestive heart failure, respiratory failure, lower extremity embolization, new dialysis requirement, intestinal ischemia, and return to the operation room. BMI = Body mass index; COPD = Chronic obstructive pulmonary disease; MCC = Major complication or comorbidity; DRG = Diagnosis-related group; SD = standard deviation
The rate of perioperative complications was 6.6% for EVR vs. 38.0% for open surgery (p<0.001). Median length of stay was 1 day for EVR and 7 days for open repair (mean (SD): 3.0 (5.3) days EVR vs. 8.9 (9.4) days open; p<0.001). ICU stay was also significantly longer for open repair patients (mean (SD): 0.6 (1.7) days EVR vs. 4.1 (6.7) days open). In-hospital mortality was significantly lower for EVR recipients (0.4% EVR vs. 3.5% open; p<0.001).
Index surgery spending
Medicare’s median payment for index EVR surgery was $25,924 (interquartile range (IQR): $22,280-$32,556) while that for open repair was $31,442 (IQR: $24,669-$40,419). Payment for index surgery varied primarily based on DRG assignment. For example, the median payment for open repair patients without major complication or comorbidity (DRG 238/111) was $24,805 vs. $39,539 for open repair with major complication or comorbidity (DRG 237/110). Furthermore, 46.1% of open repair patients were assigned the more complex DRG, compared to only 8.4% of EVR (p<0.001). About 5% of both EVR and open surgery patients were assigned a DRG not typically used for AAA repair. Spending on index surgery by approach and DRG assignment is summarized in Table 2.
Table 2.
Medicare payments for index AAA repair stratified by diagnosis-related group (DRG) assignment
| n (%) | Median payment (Quartile 1- Quartile 3), $ | p value* | Mean payment (SD), $ | p value | |
|---|---|---|---|---|---|
| Without MCC (DRG 238/111) | <0.001 | <0.001 | |||
| EVR | 5,869 (86.3) | $25,566 ($22,362 – $31,217) | $26,949 ($6,786) | ||
| Open | 941 (49.8) | $24,805 ($21,325 – $30,072) | $25,863 ($5,476) | ||
| With MCC (DRG 237/110) | 0.115 | 0.007 | |||
| EVR | 571 (8.4) | $40,791 ($33,906 – $48,900) | $44,458 ($17,964) | ||
| Open | 870 (46.1) | $39,539 ($32,665 – $45,242) | $41,933 ($17,009) | ||
| Other DRG† | <0.001 | <0.001 | |||
| EVR | 364 (5.3) | $2,964 ($2,166 – $20,756) | $18,682 ($39,541) | ||
| Open | 78 (4.1) | $109,466 ($53,274 – $174,359) | $129,249 ($105,031) | ||
| All Patients | <0.001 | <0.001 | |||
| EVR | 6,804 | $25,924 ($22,280 – $32,556) | $27,976 ($13,361) | ||
| Open | 1,889 | $31,442 ($24,669 – $40,419) | $37,534 ($31,974) |
Crude difference in median payment using quantile regression
See Supplemental Material, Table H, for description of other DRG codes.
MCC = Major complication or comorbidity
Disease-related reinterventions
The rate of all-cause readmissions within 90 days of discharge was 12.9% for EVR patients and 17.8% for open repair patients (p<0.001). Median payments for 90-day readmissions was similar ($10,365 EVR vs. $10,766 open). (Table 3). Through last follow-up, 513/6,775 (7.6%) at-risk EVR patients underwent 614 AAA-related reinterventions while 113/1,822 (6.2%) at-risk open repair patients underwent 136 reinterventions (4.0 AAA-related reinterventions per 100 person-years EVR vs. 2.1 per 100 person-years open; p=0.041). The median payment for AAA-related reinterventions was $24,401 following EVR and $31,776 following open repair. Conversely, the rate of abdominal wall operations was 0.6 per 100 person-years following EVR and 1.5 per 100 person-years following open repair (p<0.001), with median payments of $16,977 and $17,015, respectively (Table 3). On average, spending on AAA-related reinterventions for EVR patients was offset by additional spending on abdominal wall operations for open repair patients; however, the financial burden of AAA-related reinterventions was far greater overall (Table 4).
Table 3.
Rates of and Medicare payments for disease-related and other inpatient events during follow-up
| Event | EVR | Open repair | P value* |
|---|---|---|---|
| N at risk (surviving index operation) | 6,775 | 1,822 | |
| Total person-years of follow-up | 15,455.1 | 6,392.6 | |
| DISEASE-RELATED EVENTS | |||
| 90-day readmission | |||
| % of patients (n patients) | 12.9 (874) | 17.8 (325) | <0.001 |
| Median payment per readmission, $ | $10,365 | $10,766 | 0.552 |
| AAA-related reintervention | |||
| n events (n patients) | 614 (513) | 136 (113) | |
| Events per 100 person-years | 4.0 | 2.1 | 0.041 |
| Median payment per event, $ | $24,401 | $31,776 | <0.001 |
| Abdominal wall operation | |||
| n events (n patients) | 95 (91) | 95 (88) | |
| Events per 100 person-years | 0.6 | 1.5 | <0.001 |
| Median payment per event, $ | $16,977 | $17,015 | 0.948 |
| Surveillance imaging studies | |||
| % patients with ≥1 study (n patients) | 96.8 (6,559) | 72.3 (1,317) | |
| Imaging studies per person-year | 1.8 | 0.7 | <0.001 |
| Median payment per imaging study, $ | $210 | $145 | <0.001 |
| OTHER EVENTS | |||
| Other vascular procedures | |||
| n events (n patients) | 197 (188) | 94 (82) | |
| Events per 100 person-years | 1.3 | 1.5 | <0.001 |
| Median payment per event, $ | $16,216 | $14,040 | 0.212 |
| Other non-vascular procedures | |||
| n events (n patients) | 252 (211) | 126 (88) | |
| Events per 100 person-years | 1.6 | 2.0 | <0.001 |
| Median payment per event, $ | $20,767 | $24,386 | 0.216 |
| Admissions > 90 days post-discharge | |||
| n events (n patients) | 6,226 (2,532) | 2,484 (844) | |
| Events per 100 person-years | 40.3 | 38.9 | <0.001 |
| Median payment per event, $ | $9,178 | $9,370 | 0.438 |
Counts of AAA-related reinterventions, abdominal wall operations, surveillance images, other vascular non-vascular procedures and admissions > 90 days post-discharge compared using unadjusted Poisson regression. Percent of patients with 90-day readmission compared using chi-squared test.
