Abstract
Objective:
To test the hypothesis that factor eight inhibitor bypassing activity (FEIBA) can be used to control bleeding following left ventricular assist device (LVAD) implantation without increasing the 14-day composite thrombotic outcome of pump thrombus, ischemic cerebrovascular accidents, pulmonary embolism, and deep venous thrombosis.
Design:
Retrospective cohort study.
Setting:
Academic hospital.
Participants:
Three hundred nineteen consecutive patients who underwent LVAD implantation (December 1, 2009 to December 30, 2018).
Intervention:
FEIBA administered to control perioperative hemorrhage.
Measurements and Main Results:
The 82 patients (25.7%) in the FEIBA cohort had more risk factors for perioperative hemorrhage, such as lower preoperative platelet count (169 ± 66 v 194 ± 68 × 103/mL, p = 0.004), prior cardiac surgery (36.6% v 21.9%, p = 0.008), and longer cardiopulmonary bypass (CPB) time (100.3 v 75.2 minutes, p = 0.001) than the 237 controls. After 16.6 units (95% CI: 14.3–18.9) of blood products were given, 992 units (95% CI: 821–1163) of FEIBA were required to control bleeding in the FEIBA cohort. Compared to the controls, there were no differences in the 14-day composite thrombotic outcome (11.0% v 7.6%, p = 0.343) or mortality rate (3.7% v 1.3%, p = 0.179). Multivariate logistical regression identified preoperative international normalized ratio (odds ratio [OR]: 1.30, 95% CI: 1.04–1.62) and CPB time (OR: 1.11, 95% CI: 1.02–1.20) as risk factors for 14-day thrombotic events, but FEIBA usage was not associated with an increased risk.
Conclusions:
In this retrospective cohort study, the use of FEIBA (~1,000 units, ~13 units/kg) to control perioperative hemorrhage following LVAD implantation was not associated with increases in mortality or composite thrombotic outcome.
Keywords: left ventricular assist device, LVAD, FEIBA, factor 8 inhibitor bypass activity, procoagulants, perioperative hemorrhage, thrombotic events, thrombosis, CVA, DVT, PE, pump thrombus
There is a delicate balance between controlling refractory bleeding following left ventricular assist device (LVAD) insertion and maintaining adequate anticoagulation to prevent pump thrombus and other serious thrombotic events. Despite major advances in the safety and durability of mechanical circulatory support, both hemorrhagic and thrombotic events remain common, and carry significant morbidity and mortality.1–7 The highest risk of these events appears to occur during the perioperative period.8,9
Standard treatment of coagulopathy after cardiac surgery primarily consists of blood product transfusion and antifibrinolytics, which carry their own inherent risks of volume overload and thrombosis to the already critically ill patient.10–12 The optimal treatment for coagulopathy in LVAD patients has been elusive, at least in part due to the significant heterogeneity of pathophysiologic mechanisms leading to the coagulopathy seen after cardiac surgery.6,12,13 In the treatment of refractory hemorrhage, some centers have begun using “bypassing agents” as rescue therapy. Of these, a majority of reports related to the off-label use of recombinant Factor VIIa (NovoSeven, Novo Nordisk Inc., Plainsboro, NJ), with multiple studies noting substantial risk, and relative paucity of data showing its effectiveness to reduce bleeding.14–21 Comparatively, few studies have examined the use of factor 8 inhibitor bypassing activity (FEIBA, Baxalta Inc., Takeda Pharmaceutical Company Limited, Lexington, MA) in controlling coagulopathy after cardiac surgery, with only a single study of 25 cardiac surgery patients (five LVAD) examining use in LVAD implantation.22
FEIBA was developed to control coagulopathy in patients with hemophilia, to bypass factor inhibitors and directly activate the clotting cascade.23,24 FEIBA is categorized as an activated prothrombin complex concentrate and contains factors II, VII, IX, and X, with small amounts of factors IIa, IXa, and Xa, and larger amounts of VIIa, as well as proteins C and S.25,26 FEIBA works at multiple sites in the coagulation cascade, but the most widely accepted mechanism of action is that FEIBA aids in generation of the tenase complex (VIIIa and IXa) and the prothrombinase complex (Xa, Va, Ca2+, phospholipid) to facilitate thrombin generation, thus promoting hemostasis.25,26 The direct action of thrombin generation is appealing in refractory postoperative hemorrhage thought to be due to coagulopathy when large amounts of blood products have been ineffective in achieving adequate hemostasis. Additionally, FEIBA contains proteins C and S, which in hemophilia is thought to provide a balance of natural anticoagulants that may safeguard against thrombosis.26
