Abstract
Purpose
This study aims to elucidate the role and mechanism of survivin and FOXP1 in scarring after glaucoma surgery and to evaluate the prevention and treatment of excessive wound healing and scar formation in an in vitro model of glaucoma filtration surgery.
Methods
Human Tenon's capsule fibroblasts (HTFs) were used with TGF-β to establish an in vitro cell model after glaucoma, observe survivin expression in the cell model, and observe HTFs proliferation after treatment with survivin inhibitor YM155 and the expression of α-SMA and collagen type I. In addition, the effects of survivin and cell proliferation in HTFs after knockdown of FOXP1 were observed by Western blot analysis.
Results
Survivin was upregulated in HTFs after glaucoma surgery, and it could promote the cell proliferation of HTFs. After treatment with its inhibitor YM155, the cell proliferation of HTFs was inhibited, and the expression of α-SMA and collagen type I were decreased. The results showed that in knockdown of FOXP1, the expression of survivin was downregulated, and the cell proliferation of HTFs was significantly reduced.
Conclusions
This study demonstrates that targeting survivin with an inhibitory YM155 reduced fibrosis and the extracellular matrix (ECM), and it was regulated by the FOXP1 transcription factor. These results suggest that survivin could be a potential target for treating scar formation after glaucoma surgery.
Translational Relevance
Together with the results from previous survivin and FOXP1 preclinical studies, these data support the evaluation of this gene therapy candidate in clinical trials as a potential durable treatment for antiscarring of glaucoma surgery.
Keywords: glaucoma filtration surgery, survivin, fibrosis, human Tenon's capsule fibroblasts
Introduction
Glaucoma is the second most common blind eye disease worldwide. It is a neurodegenerative disease characterized by optic neuropathy and gradual loss of retinal ganglion cells, characteristic visual field defects, and increased pathological intraocular pressure is its main risk factor.1 Using drugs to control intraocular pressure is inadvisable good. Using surgery to reduce pathological high intraocular pressure to preserve the existing visual field of retinal ganglion cells and defects is the last line of defense for glaucoma treatment. Therefore, glaucoma filtering surgery remains among the most effective treatments presently.2 However, the excessive hyperplasia of scar tissue after the glaucoma filtering operation hinders draining the filtering channel,3 thereby inducing operation failure—a frequent problem for clinicians.
Wound healing after glaucoma filtration follows the general principles of wound healing,4 that is, an inflammatory reaction occurs in the early stages of the process, and different growth factors and cytokines (such as TGF-β1, TGF-β2, and TGF-β3) are released. Immediately after fibroblast activation, proliferation and migration, angiogenesis, and collagen deposition, which participate in tissue repair, then fibroblasts transform into myofibroblasts, and with the participation of collagen, scar tissue is formed, reducing filtration of the drainage of the channel aqueous humor.5 Presently, preventive treatment is often performed using intra-operative drugs, such as mitomycin-C, 5-fluorouracil (5-FU), and other antimetabolites, but the effect is harmful, and it has serious complications or side effects, such as filtering bleb leakage and filtering bleb infection, low intraocular pressure, endophthalmitis, and corneal epithelial toxicity.6 Recent international studies on inhibiting postoperative glaucoma scarring mainly focus on the application of anti-vascular endothelial growth factor (VEGF) and anti-TGF-β drugs, and so on. However, whether the antiscarring effect is better in anti-VEGF and anti-TGF-β than in mitomycin-C and 5-FU is yet to be ascertained.7,8
Survivin is a protein screened and cloned in the human genome by Ambrosini et al. of Yale University in 1997.9 It contains a single N-terminal baculovirus IAP repeat sequence and has a unique zinc finger structure. It is an apoptotic inhibitor protein and the smallest member of the family.10 It is highly expressed in most tumor tissues and fibrotic diseases11,12 and can inhibit apoptosis, promote proliferation and blood vessel growth, and enhance invasion.13,14 Yu-xue Xu et al.15 found that survivin is highly expressed in pterygium tissue epithelial cells and can promote the proliferation of pterygium tissue cells and aggravate the growth of pterygium. Survivin has become a prognostic marker of cell hyperproliferative diseases. FOXP1 belongs to the FOXP family of transcription factors, among which FOXP1, FOXP2, and FOXP4 are involved in the development of the lungs, intestines, heart, and central nervous system.16 FOXP1 plays a significant role in regulating gene transcription. According to our research results, FOXP1 may be involved in the process of fibroblast proliferation, resulting in scarring as well as embryo development, immune system regulation, and cancer progression,17,18 in which FOXP1 can regulate B lymphocyte development.19 At the same time, FOXP1 can enhance the activity of breast cancer cells and more.20 Studies have found that FOXP1 is lowly expressed in degenerated tendon tissues. Highly expressed FOXP1 inhibits the apoptosis of tendon cells and promotes cell proliferation and differentiation.21 Kamran et al. determined that the expression of survivin is regulated by the transcription factor FOXP1 during the proliferation of gastric cancer tumor cells.22
Therefore, to explore whether survivin plays an important role in the pathological process of scarring in glaucoma surgery and to explore its action mechanism, this study used TGF-β to induce the proliferation of human Tenon's capsule fibroblasts (HTFs) to simulate the proliferation of cells during scar formation after glaucoma filtering and to explore survivin. The role and mechanism in this pathological process provides new ideas for the prevention and targeted therapy of scars after glaucoma filtration.