Table 4.
Payments on disease-related and non disease-related events per person-year of follow-up
| EVR | OPEN | DIFFERENCE (OPEN VS EVR) | |
|---|---|---|---|
| Person-years of follow up | 15,455.1 | 6,392.6 | |
| AAA-related reintervention | $1,121.03 | $872.64 | −$248.39 |
| Abdominal wall operation | $145.28 | $400.18 | $254.90 |
| Surveillance imaging | $401.18 | $122.62 | −$278.56 |
| TOTAL DISEASE-RELATED | $1,667.49 | $1,395.44 | −$272.05 |
| Vascular operations | $304.63 | $333.79 | $29.16 |
| Other non-vascular operations | $480.70 | $538.73 | $58.02 |
| Inpatient admissions > 90 days post-discharge | $4,532.12 | $4,336.75 | −$195.37 |
| TOTAL NON DISEASE-RELATED | $5,317.45 | $5,209.26 | −$108.19 |
| TOTAL PAYMENTS | $6,984.94 | $6,604.70 | −$380.23 |
Surveillance imaging
Almost all EVR patients (96.8%) underwent at least one surveillance image following surgery, while 27.7% of open repair patients had no subsequent imaging. The intensity of surveillance imaging was significantly higher for EVR patients as well (1.8 imaging studies per person-year of follow-up after EVR vs. 0.7 studies per person-year after open; p<0.001) (Table 3). This amounted to $401 spent on surveillance imaging per person-year of follow up in EVR patients versus $122 per person-year of follow up in open repair patients (Table 4). Approximately one third of open repair patients received surveillance imaging during each year of follow-up, while the proportion of EVR patients with surveillance imaging declined from 80% in the first two postoperative years to 60% by year 7 (Supplemental Table I). CT was the most common imaging modality (61.5% of EVR images, 57.5% of open images), followed by ultrasound (37.4% of EVR images, 40.3% of open images) and MRI (1.2% of EVR images, 2.2% of open images); choice of imaging modality differed by surgical group (p<0.001). Ultrasound was the least expensive study on average ($110), while CT scans ($254) and MRIs ($294) were more expensive.
Non-disease related spending
AAA surgery recipients underwent other vascular and nonvascular procedures during follow-up categorized as unrelated to their index operation (Table 3). The rate of non-AAA related vascular operations was slightly lower for EVR patients (1.3 per 100 person-years EVR vs. 1.5 per 100 person-years open; p<0.001). EVR patients similarly underwent fewer nonvascular procedures (1.6 per 100 person-years EVR vs. 2.0 per 100 person-years open; p<0.001).
Inpatient admissions more than 90 days after discharge from the index operation were common. The rate of admissions, not including those for disease-related reinterventions or other vascular and nonvascular procedures described above, was 40.3 per 100 person-years for EVR patients and 38.9 per 100 person-years for open repair patients (p<0.001). Median spending on these admissions was roughly $9,000 in both groups.