The aim of this retrospective study was to examine the use of FEIBA as a rescue hemostatic agent for control of perioperative hemorrhage in LVAD patients who are at high risk for thromboses. The authors hypothesized that FEIBA could be used to control perioperative hemorrhage following LVAD implantation without increasing the risk of developing the composite thrombotic outcome, which was defined by the 14-day postoperative incidence of an ischemic cerebrovascular accident (iCVA), combination CVA (described as iCVA with hemorrhagic conversion), pulmonary embolus (PE), deep venous thrombosis (DVT), and pump thrombus. Secondary outcomes included 14-day mortality, individual rates of iCVA, combination CVA, PE, DVT, pump thrombus, and incidence of major bleeding as measured by gastrointestinal bleeds and intracranial hemorrhages (ICH). Additionally, the authors examined if there was a correlation between FEIBA dosage and thrombotic complications or timing of thromboses.
Methods
This was a single-center, retrospective cohort study of patients who underwent LVAD implantation (n = 319) between December 1, 2009 and December 30, 2018. The protocols used in this study were approved by the institutional review board (# 47223). Study data were collected and managed using Research Electronic Data Capture electronic data capture tools hosted at Stanford University. Research Electronic Data Capture is a secure, Health Insurance Portability and Accountability Act–compliant, web-based software platform designed to support data capture for research studies. Inclusion criteria were all patients aged 18-to-90 years who underwent LVAD implantation during this period. Outcome data were verified by review of the electronic medical record of all patients included in the study. The FEIBA cohort was defined by those who were administered FEIBA intraoperatively and/or within the first 48 postoperative hours. Controls were defined as all LVAD patients who did not receive FEIBA or recombinant factor 7 (rFVIIa). Of note, two patients in the control group received prothrombin complex concentrate (PCC) for preoperative warfarin reversal. At the authors’ institution, FEIBA administration is not yet protocolized and is administered as a hemostatic rescue agent at the discretion of the surgeon, anesthesiologist, or critical care physician when there is evidence of ongoing coagulopathic hemorrhage despite attempts to correct coagulopathy by transfusion of platelets, fresh frozen plasma (FFP), and cryoprecipitate (cryo). Blood product administration was collected intraoperatively and from postoperative day (POD) zero-to-seven for packed red blood cells (pRBC), FFP, platelets, and cryo. The distinction between blood products given during POD zero-two and POD three-seven was used to provide insight into different phases of the immediate postoperative period when some patients still are being treated with FEIBA and require additional blood products for persistent bleeding thought to be secondary to coagulopathy. Additionally, the dosage of FEIBA was collected to explore if there was an association between dose of FEIBA and thrombotic outcomes.
The primary composite outcome was the 14-day incidence of serious thrombotic events as described above. Individual secondary outcomes, such as iCVA and combination CVA (combo CVA), were defined as radiologically (computed tomography [CT] or magnetic resonance imaging) confirmed acute ischemic stroke with or without hemorrhagic conversion. Pump thrombus was defined as thrombus requiring pump exchange. Diagnosis of PE was confirmed by CT angiography of the chest and all DVTs were diagnosed by ultrasonography. Additional, secondary outcomes included 30-day mortality and hemorrhagic complications including gastrointestinal bleeds and ICH. GI bleed was diagnosed if confirmed by esophagogastroduodenoscopy, colonoscopy, or documentation of high clinical suspicion by gastroenterology consultation. ICH also was diagnosed radiologically by CT scan.
