Abstract
Vitamin E, discovered in 1922, is essential for pregnant rats to carry their babies to term. However, one hundred years later, the molecular mechanisms for the vitamin E requirement during embryogenesis remain unknown. Vitamin E’s role during pregnancy has been difficult to study and thus, a vitamin E-deficient (E–) zebrafish embryo model was developed. Vitamin E deficiency in zebrafish embryos initiates lipid peroxidation, depletes a specific phospholipid [docosahexaenoic acid-phosphatidyl choline (DHA-PC)], causes secondary deficiencies of choline, betaine and critical thiols (such as glutathione), and dysregulates energy metabolism. Vitamin E deficiency not only distorts the carefully programmed development of the nervous system, but it leads to defects in several developing organs. Both the α-tocopherol transfer protein (α-TTP) and vitamin E are necessary for embryonic development, neurogenesis and cognition in this model and likely in human embryos. Elucidation of the control mechanisms for the cellular and metabolic pathways involved in the molecular dysregulation caused by vitamin E deficiency will lead to important insights into abnormal neurogenesis and embryonic malformations.
Keywords: alpha-tocopherol, neural tube defects, neural crest cells
Introduction
Vitamin E (vitamin E, α-tocopherol) was discovered about 100 y ago because it is required by pregnant rats to bring their fetuses to term (1); it is still unknown as to whether vitamin E is needed for a specific function by the mother, the placenta, or the developing embryo. It is well accepted that vitamin E functions as an antioxidant by scavenging lipid peroxyl radicals and preventing the propagation of lipid peroxidation (Figure 1) (2), but it is unclear how the antioxidant function relates to the deficiency symptoms. Vitamin E deficiency in humans is well known to cause ataxia, which is a result of the dying back of the sensory neurons of the peripheral nervous system (3, 4). Further, long-term (decades) α-tocopherol supplementation can prevent progression of the degenerating nervous system caused by vitamin E deficiency (5).
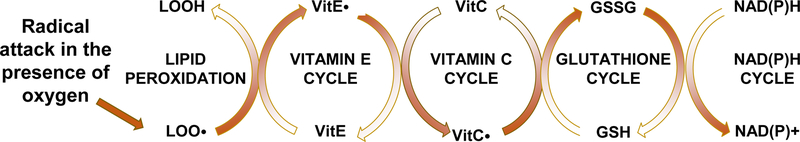
Figure 1. Vitamin E interactions with lipid peroxidation and antioxidants.
Vitamin E intercepts peroxyl radicals (LOO.), but becomes a radical itself (vitamin E.), which is reduced by VitC, oxidizing it. Glutathione reduces the VitC. and becomes oxidized itself. The GSSG is then enzymatically reduced by glutathione reductase. Thus, the reversal of the oxidation entire process is energy (NADPH) dependent.
Perhaps the most important vitamin E-related discovery in the past century has been the existence of the alpha-tocopherol transfer protein (α-TTP) (6–8). α-TTP facilitates the hepatic secretion of α-tocopherol, but not other forms of vitamin E [β-, γ-, δ-tocopherols, or α-, β-, γ-, δ-tocotrienols, or synthetic-α-tocopherol (2S-α-tocopherols)] into the circulation (9, 10). Thus, the liver via α-TTP maintains not only the plasma but the entire body α-tocopherol supply (11). In addition to the α-TTP gene initially being identified in humans, the National Center for Biotechnology Information (NCBI) lists 322 jawed vertebrates, including bony fishes, amphibians, birds and mammals, which have been reported to have the α-TTP gene (https://www.ncbi.nlm.nih.gov/gene/7274/ortholog/?scope=7776&term=TTPA). Apparently, the gene product, α-TTP, is critical for vertebrates.
Vitamin E background information
The vitamin E Dietary Reference Intake (DRI) for adult men and women (and individuals 14–18 y) was set in 2000 in the United States at a daily estimated average requirement (EAR) of 12 mg α-tocopherol and a recommended dietary allowance RDA of 15 mg (12). Plants synthesize eight different forms of vitamin E (13), which all have antioxidant activity. The vitamin E forms are not interconvertible by animals or humans, only plants have the appropriate enzymes (13). However, the human body preferentially retains α-tocopherol, α-tocopherol is the only form that has been shown to reverse clinical vitamin E deficiency symptoms. Therefore, only α-tocopherol meets human vitamin E requirements(12).
Human vitamin E deficiency is based on circulating α−tocopherol concentrations (<12 μmol/L serum or plasma). An increase in the prevalence of human vitamin E deficiency has been reported, based on low circulating α−tocopherol concentrations (14), may be a result of increased consumption of vegetable oil that has become rancid through multiple frying uses (15) or other causes of rancidity (lipid peroxidation). Vitamin E food sources in addition to vegetable oils include, nuts and seeds, and green/leafy vegetables (12).