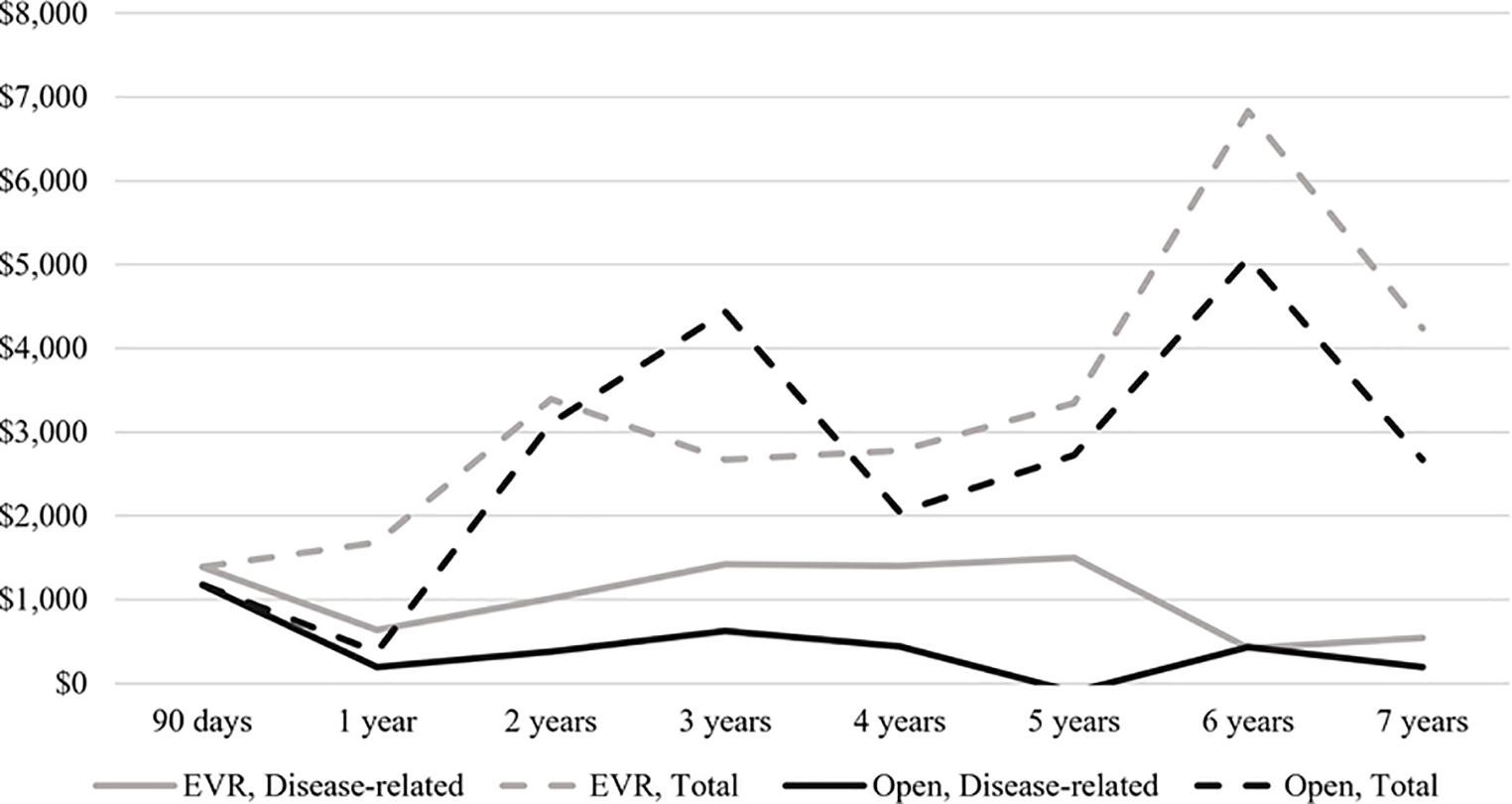
Cumulative disease-related spending
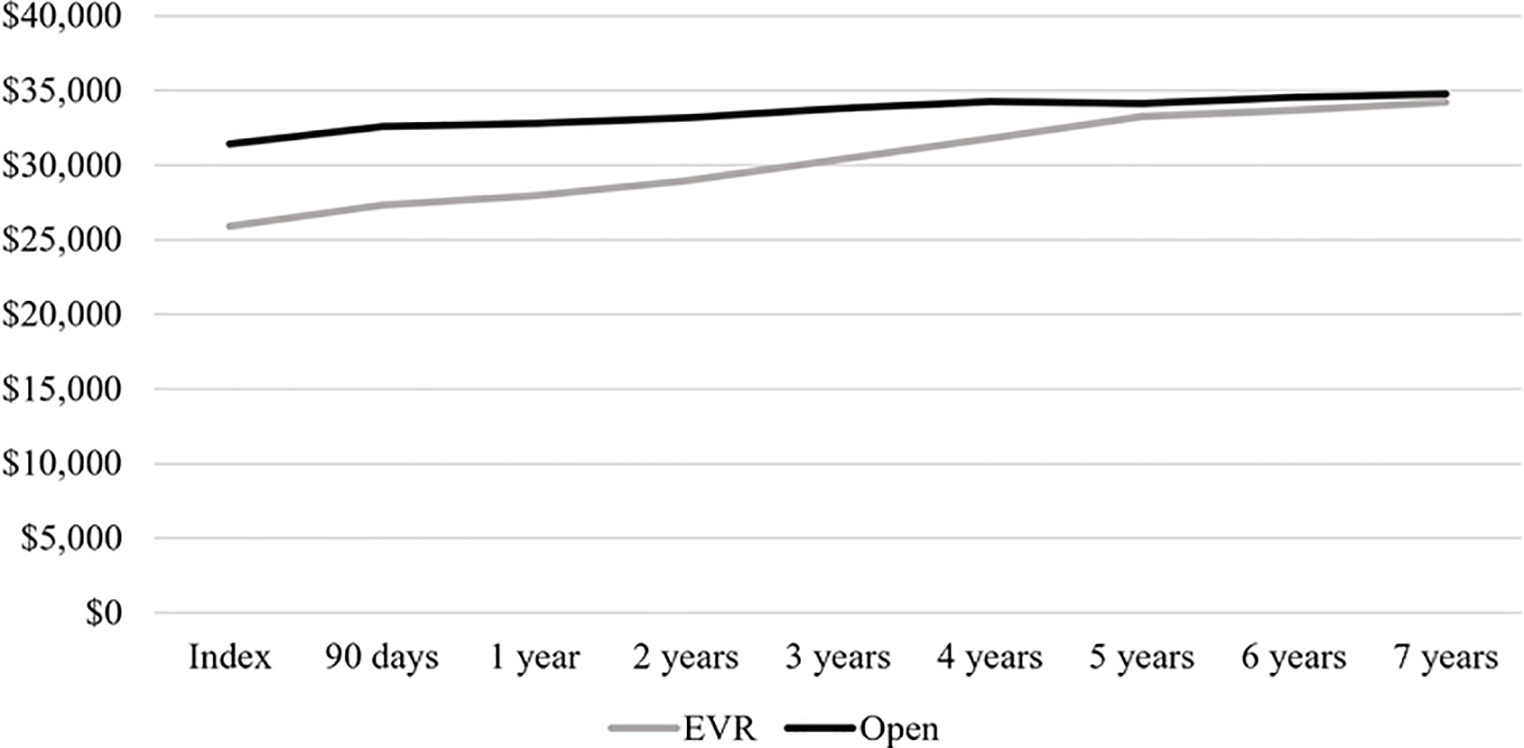
Longitudinal analysis of spending after index AAA repair is shown in Figure 3A. Spending in the 90 days following surgery was similar between groups ($1,387 EVR vs. $1,174 open). Median disease-related spending was greatest in the third year following open repair, at $621. By comparison, disease-related spending for EVR recipients was between $1,400 and $1,500 in postoperative years 3–5. In cumulative terms, disease-related follow-up spending at 5 years was $7,355 for EVR patients compared to $2,706 for open repair patients (Figure 3B).