Data were analyzed using Stata version 16.0 (StataCorp, LLC, College Station, TX) and GraphPad Prism version 9.0.2 (GraphPad Software, La Jolla, CA). Categorical variables were analyzed by Fisher exact for n ≤five and chi-squared for n > five. Continuous variables were analyzed by two-sided t test with standard deviation. A two-way analysis of variance was used to assess differences in blood product administration over time. Receiver operator characteristic curves were used to evaluate the area under the curve for variables predicting the composite outcome, such as FEIBA dosage. Univariate logistical regression was performed to analyze individual variable’s effect on the composite outcome. Multivariate logistical regression was used to examine variables for the odds of affecting the composite thrombotic outcome. The multivariate logistical regression model included factors that significantly differed between the control and FEIBA cohorts, variables which univariate logistical regression was predicted to affect the odds of the composite outcome, and factors that are known to alter thrombotic outcomes. An exhaustive subtractive method was used to develop the model using receiver operator curve analysis to maximize the area under the curve (AUC) for the model and best Akaike information criterion, while balancing the inclusion of covariates associated with LVAD outcomes. The final model included body mass index, cardiopulmonary bypass (CPB) time, preoperative platelet count and international normalized ratio, prior cardiac surgery, preoperative Impella placement, Interagency Registry for Mechanically Assisted Circulatory Support profile to control for risk, type of LVAD implanted (HeartWare, HeartMate II, or Heartmate III), and FEIBA treatment. Clinically relevant differences included the fraction of CPB time in ten-minute intervals and platelet count changes of 20,000. Patients without all of the model variables were excluded from the regression analysis (a total of 302 patient included). Time-to-event analysis was conducted using a survival proportion method with log-rank test to examine the effect of time from surgery to composite thrombotic outcomes between the FEIBA and control cohorts. The authors performed a post hoc power calculation for a two-sample (control n = 237, FEIBA n = 82) comparison with a standard deviation of 5% and alpha of 0.05 for the 14-day composite thrombotic outcome (control = 7.6%, FEIBA = 11.0%), which estimated the power of the study at 98.2% for being able to predict a significant difference in thrombotic events with FEIBA treatment.
Results
Patient Characteristics and Surgical Risk Factors
Of the 319 patients who underwent LVAD implantation at the authors’ institution from December 1, 2009 to December 30, 2018, 82 patients (25.7%) received FEIBA between POD zero and two (43 patients intraoperatively, 11 patients both intraoperatively and in the ICU during POD zero-two, and 28 patients only in the ICU during POD zero-two). FEIBA patients did not differ significantly compared to controls who did not require FEIBA for perioperative hemorrhage control with respect to age, sex, and comorbidities including type 2 diabetes mellitus, atrial fibrillation, smoking history, and prior stroke (Table 1). Patients who received FEIBA had a lower body mass index compared to controls (26.0 v 28.0; p = 0.017). The FEIBA cohort had more risk factors for perioperative hemorrhage compared to controls, such as lower preoperative platelet count (169 ± 68.0 v 194 ± 65.9 × 103 platelets/mL; p = 0.004), history of prior cardiac surgery× [30 (36.6%) v 52 (21.9%); p = 0.008], preoperative Impella (ABIOMED Inc., Danvers, MA) [11 (13.4%) v 15 (6.3%); p = 0.043], and longer CPB times (100.3 v . 75.2 minutes; p = 0.001) (Table 1).
Table 1.