α-Tocopherol absorption and transport have been studied using stable isotopes over the past 30 y. Recent studies show that intestinal absorption is about 55%, α-tocopherol is then transported in chylomicrons from the intestine to the liver where it is preferentially secreted from the liver in newly synthesized lipoproteins into the plasma (16). The α-tocopherol secretion from the liver mediated by α-TTP is the mechanism by which the plasma becomes α-tocopherol-enriched relative to the other forms of vitamin E (9, 17). Notably, the intestine does not require for α-tocopherol absorption and secretion in chylomicrons (10). Thus, the hepatic α-TTP is critical for plasma α-tocopherol enrichment.
Vitamin E and the nervous system
The clearest example of vitamin E deficiency in humans is caused by defective α-TTP and results in the disorder, Ataxia with vitamin E Deficiency [(AVED), OMIM #277460)]. AVED is characterized by degeneration of sensory neurons, a progressive dying back of peripheral nerves, which causes a spinocerebellar ataxia with Purkinje cell death (18, 19). The defective α-TTP in AVED causes low circulating α-tocopherol (<1 μmol/L plasma) and low peripheral nerve and adipose tissue α-tocopherol concentrations {Traber, 1987 #455}. In addition, the ataxia may also be a result of impaired α-tocopherol trafficking in the brain because α-TTP is located in the brain in Bergmann glial cells surrounding Purkinje cells (21, 22), suggesting α-TTP in glial cells traffics vitamin E to neurons in the brain.
Although it is clear the human body needs α-tocopherol and that other forms of vitamin E do not fulfill this vitamin function because α-TTP does not maintain them, it remains unclear as to why the embryo specifically needs α-tocopherol and what is its molecular function. Critically, humans with deficient plasma α−tocopherol concentrations (<12 μmol/L plasma) experience greater rates of miscarriage during early pregnancy (23), suggesting embryonic defects due to vitamin E deficiency. The Traber laboratory has been trying to address these questions by using the premier model of vertebrate embryogenesis, the zebrafish embryo.
Zebrafish embryo model system
Zebrafish embryos are widely used for investigating the molecular mechanisms of vertebrate development because the transcriptional networks, molecular responses and physiology are evolutionary conserved and similar to those in humans (24–26). Zebrafish are also highly relevant for antioxidant research because they require the same dietary antioxidants as do humans, specifically both vitamins E and C (27, 28). Thus, the vitamin E-deficient zebrafish embryo model allows evaluation of developmental dysregulation in a highly relevant model to establish the mechanisms for the embryonic vitamin E requirement.
The Traber laboratory group has pioneered the use of vitamin E deficient or sufficient (E– or E+) diets fed to adult zebrafish that are spawned to obtain E– and E+ embryos (29, 30). These E– and E+ fish lay and fertilize eggs in similar numbers (31). Biological variables, such as sex, age, weight, and underlying health conditions (with the exception of vitamin E deficiency) are similar between the groups. Vitamin E deficiency causes >70% of the E– embryos to die or be malformed by 72 hours post-fertilization (hpf) (32) with significant histologic abnormalities as early as 12 hpf (33), although the E+ and E– embryos appear phenotypically normal at 24 hpf (32). Each embryo is a self-contained unit that does not require food until after 120 hpf. Thus, embryonic vitamin E deficiency can be studied as a progression of deleterious outcomes as the embryo progresses through the various developmental stages impacted by increasing lipid peroxidation.
Metabolic consequences of vitamin E-deficiency in zebrafish embryos
Potentially, one reason cells in E– embryos die is due to increased lipid peroxidation because it is a self-propagating cycle that generates toxic compounds causing cell death (34, 35), while vitamin E prevents propagation of lipid peroxidation (36–38). Thus, inadequate vitamin E in lipid peroxidation-susceptible cells, such as neural crest cells (39–42), could be a cause for cell death.
