Abstract
Human immunodeficiency virus type 1 (HIV-1) RNA measurements were evaluated within an externally controlled multilaboratory program. Three external standards (1.5 × 103 to 1.5 × 106 copies/ml) were included in 814 assay runs by four laboratories. Results indicate that HIV-1 RNA levels can be measured with a precision equal to that of the pre-highly active antiretroviral therapy era (standard deviations, ±0.16 to 0.25 log10 units).
The Women's Interagency HIV-1 Study (WIHS), a prospective multicenter study established in August 1993 to carry out comprehensive investigations of the impact of human immunodeficiency virus (HIV) infection on women, utilizes nucleic acid sequence-based amplification technology (NASBA) and NucliSens assays (Organon Teknika Corporation [OTC], Durham, N.C.) to quantify HIV type 1 (HIV-1) RNA (1). Decreasing viral loads resulting from the introduction of highly active antiretroviral therapy (HAART) have necessitated the use of more sensitive HIV-1 RNA assays (17; NucliSens: Synergy of Strengths, OTC pamphlet, OTC, 1997; Amplicor HIV-1 Monitor Assay, Roche pamphlet, Roche Diagnostic Corporation, Durham, N.C., 1999).
Conflicting estimates of the contribution of biological and assay variability to the total variance of consecutive HIV-1 RNA measurements have been reported (2, 9, 11). Findings indicate that approximately two-thirds of total variance is due to biological fluctuation and that one third is due to assay variance (2, 5). The increasing number of multilaboratory-based studies has made differences between laboratories another important issue. A study by Skidmore et al. found high interlaboratory variability, indicating a need for an external quality assurance-quality control system for HIV-1 RNA measurements (15).
The WIHS HIV-1 RNA viral load data are unique because of the long observation period and the homogeneity of material used for the external standards. This report clarifies the key issues of laboratory differences and systematic fluctuations over time that affect the nonbiological variability component of HIV-1 RNA viral load measurements.
In the WIHS, four laboratories (all certified by the Virology Quality Assurance Laboratory [VQA], Rush Presbyterian/St. Luke's Medical Center, Chicago, Ill.) performed HIV-1 RNA testing using three versions of OTC assays and a common set of HIV-1 RNA external standards. The standard consisted of HIV-1-negative plasma spiked with HIV-1 virus (4, 5). NASBA assays (0.1-ml input [NASBA0.1 ml]; lower detection limit [LDL], >4,000 copies/ml) were run with sets of 1.5 × 106, 1.5 × 105, and 1.5 × 104 (∼106.18, 105.18, and 104.18, respectively) HIV-1 RNA copies/ml. The corresponding values for standards for both versions of NucliSens (0.2-ml input; LDL, >400 copies/ml; and 1.0-ml input; LDL, >80 copies/ml) were 1 log unit lower. The manufacturer retrained laboratory technicians prior to the distribution of NucliSens with reformulated enzymes (October 1998).
We used the mean square error from a one-way analysis of variance to obtain estimates for the intra-assay variance of the log10 unit of the absolute copy number for each external standard. We used Bartlett's test to compare variances between labs (14). To evaluate systematic fluctuations over time, we fit smooth curves to scatter plots of all log-transformed results for each external standard using the S-plus lowess function (6, 16). P values indicate the statistical significance of the observed nonlinear trends.
Assay format and input volume.
Table 1 shows overall means and standard deviations (SD) of the log-transformed results of the external standards for each assay-input volume combination. Observed variability was lowest for NucliSens1.0 ml and was similar for NASBA0.1 ml.
TABLE 1.
HIV-1 RNA results from each VQA control-OTC assay combination
| Test (no. of runs) | Mean ± SD (coefficient of variation) of log10-transformed results for indicated VQA control targetsa
|
||
|---|---|---|---|
| 104.18 copies/ml for NASBA and 103.18 copies/ml for NucliSens (lowest range) | 105.18 copies/ml for NASBA and 104.18 copies/ml for NucliSens (middle range) | 106.18 copies/ml for NASBA and 105.18 copies/ml for NucliSens (highest range) | |
| NASBA0.1 ml (474) | 4.16 ± 0.24 (5.8%) | 5.20 ± 0.17b (3.3%) | 6.16 ± 0.17b (2.8%) |
| NucliSens0.2 ml (178) | 3.11 ± 0.34 (10.9%) | 4.17 ± 0.18 (4.3%) | 5.16 ± 0.18 (3.5%) |
| NucliSens1.0 ml (162) | 3.20 ± 0.16 (5.0%) | 4.18 ± 0.11 (2.6%) | 5.15 ± 0.11 (2.1%) |
SD were obtained separately for low-range and combined middle- and high-range results from each assay as the mean square errors from one-way analysis of variance.
