Abstract
Background.
In the United States, human papillomavirus (HPV) vaccination has been recommended since 2011 for boys aged 11–12 years, with catch-up vaccination recommended through age 26 years for previously unvaccinated men who have sex with men (MSM).
Methods.
During 2016–2018, a cross-sectional study enrolled MSM and transgender women aged 18–26 years in Seattle, Washington. Participants submitted self-collected penile swab specimens for HPV genotyping. HPV vaccination history was self-reported. We compared HPV prevalence among vaccinated participants with that in participants with no or unknown vaccination history, using log-binomial regression to estimate adjusted prevalence ratios and confidence intervals.
Results.
Among 687 participants, 348 (50.7%) self-reported ever receiving ≥1 HPV vaccine dose; the median age at first HPV vaccination was 21 years, and the median age at first sex, 17 years. Overall, the prevalence of penile quadrivalent HPV vaccine (4vHPV)–type HPV was similar in vaccinated participants (12.1%) and participants with no or unknown vaccination (15.6%) (adjusted prevalence ratio, 0.69 [95% confidence interval, .47–1.01]). However, the prevalence was significantly lower in participants vaccinated at age ≤18 years than in those with no of unknown vaccination (0.15 [.04–.62]), corresponding to a vaccine effectiveness of 85% against 4vHPV-type HPV.
Conclusions.
Results suggest that HPV vaccination is effective in preventing penile HPV infections in young MSM when administered at age ≤18 years.
Keywords: papillomavirus infections, papillomavirus vaccines, sexual and gender minorities, vaccination
Human papillomavirus (HPV) infections in males are etiologically linked to anogenital warts and cancers of the penis, anus, and oropharynx [1]. Compared with men who have sex with women (MSW), men who have sex with men (MSM) are at increased risk for anal HPV infections and anal cancer [2–4], and at similar risk for anogenital warts and penile HPV infections [5–7].
A clinical trial of 16–26-year-old males demonstrated quadrivalent HPV vaccine (4vHPV) efficacy against HPV type 6, 11, 16, or 18 anogenital infections (detected in the penis, scrotum, or perineal/perianal regions) and anogenital lesions (inclusive of external anogenital warts and penile, perineal, and perianal neoplasias or cancers) [8]. Furthermore, substudy analyses of MSM participants demonstrated 94.4% (95% confidence interval [CI], 64.4%–99.9%) efficacy against 6-month persistent HPV type 6, 11, 16, or 18 anogenital infections in the per-protocol population, and 43.6% (19.5%–60.8%) efficacy in the intention-to-treat population [9]. Trial participation, however, was restricted to males reporting a lifetime number of ≤5 sex partners, and results may not translate to the broader population of MSM or transgender women.
Prophylactic HPV vaccines have been approved by the US Food and Drug Administration for the prevention of anogenital warts and cervical, vulvar, vaginal, anal, and oropharyngeal cancers [10]. (There is no Food and Drug Administration indication for the prevention of penile cancers; the HPV vaccine efficacy trial [9] that included penile outcomes was not powered for efficacy against penile intraepithelial neoplasias.)
Since 2011, the US Advisory Committee on Immunization Practices has recommended routine HPV vaccination for boys at age 11 or 12 years, with catch-up vaccination recommended through age 21 years for most men, and through age 26 years for MSM, transgender people [11], and women [12]. The 2011 Advisory Committee on Immunization Practices recommendations were made when only 4vHPV was licensed for use in males; recommendations were updated in 2015 to include nonavalent HPV vaccine (9vHPV) after that vaccine was licensed [11].
Uptake of these vaccines in males, and MSM in particular, is suboptimal but has increased over time. The proportion of males aged 13–17 years who received ≥1 dose of HPV vaccine increased from 8.3% in 2011 to 69.8% in 2019, based on National Immunization Survey–Teen data [13]. Among 18–26-year old MSM, ≥1-dose coverage increased from 1.2% in 2011 to 32.8% in 2017, based on National HIV Behavioral Surveillance data [14]. The increasing HPV vaccine coverage in MSM has enhanced feasibility to evaluate HPV vaccine effectiveness outside clinical trials.
To generate real-world data on HPV vaccine effectiveness in MSM, we conducted the Vaccine Impact in Men (VIM) study in young adult MSM and transgender women. Our group previously evaluated vaccine effectiveness against anal or oral 4vHPV-type HPV among MSM in 3 US cities [15]. In the current report, we present results on vaccine effectiveness against penile HPV detection among MSM who participated in the VIM study in Seattle, Washington. The ability to prevent penile HPV infections in MSM and transgender women has implications for reducing the incidence of anogenital warts and penile cancers in these populations, as well as for decreasing HPV transmission.
METHODS
Study Population
During 2016–2018, 18–26-year-old gay, bisexual, and other MSM, and transgender women were enrolled in Seattle as part of the multicenter, cross-sectional VIM study [15]. Persons seeking counseling, testing, or treatment for sexually transmitted diseases (STDs) or human immunodeficiency virus (HIV) were approached by clinicians or STD testers at the Public Health–Seattle & King County Sexual Health Clinic and Gay City Health Project, a community HIV/STD testing site. People were eligible to enroll if they (1) were assigned male sex at birth, (2) were 18–26 years of age, and (3) reported a history of anal or oral sex with a male partner, identified as gay/homosexual or bisexual, and/or intended to have sex with a male partner in the future. Eligible people were excluded if they required language translation services for clinical services or if they did not provide written informed consent. The research study protocol was reviewed and approved by the University of Washington Institutional Review Board, authorized as the the reviewing institutional review board by the Centers for Disease Control and Prevention.
