Abstract
Background:
MRI-guided focused ultrasound (FUS) has established short-term efficacy in tremor relief.
Objective:
We report our long-term experience of treating tremor with unilateral FUS unilateral VIM-thalamotomy in tremor dominant Parkinson’s disease (TDPD) patients.
Methods:
We report outcome of FUS thalamotomy in TDPD patients with 1–5 years of follow-up. Outcomes: tremor reduction assessed with Clinical Rating Scale for Tremor (CRST) and Unified Parkinson’s Disease Rating Scale (UPDRS part III) overall and in the treated hemibody and safety.
Results:
Twenty-six TDPD patients completed 1–5 years of follow-up (median follow-up 36 months, range 12–60 months). Median age was 60 years (range 46–79), with median disease duration of 6 years (range 2–16). Immediately, treatment resulted in 100%improvement in tremor in the treated arm in 23 patients and 90%improvement in 3 patients. In 15 patients with leg tremor, 2 patients with chin tremor and 1 patient with head tremor, tremor was significantly improved. Up to 5 years, median CRST score, median UPDRS score, overall and in treated hemibody, decreased significantly as compared with baseline (p < 0.0001). In 2 patients tremor returned completely and in 8 patients there was partial return of tremor. Adverse events were mild and resolved within 3 months. At baseline 4 patients were not receiving any medication vs. 3 at last follow-up and 15 were not taking levodopa vs.9 at last follow-up.
Conclusion:
Unilateral FUS VIM-thalamotomy in TDPD patients was effective and safe and provided long-term tremor relief in most patients. FUS thalamotomy for tremor may delay initiation of levodopa treatment.
Keywords: Parkinson’s disease, treatment, tremor, ultrasound, surgery
INTRODUCTION
Tremor is one of the major hallmarks of Parkinson’s disease (PD), and although it has been recognized as such since James Parkinson described paralysis agitans [1], its pathogenesis is less well understood than other manifestations and it responds only partly to medications such as antimuscarinic or dopaminergic drugs. Tremor in essential tremor (ET) patients, that was not relieved with medication was suppressed by thalamic lesioning, particularly with Focused ultrasound (FUS) VIM-thalamotomy [2–8]. This is lesioning therapy that is quickly gaining popularity as the treatment of choice for severe ET cases and has therefore been studied in tremor dominant PD (TDPD) patients [9–13].
Several groups have reported their short term positive results with this technology, but longer follow up data are not yet available. We wish to report our experience with 26 consecutive TDPD patients followed up for 1–5 years.
METHODS
Patients
TDPD patients, who regarded their arm tremor as disabling in daily life activities, were treated with FUS VIM-thalamotomy at Rambam Health Care Campus, Haifa, Israel. Patients were eligible for FUS only after a Movement Disorders Neurologist (IS or MN) verified that they were given optimal pharmacotherapy for tremor relief. Patients who refused to take levodopa or could not tolerate it, were also eligible for FUS. Patients who suffered from motor fluctuations and had tremor in the “on” state were eligible for therapy while those with tremor mostly in the “off” state were offered other treatment options and were not eligible for FUS.
Contraindications for the procedure included, current anticoagulant or anti-aggregant therapy, brain tumors, vascular brain malformations, unstable medical conditions, and contraindications for MRI, including claustrophobia.
All patients were offered DBS, when eligible, or FUS, and those who preferred FUS are included in this report.
Assessments
The treatment aim was to improve daily function that was disrupted by arm tremor and could not be controlled with medication. Patients who were taking medication, were examined at baseline after taking their morning medications, in the “on” state. Patients were examined on the day before the procedure and during follow-up visits at 1 month, 6 months, 1 year, and yearly thereafter. The data from was collected prospectively as part of an open label study.
Changes in tremor were assessed using the Clinical Rating Scale for Tremor (CRST) [14] (ranging from 0 to 156 with higher scores indicating more severe tremor). Changes in the treated hemibody were assessed using the hemi-CRST (items 5–6, 8–9, 11–15, ranging from 0 to 36). Re-emergent tremor was rated as rest tremor. Changes in motor function were assessed by the Unified Parkinson’s Disease Rating Scale (UPDRS) part III (ranging from 0 to 108 with higher scores indicating worse motor function) and in the treated hemibody with hemi-UPDRS (Items 20–26, ranging from 0 to 28). Additionally, a PDQ39 QoL questionnaire was used to assess QoL (ranging from 0 to 156 with higher scores indicating worse QoL). UPDRS and CRST were performed by a Movement Disorders Neurologist (IS or MN) and the PDQ39 questionnaire was completed by the patient.