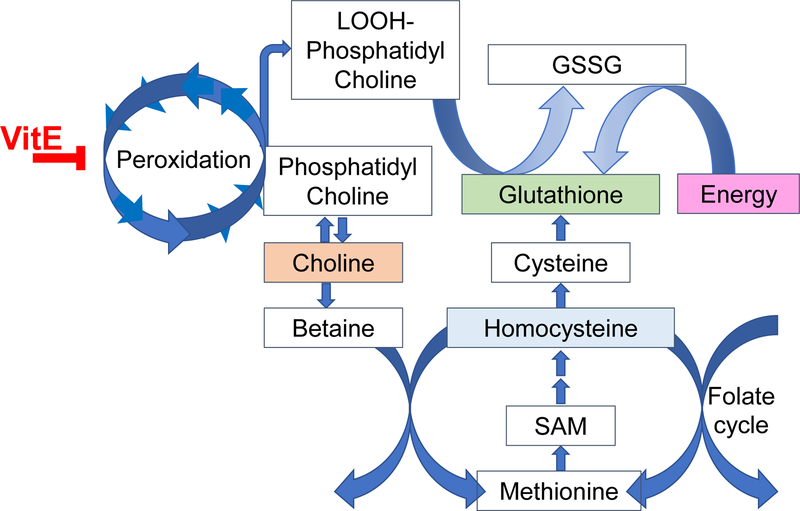
Both targeted and untargeted mass spectrometry approaches (metabolomics and lipidomics) were used to determine why the E– embryo dies. McDougall et al (32) discovered that vitamin E deficiency causes a fatal depletion of energy producing nutrients (e.g. glucose for NADPH production via the Pentose Phosphate Pathway) and that glucose repletion of the embryo by injection at an early stage could be used for rescue. Additionally, the E– embryos at 24 h post-fertilization were hypermetabolic, based on their oxygen consumption(32, 43). Thus, metabolic adaptation and compensation occur in the developing E– embryo to alleviate molecular, morphologic and biochemical phenotypes caused by the inadequate vitamin E supply. In support of this statement, E– embryos demonstrated a dysregulation of a complex, interwoven set of metabolic networks (Figure 2) (32, 43, 44). Further, quantitative measurements of glutathione, other thiols and methyl (1-C) donating molecules demonstrated that vitamin E deficiency leads to metabolic dysregulation, likely caused initially by lipid peroxidation of phosphatidyl choline-docosahexanoic acid (DHA-PC) (43, 45, 46), resulting in choline depletion and increased betaine production (46). Vitamin E-deficiency also dysregulates the methionine cycle (46). These pathways are interconnected with the folate cycle, and it is well-appreciated that inadequate folate causes neural tube defects, in humans and in zebrafish (47, 48). During vitamin E deficiency, the depleted molecule is likely a thiol, probably glutathione, which then appears to dysregulate the balance of cysteine homostasis, both through generation from cystathionine and through the Xc- antiporter (49). Additionally, methyl groups donors, such as S-adenosyl methionine are dysregulated, possibly causing epigenetic dysregulation (45).
Figure 2. Vitamin E interactions with lipid peroxidation and dysregulation of metabolism.
In the absence of vitamin E, lipid peroxidation becomes a chain reaction and depletes critical phospholipids, such as phosphatidyl choline (Oxidized phosphatidyl choline shown as a as a lipid hydroperoxide-phosphatidyl choline, LOOH-phosphatidyl choline). To replace these molecules, choline is needed, but choline is also needed via betaine for maintenance of the methionine cycle. Critically, lipid hydroperoxides (LOOH) also consume thiols, such as glutathione, which must be synthesized from the limited amino acid, cysteine. To maintain cysteine, the cell depends both on the methionine cycle as well as the Xc- antiporter. Thus, with inadequate vitamin E, multiple overlapping pathways become depleted and dysregulated.
The critical role of vitamin E as an antioxidant and the relationship between lipid peroxidation, glutathione and other thiols, taken together suggest that the abnormalities and lethality observed in the E– embryos are a result of lipid peroxidation-dependent death mechanisms, such as ferroptosis, as has been described for liver (36). Vitamin E deficiency impairs the zebrafish embryo at a time prior to when a woman knows she is pregnant, very similarly to the actions of folic acid deficiency on neural tube development. Although overt vitamin E deficiency is rare, the prevalence of vitamin E deficiency (serum α-tocopherol concentrations 12 μmol/L) in Bangladesh is estimated at 70% of women (23). Bangladeshi women with low α-tocopherol concentrations were ∼1.8 times more likely to miscarry (23). Additionally, Balogun et al in a Cochrane Database Systematic review (50) reported that “There was evidence of a decrease in the risk for stillbirth among women receiving multivitamins plus iron and folic acid compared to iron and folate only groups (RR 0.92, 95% CI 0.85 to 0.99, 10 trials, 79,851 women; high-quality evidence).” The beneficial results from supplementation with both multivitamins plus folate and iron supports the idea that the stillbirth in humans induced by vitamin E inadequacy can be reversed by multivitamins containing vitamin E, but further research is needed. Additionally, vitamin E deficiency in zebrafish embryos induces secondary deficiencies of DHA, choline, and glucose. Choline depletion may be the most important for humans because people do not consume sufficient choline (25). Choline is a methyl donor that works in concert with folic acid and other B-vitamins and there is cross-talk between methylation status and energy homeostasis (51).
Vitamin E and neurogenesis
In early studies characterizing vitamin E deficiency in rodents, abnormalities were described to include exencephaly (52, 53), dorsal root ganglia degeneration and defective blood brain barrier formation (53). Importantly, neural tube defects were also described in vitamin E-deficient mice (54–58). Vitamin E protects zebrafish and rodent embryos at embryological states in which neural tube defects occur in human embryos 18–19 hpf in zebrafish,(59) 9–12 days in rats(60) and 22–30 days in humans,(61–63) (Table 1).
Table 1.