Data Collection
All study procedures were completed on the same day as enrollment, either before or after non–study-related counseling, testing, or treatment services. Self-reported information on age, race and ethnicity, gender identity, sexual orientation, circumcision status, lifetime number of sex partners, age at first sex, history of preexposure prophylaxis (PrEP) to prevent HIV infection, HIV status, and HPV vaccination history were collected from clinic computer-assisted self-interview data and a study-specific self-completed paper questionnaire. HPV vaccination history questions asked whether participants had ever received any HPV vaccine, how many doses were received, age at first dose, and date of first dose. Participants were not asked to recall whether they had received 4vHPV or 9vHPV.
Participants in Seattle self-collected a penile swab specimen in addition to the 3 biologic specimens obtained at all VIM study sites (self-collected anal swab sample, self-collected oral rinse sample, and serum sample, described elsewhere [15]). A clinician or STD tester provided each participant with illustrated instructions and reviewed the procedure for self-collecting a penile swab specimen [16]. After washing or sanitizing their hands, participants used a Dacron swab (Puritan Medical Products) to rub all external surfaces of the penis for a total of 10 seconds. Uncircumcised participants were instructed to pull the foreskin back before swabbing. Participants placed the swab into a specimen collection vial containing 1 mL of Digene Specimen Transport Medium (Qiagen).
Specimens were stored at −20° C and shipped on dry ice to the Centers for Disease Control and Prevention laboratory for HPV testing. Extracted DNA was tested for HPV using the L1-consensus polymerase chain reaction–based Roche Linear Array HPV genotyping assay (Roche Diagnostics) for 37 types (HPV types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39), using methods described elsewhere [17]. Specimens were considered inadequate if results were negative for β-globin and negative for all 37 HPV types. Results from research-use only tests were not provided to participants.
Statistical Analysis
We dichotomized self-reported HPV vaccination history as “yes” versus “no” or “don’t know” based on the response to the question “Have you ever received any vaccine against human papillomavirus (HPV)?” We also categorized age at first vaccine dose (≤18 years, >18 years, or unknown), timing of age at first vaccine dose relative to age at first sex with any partner (younger than age at first sex, at or older than age at first sex, or unknown), and number of doses received (1, 2, ≥3, or unknown).
We used χ2, Wilcoxon rank-sum, or Fisher exact tests to compare characteristics of participants by self-reported vaccination history (yes vs no or don’t know). We also used χ2 or Fisher exact tests to identify characteristics associated with penile HPV detection (≥1 of any HPV type, ≥1 4vHPV type [types 6, 11, 16, and/or 18], or ≥1 non-4vHPV type). We calculated penile HPV prevalence (≥1 of any HPV type, ≥1 4vHPV type, ≥1 9vHPV type [types 6, 11, 16, 18, 31, 33, 45, 52, and/or 58], ≥1 additional 9vHPV type not targeted by 4vHPV [types 31, 33, 45, 52, and/or 58], ≥1 non-4vHPV type, ≥1 non-9vHPV type, and individual HPV vaccine types) and 95% CIs by self-reported HPV vaccination history.
We also used log-binomial regression to calculate crude prevalence ratios and adjusted prevalence ratios (aPRs) and 95% CIs for associations between self-reported HPV vaccination history and penile HPV detection (≥1 4vHPV or ≥1 non-4vHPV type). In addition to dichotomous HPV vaccination history, we evaluated age at first dose in a separate model. Variables significantly associated with HPV vaccination history in bivariate analyses (P < .10) were included in the adjusted models. Vaccine effectiveness against penile HPV was calculated as (1 − aPR) × 100. All analyses were performed using Stata version 15 software (StataCorp).
RESULTS
A total of 751 participants were enrolled in Seattle. One participant who did not submit a penile swab specimen, 49 with inadequate penile swab specimens (6.5% of tested specimens), and 14 with missing HPV vaccination history data were excluded, leaving 687 participants in the analysis. Among these participants, the mean age (standard deviation) was 23.0 (2.2) years. Just over half of participants (50.9%) self-identified as non-Hispanic white, 19.7% as Hispanic, 17.3% as Asian or Pacific Islander, and 6.3% as non-Hispanic black; 5.8% reported another race or >1 race or did not respond (Table 1). Most participants reported their gender identity as male (96.8%), with a small number (0.9%) identifying as female or transgender female. Most (85.3%) identified as gay or homosexual, <1% identified as straight or heterosexual, and 14.1% identified as other sexual orientation or did not respond. Almost two-thirds (64.3%) were circumcised. In total, 21.8% of participants self-reported ever taking PrEP, and 2.2% self-reported being HIV positive. The median age at first sex with any partner was 17 years, and most participants (87.8%) reported >5 sex partners in their lifetime.
Table 1.