Adjusted for the laboratory.
In a retrospective analysis of assay run acceptability, we defined run rejection criteria as (i) two results exceeding the target value by more than 2 SD, (ii) one result exceeding the target value by more than 3 SD, or (iii) an R square of <0.95 (8). Overall rejection rates were 6% for NASBA0.1 ml, 3% for NucliSens0.2 ml, and <1% for NucliSens1.0 ml (data not shown).
Fluctuations in HIV concentrations greater than 0.5 log10 unit have been described as clinically relevant (7, 10, 12, 13). The NASBA0.1 ml result was more than ± 0.5 log unit from the target of 104.18 copies/ml for 23 of 474 runs (5%) compared to 0 of 178 runs for NucliSens0.2 ml and 0 of 162 runs for NucliSens1.0 ml (P < 0.0001). NucliSens0.2 ml missed the target of 103.18 copies/ml 26 times (15%) compared to 0 times for NucliSens1.0 ml (P < 0.0001).
Laboratory differences.
There were statistically significant differences in levels of variance between labs for the lowest-range standard of all three assay-input volume combinations and for the highest-range standard for NucliSens1.0 ml (data not shown).
Variability over time.
Table 2 summarizes SD for 6-month time intervals corresponding to WIHS visit windows for each assay-input volume combination and VQA external standard. Within each combination and for all standards, the SD was stable and there were no statistically significant trends except with the lowest-range control value for NucliSens0.2 ml.
TABLE 2.
Longitudinal trends in SD of log10 HIV-1 RNA results from each VQA control-OTC assay combinationa
| Time interval | NASBA0.1 ml SD for VQA control target:
|
NucliSens0.2 ml SD for VQA control target:
|
NucliSens1.0 ml SD for VQA control target:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 104.18 | 105.18 | 106.18 | 103.18 | 104.18 | 105.18 | 103.18 | 104.18 | 105.18 | |
| April–October 1996 | 0.29 | 0.19 | 0.19 | NDb | ND | ||||
| October 1996–April 1997 | 0.23 | 0.15 | 0.18 | ND | ND | ||||
| April–October 1997 | 0.22 | 0.17 | 0.15 | ND | ND | ||||
| October 1997–April 1998 | 0.24 | 0.18 | 0.15 | 0.31 | 0.20 | 0.15 | ND | ||
| April–October 1998 | 0.18 | 0.17 | 0.10 | 0.35 | 0.17 | 0.17 | ND | ||
| October 1998–April 1999 | ND | 0.36 | 0.19 | 0.18 | 0.19 | 0.10 | 0.12 | ||
| April–October 1999 | ND | ND | 0.15 | 0.12 | 0.09 | ||||
| October 1999–January 2000 | ND | ND | 0.17 | 0.10 | 0.11 | ||||
| Overall | 0.24 | 0.18 | 0.18 | 0.34 | 0.18 | 0.17 | 0.16 | 0.11 | 0.10 |
Control target values are numbers of copies per milliliter.
ND, not determined. This assay was not used during the given time period.
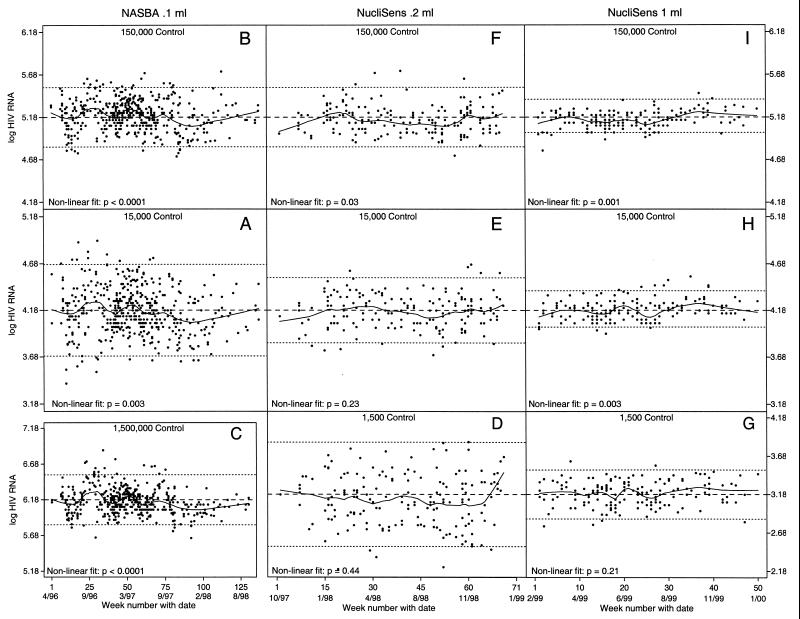
For NASBA0.1 ml (April 1996 to October 1998), the observed nonlinear trends were statistically significant for all standards (lowest range, P = 0.003 [Fig. 1A]; middle range, P < 0.0001 [Fig. 1B]; highest range, P < 0.0001 [Fig. 1C]). NucliSens0.2 ml (October 1997 to January 1999) demonstrated a marginally significant trend in the highest-range standard (Fig. 1F; P = 0.03). NucliSens1.0 ml (February 1999 to January 2000) demonstrated significant trends in the middle and high-range standards (Fig. 1H, P = 0.003, and I, P = 0.001, respectively).