Adverse events were documented by the neurologists after a thorough neurological examination and rated according to the Clavien-Dindo criteria (range 1 to 5, higher scores representing more severe events) [15].
FUS treatment
In all patients the primary aim of treatment was to relieve arm tremor. In patients with other bothersome tremors such as leg, chin, and head tremor, we aimed to relieve these tremors as well as described previously [5, 16]. In brief, FUS thalamotomy of the VIM nucleus was performed in the MRI suite using a 3-Tesla MRI (MR750, GE) and a helmet like device, ExAblate Neuro® (Software version 7.0, 650-kHz system, Insightec). The treatment comprised of multiple sonications, where ultrasound waves were focused on a predefined small target inside the brain repeatedly for different lengths of time while gradually increasing the energy. Heating the target created a lesion. The target chosen by our team for sonication was the VIM nucleus of the thalamus with the initial target coordinates located anterior to the PC at 25%of the AC-PC length and 14 mm lateral to the AC-PC line. When there was third ventricle enlargement, the initial target was midway between 14 mm lateral to the midline and 11.5 mm lateral to the third ventricle wall. When the leg tremor, chin tremor and head tremor were targeted, adjustments were made according to the homunculus of the VIM [17].
Skull density ratio (SDR) was calculated to assess ultrasound permeability through the skull. All patients had an SDR of 0.30 or above which was considered by the treatment team suitable for treatment [18].
This study was approved by the center’s institutional review board.
Statistical analysis
Differences in CRST, UPDRS, hemi-CRST, hemi-UPDRS, and PDQ-39 scores at each follow-up time-point were calculated as simple individual score differences at that time minus the pretreatment score. Therefore, negative scores indicate improvement. Percent changes at each landmark follow-up time point for CRST, were calculated as difference in scores after versus before the treatment, divided by the score baseline score, multiplied by 100. Most of these changes were not normally distributed and had high degrees of kurtosis and/or skewness. Therefore, median changes and their ranges are reported along with Wilcoxon signed-rank tests of changes, to evaluate significance of difference from pretreatment. In order to maximize sensitivity in this statistically small cohort, no adjustments were made for multiple comparisons. Correlation between outcome parameters, particularly on related scales such as tremor, should also be considered in evaluating these results. Other analyses employed median tests and Spearman correlation to analyze possible influences of age, duration of disease and treatment parameters on outcome. JMP (SAS Institute, Cary, NC) was used for statistical analyses.
RESULTS
Patients
Between February 2014 and May 2020, 26 TDPD patients underwent unilateral FUS and completed follow-up of 1–5 years. Median follow-up after FUS thalamotomy in these 26 patients was 36 months (range 12 to 60 months). Three patients were lost to follow-up (1 after 12 months and 2 after 24 months).
The median disease duration of TDPD before FUS was 6 years (range 2 to 16 years) with a median Hoehn and Yahr stage of 1.5 (range 1 to 4). Two patients developed PD after having been diagnosed with essential tremor for many years. At the time of thalamotomy patients’ median age was 60 years (range 46 to 79 years), 20 patients were male and 24 were right-handed.
Treatment parameters
SDR ranged from 0.30–0.55 with a median of 0.45. The treatment time (from first to last sonication) was 63–260 minutes (median 132 minutes). We administered a median of 18 sonications (range 12 to 45), with a median maximal sonication time of 17 seconds (range 12–39 seconds). The median maximal energy used was 15,300J (range, 5,850 to 36,520J), which resulted in a median maximal temperature of 57°C (range, 50.0 to 60.0°C).
Tremor and motor assessments
Treatment was designed to alleviate tremor in the dominant arm in 23 patients, 21 of them right-handed while 3 patients chose to treat the left, non-dominant arm due to severe disabling tremor. Treatment resulted in immediate complete cessation of rest tremor and action tremor at the end of the procedure, in the treated arm in 23 patients and 90%improvement in tremor in the remaining 3 patients. In 15 patients with leg tremor (14 right dominant), leg tremor was abolished in 10 patients and reduced by 80%in 5 other patients. In 3 patients with lip tremor and 1 with head tremor, we successfully alleviated these tremors as well.