Characteristics of Participating Transgender Women and Men Who Have Sex With Men, by Self-Reported Human Papillomavirus Vaccination History—Seattle, Washington, 2016–2018
| Participants, No. (%)a | ||||
|---|---|---|---|---|
| History of ≥1 HPV Vaccine Dose | ||||
| Characteristic | Total | No or Don’t Knowb | Yes | P Value |
| Total | 687 (100) | 339 (100) | 348 (100) | … |
| Age, y | ||||
| 18–21 | 178 (25.9) | 77 (22.7) | 101 (29.0) | .06c |
| 22–26 | 509 (74.1) | 262 (77.3) | 247 (71.0) | |
| Gender identity | ||||
| Male | 665 (96.8) | 326 (96.2) | 339 (97.4) | .59c |
| Female/transgender female | 6 (0.9) | 4 (1.2) | 2 (0.6) | |
| Other/unknown | 16 (2.3) | 9 (2.7) | 7 (2.0) | |
| Sexual orientation | ||||
| Gay/homosexual | 586 (85.3) | 296 (87.3) | 290 (83.3) | .26c |
| Straight/heterosexual | 4 (0.6) | 1 (0.3) | 3 (0.9) | |
| Other/unknown | 97 (14.1) | 42 (12.4) | 55 (15.8) | |
| Race and/or ethnicity | ||||
| Non-Hispanic white | 350 (50.9) | 172 (50.7) | 178 (51.1) | .80c |
| Non-Hispanic black | 43 (6.3) | 24 (7.1) | 19 (5.5) | |
| Asian/Pacific Islander | 119 (17.3) | 58 (17.1) | 61 (17.5) | |
| Hispanic | 135 (19.7) | 63 (18.6) | 72 (20.7) | |
| Other/unknownd | 40 (5.8) | 22 (6.5) | 18 (5.2) | |
| Circumcised | ||||
| Yes | 444 (64.6) | 213 (62.8) | 231 (66.4) | .52c |
| No | 235 (34.2) | 121 (35.7) | 114 (32.8) | |
| Unknown | 8 (1.2) | 5 (1.5) | 3 (0.9) | |
| History of PrEP for HIV prevention | ||||
| Yes | 150 (21.8) | 52 (15.3) | 98 (28.2) | <.01c |
| No or unknown | 537 (78.2) | 287 (84.7) | 250 (71.8) | |
| Most recent HIV test result | ||||
| Negative or unknown | 672 (97.8) | 337 (99.4) | 335 (96.3) | .007e |
| Positive | 15 (2.2) | 2 (0.6) | 13 (3.7) | |
| Lifetime no. of sex partners of any sex | ||||
| ≤5 | 47 (6.8) | 21 (6.2) | 26 (7.5) | .03c |
| 6–10 | 83 (12.1) | 37 (10.9) | 46 (13.2) | |
| 11–20 | 149 (21.7) | 86 (25.4) | 63 (18.1) | |
| >20 | 371 (54.0) | 171 (50.4) | 200 (57.5) | |
| Other/unknown | 37 (5.4) | 24 (7.1) | 13 (3.7) | |
| Lifetime no. of sex partners of any sex,f mean/median (IQR) | 45.3/25 (12–50) | 37.2/23 (13–41) | 52.8/29.5 (12–55) | .06g |
| Age at first sex with any partner,h mean/median (IQR), y | 16.8/17 (15–18) | 16.8/17 (15–18) | 16.8/17 (15–18) | .96g |
| Age at first HPV vaccination | ||||
| ≤18 y | … | … | 83 (23.9) | … |
| >18 y | … | … | 217 (62.4) | |
| Unknown | … | … | 48 (13.8) | |
| Age at first HPV vaccination, mean/median (IQR), yi | … | … | 20.4/21 (18–23) | … |
| First HPV vaccination relative to age at first sex | ||||
| Before first sex | … | … | 37 (10.6) | … |
| At or after first sex | … | … | 263 (75.6) | |
| Don’t know | … | … | 48 (13.8) | |
| No. of HPV vaccine doses received (by self-report) | ||||
| 1 | … | … | 50 (14.4) | … |
| 2 | 65 (18.7) | |||
| 3 | 179 (51.4) | |||
| Don’t know | 54 (15.5) | |||
Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; IQR, interquartile range; PrEP, preexposure prophylaxis.
Data represent no. (%) of participants unless otherwise specified.
Includes 180 participants who reported “no” and 159 who reported “don’t know.”
Based on Pearson χ2 test.
Includes American Indian, Alaska Native, other race, >1 race, or unknown.
Based on Fisher exact test.
Includes 650 participants (315 in the “no or don’t know” group and 335 in the “yes” group).
Based on Wilcoxon rank-sum test.
Includes 685 participants (338 in the “no or don’t know” group and 347 in the “yes” group).
Includes 300 participants.
About half of participants (n = 348 [50.7%]) self-reported ever having received an HPV vaccine, and the other half reported either that they had not been vaccinated (n = 180 [26.2%]) or that they did not know whether they had been vaccinated (n = 159 [23.1%]) (Table 1). Among vaccinated participants, the median age at first HPV vaccine dose was 21 years, and only 10.6% reported that their age at first vaccine dose was younger than that at first sex. More than half (51.4%) of vaccinated participants reported receiving 3 doses, 33.0% reported 1 or 2 doses, and 15.5% did not know how many doses they had received.
Age, PrEP history, HIV positivity, and lifetime number of sex partners differed significantly by HPV vaccination history (Table 1). Vaccinated participants were more likely than those with no or unknown vaccination history to be 18–21 years of age (29.0% vs 22.7%), report ever taking PrEP (28.2% vs 15.3%), or report being HIV positive (3.7% vs 0.6%). The median lifetime number of sex partners was 29.5 in vaccinated participants versus 23 in those with no or unknown vaccination history. Other characteristics did not differ significantly by vaccination history.