Figure 3.
Disease-related and total follow-up spending on EVR and open AAA repair patients. A, Interval follow-up spending, median $. B, Cumulative follow-up spending, median $.
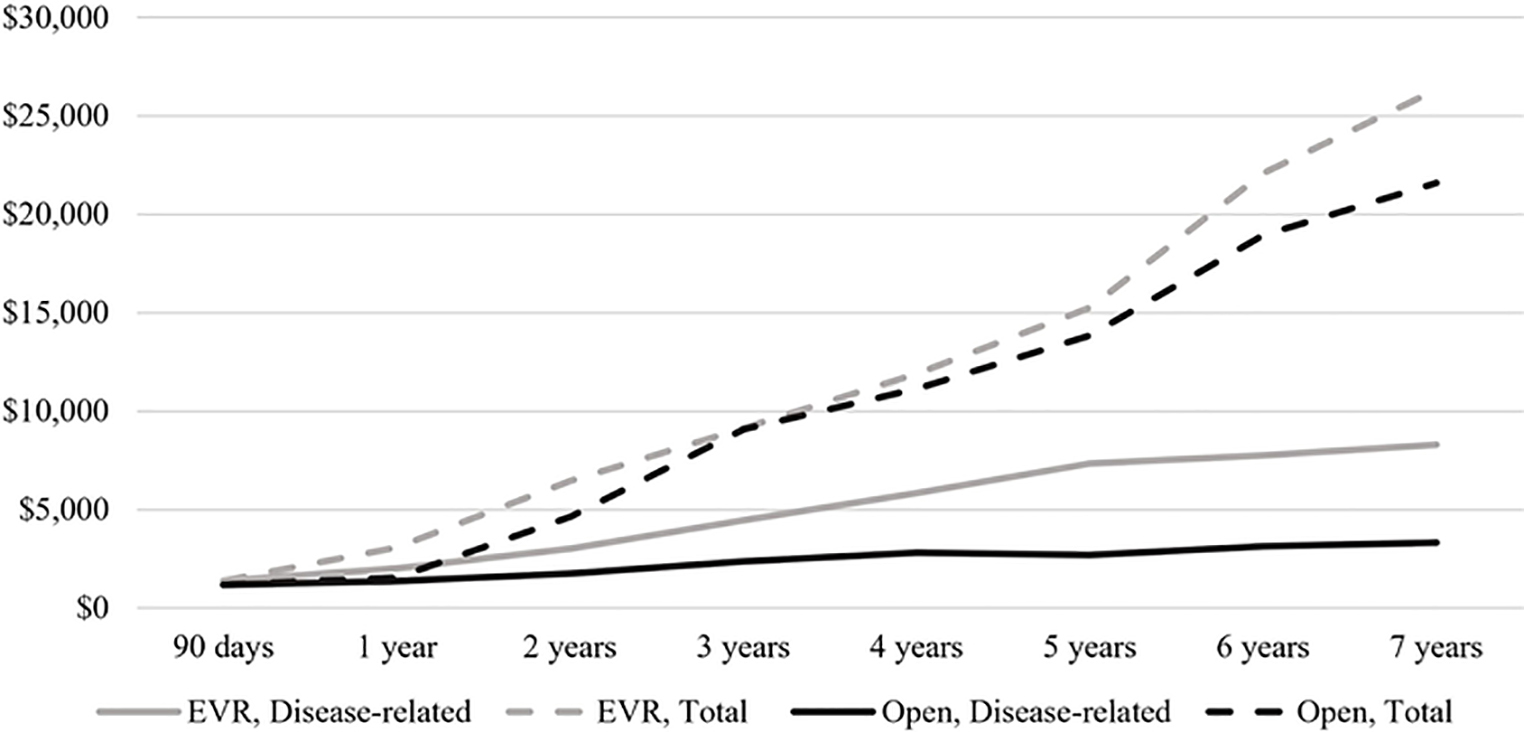
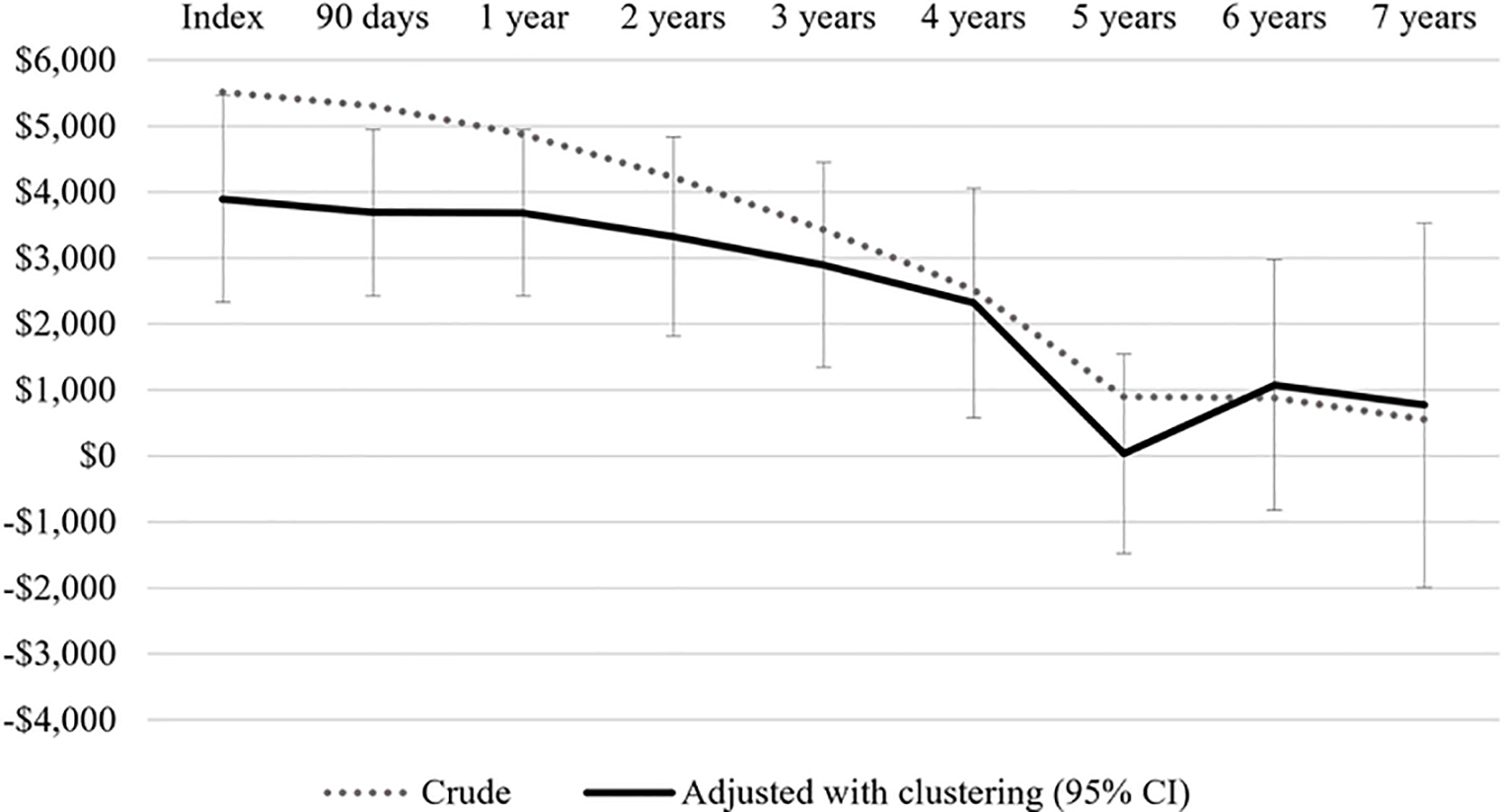
Finally, we considered cumulative spending on disease-related care including index surgery (Figure 4A). While EVR patients incurred significantly less spending through 90 days (crude: -$5,307, CI -$5,945 to +$4,670; adjusted: -$3,690, CI -$4,952 to +$2,427), there was no difference in spending between the procedural cohorts at 5–7 years of follow-up. At 5 years of follow-up, the crude difference in median spending was -$896 for EVR vs. open patients (CI: -$2,188 to +$396); adjusting for patient factors and center-level effects, this difference was -$33 for EVR patients (CI: -$1,543 to +$1,476) (Figure 4B). A sensitivity analysis among only those patients having surgery between 2011–2015 demonstrated a similar trajectory, with significantly less spending on EVR patients through 3 years but no significant difference between the procedural cohorts at 4–5 years of follow-up.
Figure 4.
Cumulative disease-related spending on EVR and open AAA repair patients. A, Cumulative disease-related spending, median $. B, Crude and adjusted difference in disease-related spending, $*.
*Positive $ value reflects higher spending for open AAA repair patients. Adjustment using quantile regression accounting for patient-level factors and clustering at the level of index surgical center. 95% confidence interval (CI) shown for final model.
Discussion
We performed a longitudinal analysis of spending on endovascular and open AAA repair patients using Medicare claims linked to the Vascular Quality Initiative clinical registry. Index operations comprised the vast majority of spending for most AAA repair patients. Driven by a higher rate of postoperative complications, average spending on index open AAA repair was approximately $5,000 more per patient than for EVR. EVR patients subsequently received more frequent and expensive surveillance imaging and reinterventions and accrued roughly $5,000 in incremental disease-related spending by 5 years following surgery. After adjusting for patient factors and center-level effects, there was no difference in cumulative spending between the procedural cohorts at 5–7 years of follow-up.