Comparisons of timing of developmental stages between zebrafish, rats and humans.
| Developmental Stage | Zebrafish | Rat | Human |
|---|---|---|---|
|
| |||
| Blastula/Blastocyst | 2–5 h | 3–5 d | 4–6 d |
| Implantation | n/a | 6 d | 8–10 d |
| Neural Plate | 10 h | 9.5 d | 17–19 d |
| Neural Tube | 18–19 h | 9–12 d | 22–30 d |
| First Heartbeat | 24 h | 10.2 d | 22 d |
| Birth/Hatching | 48–72 h | 21 d | 253 d |
Neural crest cells are also important for evaluation of the impact of vitamin E deficiency during embryonic development. Neural crest cells are stem cells that differentiate into precursors of the peripheral nervous system, as well as the cardiovascular system, craniofacial skeleton and pigment epithelia (64). They migrate and differentiate into distinct populations along the embryo body axis during embryogenesis. Neural crest cells have a limited supply of nutrients for their migration through the embryo and are, thus, especially susceptible to oxidative damage (39). Studies in E– embryos indicated these cells need more vitamin E antioxidant protection (33). Specifically, neural crest cell formation is the result of a well-orchestrated gene regulatory network (65). SRY-related HMG-box 10 (SOX10) protein, a transcription factor, has a direct role in sensory neuron specification (66) and most peripheral nervous system neurons and glia are neural crest cell-derived and express Sox10 (65). These peripheral nervous system sites are also the most susceptible to damage in vitamin E deficient humans (67). Importantly, E– zebrafish embryos demonstrated fewer cells expressing Sox10 (33). Collectively these data suggest that impaired neurodevelopment and degeneration are associated with neural crest cell abnormalities and cells derived from neural crest cells. Apparently, metabolic adaptation of the neural crest cells to vitamin E deficiency limits their migration, proliferation, differentiation, and survival.
Requirement for α-TTP and α-tocopherol in embryonic development
α-TTP was reported in the developing human embryo at 5 to 12 weeks of gestation, specifically, α-TTP is expressed in the yolk sac (68). Thus, zebrafish embryos with their yolk sac and ease of visualization make a good model for these studies. The zebrafish embryo Ttpa mRNA increases 7-fold by 12 hpf and remains elevated at 24–36 hpf, while its knockdown causes embryonic 100% mortality by 24 hpf (69). Further, α-TTP is essential for normal neural plate and neural tube formation (69). Ttpa is also found in developing brain, eyes and tail bud (69). Thus, vitamin E uptake and trafficking occurs in the nervous system prior to development of the liver or circulatory system, suggesting it is critical in the nervous system for delivery of vitamin E to specific regions. Ttpa is also highly expressed at the leading edges of the brain cavities during brain ventricle formation (33). Importantly, in E– embryos the development of the brain, the migration of neural stem cells and the formation of the spinal cord were impaired (33). Taken together these data show that both α-TTP and vitamin E are critical molecules during embryonic development, especially during neurulation (neural plate and tube formation) (69) and neural crest cell migration (33).
Neurodegeneration and cognition
Recent developments in neuroscience have shown that the human hippocampus, the site of memory and learning, undergoes neurogenesis in adults, but declines with aging, which may be linked to cognitive impairments (70). In 2015, 46.8 million people worldwide were living with dementia and this number will reach 131.5 million in 2050 (71) Brain neurodegenerative disorders (e.g. Alzheimer’s disease and -related diseases, and Down syndrome) are associated with (1) cognitive decline,(72, 73) (2) increased lipid peroxidation,(74) (3) changes in metabolic function,(75) and (4) mitochondrial dysfunctions and metabolic reprogramming (76, 77) The research community has focused on damaged proteins, but lipid peroxidation may be more dangerous in the brain because it is a self-propagating cycle that generates radicals and toxic lipid oxidation end-products (e.g. reactive aldehydes) that can damage proteins, DNA, etc. Our discoveries in adult zebrafish also show that low brain α-tocopherol is associated with a nearly 60% depletion of 19 brain lysophosphatidyl cholines (lysoPL, combined P=0.0003), especially 3 lysoPL containing DHA: lysoP-choline, -ethanolamine, -serine (78). The wide variety of lysoPLs that are depleted suggests that the entire lysoPL substrate population is affected. LysoPL are needed for phospholipid remodeling during membrane synthesis, repair and replacement (79). The brain acquires DHA as lysoPL-DHA (80). A transporter from the major facilitator superfamily, MFSD2A, which is critical to maintain the blood brain barrier (81), facilitates brain lysoPL-DHA uptake (82). The MFSD2A transporter is a critical mechanism for lysoPL-DHA (80) delivery to the brain (82), resolving a long-standing mystery of how the brain acquires DHA (83). Importantly, lysoPL-DHA depletion is linked to Alzheimer Disease (84).
Protection from lipid peroxidation is provided by a network of antioxidants, including vitamin E and glutathione (85), and is dependent on energy production [the reduced form of nicotinamide adenine dinucleotide phosphate, NADP(H)] (32). The brain is particularly susceptible to lipid peroxidation due to its high concentration of polyunsaturated lipids [e.g. phosphatidyl choline with docosahexaenoic acid, DHA-PC (18:0/22:6)] (86, 87). DHA-PC is a significant membrane component in the brain(88) and a human serum biomarker of Alzheimer disease (89). To replace peroxidized DHA-PC requires (1) GSH to detoxify the oxidized lipids(85) and (2) choline(90), a one carbon (1-C) donor for DHA-PC synthesis (90, 91). The metabolic connection linking choline and 1-C donors is through homocysteine.