Penile HPV DNA was detected in 333 participants (48.5%), with 95 (13.8%) positive for ≥1 4vHPV type and 133 (19.4%) positive for ≥1 9vHPV type (Figure 1). The prevalence of each individual HPV vaccine type was 6.3% for HPV-6, 5.2% for HPV-16, 3.9% for HPV-45, 2.2% for HPV-18, and <2% for HPV-11 and each of the additional 9vHPV types. The prevalence of any penile HPV was significantly higher in participants who were aged 22–26 years, reported ever taking PrEP, or reported more sex partners in their lifetime (Table 2). Similar associations were observed for the prevalence of ≥1 4vHPV type or ≥1 non-4vHPV type, although associations with older age were not statistically significant. Observed differences in prevalence for other characteristics (race/ethnicity, gender identity, sexual orientation, and HIV status) were not statistically significant.
Figure 1.
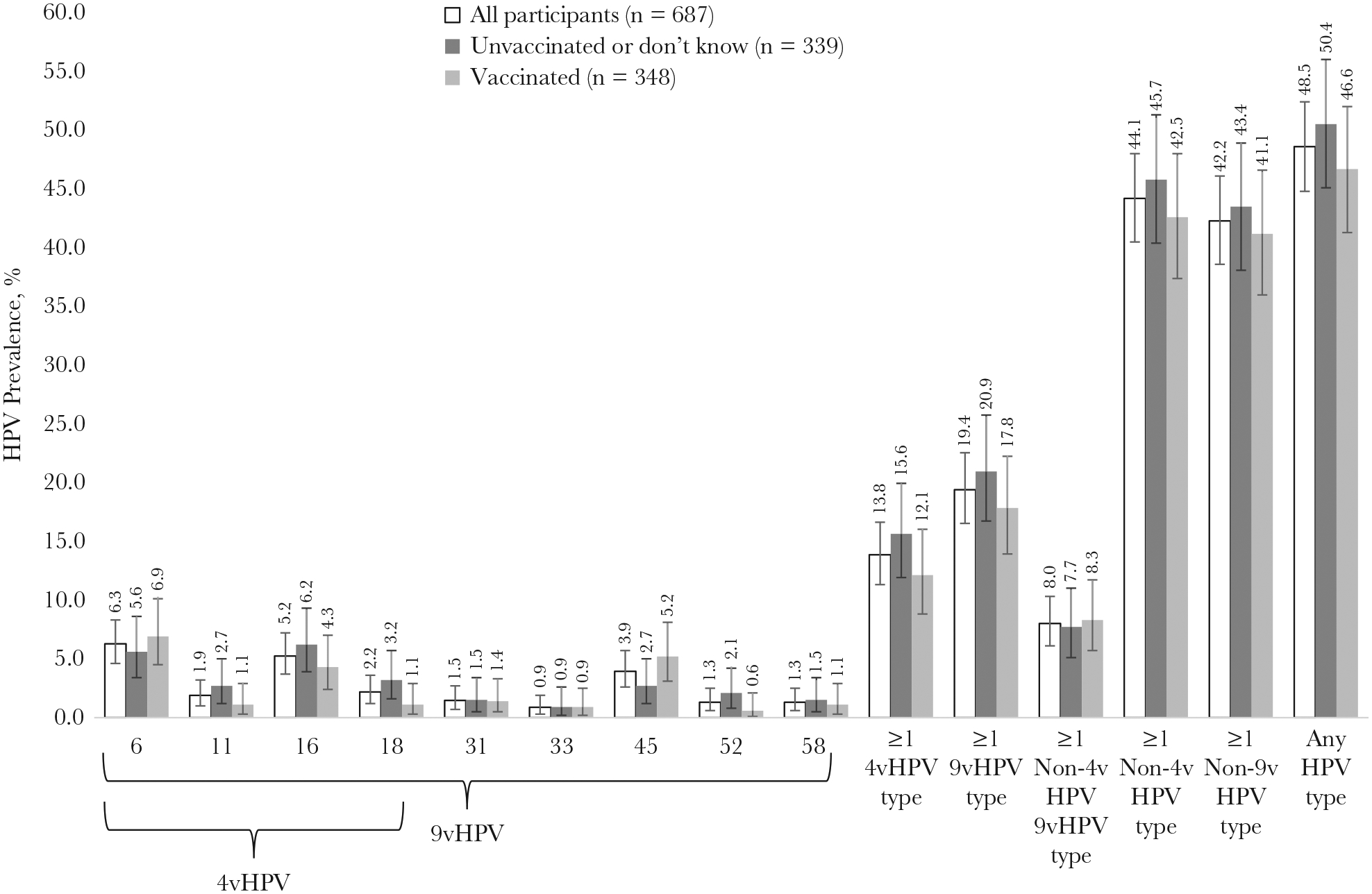
Human papillomavirus (HPV) prevalence in penile swab specimens from 18–26-year-old transgender women and men who have sex with men, overall and by self-reported HPV vaccination history (Seattle, Washington, 2016–2018). Error bars show 95% confidence intervals. Abbreviations: 4vHPV, quadrivalent HPV vaccine; 9vHPV, nonavalent HPV vaccine.
Table 2.