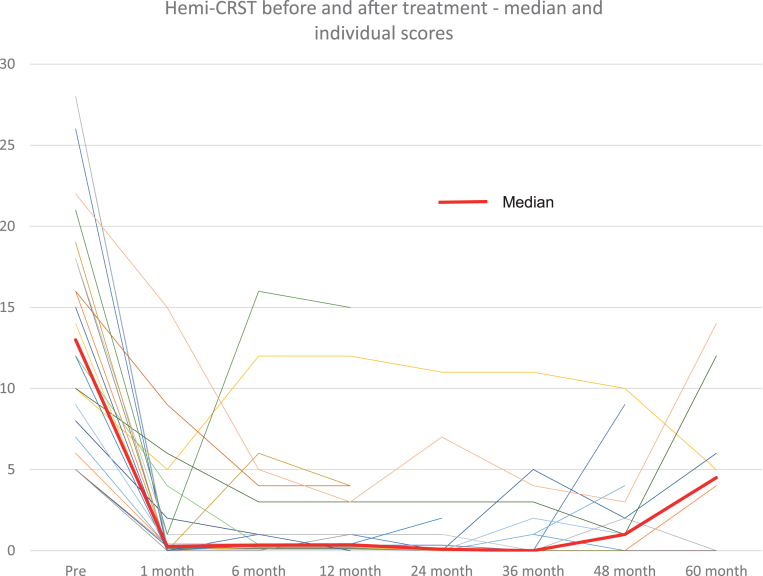
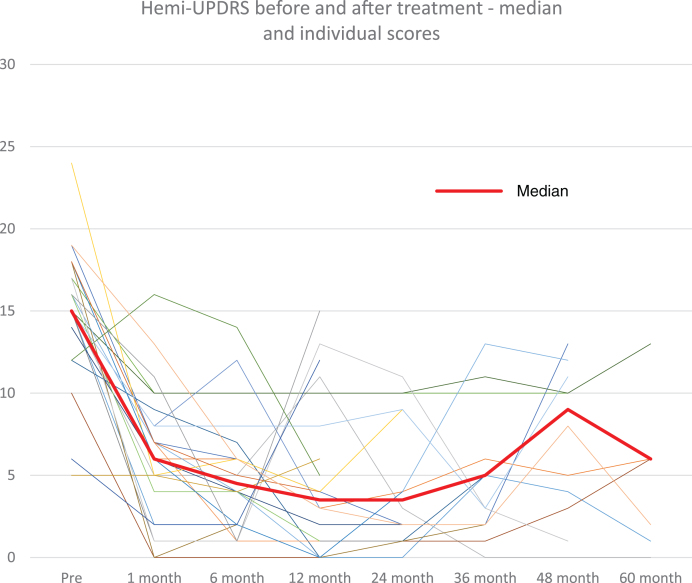
At 1-month post-treatment, the median CRST score decreased by 12 points (n = 37, range –28 to –4 points) from baseline (median 13.0, range 5–28 points, p < 0.0001) (Table 1). The significant tremor suppression persisted during follow-up visits up to 5 years (6 months, n = 22, p < 0.0001; 1 year, n = 23, p < 0.0001; 2 years, n = 15, p < 0.0001; 3 years, n = 15, p = 0.023; 4 years, n = 12, p = 0.012; 5 years, n = 7, p = 0.031) (Table 1). Similarly, at 1 month, the median hemi-CRST decreased by 18 points (range –66-0, p < 0.0001). The hemi-CRST score also remained significantly decreased over time (Table 1, Fig. 1). Of 26 patients, at last follow-up visit 11 At one month, median hemi-UPDRS score decreased by 10 points (range –19-4 points, p < 0.0001) (Table 1). Total UPDRS part III showed similar significant changes as well. (Table 1). The median UPDRS part III and hemi-UPDRS remained significantly decreased over time up to 5 years (Table 1, Fig. 2). Before FUS treatment, the median score for rest tremor, item 20 of the UPDRS was 3 while at last follow-up visit, the median score was 0 (range 0–4) with a score of 0 on this item recorded in 20 patients (77%). The median score before treatment for action tremor, item 21 of the UPDRS was 3 and at last follow-up the median score was 0 (range 0–2), with a score of 0 recorded in 18 patients (69%). Of the 7 patients that have been followed for 5 years, 5 patients still have a score of 0 on items 20 and 6 patients still have a score of 0 on item 21.