Materials and Methods
Cell Culture
With the approval of the Ethics Committee of the Third Affiliated Hospital of Zunyi Medical University and the informed consent of the patients and their families, the Tenon's capsule tissues of 4 patients with glaucoma filtration surgery were put into 15% fetal bovine serum (FBS; Gibco, USA), and blue chain cells were obtained by culturing in DMEM culture medium of DMEM (Beijing Soleibao Company, China).
CCK-8
To conduct cell pretreatment according to the experimental groups, add 10 µL CCK-8 to each well, incubate at 37°C for 4 hours, and measure the absorbance OD 450 of each well with a microplate reader.
Hematoxylin and Eosin Staining
The cells were fixed with 4% paraformaldehyde for 15 minutes, and Mayer hematoxylin (H9627; Sigma, Shanghai, China) was added dropwise and stained on the coverslip for 5 minutes with 1% water-soluble eosin (71014544; Sinopharm Group, Beijing, China). After staining for 2 to 4 minutes, the slides were mounted with neutral gum (10004160; Sinopharm Group) after treatment with absolute ethanol (10009218; Sinopharm GroupChina) and xylene (10023418; Sinopharm Group).
Immunohistochemistry
Fix the cells with 4% paraformaldehyde for 15 minutes, 0.5% Triton X-100 (prepared in phosphate-buffered saline [PBS]) for 20 minutes at room temperature, and drop 3% hydrogen peroxide on the slide to block endogenous peroxidase, then incubate at room temperature for 15 minutes, add the diluted normal goat serum dropwise, block at room temperature for 30 minutes, add a sufficient amount of diluted primary antibody, and put it in a humid box, incubate overnight at 4°C, add dropwise HRP-labeled goat anti-rabbit/mouse secondary antibody, incubate at room temperature or 37°C for 30 minutes, add freshly prepared DAB color developing solution, Harris hematoxylin counterstain for about 2 minutes, and put the slides in 70% alcohol-80% alcohol-90% alcohol-95% alcohol-none. Water ethanol Ⅰ-anhydrous ethanol Ⅱ-xylene Ⅰ-xylene Ⅱ was dehydrated and transparent and placed in each reagent for 2 minutes. Finally, the slides were air-dried in the fume hood, neutral gum drops were placed on the slides to seal them, and the images were observed or collected under a microscope.
Immunofluorescence
The cells were fixed with 4% paraformaldehyde for 15 minutes, 0.5% Triton X-100 (prepared in PBS) was permeated for 20 minutes at room temperature, normal goat serum was added dropwise to the slide, and adequate diluted primary antibody was added dropwise and placed. Then put the cells into the wet box, add the diluted fluorescent secondary antibody dropwise, add DAPI dropwise, and incubate for 5 minutes in the dark, mount the slide with the mounting solution containing anti-fluorescence quencher, and then observe and collect the image under a fluorescence microscope.
Scratch Test
Cells were pretreated according to experimental groups. Use a pipette tip to compare with a ruler for effective hanging to the horizontal scratch on the back, wash the cells with PBS three times, remove the marked cells, add serum-free medium, and take a 0 hour photograph simultaneously. Cultivate for 24 hours to take pictures.
Quantitative Polymerase Chain Reaction
The Trizal method was used to extract total RNA, and cDNA was synthesized according to the operating instructions of PrimeScript RT kit (Takara, Dalian, China), and Primer version 5.0 software was used to design specific polymerase chain reaction (PCR) primer sequences. Following the manufacturer's instructions, real-time polymerase chain reaction (RT-PCR) was performed using a real-time fluorescent quantitative PCR instrument (ABI, QuantStudio 6).
Primers used in RT-PCR
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| Homo GAPDH | Forward | TCAAGAAGGTGGTGAAGCAGG |
| Reverse | TCAAAGGTGGAGGAGTGGGT | |
| Homo TGF-β1 | Forward | CAGCAACAATTCCTGGCGATACCT |
| Reverse | CGCTAAGGCGAAAGCCCTCAAT | |
| Homo Survivin | Forward | CCCTTTCTCAAGGACCACCGCATCT |
| Reverse | CTCGTTCTCAGTGGGGCAGTGGATG | |
| Homo α-SMA | Forward | TCATGGTCGGTATGGGTCAG |
| Reverse | CGTTGTAGAAGGTGTGGTGC | |
| Homo FN | Forward | AGCGAGCATCCCCCAAAGTT |
| Reverse | GGGCACGAAGGCTCATCATT | |
| Homo Collagen I | Forward | TGGAGAGGAAGGAAAGCGAG |
| Reverse | ACCAGCTTCACCAGGAGATC | |
| Homo FOXP1 | Forward | GCATCTCATAAACCATCAGCCCTCT |
| Reverse | ATCTTCGTCTCAGCAACTGCTCC |
Western Blotting Analysis
Lyse with PMSF (ST506; Biyuntian, Shanghai, China) lysis buffer (1 mL lysis buffer plus 10 µL PMSF) for 30 minutes, centrifuge at 12,000 rpm at 4°C for 5 minutes, and determine OD568 with a DG-3022A microplate reader following the manufacturer's operating requirements. Then, calculate the protein concentration. The extracted protein supernatant was mixed with a 5 times protein loading buffer (mixed by volume ratio 4:1) for protein denaturation. Put the protein sample in 5% concentrated gel (water, 30% acrylamide Gel preparation in 1.0 Mtris-HCl [pH 6.8], 10% SDS, APS, and TEMED), electrophorese in 6%, 10%, and 12% separation gels, respectively, and perform on PVDF membrane (IPVH00010; Millipore, Burlngton, MA, USA). Transfer the membrane, add the corresponding primary antibody for incubation, react with the HRP-labeled secondary antibody, and perform color exposure.