Prevalence of Any Human Papillomavirus (HPV), Quadrivalent HPV Vaccine (4vHPV)–Type HPV or Non–4vHPV-Type HPV in Penile Swab Specimens, by Characteristics of Participating Transgender Women and Men Who Have Sex With Men—Seattle, Washington, 2016–2018
| Participants by HPV Type | |||||||
|---|---|---|---|---|---|---|---|
| Any HPV Type | ≥1 4vHPV Typea | ≥1 Non-4vHPV Typeb | |||||
| Characteristic | Total Participants, No. | No. (%) | P Value | No. (%) | P Value | No. (%) | P Value |
| Total | 687 | 333 (48.5) | … | 95 (13.8) | … | 303 (44.1) | … |
| Age, y | |||||||
| 18–21 | 178 | 74 (41.6) | .03c | 21 (11.8) | .36c | 69 (38.8) | .10c |
| 22–26 | 509 | 259 (50.9) | 74 (14.5) | 234 (46.0) | |||
| Gender identity | |||||||
| Male | 665 | 323 (48.6) | .24c | 93 (14.0) | .61c | 294 (44.2) | .36c |
| Female/transgender female | 6 | 1 (16.7) | 0 (0.0) | 1 (16.7) | |||
| Other/unknown | 16 | 9 (56.3) | 2 (12.5) | 8 (50.0) | |||
| Race and/or ethnicity | |||||||
| Non-Hispanic white | 350 | 164 (46.9) | .16c | 52 (14.9) | .26c | 147 (42.0) | .10c |
| Non-Hispanic black | 43 | 28 (65.1) | 8 (18.6) | 25 (58.1) | |||
| Asian/Pacific Islander | 119 | 53 (44.5) | 15 (12.6) | 46 (38.7) | |||
| Hispanic | 135 | 66 (48.9) | 12 (8.9) | 22 (55.0) | |||
| Other/unknownd | 40 | 22 (55.0) | 8 (20.0) | 63 (46.7) | |||
| Sexual orientation | |||||||
| Gay/homosexual | 586 | 280 (47.8) | .34f | 76 (13.0) | .19f | 255 (43.5) | .54f |
| Straight/heterosexual | 4 | 1 (25.0) | 0 (0.0) | 1 (25.0) | |||
| Other/unknown | 97 | 52 (53.6) | 19 (19.6) | 47 (48.5) | |||
| Circumcisede | |||||||
| Yes | 444 | 208 (46.8) | .30c | 69 (15.5) | .08c | 185 (41.7) | .11c |
| No | 235 | 120 (51.1) | 25 (10.6) | 113 (48.1) | |||
| Lifetime no. of sex partners of any sex | |||||||
| ≤5 | 47 | 16 (34.0) | <.001c | 2 (4.3) | <.01c | 16 (34.0) | <.01c |
| 6–10 | 83 | 26 (31.3) | 3 (3.6) | 25 (30.1) | |||
| 11–20 | 149 | 66 (44.3) | 18 (12.1) | 60 (40.3) | |||
| >20 | 371 | 198 (53.4) | 64 (17.3) | 179 (48.2) | |||
| Other/unknown | 37 | 27 (73.0) | 8 (21.6) | 23 (62.2) | |||
| History of ever taking PrEP for HIV prevention | |||||||
| No or unknown | 537 | 247 (46.0) | .01c | 65 (12.1) | .01c | 224 (41.7) | .02c |
| Yes | 150 | 86 (57.3) | 30 (20.0) | 79 (52.7) | |||
| Most recent HIV test result | |||||||
| Negative or unknown | 672 | 323 (48.1) | .19f | 91 (13.5) | .14f | 293 (43.6) | .11f |
| Positive | 15 | 10 (66.7) | 4 (26.7) | 10 (66.7) | |||
Abbreviations: 4vHPV, quadrivalent HPV vaccine; HIV, human immunodeficiency virus; HPV, human papillomavirus; PrEP, preexposure prophylaxis.
4vHPV types include types 6, 11, 16, and 18.
Non-4vHPV types include types 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39.
Based on Pearson χ2 test.
Including American Indian, Alaska Native, other race, >1 race, or unknown.
Excludes 8 participants with unknown circumcision status.
Based on Fisher exact test.
The prevalence of ≥1 non-4vHPV-type HPV was similar in vaccinated participants (42.5%) and participants with no or unknown vaccination history (45.7%) (Figure 1) (aPR 0.89 [95% CI, .76–1.05) (Table 3). The prevalence of ≥1 4vHPV type was also similar in vaccinated (12.1%) versus unvaccinated participants or those with unknown vaccination history (15.6%) (aPR, 0.69 [95% CI, .47–1.01]).
Table 3.