Since homocysteine is an oxidation product and vitamin E is an antioxidant, oxidative damage has long been a focus in Alzheimer’s Disease research. Homocysteine elevation has been long recognized as a risk factor for dementia(92), is used as a biomarker to predict Alzheimer disease pathology in humans(93), and homocysteinemia is decreased by increased B-vitamin intakes (94). Nonetheless, clinical trials using B-vitamin supplements have shown no benefit for improving cognitive impairment or dementia(95), despite slowing of brain shrinkage (96). Could hyperhomocysteinemia be a result of inadequate brain vitamin E? Human clinical trials have shown that vitamin E supplements slowed the onset of dementia in patients with Alzheimer’s Disease (97, 98). However, meta-analyses of vitamin E supplements used in a number of Alzheimer’s Disease trials have shown no statistical benefit(99, 100). By contrast, low blood vitamin E levels are associated with high AD incidence (101). Importantly, improved cognition and less brain shrinkage were associated with long-term dietary patterns that increase blood levels of both B-vitamins and vitamin E (101, 102). Based on the molecular interrelationships between vitamin E and B-vitamins in neurodegeneration, it is clear that chronic poor dietary choices such as those low in vitamins B and E, can promote neurodegeneration and cognitive decline.
Conclusions
Significant progress has been made in understanding the role of vitamin E in embryogenesis. The Traber lab has taken on these investigations because: (1) vitamin E is critical during neuro-embryogenesis; (2) the mechanisms by which vitamin E prevents defects during neural differentiation are heretofore unstudied; (3) the pathophysiological mechanisms of embryonic neurodegeneration are investigated in a highly relevant paradigm since women are increasingly consuming inadequate amounts of vitamin E (14, 103); and (4) the role of lipid peroxidation, as a mediator of embryonic neurodegeneration has largely been overlooked, although the embryonic environment is recognized to be under oxidative stress (104) and redox status is tightly regulated (105).
The Traber lab has focused on the unknown mechanism of an antioxidant vitamin in a vertebrate embryo to prevent nervous system abnormalities. Elucidation of the cellular and metabolic pathways involved in the molecular dysregulation caused by vitamin E deficiency will lead to important insights into abnormal neurogenesis and embryonic malformations. Identification of vitamin E-dependent pathways is necessary to provide critical knowledge necessary for effective progress in public policy concerning nutritional and therapeutic interventions to prevent malformations, such as neural tube defects during early embryonic development and potentially associated miscarriages (23).
Importantly, for human disease pathophysiology, the brain is particularly susceptible to lipid peroxidation due to its high concentration of polyunsaturated lipids, especially DHA-PC (86, 87). Based on the molecular interrelationships between vitamin E and B-vitamins in neurodegeneration, it is clear that chronic poor dietary choices may exacerbate deficiencies that can promote neurodegeneration and cognitive decline. What is less clear is whether any dietary changes can reverse damage or improve cognition.
Acknowledgements
MGT is supported in part by the Ava Helen Pauling Endowment to the Linus Pauling Institute.
Financial Support
The research reported in this publication was partially supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award P30ES030287. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The author declares no competing financial and/or non-financial interests in relation to the work described.
References
- 1.Evans HM & Bishop KS (1922) On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56, 650–651. [DOI] [PubMed] [Google Scholar]
- 2.Niki E (2014) Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med 66, 3–12. [DOI] [PubMed] [Google Scholar]
- 3.Sokol RJ, Bove KE, Heubi JE et al. (1983) Vitamin E deficiency during chronic childhood cholestasis: presence of sural nerve lesion prior to 2 1/2 years of age. J Pediatr 103, 197–204. [DOI] [PubMed] [Google Scholar]
- 4.Traber MG (2014) Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 5, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohlschutter A, Finckh B, Nickel M et al. (2020) First Recognized Patient with Genetic Vitamin E Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neurodegener Dis 20, 35–38. [DOI] [PubMed] [Google Scholar]
- 6.Ben Hamida C, Doerflinger N, Belal S et al. (1993) Localization of Friedreich ataxia phenotype with selective vitamin E deficiency to chromosome 8q by homozygosity mapping. Nat Genet 5, 195–200. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Arai H, Miyata A et al. (1993) Primary structure of alpha-tocopherol transfer protein from rat liver. Homology with cellular retinaldehyde-binding protein. J Biol Chem 268, 17705–17710. [PubMed] [Google Scholar]
- 8.Ouahchi K, Arita M, Kayden H et al. (1995) Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nature Genetics 9, 141–145. [DOI] [PubMed] [Google Scholar]
- 9.Traber MG, Burton GW, Ingold KU et al. (1990) RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res 31, 675–685. [PubMed] [Google Scholar]
- 10.Traber MG, Sokol RJ, Burton GW et al. (1990) Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest 85, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard SW, Terasawa Y, Farese RV, Jr. et al. (2002) Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein--null mice. Am J Clin Nutr 75, 555–560. [DOI] [PubMed] [Google Scholar]
- 12.Food and Nutrition Board & Institute of Medicine (2000) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): National Academy Press. [PubMed] [Google Scholar]
- 13.Mene-Saffrane L & DellaPenna D (2010) Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol Biochem 48, 301–309. [DOI] [PubMed] [Google Scholar]