Given dramatic reductions in short-term morbidity and mortality as compared to open surgery, EVR quickly became the predominant method for AAA repair in the United States.1 However, the high cost of endografts, potential need for reintervention, and studies demonstrating equivalent or superior long-term mortality with open repair, has led to cost-effectiveness concerns with an endovascular-first approach.4,18 Highlighting this issue, 2018 draft guidelines from the UK National Institute for Health and Care Excellence (NICE) outlined a recommendation that patients with unruptured infrarenal AAA should not be offered EVR if open surgical repair is suitable.19 This recommendation was based, in part, on a statement that EVR entails higher net costs than open surgical repair. Final NICE guidelines have yet to be submitted; clearly, there is a need to better understand both the long-term outcomes and costs of care for AAA surgery patients. Our analysis furthers this effort, and has important implications for clinicians, health services researchers, device manufacturers, policymakers and patients.
First, this analysis of Medicare data allows for long-term characterization of health care utilization and expenditure following AAA repair. We differentiated between AAA-related reinterventions, which were more common among EVR recipients, and abdominal wall procedures, which were more common among open repair patients. Our reported rate of abdominal wall operations is consistent with a prior study of the Medicare population, which reported rates of incisional hernia repair of 1.2 per 100 person-years for open repair and 0.3 per 100 person-years for EVR. 20 The rate of repeat AAA repair was lower in that study (1.0 per 100 person-years EVR vs. 1.2 per 100 person-years open), reflecting a narrower code list used for event detection. The cost attributed to these reinterventions by studies to date has ranged from $7,000 to $17,000, and often were not stated explicitly.7 These estimates may grossly underestimate the financial impact of reintervention, which was comparable to index surgery in our analysis.
Incremental spending on abdominal wall procedures for open repair patients was nearly equivalent to incremental spending on AAA-related reinterventions for EVR patients. This supports surveillance imaging as a key driver of higher disease-related spending for EVR patients in follow-up. Current guidelines from the Society for Vascular Surgery recommend a CT at 1 month and, if normal, support a transition to ultrasound for annual surveillance after EVR; CT at 5 year intervals is recommended following open repair.5 We found EVR recipients received surveillance imaging at more than twice the rate of open repair patients and underwent a slightly greater proportion of CT scans. At the same time, compliance with surveillance imaging is an established concern in the EVR population, with 20 to 40% of EVR patients in our analysis having no surveillance study in a given 12 month interval.21 Suboptimal surveillance in EVR patients, and the perpetual nature of this imaging in current guidelines, ensures that surveillance practices will remain a focus in the debate over the long-term cost of care for AAA surgery patients.
Our study was not designed to address the challenge of quantifying the utility of changing health states that accompany the varied natural histories following AAA repair. Review of prior cost-effectiveness analyses comparing EVR and open repair does help place our findings in context. In 2016, van Bochove and colleagues published a systematic review of cost-effectiveness studies comparing open and endovascular AAA repair.7 Most of the 13 studies they reviewed found EVR to be both more expensive (range of incremental costs of EVR: -$5,019 to +$14,576) and more effective (range of incremental quality-adjusted life years: −0.042 to +0.42 years) than open repair, with six of the 13 analyses finding EVR to be cost-effective. These studies assumed variable time horizons from 12 months to lifetime follow-up, three used cost estimates based in the United States, and only one of those three studies was conducted in the last 15 years.22,23 Studies included in this review estimated disease-related follow-up costs ranging from $5,000-$8,000 for EVR patients and $2,000-$3,000 for open repair patients.7 Our analysis yielded 5-year disease-related spending of $7,355 after EVR and $2,706 after open repair.
A contemporary cost-effectiveness analysis comparing EVR and open AAA repair in the United States comes from the Open vs. Endovascular Repair Study of 881 elective AAA repairs performed at Veterans Affairs medical centers between 2002 and 2008.9 Mean follow-up in this study was 5.2 years. AAA-related costs were no different between endovascular and open repair (median $42,375 EVR vs. $37,397 open) and reflected about 40% of total healthcare costs, which were also not significantly different between groups (median $106,905 EVR vs. $110,484 open repair). The authors concluded that for patients who are candidates for both procedures, selection of either approach was reasonable. Furthering the argument for equipoise between EVR and open AAA repair, the same group recently presented outcomes at 14 years of follow-up, finding no difference in long-term overall survival.18 The true cost-effectiveness of EVR likely rests with individual willingness to pay thresholds and the relative utility of the short-term risks of open repair weighed against the follow-up requirements and long-term risks of endovascular repair. These real tradeoffs should prompt providers to explore ways to align surgical decision-making with individual patient preferences. A randomized clinical trial evaluating the utility of a decision aid for patients facing AAA surgery is underway.24
Medicare claims are a critical tool for exploring utilization and spending in the AAA surgical population given that the vast majority of these patients are Medicare beneficiaries.25 This is especially timely in the United States amidst mounting calls for single-payer or “Medicare for All” healthcare reform. Medicare spending is not the same as cost, but DRG-based reimbursement – used by Medicare for most reimbursement of inpatient care – is designed to reflect cost.26 In this study, Medicare spending can be interpreted as a surrogate for cost from a societal payer perspective. A comparison to direct cost estimates in other studies is also useful from a provider perspective. Gupta and colleagues recently performed a real-world analysis of EVR vs. open repair costs for nonruptured AAA using the Premier Healthcare Database.11 The authors, who also adjusted to 2015 dollars, reported the mean total cost of index admission was lower for EVR ($32,052 EVR vs. $36,091 open, p<0.001). This suggests a potential financial loss for EVR and gain for open repair based on Medicare’s reimbursement in the present study.