FIG. 1.
Scatter plots with nonlinear smooth curves of all log-transformed results over the observation period of each assay for each of the three VQA controls (solid line). Each dot represents the result from one run. Nominal target values (dashed lines) and values 2 SD above and below the nominal value (dotted lines) give context to the measurement fluctuations over time. Nonlinear P values indicate the strength (fit) of the association between time and the control result. (C) 1.5 × 106 copies/ml; (B, F, and I) 1.5 × 105 copies/ml; (A, E, and H) 1.5 × 104 copies/ml; (D and G) 1.5 × 103 copies/ml.
While we observed no gain in precision in the transition from NASBA0.1 ml to Nuclisens0.2 ml, there was a gain in precision and reproducibility for all standards in the transition from Nuclisens0.2 ml to Nuclisens1.0 ml. This was most likely the cumulative result of kit reformulation and increased input volume (M. Cronin, OTC, personal communication, 2000; Introducing a New Family of Assays Based on NASBA, OTC pamphlet, 1997; NucliSens: Synergy of Strengths, OTC pamphlet, 1997).
Theoretically, the observed systematic decrease in results over the 2.5-year observation period for NASBA0.1 ml may be an indication of the deterioration of the virus in the viral stock used for VQA standards over time. However, an internal VQA study to address this possibility found no indication of deterioration of the virus in quality assurance panels (3). The observed increase in the level of HIV-1 RNA over the 1-year observation period for NucliSens1.0 ml also argues against deterioration of the virus in the viral stock. All statistically significant longitudinal trends were well within 2 SD, indicating no biologically relevant bias. We conclude that it is not necessary to make an intra-assay adjustment for time.
We have described the performance over a period spanning the introduction of HAART of three versions of OTC assays within an externally controlled multilaboratory program. NASBA technology is invariant to specimen type, an advantage that makes this assay valuable to clinicians and researchers testing nonplasma body fluids for HIV-1 RNA. Our data demonstrate that the precision of the OTC assays is comparable to that of other assays not invariant to specimen type. We also demonstrated that patient viral load levels in the HAART era can be measured with the same precision as or with better precision than in the pre-HAART era, despite an average HIV-1 RNA decrease of 2 log10 units. We have shown that HIV-1 RNA measurements across multiple WIHS sites over a period of 4 years were comparable despite the introduction of HAART, different versions of the assay, and differences between labs.
Acknowledgments
Data in this manuscript were collected by the WIHS Collaborative Study Group, which is made up of the following consortia or centers (principal investigators): New York City/Bronx, N.Y., Consortium (Kathryn Anastos); Brooklyn, N.Y. (Howard Minkoff); Washington, D.C., Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Herminia Palacio); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and the Data Coordinating Center (Alvaro Muñoz, Stephen J. Gange).
The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute, the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Dental and Craniofacial Research, the Agency for Health Care Policy and Research, and the Centers for Disease Control and Prevention (grants U01-AI-35004, U01-AI-31834, U01-AI-34994, AI-34989, U01-HD-32632 [NICHD], U01-AI-34993, and U01-AI-42590). The VQA program is funded by the Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, contract NO1-A1-85354.