Table 1.
Median change of tremor and motor scores before and after FUS
| Baseline (N = 26) | 1 month (N = 26) | 6 months (N = 22) | 12 months (N = 23) | 24 months (N = 15) | 36 months (N = 15) | 48 months (N = 12) | 60 months (N = 7) | |
| UPDRS | 22.5 (9–43) | –10 (–34 to 6) | –16 (–34 to 16) | –18 (–31 to 47) | –15 (–33 to –1) | –16 (–29 to 0) | –10 (–30 to –4) | –19 (–31 to –5) |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.0002 | p = 0.0005 | p = 0.016 | ||
| Hemi–UPDRS | 15 (5–24) | –9 (–19 to 4) | –12 (–18 to 2) | –12 (–20 to 6) | –12 (–17 to 2) | –10 (–17 to 2) | –8 (–16 to –3) | –8 (–17 to –4) |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.0005 | p = 0.016 | ||
| CRST | 20 (9–70) | –15 (–66 to –5) | –17.5 (–64 to –5) | –17 (–63 to –5) | –20 (–69 to –6) | –14 (–70 to 6) | –15 (–54 to 6) | –14 (–54 to 3) |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.015 | p = 0.013 | p = 0.031 | ||
| Hemi-CRST | 13 (5–28) | –12 (–28 to –4) | –13 (–28 to 2) | –13 (–28 to 2) | –14 (–28 to 1) | –13 (–28 to 1) | –14.5 (–28 to 0) | –8 (–28 to 1) |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p = 0.0002 | p = 0.0010 | p = 0.031 | ||
| PDQ39 | 32 (17–79) | –18* (–42 to 27) | –19 (–61 to 9) | –15 (–66 to 11) | –7 (–43 to 15) | –8 (–46 to 24) | –6 (–48 to 29) | –11 (–47 to 24) |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | NS | NS | NS | NS | ||
| Levodopa equivalent | 300 (0–1050) | 0 (–200 to 141) | 0 (–200 to 582) | 0 (–200 to 985) | 66 (–250 to 1676) | 199 (–250 to 1076) | 122.5 (–250 to 1676) | 151 (0 to 1476) |
| NS | NS | NS | NS | p = 0.0049 | p = 0.037 | p = 0.031 |
Baseline scores are reported as raw values. For all other outcome parameters median change from baseline (follow-up –baseline) and range of change are reported. UPDRS is the Unified Parkinson’s Disease Rating Scale part III, Hemi-UPDRS is-the treated hemibody UPDRS, CRST is Clinical Rating Scale for Tremor, and Hemi-CRST is the treated hemibody CRST. Negative values indicate improvement on all scales. p values are based on Wilcoxon signed rank test of changes (follow-up –baseline). *N = 25 for this item.
Fig. 1.
Hemi-CRST score at baseline and at follow-up visits for all patients (thin lines) and median score (thick line).
Fig. 2.
Hemi-UPDRS score at baseline and at follow-up visits for all patients (thin lines) and median score (thick line).
During follow-up, tremor returned on the treated side to the same degree as before the procedure in 1 patient after 5 years (4%). In 5 patients (19%) that initially had complete cessation of tremor after FUS, there was partial return of tremor, which was, however, still less pronounced than before treatment (median reduction of hemi-CRST score compared with baseline in these 8 patients, –10, range –3 to –17)
Statistical analysis revealed that age, duration of disease and SDR did not affect tremor outcome.
Quality of life
Patients’ ratings of their quality of life, assessed by PDQ39 score showed significant improvement after MRgFUS from a median baseline score of 34 (range 17–93) before treatment to a median score of 21 (range 1–78) 1 month after treatment (p < 0.0001). The improvement in quality of life scores were significantly improved at 6 months (median score 22, range 1–83, p < 0.0001) and 1 year (median score 20, range 1–76, p < 0.0001). At later time points the improvement in PDQ39 scores was not statistically significant when compared with baseline.