Medicare has traditionally reimbursed EVR and open AAA repair using the same set of DRG codes, divided into cases with or without major complication or comorbidity. As a result, differences in per patient spending by Medicare is functionally driven by DRG code assignment for the index hospital stay. In our analysis, only 1 in 10 index EVR procedures were billed with MCC, compared to half of open procedures. For provider systems, potential coding inaccuracy represents an important focus in ensuring needed services remain financially viable.27 Many also felt the decision to lump EVR in with open repair DRG groups was untenable due in large part to high graft device costs, and CMS introduced new DRGs specific to EVR in 2016.28 These changes are estimated to increase reimbursement for EVR with and without MCC by 24% and 14%, respectively.29 Evolving device technologies, perioperative care strategies and reimbursement schemes will perpetuate uncertainty about the most cost-effective approach to elective AAA repair.
Using Medicare claims to evaluate and compare healthcare utilization and spending was subject to limitations. We relied on administrative coding for event detection. Coding error and heterogeneity make it difficult to attribute disease-related healthcare spending with both sensitivity and specificity. For example, our use of ICD-9 codes for detecting index and reintervention events, based on a previously developed VQI-Medicare matching algorithm, may be more sensitive but less specific than using CPT codes assigned by the surgeon. Most EVR procedures occurred in the final three years of our study period, leading to significantly higher censorship in the EVR cohort. As a result, longer term data in the EVR cohort may not reflect contemporary outcomes with this approach. Sample size constraints limited our analysis to seven years of follow-up, so our findings may not be generalizable to young, non-Medicare patients with longer life expectancies. With the exception of surveillance imaging, we did not evaluate spending beyond the inpatient setting. In the Veterans Affairs cost-effectiveness study, outpatient visits accounted for only 2% of AAA-related costs, but nearly 40% of non-AAA related costs.9 Our primary spending outcomes were confounded by center-level adjustments to DRG base payments based on regional price, teaching hospital status and share of low-income patients. The difference in spending we observed between EVR and open index surgery within a given DRG suggests that EVR is performed disproportionately in more urban settings and teaching hospitals, where adjusted reimbursement tends to be higher. To mitigate this effect, we performed clustering at the level of index surgical center in our final model of incremental spending.
Conclusion
Medicare’s increased spending on index open AAA repair is offset by higher disease-related spending on endovascular repair patients during follow-up. If long-term cost and outcomes of EVR and open repair remain in relative equipoise, maximizing value of AAA surgical care may best be accomplished at the patient level by tailoring selection of surgical approach to individual preferences.
Supplementary Material
What is known.
Endovascular repair (EVR) of abdominal aortic aneurysms (AAA) is the procedure of choice for elective repair in the United States due to decreased short term morbidity and mortality versus open surgical repair.
Citing long term cost-effectiveness concerns, 2018 draft guidelines from the UK National Institute for Health and Care Excellence outlined a recommendation that patients with unruptured infrarenal AAA should not be offered EVR if open repair is suitable.
What the study adds.
In the Medicare population, increased upfront spending on open repair due to longer and more complicated hospitalizations is gradually offset by increased disease-related care for EVR patients in follow-up.
Higher spending on abdominal wall procedures for open repair patients and AAA-related reinterventions for EVR patients was similar, highlighting surveillance imaging as a key variable when comparing long term care.
Relative cost equipoise between these alternative approaches to AAA repair over time should prompt providers to explore ways for best aligning surgical decision-making with individual patient preferences.
Acknowledgements
We gratefully acknowledge the administrative support of Kayla Moore in executing this project.
Sources of Funding
This work was supported by the Office of Academic Affiliations, Department of Veteran’s Affairs.
Footnotes
Disclosures
None.
References
- 1.Dua A, Kuy S, Lee CJ, Upchurch GR, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–1517. [DOI] [PubMed] [Google Scholar]
- 2.Clair DG, Gray B, O’Hara PJ, Ouriel K. An evaluation of the costs to health care institutions of endovascular aortic aneurysm repair. J Vasc Surg. 2000;32:148–152. [DOI] [PubMed] [Google Scholar]
- 3.Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg. 2013;100:863–872. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. [DOI] [PubMed] [Google Scholar]
- 5.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schemerhorn ML, Starnes BW. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77.e72. [DOI] [PubMed] [Google Scholar]
- 6.Garg T, Baker LC, Mell MW. Postoperative Surveillance and Long-term Outcomes After Endovascular Aneurysm Repair Among Medicare Beneficiaries. JAMA Surg. 2015;150:957–963. [DOI] [PubMed] [Google Scholar]