REFERENCES
- 1.Barkan S E, Melnick S L, Preston-Martin S, Weber K, Kalish L A, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women's Interagency HIV Study WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 2.Bartlett J A, DeMasi R, Dawson D, Hill A. Variability in repeated consecutive measurements of plasma human immunodeficiency virus RNA in persons receiving stable nucleoside reverse transcriptase inhibitor therapy or no treatment. J Infect Dis. 1998;178:1803–1805. doi: 10.1086/314503. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla D. 180-Day stability of stored RNA proficiency panels. VQA Program's Report Quarterly. Chicago, Ill: Virology Quality Assurance Laboratory, Rush Presbyterian/St. Luke's Medical Center; 2000. [Google Scholar]
- 4.Brambilla D, Leung S, Lew J, Todd J, Herman S, Cronin M, Shapiro D E, Bremer J, Hanson C, Hillyer G V, McSherry G D, Sperling R S, Coombs R W, Reichelderfer P S. Absolute copy number and relative change in determinations of human immunodeficiency virus type 1 RNA in plasma: effect of an external standard on kit comparisons. J Clin Microbiol. 1998;36:311–314. doi: 10.1128/jcm.36.1.311-314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambilla D, Reichelderfer P S, Bremer J W, Shapiro D E, Hershow R C, Katzenstein D A, Hammer S M, Jackson B, Collier A C, Sperling R S, Fowler M G, Coombs R W. The contribution of assay variation and biological variation to the total variability of plasma HIV-1 RNA measurements. The Women Infant Transmission Study Clinics. Virology Quality Assurance Program. AIDS. 1999;13:2269–2279. doi: 10.1097/00002030-199911120-00009. [DOI] [PubMed] [Google Scholar]
- 6.Chambers J M, Hastie T J, editors. Statistical models in S. New York, N.Y: Chapman and Hall; 1992. [Google Scholar]
- 7.Clerici M, Sarin A, Coffman R L, Wynn T A, Blatt S P, Hendrix C W, Wolf S F, Shearer G M, Henkart P A. Type 1/type 2 cytokine modulation of T-cell programed cell death as a model for human immunodeficiency virus pathogenesis. Immunology. 1994;91:11811–11815. doi: 10.1073/pnas.91.25.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantine N T, Callahan J D W. Retroviral testing. Essentials for quality control and laboratory diagnosis. Boca Raton, Fla: CRC Press, Inc.; 1992. Quality control and quality assurance; pp. 121–167. [Google Scholar]
- 9.Deeks S G, Coleman R L, White R, Pachl C, Schambelan M, Chernoff D N, Feinberg M B. Variance of plasma human immunodeficiency virus type 1 RNA levels measured by branched DNA within and between days. J Infect Dis. 1997;176:514–517. doi: 10.1086/517278. [DOI] [PubMed] [Google Scholar]
- 10.Gerondelis P, Archer R H, Palaniappan C, Reichman R C, Fay P J, Bambara R A, Demeter L M. The P236L delavirdine-resistant human immunodeficiency virus type 1 mutant is replication defective and demonstrates alterations in both RNA 5′-end- and DNA 3′-end-directed RNase H activities. J Virol. 1999;73:5803–5813. doi: 10.1128/jvi.73.7.5803-5813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H J, Pedneault L, Hollinger F B. Intra-assay performance characteristics of five assays for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:835–839. doi: 10.1128/jcm.36.3.835-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellors J W, Munoz A, Giorgi J V, Margolick J B, Tassoni C J, Gupta P, Kingsley L A, Todd J A, Saah A J, Detels R, Phair J P, Rinaldo C R J. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Mellors J W, Rinaldo C R J, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. . (Erratum, 275:14, 1997.) [DOI] [PubMed] [Google Scholar]
- 14.Pollard J H. A handbook of numerical and statistical techniques. New York, N.Y: Cambridge University Press; 1977. pp. 195–196. [Google Scholar]
- 15.Skidmore S J, Zuckerman M, Parry J V. Accuracy of plasma HIV RNA quantification: a multicentre study of variability. J Med Virol. 2000;61:417–422. doi: 10.1002/1096-9071(200008)61:4<417::aid-jmv2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Venables W N, Ripley B D. Modern applied statistics with S-Plus. New York, N.Y: Springer; 1997. Modern regression; pp. 323–331. [Google Scholar]
- 17.Witt D J, Kemper M, Stead A, Ginocchio C C, Caliendo A M. Relationship of incremental specimen volumes and enhanced detection of human immunodeficiency virus type 1 RNA with nucleic acid amplification technology. J Clin Microbiol. 2000;38:85–89. doi: 10.1128/jcm.38.1.85-89.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche Molecular Diagnostics. Amplicore HIV-1 Monitor Assay. 2-1999. Roche Diagnostic Corporation. Ref Type: Pamphlet