Statistical Analysis
SPSS version 18.0 software was used for data processing. All measurement data of the experimental data were tested by PP chart to be normal distribution, expressed as mean ± standard deviation (x ± s). The sample mean of each group was verified by Levene's test to confirm the variance's uniformity. The independent sample t-test was used to compare the means. Graphad Prism version 8.0 was used for graph creation, and P < 0.05 is considered statistically significant.
Results
Cell Culture and Identification
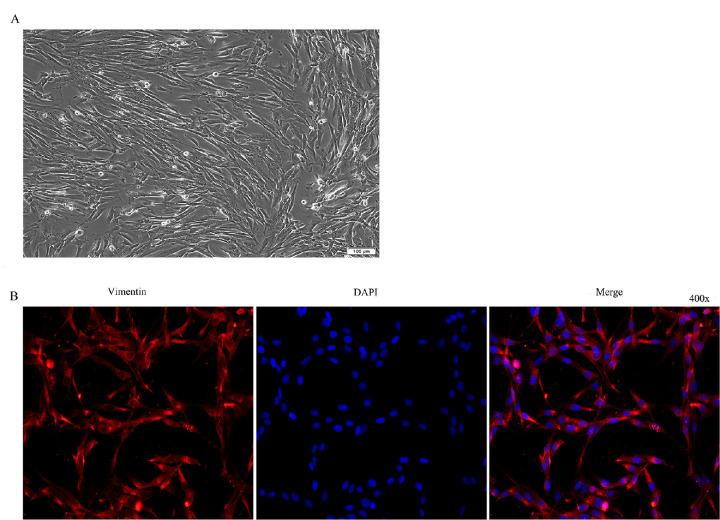
To identify the cultured cells as HTFs, the cells were observed by morphology and identified by immunofluorescence. The cultured cells were long fusiform with large nucleolus and positive expression of vimentin antibody, indicating that the cultured cells were HTFs (Fig. 1).
Figure 1.
Culture and identification of HTFs. (A) Morphological observation of primary cultured cells of HTFs (100 × inverted microscope). (B) Immunofluorescence identification (400 × inverted fluorescence microscope). Note: Fluorescence (Cy3) labeled goat anti-rabbit IgG labeling (red), DAPI staining (blue).
Survivin Expression in HTFs Treated With TGF-β1
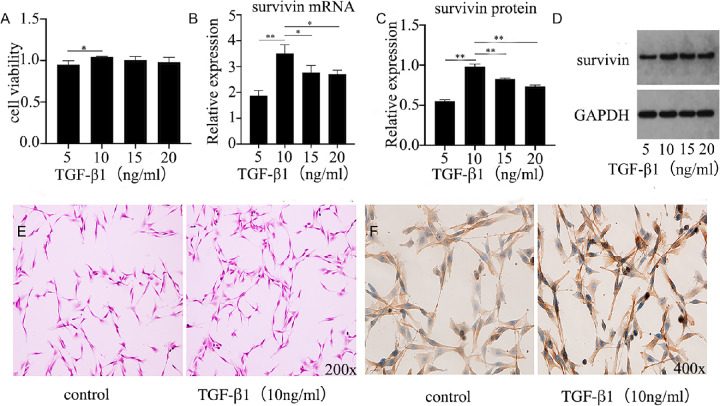
The experiment used different concentrations of TGF-β1 (5, 10, 15, and 20 ng/mL) to act on HTFs for 48 hours to simulate the process of scar formation after glaucoma filtration. The growth status of cells are found in the middle, take them and explore the expression of survivin and related mechanisms. The experiment first used the CCK-8 method to study the proliferation activity of cells controlled by different concentrations of TGF-β1 (Fig. 2A). The results showed that the proliferation activity of the cells was the most significant at a concentration of 10 ng/mL. This result supports those of previous researchers.23 Concurrently, at this concentration, the expression of survivin mRNA and protein was also the most significant. Compared with other concentrations (5, 15, and 20 ng/mL), the difference was statistically significant (P < 0.05) (Figs. 2B–D). It is suggested that survivin may contribute to the proliferation of HTFs. Meanwhile, to further study the cell morphology and localization expression of survivin protein during the proliferation of HTFs, the staining and immunohistochemistry were performed on HTFs treated with or without 10 ng/mL TGF-β1 (Figs. 2E–F). The results showed that the two groups of cells were long fusiform, with large nucleoli and swirling arrangements, suggesting that there was no significant difference in cell morphology during the proliferation of HTFs. Survivin protein was expressed in both the nucleus and cytoplasm, mainly in the cytoplasm. Survivin protein was more expressed in the presence of TGF-β1, suggesting that survivin produces more protein in the cytoplasm and participates in the proliferation process of HTFs.24 Survivin is expressed in both cytoplasm and mitochondria; nuclear survivin is involved in regulating cell division, and cytoplasmic survivin is involved in inhibiting apoptosis.
Figure 2.