Patient Characteristics and Surgical Risk Factors
| Preoperative Characteristics | Control (n = 237) | FEIBA (n = 82) | p Value |
|---|---|---|---|
| Male (%) | 176 (74.3) | 68 (82.9) | 0.111 |
| Age (y) | 56.2 ± 12.6 | 56.4 ± 14.4 | 0.909 |
| BMI (kg/m2) | 28.0 ± 6.3 | 26.0 ± 6.3 | 0.017* |
| Creatinine (mg/dL) | 1.45 ± 0.6 | 1.52 ± 1.1 | 0.639 |
| Diabetes (%) | 83 (35.0) | 28 (34.1) | 0.864 |
| Atrial fibrillation (%) | 148 (62.4) | 52 (63.4) | 0.778 |
| Liver disease (%) | 31 (13.1) | 13 (15.9) | 0.504 |
| Hx of tobacco (%) | 110 (46.4) | 43 (52.4) | 0.330 |
| Prior CVA (%) | 42 (17.7) | 12 (14.6) | 0.538 |
| Platelets (103/mL) | 194.1 ± 65.9 | 169.0 ± 68.0 | 0.004† |
| INR | 1.5 ± 1.3 | 1.5 ± 0.6 | 0.814 |
| Surgical factors | |||
| Prior cardiac surgery (%) | 52 (21.9) | 30 (36.6) | 0.008† |
| Preop ECMO (%) | 5 (2.1) | 5 (6.1) | 0.074 |
| Preop IABP (%) | 37 (15.6) | 13 (15.9) | 0.959 |
| Preop Impella (%) | 15 (6.3) | 11 (13.4) | 0.043* |
| CPB time (min) | 75.2 ± 38.1 | 100.3 ± 60.4 | <0.001‡ |
| LVAD type | <0.001‡ | ||
| HeartWare (%) | 141 (59.5) | 30 (36.6) | <0.001‡ |
| HeartMate II (%) | 83 (35.0) | 48 (58.5) | <0.001‡ |
| HeartMate III (%) | 12 (5.1) | 4 (4.9) | 0.947 |
| Preoperative echo | |||
| LVEF | 20.6 ± 5.9 | 20.1 ± 6.6 | 0.534 |
| RVSP | 51.1 ± 13.5 | 49.7 ± 13.2 | 0.432 |
| TAPSE | 1.84 ± 2.1 | 1.36 ± 0.5 | 0.084 |
NOTE. LVAD was analyzed by chi-square for individual type and by chi-square for the group. Categorical variables analyzed by Fisher exact test for n ≤five and chi-square test for n > five. Continuous variables analyzed by two-sided t test with SD (± SD).
Abbreviations: BMI, body mass index; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; echo, echocardiogram; ECMO, extracorporeal membrane oxygenation; Hx of tobacco, history of tobacco smoking; IABP, intra-aortic balloon pump; INR, international normalized ratio; LVAD, left ventricular device; LVEF, left ventricular ejection fraction; preop, preoperative; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
p value < 0.050.
p value < 0.010.
p value < 0.001.
The cohorts were similar in preoperative cardiac function as assessed by echocardiographic parameters (Table 1) and Interagency Registry for Mechanically Assisted Circulatory Support profile (Supplementary Table 1). There were no differences between preoperative antiplatelet and anticoagulation therapy (Supplementary Table 1). Patients did differ between LVAD type, with more control patients receiving HeartWare (Medtronic, Dublin, Ireland) devices (141 [59.5%] v 30 [36.6%]; p < 0.001) and more FEIBA patients receiving HeartMate II (Abbott Laboratories, Abbott Park, IL) devices (48 [58.5%] v 83 [35.0%]; p < 0.001) (Table 1).
Blood Product Usage
The FEIBA cohort was transfused 5.57 (±4.92) units of pRBCs and 6.48 (±4.60) units of FFP before FEIBA administration. Additionally, 2.66 (±1.58) units of platelets and 1.90 (±2.86) bags of cryoprecipitate, which contain tenpooled units at the authors’ institution, were given prior to FEIBA. This resulted in an average of 16.6 (±10.4) units of blood products given before FEIBA was administered in the FEIBA cohort to control perioperative hemorrhage (Supplementary Table 2).