- 7.van Bochove CA, Burgers LT, Vahl AC, Birnie E, van Schothorst MG, Redekop WK. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm. J Vasc Surg. 2016;63:827–838 e822. [DOI] [PubMed] [Google Scholar]
- 8.Prinssen M, Buskens E, de Jong SE, Buth J, Mackaay AJ, van Sambeek MR, Blankensteijn JD; DREAM trial participants. Cost-effectiveness of conventional and endovascular repair of abdominal aortic aneurysms: Results of a randomized trial. J Vasc Surg. 2007;46:883–890.e881. [DOI] [PubMed] [Google Scholar]
- 9.Lederle FA, Stroupe KT, Kyriakides TC, Ge L, Freischlag JA, Open vs Endovascular Repair Veterans Affairs Cooperative Study G. Long-term Cost-effectiveness in the Veterans Affairs Open vs Endovascular Repair Study of Aortic Abdominal Aneurysm: A Randomized Clinical Trial. JAMA Surg. 2016;151:1139–1144. [DOI] [PubMed] [Google Scholar]
- 10.Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess. 2018;22:1–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta AK, Alshaikh HN, Dakour-Aridi H, King RW, Brothers TE, Malas MB. Real-world cost analysis of endovascular repair versus open repair in patients with nonruptured abdominal aortic aneurysms. J Vasc Surg. 2020;71:432–443.e4. [DOI] [PubMed] [Google Scholar]
- 12.Hayter CL, Bradshaw SR, Allen RJ, Guduguntla M, Hardman DTA. Follow-up costs increase the cost disparity between endovascular and open abdominal aortic aneurysm repair. J Vasc Surg. 2005;42:912–918. [DOI] [PubMed] [Google Scholar]
- 13.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. [DOI] [PubMed] [Google Scholar]
- 14.Hoel AW, Faerber AE, Moore KO, Ramkumar N, Brooke BS, Scali ST, Sedrakyan A, Goodney PP. A pilot study for long-term outcome assessment after aortic aneurysm repair using Vascular Quality Initiative data matched to Medicare claims. J Vasc Surg. 2017;66:751–759.e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Columbo JA, Kang R, Hoel AW, Kang J, Leinweber KA, Tauber KS, Hila R, Ramkumar N, Sedrakyan A, Goodney PP. A comparison of reintervention rates after endovascular aneurysm repair between the Vascular Quality Initiative registry, Medicare claims, and chart review. J Vasc Surg. 2019;69:74–79.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consumer Price Index for All Urban Consumers: Medical care services; [CUSR0000SAM2] https://fred.stlouisfed.org/series/CUSR0000SAM2. Accessed December 15, 2018. [Google Scholar]
- 17.Olsen MA, Tian F, Wallace AE, Nickel KB, Warren DK, Fraser VJ, Selvam N, Hamilton BH. Use of Quantile Regression to Determine the Impact on Total Health Care Costs of Surgical Site Infections Following Common Ambulatory Procedures. Ann Surg. 2017;265:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederle FA, Kyriakides TC, Stroupe KT, Freischlag JA, Padberg FT, Mastumura JS, Huo Z, Johnson GR. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2019;380:2126–2135. [DOI] [PubMed] [Google Scholar]
- 19.Abdominal aortic aneurysm: diagnosis and management. NICE guideline, Draft for consultation 2018; https://www.nice.org.uk/guidance/gid-cgwave0769/documents/short-version-of-draft-guideline. Accessed April 25, 2019.
- 20.Jackson RS, Chang DC, Freischlag JA. Comparison of Long-term Survival After Open vs Endovascular Repair of Intact Abdominal Aortic Aneurysm Among Medicare Beneficiaries. JAMA. 2012;307:1621–1628. [DOI] [PubMed] [Google Scholar]
- 21.Wanken ZJ, Trooboff SW, Gladders B, Columbo JA, Ramkumar N, Austin AM, Stone DH, Mell MW, Sedrakyan A, Goodney PP. Characterization of Endovascular Abdominal Aortic Aneurysm Repair Surveillance in the Vascular Quality Initiative. Circulation. 2020;141:866–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel ST, Haser PB, Bush HL, Kent KC. The cost-effectiveness of endovascular repair versus open surgical repair of abdominal aortic aneurysms: A decision analysis model. J Vasc Surg. 1999;29:958–972. [DOI] [PubMed] [Google Scholar]
- 23.Bosch JL, Kaufman JA, Beinfeld MT, Adriaensen MEAPM, Brewster DC, Gazelle GS. Abdominal Aortic Aneurysms: Cost-effectiveness of Elective Endovascular and Open Surgical Repair. Radiology. 2002;225:337–344. [DOI] [PubMed] [Google Scholar]
- 24.Preferences for Open Vs. Endovascular Repair for Abdominal Aortic Aneurysm. https://ClinicalTrials.gov/show/NCT03115346. Accessed September 21, 2019.
- 25.Suckow BD, Goodney PP, Columbo JA, Kang R, Stone DH, Sedrakyan A, Cronenwett JL, Fillinger MF. National trends in open surgical, endovascular, and branched-fenestrated endovascular aortic aneurysm repair in Medicare patients. J Vasc Surg. 2018;67:1690–1697.e1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhardt UE. The Pricing Of U.S. Hospital Services: Chaos Behind A Veil Of Secrecy. Health Affairs. 2006;25:57–69. [DOI] [PubMed] [Google Scholar]
- 27.Stone DH, Horvath AJ, Goodney PP, Rzucidlo EM, Nolan BW, Walsh DB, Zwolak RM, Powell RJ. The financial implications of endovascular aneurysm repair in the cost containment era. I Vasc Surg. 2014;59:283–290.e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayub S, Scali ST, Richter J, Huber TS, Beck AW, Fatima J, Berceli SA, Upchurch GR, Arnaoutakis D, Back MR, Giles KA. Financial implications of coding inaccuracies in patients undergoing elective endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2019;69:210–218. [DOI] [PubMed] [Google Scholar]
- 29.Center for Medicare and Medicaid Services EVAR Reimbursement. https://www.codingstrategies.com/pdf/Center%20for%20Medicare%20and%20Medicaid%20Services%20EVAR%20Reimbursement.pdf. Accessed April 27, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.