TGF-β1 promoted the proliferation and survivin expression of HTFs. (A) Proliferation activity of HTFs at different concentrations of TGF-β1. (B–D) Expression of survivin mRNA and WB under different concentrations of TGF-β1. The data provided are mean ± SD for three independent experiments. *P < 0.05, **P < 0.01. (E) H&E staining observation of HTFs. (F) Immunohistochemical observation of HTFs.
Toxicity of Survivin Inhibitor YM155 Against HTFs
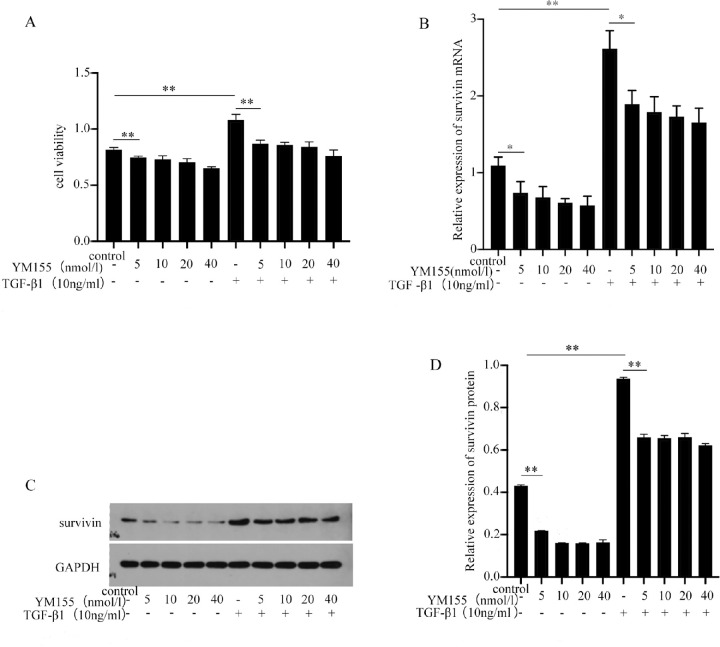
Survivin inhibitors are widely used. The first confirmed survivin inhibitor, YM155, can target the survivin promoter, inhibit the transactivation of survivin, and reduce the expression of survivin protein.25,26 We have confirmed that the proliferation activity and survivin expression of cells were most significant for 10 ng/mL TGF-β1 (Fig. 3A). In addition, different concentrations of YM155 were used to target survivin inhibition to observe cell proliferation activity and survivin expression. To investigate the cytotoxicity of YM155 to HTFs, different concentrations of YM155 (5, 10, 20, and 40 nmol/L) were used for HTFs culture. Compared with the blank control group, the growth activity of HTFs and the expression of survivin mRNA and protein levels were significantly decreased, with statistical significance (P < 0.05) (Figs. 3B–D). No statistical significance was found when the experimental groups were compared with different concentrations of YM155 (P > 0.05). However, with the increase in YM155 concentration, cell proliferation activity and survivin expression were also decreased. These results suggest that YM155 may have a certain toxic effect on the growth of HTFs, and that its toxic effect increases with the increase in drug concentration. Concurrently, the effects of different concentrations of YM155 on the proliferation activity and survivin expression of HTFs were also investigated. However, compared with the blank control group, the proliferation activity and survivin expression level of HTFs were significantly expressed in the proliferation process, and the differences were statistically significant (P < 0.05). The trend of the results between the experimental groups agreed with the trend of YM155 cytotoxicity. These results indicate that YM155 can inhibit the proliferation activity and expression of survivin mRNA and proteins in HTFs. However, when the YM155 concentration exceeds 5 nmol/L, the effect of YM155 is nonsignificant. Following the above results, 5 nmol/L YM155 was selected for the next experiment.
Figure 3.
YM155 inhibited the proliferation and survivin expression of HTFs. (A) Different concentrations of YM155 inhibited the proliferation of HTFs (induced with or without TGF-β1). (B–D). Different concentrations of YM155 inhibited survivin mRNA and Wb expression (induced with or without TGF-β1). The data provided are mean ± SD for three independent experiments. *P < 0.05, **P < 0.01.