PD related outcomes
Before FUS, 11 patients received levodopa, 11 patients received symptomatic therapy but not levodopa and 4 patients did not receive any antiparkinsonian medication. One patient suffered from motor fluctuation before FUS. During follow-up 6 more patients that received levodopa before FUS developed motor fluctuations, one of them underwent deep brain stimulation for this complication 24 months after FUS and was lost for further follow-up. Six patients started taking levodopa during follow-up, 5 patients for rigidity (6 months n = 2, 12 months n = 1, 24 months n = 1, 60 months n = 1) and 1 patient for tremor (24 months). Three of these patients developed motor fluctuations.
At last follow-up 9 patients were not taking levodopa, 3 patients that were not taking any antiparkinsonian medications (12 months n = 2, 36 months n = 1) and six patients were not taking other antiparkinsonian medications (12 months n = 1, 24 months n = 1, 36 months n = 1, 48 months n = 3).
Safety
All adverse events appeared during the first week, were mild, Level 1 [15] and resolved within three months. Adverse events during the treatment that resolved included headache (n = 9), vertigo (n = 8), dizziness (n = 3), hand/scalp heat (n = 3), lip/tongue paresthesiae (n = 2), and hand paresthesiae (n = 1). Adverse events that appeared after the procedure and resolved included: objective unsteadiness on tandem gait (n = 5, for 1–4 weeks), subjective unsteadiness of gait (n = 1, for 7 days), arm ataxia (n = 2, for 1–4 weeks), asthenia (n = 2, for 1–4 weeks), mild right hemiparesis (for 3 months), hypogeusia (n = 1, for 3 months) and scalp numbness (n = 1, for one week). None of the adverse events were persistent.
DISCUSSION
In this paper, we confirm that the beneficial effects of FUS already shown by us to be effective for a short period [11] are maintained over several years without demonstrating the development of tolerance or adverse effects. This is not trivial since PD is a progressive neurodegenerative disease and the clinical manifestations are expected to increase over time. In this respect our results are similar to those seen in ET [2–6]. The beneficial effects were recorded by us using the accepted tremor scales but also extended to QoL. The effect on QoL was very marked shortly after the FUS procedure but was reduced later, not due to re-emergence of the tremor but probably because of the accumulation of other PD manifestations, as expected [19].
In our hands FUS had a very small number of AE, less than in other series, probably because of our large experience with the procedure.
In the present paper we have not addressed the effects of FUS on other manifestations of the disease which will be reported separately.
The fact that lesioning the thalamus can suppress tremor in TDPD is by itself not new and has already been demonstrated by Hassler et al. (1954) [20] and selective lesioning of the VIM of the thalamus by Narabayashi and Ohye (1980) [21]. Since the effect is not specific to TDPD it is likely that the thalamus is not involved in the generation of the tremor but rather is part of an efferent pathway to the cortex.
Although the tremor is only part of the motor and nonmotor spectrum of dysfunction in PD, FUS should be looked at as a symptomatic treatment for those PD patients in whom the tremor is functionally disabling. Its main limitation is the fact that it is currently limited to one side, but it’s suppression can allow the use of the dominant hand and thus facilitate activities of daily living. Few reports on bilateral treatments and studies conducted in this field may extend the use of FUS in TDPD [22].
Our study was open labeled, since it is unethical and impractical to conduct such studies in a double blind manner for several years. A shorter double blind study was reported by Bond et al. [10] and found that active treatment was superior to sham procedure at 3 months. The 12-months follow-up was open and only for the active treatment arm and showed efficacy as well.
Another option for treatment of tremor in TDPD is DBS, which has similar effects to FUS [23–25]. Patients that chose to undergo DBS instead of FUS were not included in this report which is an intrinsic selection bias. DBS is advantageous because it also improves other PD symptoms but of course carries the risks involved with invasive intracranial surgery [26]. Therefore, FUS seems to be a good treatment alternative for a subgroup of PD patients, those in whom the tremor is disabling and those who are reluctant to undergo DBS.
A high proportion (58%) of patients treated with FUS did not receive levodopa before the procedure because they refused to try this medication for fear of adverse effects. At last follow-up visit nine patients (35%) were not receiving levodopa and 3 were not taking any medication. Thus, we suggest that treating tremor with FUS may delay the need for levodopa in some patients and thus delay onset of levodopa related adverse events. This observation, if verified, may have major implications on the question of when to begin levodopa treatment in TDPD patients.