Prevalence of Quadrivalent Human Papillomavirus (HPV) Vaccine (4vHPV)–Type HPV and Non–4vHPV-Type HPV Detected in Penile Swab Specimens, by HPV Vaccination History, Among 18–26-Year-Old Transgender Women and Men Who Have Sex with Men—Seattle, Washington, 2016–2018
| Participants With ≥1 4vHPV Typea | Participants With ≥1 Non-4vHPV Typeb | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Total Participants, No. | No. (%) | PR (95% CI) | aPRc (95% CI) | No. (%) | PR (95% CI) | aPRc (95% CI) |
| HPV vaccination history, self-reportedd | |||||||
| No/don’t know | 339 | 53 (15.6) | Reference | Reference | 155 (45.7) | Reference | Reference |
| Yes | 348 | 42 (12.1) | 0.77 (.53– 1.12) | 0.69 (.47–1.01) | 148 (42.5) | 0.93 (.79–1.10) | 0.89 (.76–1.05) |
| Age at first HPV vaccination | |||||||
| ≤18 y | 83 | 2 (2.4) | 0.15 (.04–.62) | 0.15 (.04–.62) | 27 (32.5) | 0.71 (.51–.99) | 0.75 (.54–1.05) |
| >18 y | 217 | 32 (14.7) | 0.94 (.63–1.41) | 0.80 (.52–1.22) | 101 (46.5) | 1.02 (.85–1.22) | 0.93 (.77–1.12) |
| Unknown | 48 | 8 (16.7) | 1.07 (.54–2.10) | 1.00 (.51–1.96) | 20 (41.7) | 0.91 (.64–1.30) | 0.94 (.67–1.31) |
| Age, y | |||||||
| 18–21 | 178 | 21 (11.8) | Reference | Reference | 69 (38.8) | Reference | Reference |
| 22–26 | 509 | 74 (14.5) | 1.23 (.78–1.94) | 1.05 (.67–1.64) | 234 (46.0) | 1.19 (.96–1.46) | 1.11 (.91–1.36) |
| History of ever taking PrEP for HIV prevention | |||||||
| No or unknown | 537 | 65 (12.1) | Reference | Reference | 224 (41.7) | Reference | Reference |
| Yes | 150 | 30 (20.0) | 1.65 (1.12–2.45) | 1.56 (1.04–2.35) | 79 (52.7) | 1.26 (1.05–1.51) | 1.22 (1.01–1.47) |
| Most recent HIV test result | |||||||
| Negative or unknown | 672 | 91 (13.5) | Reference | Reference | 293 (43.6) | Reference | Reference |
| Positive | 15 | 4 (26.7) | 1.97 (.83–4.66) | 2.16 (.91–5.12) | 10 (66.7) | 1.53 (1.06–2.21) | 1.62 (1.30–2.02) |
| Lifetime no. of sex partners of any sex | |||||||
| ≤5 | 47 | 2 (4.3) | Reference | Reference | 16 (34.0) | Reference | Reference |
| 6–10 | 83 | 3 (3.6) | 0.85 (.15–4.90) | 0.81 (.14–4.62) | 25 (30.1) | 0.88 (.53–1.48) | 0.85 (.51–1.43) |
| 11–20 | 149 | 18 (12.1) | 2.84 (.68–11.79) | 2.56 (.63–10.48) | 60 (40.3) | 1.18 (.76–1.84) | 1.13 (.72–1.75) |
| >20 | 371 | 64 (17.3) | 4.05 (1.03–16.02) | 3.33 (.85–13.11) | 179 (48.2) | 1.42 (.94–2.14) | 1.27 (.84–1.92) |
| Other/unknown | 37 | 8 (21.6) | 5.08 (1.15–22.51) | 4.05 (.92–17.83) | 23 (62.2) | 1.83 (1.14–2.92) | 1.68 (1.06–2.66) |
Abbreviations: 4vHPV, quadrivalent HPV vaccine; aPR, adjusted prevalence ratio; CI, confidence interval; HIV, human immunodeficiency virus; HPV, human papillomavirus; PR, prevalence ratio; PrEP, preexposure prophylaxis.
4vHPV types include types 6, 11, 16, and 18.
Non-4vHPV types include types 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39.
Analyses were adjusted for age, history of ever taking PrEP for HIV prevention, HIV status, and lifetime number of sex partners. The aPRs presented for age, HIV status, and lifetime number of sex partners are from models that considered HPV vaccination history as a dichotomous variable.
Separate models were constructed considering HPV vaccination history as either a dichotomous (yes vs no/don’t know) or 4-level variable (yes with first dose at age ≤18 years, yes with first dose at age >18 years, yes with unknown age at first dose, or no/don’t know).
However, compared with participants with no or unknown vaccination history, 4vHPV-type HPV prevalence was significantly lower in participants who were first vaccinated at ≤18 years of age (aPR 0.15 [95% CI, .04–.62]). The effectiveness of ≥1 HPV vaccine dose at ≤18 years of age was 85% against 4vHPV-type HPV. Among vaccinated participants, 4vHPV-type penile HPV prevalence was 0% (0 of 37) when age at first HPV vaccine dose was younger than age at first sex, 12.9% (34 of 263) when at or older than age at first sex, and 16.7% (8/48) when age at first sex was unknown.
DISCUSSION
Results from the current study suggest that vaccinating MSM and transgender women at age ≤18 years is effective in preventing penile HPV infections. Among our study population of 18–26-year-old MSM and transgender women seeking STD/HIV services, the effectiveness of ≥1 HPV vaccine dose received at age ≤18 years was 85% against 4vHPV types. Furthermore, no 4vHPV-type HPV was detected in participants who reported that their age at first HPV vaccine dose was younger than their age at first sex. Effectiveness against penile HPV was not observed in participants who reported receiving their first dose of HPV vaccine at age >18 years. This observed lack of effectiveness with older age at vaccine administration is likely due to exposure to HPV before vaccination, as the reported median age at first HPV vaccine dose (21 years) was older than the median age at first sex with a partner (17 years) in this study. Furthermore, most participants reported >5 sex partners, and more than half reported >20 sex partners in their lifetime. In contrast, the 4vHPV clinical trial that demonstrated efficacy against 6-month persistent anogenital (penile, scrotal, perineal, or perianal) infections in MSM was restricted to participants with ≤5 sex partners in their lifetime [9].
In our study, we adjusted for variables that could have affected vaccine effectiveness, including age, lifetime number of sex partners, history of taking PrEP, and HIV status. Prevalence of non-4vHPV types can also provide information on sexual risk and differences by vaccination history. The prevalence of ≥1 non-4vHPV-type penile HPV was somewhat lower in vaccinated participants than in participants with no or unknown vaccination history, but this difference was not statistically significant. The difference in non-4vHPV type prevalence was greater when comparing participants vaccinated at age ≤18 years to those who had no or unknown vaccination history, but this was not statistically significant in the adjusted analysis. The difference in non-4vHPV type prevalence by vaccination status was not attributable to differences in prevalence of HPV types targeted by 9vHPV only. Although some participants in this study could have received 9vHPV rather than 4vHPV (9vHPV has been the only vaccine distributed in the United States since late 2016 [11]), the prevalence of any of the additional 5 types targeted by 9vHPV was similar regardless of vaccination status.