Effects of Survivin Inhibitor YM155 on TGF-β1-Treated HTFs
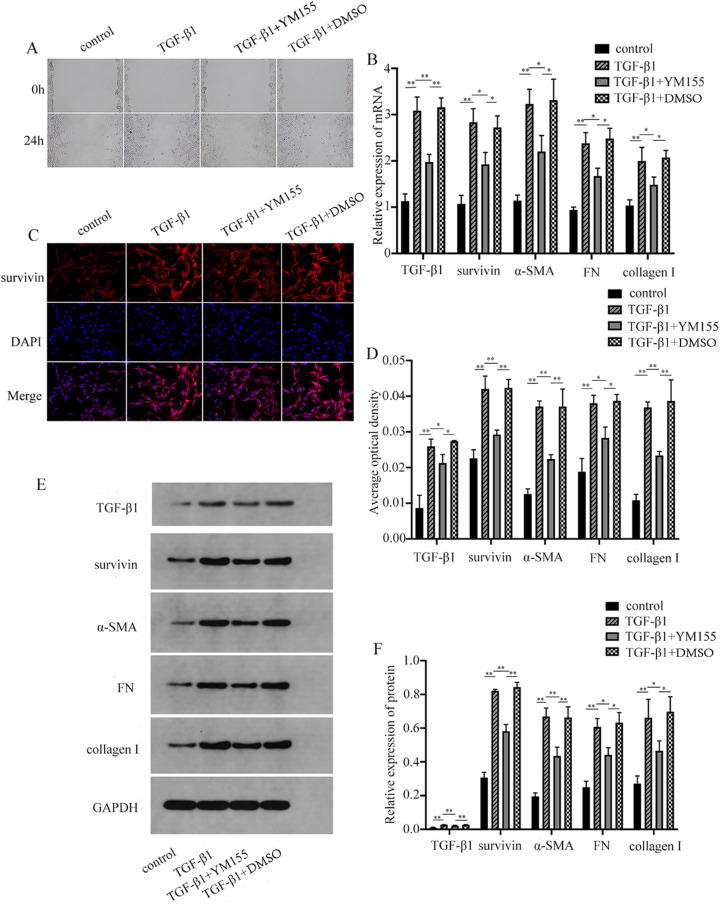
Cell migration was detected by the scratching test (Fig. 4A). The mRNA and protein levels of TGF-β1, survivin, α-SMA, collagen I, and FN were detected by PCR, immunofluorescence, and Western blot (WB) (Figs. 4B–F). The results showed that the cell migration ability of the TGF-β1 group was upregulated, whereas YM155 could inhibit the migration of HTFs, and YM155 had no effect on the cell migration ability. Meanwhile, compared with the blank control group, the mRNA and protein levels of TGF-β1, survivin, α-SMA, collagen I, and FN in the TGF-β1 group were increased, and the differences were statistically significant (P < 0.05). The mRNA and protein levels of TGF-β1, survivin, α-SMA, collagen I, and FN were all decreased, and the differences were statistically significant (P < 0.05). YM155 had no significant effect on the experimental results, and the differences were statistically insignificant (P > 0.05). It was suggested that YM155 could downregulate the migration ability of HTFs and the expressions of TGF-β1, survivin, α-SMA, collagen I, and FN. In this study, HTFs were treated with TGF-β1 to simulate the cell growth process in the scar formation process after glaucoma filtration. After the survivin inhibitor YM155 was used to treat HTFs, the expression of TGF-β1 mRNA and protein levels were also reduced, indicating that YM155 could not only inhibit cell proliferation but also inhibit cell migration. Downregulation of survivin expression also interferes with TGF-β1 expression. These results suggest that the proliferation of HTFs is due to the interaction between TGF-β1 and survivin. This agrees with the conclusion of previous researchers.27 Concurrently, recent studies have found that YM155 is a DNA destructive agent, and survivin inhibition is only a consequence of transcriptional inhibition,28 so YM155 may not be a specific inhibitor of survivin alone.
Figure 4.
YM155 inhibited the migration of HTFs and the expression of TGF-β1, survivin, α-SMA, collagen I, and FN. (A) YM155 inhibited the migration of HTFs. YM155 inhibited the expression of TGF-β1, survivin, α-SMA, FN, and collagen I. (B) RT-PCR. (C–D) Immunofluorescence. (E, F). (B) The data provided are mean ± SD for three independent experiments. *P < 0.05, **P < 0.01.
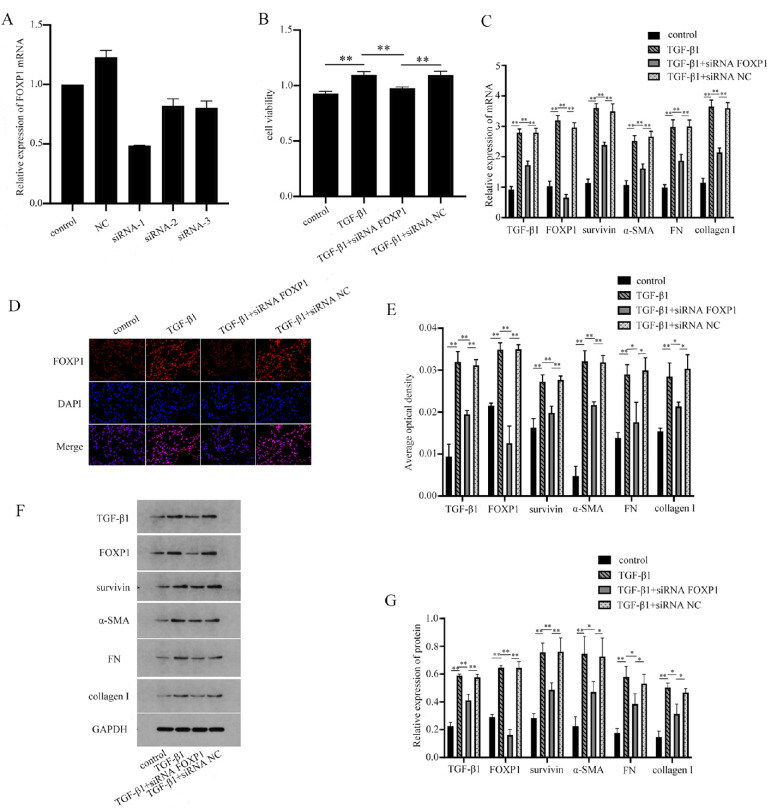
FOXP1 Plays a Role in TGF-β1-Treated HTFs Through Survivin
The proliferation activity of the cells was detected by CCK-8, and the mRNA and protein expressions of TGF-β1, FOXP1, survivin, α-SMA, collagen I, and FN were detected by PCR, immunofluorescence, and WB. Compared with the control group, both the cell proliferation activity and the mRNA and protein levels of TGF-β1, FOXP1, survivin, α-SMA, collagen I, and FN in the TGF-β1 group were increased, with statistical significance (P < 0.05) (Fig. 5). The siRNA FOXP1 inhibited the proliferation of TGF-β1-induced cells and the mRNA and protein expressions of TGF-β1, FOXP1, survivin, α-SMA, collagen I, and FN. These results suggest that transcription factor FOXP1 contributes to HTFs cell proliferation through survivin.