- 14.Peter S, Friedel A, Roos FF et al. (2015) A systematic review of global alpha-tocopherol status as assessed by nutritional intake levels and blood serum concentrations. Int J Vitam Nutr Res 85, 261–281. [DOI] [PubMed] [Google Scholar]
- 15.Fišnar J, Doležal M Réblová Z (2014) Tocopherol losses during pan-frying. European Journal of Lipid Science and Technology 116, 1694–1700. [Google Scholar]
- 16.Traber MG, Leonard SW, Ebenuwa I et al. (2019) Vitamin E absorption and kinetics in healthy women, as modulated by food and by fat, studied using 2 deuterium-labeled alpha-tocopherols in a 3-phase crossover design. Am J Clin Nutr 110, 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traber MG, Sokol RJ, Kohlschutter A et al. (1993) Impaired discrimination between stereoisomers of alpha-tocopherol in patients with familial isolated vitamin-E-deficiency. Journal of Lipid Research 34, 201–210. [PubMed] [Google Scholar]
- 18.Di Donato I, Bianchi S Federico A (2010) Ataxia with vitamin E deficiency: update of molecular diagnosis. Neurol Sci 31, 511–515. [DOI] [PubMed] [Google Scholar]
- 19.Schuelke M Ataxia with Vitamin E deficiency. GeneReviews(R). [Google Scholar]
- 20.Traber MG, Sokol RJ, Ringel SP et al. (1987) Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N Engl J Med 317, 262–265. [DOI] [PubMed] [Google Scholar]
- 21.Ulatowski L, Parker R, Warrier G et al. (2014) Vitamin E is essential for Purkinje neuron integrity. Neuroscience 260, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcos P, Gonzalez-Fuentes J, Castro-Vazquez L et al. (2018) Vitamin transporters in mice brain with aging. J Anat 232, 699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamim AA, Schulze K, Merrill RD et al. (2015) First-trimester plasma tocopherols are associated with risk of miscarriage in rural Bangladesh. Am J Clin Nutr 101, 294–301. [DOI] [PubMed] [Google Scholar]
- 24.Howe K Clark MD Torroja CF et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace TC & Fulgoni VL 3rd (2016) Assessment of total choline intakes in the United States. J Am Coll Nutr 35, 108–112. [DOI] [PubMed] [Google Scholar]
- 26.Garcia GR, Noyes PD Tanguay RL (2016) Advancements in zebrafish applications for 21st century toxicology. Pharmacol Ther 161, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drouin G, Godin JR Page B (2011) The genetics of vitamin C loss in vertebrates. Curr Genomics 12, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkwood JS, Lebold KM, Miranda CL et al. (2012) Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J Biol Chem 287, 3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebold KM, Kirkwood JS, Taylor AW et al. (2013) Novel liquid chromatography-mass spectrometry method shows that vitamin E deficiency depletes arachidonic and docosahexaenoic acids in zebrafish (Danio rerio) embryos. Redox Biol 2, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller GW, Labut EM, Lebold KM et al. (2012) Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J Nutr Biochem 23, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebold KM, Jump DB, Miller GW et al. (2011) Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio). J Nutr 141, 2113–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDougall M, Choi J, Kim HK et al. (2017) Lethal dysregulation of energy metabolism during embryonic vitamin E deficiency. Free Radic Biol Med 104, 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Head B, La Du J, Tanguay RL et al. (2020) Vitamin E is necessary for zebrafish nervous system development. Sci Rep 10, 15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang WS & Stockwell BR (2016) Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol 26, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon SJ, Lemberg KM, Lamprecht MR et al. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson BA, Tobe R, Yefremova E et al. (2016) Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 9, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zilka O, Shah R, Li B et al. (2017) On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci 3, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angeli JPF, Shah R, Pratt DA et al. (2017) Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci 38, 489–498. [DOI] [PubMed] [Google Scholar]
- 39.Eason J, Williams AL, Chawla B et al. (2017) Differences in neural crest sensitivity to ethanol account for the infrequency of anterior segment defects in the eye compared with craniofacial anomalies in a zebrafish model of fetal alcohol syndrome. Birth Defects Res 109, 1212–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai D, Dixon J, Achilleos A et al. (2016) Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat Commun 7, 10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai D & Trainor PA (2016) Face off against ROS: Tcof1/Treacle safeguards neuroepithelial cells and progenitor neural crest cells from oxidative stress during craniofacial development. Dev Growth Differ 58, 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han D, Schomacher L, Schule KM et al. (2019) NEIL1 and NEIL2 DNA glycosylases protect neural crest development against mitochondrial oxidative stress. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDougall M, Choi J, Kim HK et al. (2017) Lipid quantitation and metabolomics data from vitamin E-deficient and -sufficient zebrafish embryos from 0 to 120 hours-post-fertilization. Data Brief 11, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDougall M, Choi J, Magnusson K et al. (2017) Chronic vitamin E deficiency impairs cognitive function in adult zebrafish via dysregulation of brain lipids and energy metabolism. Free Radic Biol Med 112, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDougall M, Choi J, Truong L et al. (2017) Vitamin E deficiency during embryogenesis in zebrafish causes lasting metabolic and cognitive impairments despite refeeding adequate diets. Free Radic Biol Med 110, 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Head B, Leonard SW et al. (2021) Vitamin E deficiency dysregulates thiols, amino acids and related molecules during zebrafish embryogenesis. Redox Biol 38, 101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MS, Bonner JR, Bernard DJ et al. (2012) Disruption of the folate pathway in zebrafish causes developmental defects. BMC Dev Biol 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kao TT, Chu CY, Lee GH et al. (2014) Folate deficiency-induced oxidative stress contributes to neuropathy in young and aged zebrafish--implication in neural tube defects and Alzheimer’s diseases. Neurobiol Dis 71, 234–244. [DOI] [PubMed] [Google Scholar]