The blood product requirement intraoperatively and POD zero-two was significantly higher across all product types (pRBCs, FFP, platelets, cryoprecipitate) in patients who received FEIBA compared to controls (p < 0.001, Fig 1). The FEIBA cohort required 4.7 (±4.9) units intraoperatively and 10.1 (±7.5) units of pRBCs during POD zero-two compared to the control group that needed 1.3 (±2.7) units intraoperatively and 2.2 (±3.7) units of pRBCs (p < 0.001) during POD zero-two. After FEIBA administration, pRBC utilization was decreased (p < 0.001) from POD 0 to 2 and was similar to controls during POD 3to 7 (FEIBA: 2.0 ± 3.0 v controls: 1.4 ± 3.0, p = 0.125, Fig 1, A). Similarly, the FEIBA cohort used more FFP intraoperatively (5.8 units v 1.7 units, p < 0.001) and during POD zero-two (9.1 units v 1.7 units, p < 0.001), with no difference observed between groups during POD three-to-seven (0.4 units v 0.3 units, p = 0.541, Fig 1, B). Platelet transfusion was increased throughout the perioperative period in patients who received FEIBA: intraoperatively (2.4 units v 0.9 units, p < 0.001), during POD zero-to-two (3.7 units v 0.7 units, p < 0.001), and during POD three-to-seven (0.5 ± 1.0 units v 0.2 ± 0.9 units, p = 0.027, Fig 1, C). Cryoprecipitate usage was increased intraoperatively in patients treated with FEIBA versus controls (1.81 units v 0.34 units, respectively, p < 0.001) and POD zero-to-two (2.23 units v 0.34 units, respectively, p < 0.001), decreasing to a level similar to the controls during POD three-to-seven (0.05 units v 0.11 units, p = 0.151, Fig 1, D).
Fig 1.
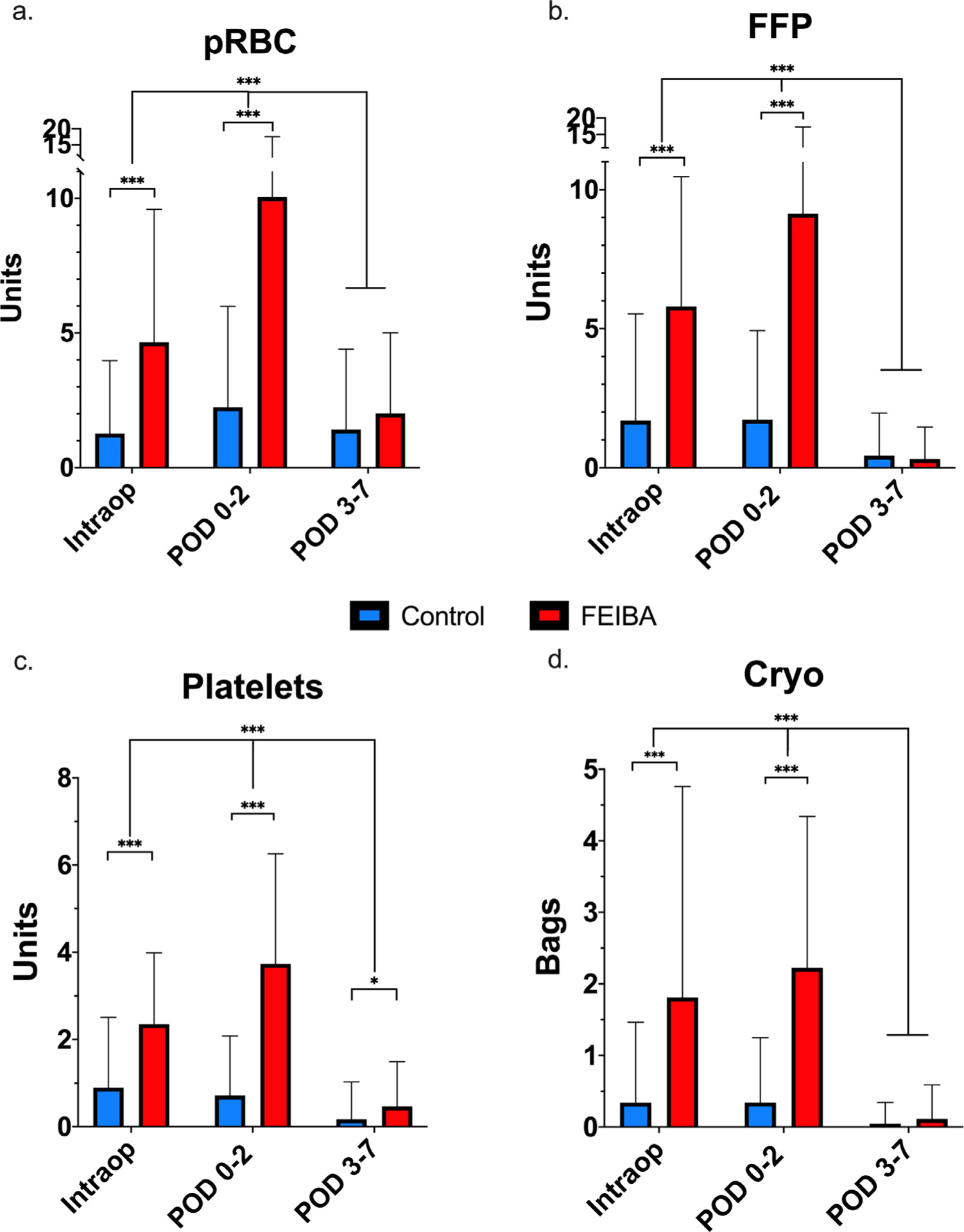
Perioperative blood product usage. FEIBA was administered intraoperatively and/or between day of surgery and postoperative day two (POD zero-two). (A) pRBC = units of packed red blood cells required intraoperatively, during POD zero-two, and POD three-seven. (B) FFP = units of fresh frozen plasma required intraoperatively, during POD zero-two, and POD three-seven. (C) Platelets = units of platelets required intraoperatively, during POD zero-two, and POD three-seven. (D) Cryoprecipitate bags (ten pooled units) required