Figure 5.
FOXP1 contributes to HTFs cell proliferation through survivin. (A) The results showed that siRNA-1 had the highest knockout efficiency. (B–G) The siRNA FOXP1 inhibited the proliferation of TGF-β1-induced cells and the mRNA and protein expressions of TGF-β1, FOXP1, survivin, α-SMA, collagen I, and FN. *P < 0.05, **P < 0.01.
Discussion
In the study of glaucoma, the purpose of glaucoma filtering surgery is to establish an effective drainage channel of aqueous humor under the conjunctiva to reduce the increase in intraocular pressure. However, postoperative TGF-β (TGF-β1, TGF-β2, and TGF-β3) high expression,29,30 HTFs proliferation, and migration,31 fibroblasts transformed into myofibroblasts,32 and accompanying the deposition of extracellular matrix proteins induces failure of the operation. Recent studies have found that survivin is also involved in the general process of wound healing.33 Because of its dual effects of promoting the cell cycle and inhibiting apoptosis, it is expressed in most tumor cells, such as non-small cell lung cancer,34 hepatocellular carcinoma,35 esophageal squamous cell carcinoma,36 colorectal cancer,37 and bladder cancer.38 In our study, survivin was seen to promote fibroblast proliferation and accelerate scar formation (Fig. 4).39 In other studies, survivin is also involved in tumor formation, posterior cataract,40 and neovascularization in proliferative diabetic retinopathy.41 It has become a predictive marker for diagnosis efficacy and also contributes to the process of vascular intimal hyperplasia and angiogenesis.42 Some researchers also cultured fibroblasts from the skin of patients with systemic sclerosis (SSC) and healthy patients, and PCR examination indicated that survivin mRNA expression in fibroblasts of patients with SSC was significantly increased,43 suggesting that survivin may play a certain role during cell fibrosis. Some researchers have found the expression of survivin in ocular fibroblast diseases, such as proliferative vitreoretinopathy44 and pterygium.45,46 Following these studies, it is indicated that survivin may be involved in the scar formation process after glaucoma filtering surgery. However, there are currently few studies on the expression of survivin during the scar formation process after glaucoma filtering surgery. Therefore, the expression of survivin in the proliferation of HTFs was first discussed in this study. The results showed that survivin was expressed in HTFs, and the expression of survivin increased with an increase in cell proliferation during the proliferation process, which was also accompanied by the high expression of extracellular matrix protein and α-smooth muscle actinin. In this experiment, the commonly used survivin inhibitor YM155 was used to inhibit survivin. Considering the possible toxic effects of YM155, different concentrations of YM155 were used in the experiment. Observably, 5 nmol/L YM155 could reduce the proliferation activity of cells (Fig. 3).47 The research results are distinct, but previous studies have evaluated the role of YM155 in tumors. This study shows that when YM155 is used in different cells, the sensitivity of the cells to drugs should be considered. Combined with recent studies, YM155 is a DNA inhibitor,48 and the inhibition of survivin is only the result of an event.49 It shows that YM155 is not a specific inhibitor of survivin, which also agrees with the YM155 cytotoxicity test results in this experiment.
In prior research on survivin, some researchers used small interfering RNA (siRNA) to interfere with survivin, but the lack of a suitable siRNA delivery carrier for siRNA seriously hinders the application of targeted survivin in clinical work.50 This experiment tried to find and verify the upstream transcription factor of survivin. In previous studies, the expression of survivin was regulated by the transcription factor FOXP1 during the proliferation of gastric cancer tumor cells.51
This is an exploratory study that suggests increased survivin in scarring after glaucoma surgery. This study shows that targeting inhibition or overexpression of FOXP1 can downregulate or upregulate survivin expression, thereby regulating the proliferation and migration of HTFs and the expression of extracellular matrix (Fig. 5). The external experimental data showed that during scar formation after glaucoma filtration, the effect of survivin could be inhibited by the influence on FOXP1 to reduce the chance of scar formation of the filtration bubble, improve the success rate of surgery, and bring more benefits to patients. Therefore, we suggest that FOXP1 and survivin may be important therapeutic targets for postoperative scar formation in glaucoma. However, this study was conducted to explore the functional and molecular mechanism of FOXP1 through survivin in scar formation after glaucoma filtration in an in vitro cell experiment, which should be further verified in vivo, and its clinical applicability studied.
Acknowledgments
Supported by The National Natural Science Foundation Project (82160200); Science and technology Fund project of Guizhou Provincial Health Commission (gzwjkj2020-1-155 and gzwkj2021-330); and Science and Technology Fund project of Zunyi (2020) No. 4, HZ(2021)241; R&D Program of The First People's Hospital of Zunyi (2020) No. 4.