Only one HPV vaccine efficacy trial has evaluated penile outcomes [9]. To our knowledge, ours is the first study in MSM to report on HPV vaccine effectiveness against penile HPV infections outside the initial clinical efficacy trial. In the general US population, an analysis of 304 men aged 18–24 years participating in the 2013–2014 National Health and Nutrition Examination Survey showed that self-reporting a history of HPV vaccination was associated with a 30% reduction in 4vHPV-type penile HPV, but the effect was not statistically significant [18].
In another study of 400 males aged 13–26 years recruited from a teen health clinic and an STD clinic in 2013–2015, 4vHPV-type HPV detection did not differ significantly between those who did (20.4%) and did not (27.9%) self-report prior HPV vaccination [19]. Penile and anal HPV test results were combined owing to insufficient study power, and no subanalysis restricted to MSM participants (representing only 10% of the study population) was reported. In addition, power was limited by a relatively small sample size and low proportions of vaccinated participants (17%–26%) in both of these studies. In Australia, a repeated cross-sectional study of MSM aged 16–20 years showed a decrease in penile 4vHPV-type HPV prevalence from 2011–2012 (12%) to 2017–2018 (6%), before and after implementation of a nationwide gender-neutral HPV vaccination program; most (75%) of the 200 participants in the postimplementation cohort self-reported a history of HPV vaccination, and no contemporaneous analysis of vaccine effectiveness was performed [20].
Data used in this analysis were collected as part of a larger study on HPV vaccine effectiveness in young MSM and transgender women. In the full VIM study population—combining data from participants aged 18–26 years in Seattle as well as Chicago, Illinois, and Los Angeles, California—we observed 29% vaccine effectiveness for ≥1 self-reported dose against anal or oral 4vHPV-type HPV [15]. Similar to the current analysis of penile HPV, the effectiveness against anal or oral HPV was greater when the first vaccine dose was administered at age ≤18 years (59% effectiveness), although a modest degree of protection was still observed among individuals vaccinated after 18 years of age (18% effectiveness). These observed differences in vaccine effectiveness against anal/oral versus penile HPV in those vaccinated after age 18 years may be due in part to study site differences in sexual behavior or median age at vaccination, with Seattle participants self-reporting a higher lifetime number of sex partners (median, 25 partners) and older age at first dose (median, 21 years) compared with Chicago and Los Angeles participants (median, 21 sex partners and 18 years at first dose).
Penile HPV prevalence (48.5% for any type) was at the upper end of the range of penile HPV estimates reported in other studies of MSM, and closer to those observed in HIV-positive versus HIV-negative MSM, despite the very low proportion of HIV-infected participants in our study. In a meta-analysis conducted among 7169 MSM from 13 countries, penile HPV prevalence was 36.2% overall, and 45.4% versus 28.6%, respectively, in HIV-positive versus HIV-negative participants [21]. Among 18–59-year-old male participants in the 2013–2014 National Health and Nutrition Examination Survey (using identical self-collection methods and the same HPV detection assay as in our study), the prevalence of any penile HPV was 42.2% [16]. Whereas anal HPV prevalence is consistently higher in MSM than in MSW [2, 3], penile HPV prevalence estimates tend to be more comparable [5, 7].
Compared with participants with no or unknown HPV vaccination history, HPV-vaccinated participants in our study were more likely to report having used PrEP, being HIV positive, and a higher lifetime number of sex partners, characteristics associated with increased HPV infection risk [16, 19, 21–24]. Similarly, data from the 2017 National HIV Behavioral Surveillance showed that HPV vaccine coverage in MSM was positively associated with HIV positivity and higher numbers of recent sex partners [14]. Studies of MSM in Australia and Canada have also demonstrated positive associations between use of PrEP and HPV vaccination [25, 26]. As observed in other studies of MSM [22] and MSW [16, 19, 23], we also found that the lifetime number of sex partners was associated with penile HPV detection; compared with participants reporting ≤10 partners, prevalence was higher in those reporting 11–20 or >20 partners. Penile HPV detection was also higher in participants who did than in those who did not report a history of PrEP. Two small studies in France demonstrated high HPV prevalence among MSM using PrEP [24, 27].
Strengths of our study include the relatively large sample size, relatively high (50%) ≥1 dose HPV vaccine coverage, and a sufficient number of penile 4vHPV-type HPV outcomes to detect differences in prevalence by HPV vaccination history. Several limitations should also be noted. First, we relied on self-report for capturing HPV vaccination history, which may have limited validity. Second, the cross-sectional nature of the study did not allow for an evaluation of HPV vaccination effectiveness against penile HPV acquisition. Third, few participants were HIV positive, thus precluding an analysis of HPV vaccine effectiveness stratified by HIV status. Finally, participants were recruited from a sexual health clinic and community HIV/STD testing site in a single US city, and few transgender women were enrolled. Therefore, results may not be generalizable to the broader population of MSM or transgender women.