Our report has limitations. This study is a prospective open label study and may therefore be biased by collection of data in an unblinded fashion. Placebo effect cannot be completely ruled out but the length of follow-up makes this unlikely. The small number of patients and the drop-out rate may have also influenced our findings. Change in medications over time may have had an effect on tremor as well though patients’ tremor in this cohort did not respond to the medications they were taking prior to the procedure and increasing the dose before the procedure did not improve tremor. It is therefore reasonable to assume, that increasing the dose of these medications after the procedure did not affect tremor as well, except in one patient that started taking levodopa after the procedure for return of tremor and reported improvement. Our study was not aimed to separate the effects of FUS from that of medication. Thus, changes in hemi-UPDRS and UPDRS immediately following FUS can be attributed to the procedure but changes over time should take into account the progressive nature of the disease and the increase in dose of medication. Our study was also not aimed to assess the reason why QoL varied over time. Thus, our explanation can only be viewed as speculative.
In conclusion, unilateral FUS VIM thalamotomy for tremor in TDPD patients is effective, safe and provides long-term tremor relief in most patients. Our results also suggest that FUS thalamotomy may delay levodopa treatment. Additional studies are needed to substantiate our results.
ACKNOWLEDGMENTS
We thank Avi and Ziva Bittan for the funding.
CONFLICT OF INTEREST
Ilana Schlesinger was employed by the Rambam Healthcare Campus, received honoraria for a lecture sponsored by Insightec, and gave expert testimony as a consultant to the Disability Rehabilitation Division of the Israeli Ministry of Defense.
Menashe Zaaroor received honoraria for a lecture sponsored by Insightec and serves as a medical expert in the field of Neurosurgery on medical committees of the Disability Rehabilitation Division of the Israeli Ministry of Defense.
Alon Sinai received funding for travel to an Insightec investigator’s meeting.
Maria Nassar, Marius Constantinescu, and Elliot Sprecher have no conflicts of interest to declare.
REFERENCES
- [1]. Hurwitz B (2014) Urban observation and sentiment in James Parkinson’s Essay on the Shaking Palsy (1817). Lit Med 32, 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, Schwartz M, Hynynen K, Lozano AM, Shah BB, Huss D, Dallapiazza RF, Gwinn R, Witt J, Ro S, Eisenberg HM, Fishman PS, Gandhi D, Halpern CH, Chuang R, Butts Pauly K, Tierney TS, Hayes MT, Cosgrove GR, Yamaguchi T, Abe K, Taira T, Chang JW (2016) A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375, 730–739. [DOI] [PubMed] [Google Scholar]
- [3]. Park YS, Jung NY, Na YC, Chang JW (2019) Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov Disord 34, 727–734. [DOI] [PubMed] [Google Scholar]
- [4]. Halpern CH, Santini V, Lipsman N, Lozano AM, Schwartz ML, Shah BB, Elias WJ, Cosgrove GR, Hayes MT, McDannold N, Aldrich C, Eisenberg HM, Gandhi D, Taira T, Gwinn R, Ro S, Witt J, Jung NY, Chang JW, Rosenberg J, Ghanouni P (2019) Three-year follow-up of prospective trial of focused ultrasound thalamotomy for essential tremor. Neurology 93, e2284–e2293. [DOI] [PubMed] [Google Scholar]
- [5]. Sinai A, Nassar M, Eran A, Constantinescu M, Zaaroor M, Sprecher E, Schlesinger I (2019) Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: A 5-year single-center experience. J Neurosurg 5, 1–8. [DOI] [PubMed] [Google Scholar]
- [6]. Boutet A, Ranjan M, Zhong J, Germann J, Xu D, Schwartz ML, Lipsman N, Hynynen K, Devenyi GA, Chakravarty M, Hlasny E, Llinas M, Lozano CS, Elias GJB, Chan J, Coblentz A, Fasano A, Kucharczyk W, Hodaie M, Lozano AM (2018) Focused ultrasound thalamotomy location determines clinical benefits in patients with essential tremor. Brain 141, 3405–3414. [DOI] [PubMed] [Google Scholar]
- [7]. Miller WK, Becker KN, Caras AJ, Mansour TR, Mays MT, Rashid M, Schwalb J (2021) Magnetic resonance-guided focused ultrasound treatment for essential tremor shows sustained efficacy: A meta-analysis. Neurosurg Rev doi: 10.1007/s10143-021-01562-w. [DOI] [PubMed]