Disclosure: X. Wang, None; Z. Huang, None; L. Zeng, None; X. Jin, None; A. Yan, None; Y. Zhang, None; W. Tan, None
References
- 1. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 2. Elisa B. Glaucoma treatment. La Revue du praticien. 2016; 66: 508–513. [PubMed] [Google Scholar]
- 3. Sharma A, Anumanthan G, Reyes M, et al.. Epigenetic Modification Prevents Excessive Wound Healing and Scar Formation After Glaucoma Filtration Surgery. Invest Ophthalmol Vis Sci. 2016; 57: 3381–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holló G. Wound Healing and Glaucoma Surgery: Modulating the Scarring Process with Conventional Antimetabolites and New Molecules. Dev Ophthalmol. 2017; 59: 80–89. [DOI] [PubMed] [Google Scholar]
- 5. Schlunck G, Meyer-Ter-Vehn T, Klink T, et al.. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res. 2016; 142: 76–82. [DOI] [PubMed] [Google Scholar]
- 6. Cabourne E, Clarke JC, Schlottmann PG, et al.. Mitomycin C versus 5-Fluorouracil for wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2015; 11: 6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng JW, Cheng SW, Wei RL, et al.. Anti-vascular endothelial growth factor for control of wound healing in glaucoma surgery. Cochrane Database Syst Rev. 2016; 1: CD009782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mead AL, Wong TTL, Cordeiro MF, et al.. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003; 44: 3394–3401. [DOI] [PubMed] [Google Scholar]
- 9. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997; 3: 917–921. [DOI] [PubMed] [Google Scholar]
- 10. Altieri DC. Survivin—The inconvenient IAP. Seminars in Cell Develop Biol. 2015; 39: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groner B, Weiss A.. Targeting Survivin in Cancer: Novel Drug Development Approaches. BioDrugs. 2014; 28: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaiswal PK, Goel A, Mittal RD. Survivin: A molecular biomarker in cancer. Indian J Med Res. 2015; 141: 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui X, Shen D, Kong C, et al.. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci Rep. 2017; 7: 40723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antonio CCH, Huang CC, Fang-Ying T, et al.. Survivin—biology and potential as a therapeutic target in oncology. Oncotargets & Ther. 2013; 6: 1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu YX, Zhang LY, Zou DL, et al.. Differential expression and function of survivin during the progress of pterygium. Invest Ophthalmol Vis Sci. 2014; 55: 8480–8487. [DOI] [PubMed] [Google Scholar]
- 16. Klimenko OV. Regulation of immune responses, apoptosis, and tumorigenesis by separate FOXP-3-dependent genes: connection with clinical manifestations. J Microbiol Immunol Infect. 2011; 44: 412–417. [DOI] [PubMed] [Google Scholar]
- 17. Brown PJ, Wong KK, Felce SL, et al.. FOXP1 suppresses immune. response signatures and MHC class II expression in activated B-cell-like diffuse large B-cell lymphomas. Leukemia. 2016; 30: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koon Henry B, Ippolito Gregory C, Banham Alison H, et al.. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets, 2007; 11: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gascoyne Duncan M, Banham Alison H. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk Lymphoma. 2017; 58: 1037–1051. [DOI] [PubMed] [Google Scholar]
- 20. Nobuhiro I, Kazuhiro I, Kuniko H-I, et al.. FOXP1 and estrogen signaling in breast cancer. Vitam Horm. 2013; 93: 203–212. [DOI] [PubMed] [Google Scholar]
- 21. Xu H, Liu F. Downregulation of FOXP1 correlates with tendon stem/progenitor cells aging. Biochem Biophys Res Commun. 2018; 504: 96–102. [DOI] [PubMed] [Google Scholar]
- 22. Kamran M, Long ZJ, Xu D, et al.. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis. 2017; 6: e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu H, Dai L, Li X, et al.. Role of the long noncoding RNA H19 in TGF-β1-induced Tenon's capsule fibroblast proliferation and extracellular matrix deposition. Exp Cell Res. 2019; 387: 111802. [DOI] [PubMed] [Google Scholar]
- 24. Li F, Yang J, Ramnath N, et al.. Nuclear or cytoplasmic expression of survivin: what is the significance. Int J Cancer. 2005; 114: 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakahara T, Takeuchi M, Kinoyama I, et al.. YM155, a Novel Small-Molecule Survivin Suppressant, Induces Regression of Established Human Hormone-Refractory Prostate Tumor Xenografts. Cancer Res. 2007; 67: 8014–8021. [DOI] [PubMed] [Google Scholar]
- 26. Miyao T, Koike H, Sekine Y, et al.. YM155 Reverses Cabazitaxel Resistance in Castration-resistant Prostate Cancer by Reducing Survivin Expression. Anticancer Res. 2020; 40: 5091–5095. [DOI] [PubMed] [Google Scholar]
- 27. Wang YF, Ma SR, Wang WM, et al.. Inhibition of Survivin Reduces HIF-1α, TGF-β1 and TFE3 in Salivary Adenoid Cystic Carcinoma. PLoS One. 2014; 9: e114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glaros TG, Stockwin LH, Mullendore ME, Smith B, Morrison BL, Newton DL. The “survivin suppressants” NSC 80467 and YM155 induce a DNA. damage response. Cancer Chemother Pharmacol. 2012; 70: 207–212. [DOI] [PubMed] [Google Scholar]