In conclusion, our findings contribute real-world data on HPV vaccine effectiveness in MSM and transgender women. When initiated at ≤18 years of age, HPV vaccination was protective against penile 4vHPV-type HPV, and no penile 4vHPV-type HPV was detected in participants who were vaccinated before sexual debut. No protective effect was observed with HPV vaccine initiation after 18 years of age, likely attributable to HPV exposure before vaccination in this highly sexually active population. Results highlight the importance of routine HPV vaccination in early adolescence and the need for continued public health efforts to increase adolescent HPV vaccine coverage.
Acknowledgments.
We thank Juanita Onyekwuluje and Carly Herbert for providing laboratory support for the Vaccine Impact in Men study.
Financial support.
This work was the product of a Prevention Research Center and was supported by the Centers for Disease Control and Prevention (cooperative agreement U48 DP005013-02S7).
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2018 International Papillomavirus Conference, Sydney, Australia, October 2–6, 2018.
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012; 100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 2.Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in Men (HIM) study. J Infect Dis 2011; 203:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra E, Lin C, Clifford GM. Type-specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: a systematic literature review and meta-analysis. J Infect Dis 2019; 219:590–8. [DOI] [PubMed] [Google Scholar]
- 4.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012; 13:487–500. [DOI] [PubMed] [Google Scholar]
- 5.Nyitray AG, da Silva RJ, Baggio ML, et al. The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis 2011; 38:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llata E, Stenger M, Bernstein K, et al. Prevalence of genital warts among sexually transmitted disease clinic patients— Sexually Transmitted Disease Surveillance Network, United States, January 2010 to December 2011. Sex Transm Dis 2014; 41:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstone S, Palefsky JM, Giuliano AR, et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis 2011; 203:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–85. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. Gardasil 9. 2020. https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9. Accessed 23 August 2021. [Google Scholar]
- 11.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016; 65:1405–8. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1–30. [PubMed] [Google Scholar]
- 13.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClung N, Burnett J, Wejnert C, Markowitz LE, Meites E. Human papillomavirus vaccination coverage among men who have sex with men—National HIV Behavioral Surveillance, United States, 2017. Vaccine 2020; 38:7417–21. [DOI] [PubMed] [Google Scholar]
- 15.Meites E, Winer RL, Newcomb ME, et al. Vaccine effectiveness against prevalent anal and oral human papillomavirus infection among men who have sex with men—United States, 2016–2018. J Infect Dis 2020; 222:2052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013–2014. J Infect Dis 2017; 215:1070–9. [DOI] [PubMed] [Google Scholar]
- 17.Meites E, Gorbach PM, Gratzer B, et al. Monitoring for human papillomavirus vaccine impact among gay, bisexual, and other men who have sex with men—United States, 2012–2014. J Infect Dis 2016; 214:689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwer AF, Eisenberg MC, Carey TE, Meza R. Multisite HPV infections in the United States (NHANES 2003–2014): an overview and synthesis. Prev Med 2019; 123:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandler E, Ding L, Gorbach P, et al. Epidemiology of any and vaccine-type anogenital human papillomavirus among 13–26-year-old young men after HPV vaccine introduction. J Adolesc Health 2018; 63:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow EPF, Tabrizi SN, Fairley CK, et al. Prevalence of human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: a repeated cross-sectional study. Lancet Infect doi: 10.1016/S1473-3099(20)30687-3. Published 24 May 2021. [DOI] [PubMed] [Google Scholar]
- 21.Farahmand M, Monavari SH, Tavakoli A. Prevalence and genotype distribution of human papillomavirus infection in different anatomical sites among men who have sex with men: a systematic review and meta-analysis. Rev Med Virol 2021. doi: 10.1002/rmv.2219 [DOI] [PubMed] [Google Scholar]
- 22.Woestenberg PJ, van Benthem BHB, Bogaards JA, et al. ; Medical Microbiological Laboratories, the Public Health Services. HPV infections among young MSM visiting sexual health centers in the Netherlands: opportunities for targeted HPV vaccination. Vaccine 2020; 38:3321–9. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty M, Byler T. Genital wart and human papillomavirus prevalence in men in the United States from penile swabs: results from National Health and Nutrition Examination Surveys. Sex Transm Dis 2018; 45:412–6. [DOI] [PubMed] [Google Scholar]
- 24.Cotte L, Veyer D, Charreau I, et al. Prevalence and incidence of human papillomavirus infection in men having sex with men enrolled in a pre-exposure prophylaxis study: a sub-study of the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales “Intervention Préventive de l’Exposition aux Risques avec et pour les hommes Gays” Trial. Clin Infect Dis 2021; 72:41–9. [DOI] [PubMed] [Google Scholar]
- 25.Grewal R, Deeks SL, Hart TA, et al. Human papillomavirus (HPV) vaccine uptake among a community-recruited sample of gay, bisexual, and other men who have sex with men in the three largest cities in Canada from 2017 to 2019. Vaccine 2021; 39:3756–66. [DOI] [PubMed] [Google Scholar]
- 26.Chow EP, Aung ET, Chen MY, Bradshaw CS, Fairley CK. Human papillomavirus vaccination and sexual practices among men who have sex with men in Melbourne, Australia: a cross-sectional study. Int J STD AIDS 2020; 31:312–7. [DOI] [PubMed] [Google Scholar]
- 27.Mboumba Bouassa R-S, Bélec L, Gubavu C, et al. High prevalence of anal and oral high-risk human papillomavirus in human immunodeficiency virus–uninfected French men who have sex with men and use preexposure prophylaxis. Open Forum Infect Dis 2019; 6:ofz291. [DOI] [PMC free article] [PubMed] [Google Scholar]