- [8]. Agrawal M, Garg K, Samala R, Rajan R, Naik V, Singh M (2021) Outcome and complications of MR guided focused ultrasound for essential tremor: A systematic review and meta-analysis. Front Neurol 12, 654711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Schlesinger I, Eran A, Sinai A, Erikh I, Nassar M, Goldsher D (2015) MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson’s disease. Parkinsons Dis 2015, 219149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Bond AE, Shah BB, Huss DS, Dallapiazza RF, Warren A, Harrison MB (2017) Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: A randomized clinical trial. JAMA Neurol 74, 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I (2018) Magnetic resonance-guided focused ultrasound thalamotomy for tremor: A report of 30 Parkinson’s disease and essential tremor cases. J Neurosurg 128, 202–210. [DOI] [PubMed] [Google Scholar]
- [12]. Schlesinger I, Sinai A, Zaaroor M (2017) MRI-guided focused ultrasound in Parkinson’s disease: A review. Parkinsons Dis 2017, 8124624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Moosa S, Martínez-Fernández R, Elias WJ, Del Alamo M, Eisenberg HM, Fishman PS (2019) The role of high-intensity focused ultrasound as a symptomatic treatment for Parkinson’s disease. Mov Disord 34, 1243–1251. [DOI] [PubMed] [Google Scholar]
- [14]. Stacy MA, Elble RJ, Ondo WG, Wu SC, Hulihan J (2007) Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord 22, 833–838. [DOI] [PubMed] [Google Scholar]
- [15]. Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Sinai A, Katz Y, Zaaroor M, Sandler O, Schlesinger I (2018) The role of the anesthesiologist during magnetic resonance-guided focused ultrasound thalamotomy for tremor: A single-center experience. Parkinsons Dis 2018, 9764807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Schaltenbrand G, Wahren W (1977) Atlas for Stereotaxy of the Human Brain. Thieme Medical Publishers, Stuttgart, pp, 41–47. [Google Scholar]
- [18]. Chang WS, Jung HH, Zadicario E, Rachmilevitch I, Tlusty T, Vitek S, Chang JW (2016) Factors associated with successful magnetic resonance-guided focused ultrasound treatment: Efficiency of acoustic energy delivery through the skull. J Neurosurg 124, 411–416. [DOI] [PubMed] [Google Scholar]
- [19]. Sperling SA, Shah BB, Barrett MJ, Bond AE, Huss DS, Gonzalez Mejia JA, Elias WJ (2018) Focused ultrasound thalamotomy in Parkinson disease: Nonmotor outcomes and quality of life. Neurology 91, e1275–e1284. [DOI] [PubMed] [Google Scholar]
- [20]. Hassler R, Riechert T (1954) Indikationen und Lokalisationsmethode der gezielten Hirnoperationen [Indications and localization of stereotactic brain operations]. Nervenarzt 25, 441–447. [PubMed] [Google Scholar]
- [21]. Narabayashi H, Ohye C (1980) Importance of microstereoencephalotomy for tremor alleviation. Appl Neurophysiol 43, 222–227. [DOI] [PubMed] [Google Scholar]
- [22]. Gallay MN, Moser D, Rossi F, Pourtehrani P, Magara AE, Kowalski M, Arnold A, Jeanmonod D (2016) Incisionless transcranial MR-guided focused ultrasound in essential tremor: Cerebellothalamic tractotomy. J Ther Ultrasound 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Lin F, Wu D, Yu J, Weng H, Chen L, Meng F, Chen Y, Ye Q, Cai G (2021) Comparison of efficacy of deep brain stimulation and focused ultrasound in parkinsonian tremor: A systematic review and network meta-analysis. J Neurol Neurosurg Psychiatry, doi: 10.1136/jnnp-2020-323656. [DOI] [PubMed]
- [24]. Ravikumar VK, Parker JJ, Hornbeck TS, Santini VE, Pauly KB, Wintermark M, Ghanouni P, Stein SC, Halpern CH (2017) Cost-effectiveness of focused ultrasound, radiosurgery, and DBS for essential tremor. Mov Disord 32, 1165–1173. [DOI] [PubMed] [Google Scholar]
- [25]. Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ (2015) Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord 30, 1937–1943. [DOI] [PubMed] [Google Scholar]
- [26]. Fenoy AJ, Simpson RK Jr. (2014) Risks of common complications in deep brain stimulation surgery: Management and avoidance. J Neurosurg 120, 132–139. [DOI] [PubMed] [Google Scholar]