- 29. Cordeiro MF, Reichel MB, Gay JA, et al.. Transforming growth factor-beta1, -beta2, and -beta3 in vivo: effects on normal and mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis Sci. 1999; 40: 1975–1982. [PubMed] [Google Scholar]
- 30. Cordeiro MF, Chang L, Lim KS, et al.. Modulating conjunctival wound healing. Eye. 2000; 14: 536–547. [DOI] [PubMed] [Google Scholar]
- 31. Lama PJ, Fechtner RD. Antifibrotics and Wound Healing in Glaucoma Surgery . Surv Ophthalmol. 2003; 48: 314–346. [DOI] [PubMed] [Google Scholar]
- 32. Hinz B, Mastrangelo D, Iselin CE, et al.. Mechanical Tension Controls Granulation Tissue Contractile Activity and Myofibroblast Differentiation. Am J Pathol. 2001; 159: 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh P, Fukuda S, Liu L, et al.. Survivin Is Required for Mouse and Human Bone Marrow Mesenchymal Stromal Cell Function. Stem Cells. 2018; 36: 123–129. [DOI] [PubMed] [Google Scholar]
- 34. Reck M, Kugler C, Rabe KF, Ammerpohl O, Goldmann T.. Live and let die: epigenetic modifications of Survivin and Regucalcin in non-small cell lung cancer tissues contribute to malignancy. Clin Epigenetics. 2019; 11: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang S, Zeng C, Wang D, et al.. Efficient induction of cytotoxic T lymphocytes in hepatocellular carcinoma using the HLA-A2-restricted survivin peptide in vitro. Exp Cell Res. 2019; 386: 111741. [DOI] [PubMed] [Google Scholar]
- 36. Zhou Y-X, Liu Q, Wang H, Ding F, Ma YQ. The expression and prognostic value of SOX2, β-catenin and survivin in esophageal squamous cell carcinoma. Future Oncology (London, England). 2019; 15: 4181–4195. [DOI] [PubMed] [Google Scholar]
- 37. Or C, Huang CW, Chang CC, et al.. Obatoclax, a Pan-BCL-2 Inhibitor, Downregulates Survivin to Induce Apoptosis in Human Colorectal Carcinoma Cells Via Suppressing WNT/β-catenin Signaling. Int J Molec Sci. 2020; 21: 1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jazayeri MH, Aghaie T, Nedaeinia R, et al.. Rapid noninvasive detection of bladder cancer using survivin antibody-conjugated gold nanoparticles (GNPs) based on localized surface plasmon resonance (LSPR). Cancer Immunol Immunother. 2020; 69: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garg H, Suri P, Gupta JC, et al.. Survivin: a unique target for tumor therapy. Cancer Cell Intl, 2016; 16: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jarrin M, Mansergh FC, Boulton ME, et al.. Survivin expression is associated with lens epithelial cell proliferation and fiber cell differentiation. Molec Vis, 2012; 18: 2758–2769. [PMC free article] [PubMed] [Google Scholar]
- 41. Liu N, Zhao N, Chen L, et al.. Survivin contributes to the progression of diabetic retinopathy through HIF-1α pathway. Int J Clinic Exp Pathol. 2015; 8: 9161. [PMC free article] [PubMed] [Google Scholar]
- 42. Li W, Du D, Li Y. Id-1 Promotes Reendothelialization In The Early Phase After Vascular Injury Through Activation Of NFkB/survivin Signaling Pathway Drug Design, Development Ther. 2019; 13: 3799–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mahmoudi MB, Farashahi Yazd E, Gharibdoost F, et al.. Overexpression of apoptosis-related protein, survivin, in fibroblasts from patients with systemic sclerosis. Irish J Med Sci. 2019; 188: 1443–1449. [DOI] [PubMed] [Google Scholar]
- 44. Özal SA, Gürlü VP, Türkekul K, et al.. Neferine inhibits epidermal growth factor-induced proliferation and migration of retinal pigment epithelial cells through downregulating p38 MAPK and PI3K/AKT signalling. Cutaneous Ocular Toxicol. 2020; 39: 97–105. [DOI] [PubMed] [Google Scholar]
- 45. Konstantopoulou K, Tsiambas E, Baliou E, et al.. Deregulation of p53/survivin apoptotic markers correlated to PTEN expression in pterygium neoplastic cell. J BUON. 2018; 23: 826–831. [PubMed] [Google Scholar]
- 46. Liu ZG, Xu Y, Zhang LY, et al.. Differential Expression and Function of Survivin During the Progress of Pterygium. Invest Ophthalmol Vis Sci. 2014; 55: 8480–8487. [DOI] [PubMed] [Google Scholar]
- 47. Jane EP, Premkumar DR, Didomenico JD, et al.. YM-155 Potentiates the Effect of ABT-737 in Malignant Human Glioma Cells via Survivin and Mcl-1 Downregulation in an EGFR-Dependent Context. Molec Cancer Ther. 2013; 12: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hong M, Ren MQ, Silva J, et al.. YM155 inhibits topoisomerase function. Anti Cancer Drugs. 2017; 28: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glaros TG, Stockwin LH, Mullendore ME, Smith B, Morrison BL, Newton DL. The “survivin suppressants” NSC 80467 and YM155 induce a DNA damage response. Cancer Chemother Pharmacol. 2012; 70: 207–212. [DOI] [PubMed] [Google Scholar]
- 50. Yang F, Huang W, Li Y, et al.. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials. 2013; 34: 5689–5699. [DOI] [PubMed] [Google Scholar]
- 51. Kamran M, Long ZJ, Xu D, et al.. Aurora kinase A regulates Survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis. 2017; 6: e298. [DOI] [PMC free article] [PubMed] [Google Scholar]