- 49.McBean GJ (2017) Cysteine, glutathione, and thiol redox balance in astrocytes. Antioxidants (Basel) 6, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balogun OO, da Silva Lopes K, Ota E et al. (2016) Vitamin supplementation for preventing miscarriage. Cochrane Database Syst Rev 5, CD004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisel SH (2013) Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin Chem Lab Med 51, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng DW, Chang LF Bairnson TA (1957) Gross observations on developing abnormal embryos induced by maternal vitamin E deficiency. Anat Rec 129, 167–185. [DOI] [PubMed] [Google Scholar]
- 53.Verma K & Wei King D (1967) Disorders of the developing nervous system of vitamin E-deficient rats. Acta Anat (Basel) 67, 623–635. [DOI] [PubMed] [Google Scholar]
- 54.Jishage K, Arita M, Igarashi K et al. (2001) alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. Journal of Biological Chemistry 276, 1669–1672. [DOI] [PubMed] [Google Scholar]
- 55.Santander N, Lizama C, Parga MJ et al. (2017) Deficient vitamin E uptake during development impairs neural tube closure in mice lacking lipoprotein receptor SR-BI. Sci Rep 7, 5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E, Cham CM, Veniant MM et al. (1998) Dual mechanisms for the low plasma levels of truncated apolipoprotein B proteins in familial hypobetalipoproteinemia. Analysis of a new mouse model with a nonsense mutation in the Apob gene. J Clin Invest 101, 1468–1477. [PMC free article] [PubMed] [Google Scholar]
- 57.Homanics GE, Maeda N, Traber MG et al. (1995) Exencephaly and hydrocephaly in mice with targeted modification of the apolipoprotein B (Apob) gene. Teratology 51, 1–10. [DOI] [PubMed] [Google Scholar]
- 58.Raabe M, Flynn LM, Zlot CH et al. (1998) Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci U S A 95, 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimmel CB, Ballard WW, Kimmel SR et al. (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310. [DOI] [PubMed] [Google Scholar]
- 60.Federation of American Societies for Experimental Biology. Committee on Biological Handbooks. & Altman PL(1962) Growth, including reproduction and morphological development, Biological handbooks. Washington. [Google Scholar]
- 61.O’Rahilly R (1979) Early human development and the chief sources of information on staged human embryos. Eur J Obstet Gynecol Reprod Biol 9, 273–280. [DOI] [PubMed] [Google Scholar]
- 62.Wilcox AJ, Baird DD Weinberg CR (1999) Time of implantation of the conceptus and loss of pregnancy. N Engl J Med 340, 1796–1799. [DOI] [PubMed] [Google Scholar]
- 63.Gilbert SF (2010) Developmental Biology, Ninth Edition ed. Sunderland, MA: Sinauer Associates, Inc. [Google Scholar]
- 64.Tang W, Martik ML, Li Y et al. (2019) Cardiac neural crest contributes to cardiomyocytes in amniotes and heart regeneration in zebrafish. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weider M & Wegner M (2017) SoxE factors: Transcriptional regulators of neural differentiation and nervous system development. Semin Cell Dev Biol 63, 35–42. [DOI] [PubMed] [Google Scholar]
- 66.Carney TJ, Dutton KA, Greenhill E et al. (2006) A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 133, 4619–4630. [DOI] [PubMed] [Google Scholar]
- 67.El Euch-Fayache G, Bouhlal Y, Amouri R et al. (2014) Molecular, clinical and peripheral neuropathy study of Tunisian patients with ataxia with vitamin E deficiency. Brain : a journal of neurology 137, 402–410. [DOI] [PubMed] [Google Scholar]
- 68.Jauniaux E, Cindrova-Davies T, Johns J et al. (2004) Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab 89, 1452–1458. [DOI] [PubMed] [Google Scholar]
- 69.Miller GW, Ulatowski L, Labut EM et al. (2012) The alpha-tocopherol transfer protein is essential for vertebrate embryogenesis. PLoS One 7, e47402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boldrini M, Fulmore CA, Tartt AN et al. (2018) Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22, 589–599 e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prince M, Comas-Herrera A, Knapp M et al. (2016) World Alzheimer Report 2016, pp. 140 [Alzheimer’s Disease International, editor]. London: The International Federation of Alzheimer’s Disease and Related Disorders Societies, Inc. [Google Scholar]
- 72.Barone E, Arena A, Head E et al. (2018) Disturbance of redox homeostasis in Down Syndrome: Role of iron dysmetabolism. Free Radic Biol Med 114, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butterfield DA & Boyd-Kimball D (2018) Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J Alzheimers Dis 62, 1345–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buijs M, Doan NT, van Rooden S et al. (2017) In vivo assessment of iron content of the cerebral cortex in healthy aging using 7-Tesla T2*-weighted phase imaging. Neurobiol Aging 53, 20–26. [DOI] [PubMed] [Google Scholar]
- 75.Fu W, Shi D, Westaway D et al. (2015) Bioenergetic mechanisms in astrocytes may contribute to amyloid plaque deposition and toxicity. J Biol Chem 290, 12504–12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grimm A, Friedland K Eckert A (2016) Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer’s disease. Biogerontology 17, 281–296. [DOI] [PubMed] [Google Scholar]
- 77.Demetrius LA, Magistretti PJ Pellerin L (2014) Alzheimer’s disease: the amyloid hypothesis and the Inverse Warburg effect. Front Physiol 5, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choi J, Leonard SW, Kasper K et al. (2014) Vitamin E deficiency dysregulates brain phospholipids and lysophospholipids in zebrafish in preparation.
- 79.Shindou H, Hishikawa D, Harayama T et al. (2013) Generation of membrane diversity by lysophospholipid acyltransferases. Journal of biochemistry 154, 21–28. [DOI] [PubMed] [Google Scholar]
- 80.Lagarde M, Bernoud N, Brossard N et al. (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. Journal of molecular neuroscience : MN 16, 201–204; discussion 215–221. [DOI] [PubMed] [Google Scholar]
- 81.Ben-Zvi A, Lacoste B, Kur E et al. (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen LN, Ma D, Shui G et al. (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506. [DOI] [PubMed] [Google Scholar]
- 83.Pelerin H, Jouin M, Lallemand MS et al. (2014) Gene expression of fatty acid transport and binding proteins in the blood-brain barrier and the cerebral cortex of the rat: Differences across development and with different DHA brain status. Prostaglandins, leukotrienes, and essential fatty acids. [DOI] [PubMed] [Google Scholar]
- 84.Semba RD (2020) Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv Nutr 11, 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cadenas E, Packer L Traber MG (2016) Antioxidants, oxidants, and redox impacts on cell function - A tribute to Helmut Sies. Arch Biochem Biophys 595, 94–99. [DOI] [PubMed] [Google Scholar]
- 86.Cobley JN, Fiorello ML Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15, 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Y, Song J, Wang Y et al. (2019) The potential role of ferroptosis in neonatal brain injury. Front Neurosci 13, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farkas T, Kitajka K, Fodor E et al. (2000) Docosahexaenoic acid-containing phospholipid molecular species in brains of vertebrates. Proc Natl Acad Sci U S A 97, 6362–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuki D, Sugiura Y, Zaima N et al. (2014) DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Sci Rep 4, 7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernhard W, Bockmann K, Maas C et al. (2020) Combined choline and DHA supplementation: a randomized controlled trial. Eur J Nutr 59, 729–739. [DOI] [PubMed] [Google Scholar]
- 91.Yan J, Ginsberg SD, Powers B et al. (2014) Maternal choline supplementation programs greater activity of the phosphatidylethanolamine N-methyltransferase (PEMT) pathway in adult Ts65Dn trisomic mice. FASEB J 28, 4312–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith AD & Refsum H (2016) Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36, 211–239. [DOI] [PubMed] [Google Scholar]
- 93.Dayon L, Guiraud SP, Corthesy J et al. (2017) One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: homocysteine and beyond. Alzheimers Res Ther 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cordaro M, Siracusa R, Fusco R et al. (2021) Involvements of Hyperhomocysteinemia in Neurological Disorders. Metabolites 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aisen PS, Schneider LS, Sano M et al. (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 300, 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Douaud G, Refsum H, de Jager CA et al. (2013) Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 110, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dysken MW, Sano M, Asthana S et al. (2014) Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sano M, Ernesto C, Thomas RG et al. (1997) A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 336, 1216–1222. [DOI] [PubMed] [Google Scholar]
- 99.Farina N, Isaac MG, Clark AR et al. (2012) Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev 11, CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farina N, Llewellyn D, Isaac MG et al. (2017) Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev 1, CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Wilde MC, Vellas B, Girault E et al. (2017) Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement (N Y) 3, 416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bowman GL, Silbert LC, Howieson D et al. (2012) Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology 78, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.US Department of Health and Human Services & US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Published online: 2015. [Google Scholar]
- 104.Rodriguez-Rodriguez P, Ramiro-Cortijo D, Reyes-Hernandez CG et al. (2018) Implication of oxidative stress in fetal programming of cardiovascular disease. Front Physiol 9, 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones DP & Sies H (2015) The redox code. Antioxid Redox Signal 23, 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]