intraoperatively, during POD zero-two and POD three-seven. Control = blue, FEIBA = red. Analysis performed by multiple unpaired t tests per period between controls and FEIBA, and two-way ANOVA over time. *p < 0.050, ***p < 0.001. ANOVA, analysis of variance; FEIBA, Factor Eight Inhibitor Bypassing Activity; FFP, fresh frozen plasma; pRBC, packed red blood cells.
Primary and Secondary Outcomes
Compared with controls, there was no difference in the primary composite outcome of thrombotic events in the first 14-days postoperatively in FEIBA patients (9 [11.0%] v 18 [7.6%%]; p = 0.343) (Table 2). Secondary outcomes, such as the rates of thrombotic events such as iCVA, combination CVA, ICH, GI bleed, pump thrombus, PE, and DVT, also were comparable between both groups (Table 2). Additionally, 14-day mortality rates were similar between the control and FEIBA cohorts (3 [3.7%] v 3 [1.3%]; p = 0.179) (Table 2).
Table 2.
Fourteen-Day Composite Thrombotic Events and Secondary Outcomes After FEIBA Administration for Perioperative Hemorrhage in LVAD Patients
| Primary Composite Outcome | Control (n = 237) (%) | FEIBA (n = 82) (%) | p Value |
|---|---|---|---|
| Thrombotic events | 18 (7.6) | 9 (11.0) | 0.343 |
| Secondary outcomes | |||
| Ischemic CVA | 4 (1.7) | 0 (0.0) | 0.576 |
| ICH | 3 (1.3) | 0 (0.0) | 0.572 |
| Combination CVA | 1 (0.4) | 0 (0.0) | 1.000 |
| Pump thrombus | 0 (0.0 | 0 (0.0) | 1.000 |
| Pulmonary embolism | 1 (0.4) | 0 (0.0) | 1.000 |
| Deep vein thrombosis | 13 (5.5) | 9 (11.0) | 0.091 |
| GI bleeds | 5 (2.1) | 1 (1.2) | 1.000 |
| Mortality | 3 (1.3) | 3 (3.7) | 0.179 |
NOTE. Thrombotic events, composite thrombotic outcome of ischemic cerebrovascular accident (iCVA), intracranial hemorrhage (ICH), combination iCVA with hemorrhagic conversion (combination CVA), pump thrombus, pulmonary embolism, and deep venous thrombosis in first 14 days. Categorical variables analyzed by Fischer exact test for n ≤five and chi-squared test for n > five.
Abbreviations: FEIBA, Factor Eight Inhibitor Bypassing Activity; .GI, gastrointestinal.
FEIBA Dosage
The mean intraoperative FEIBA dose of 937 units (95% CI: 717–1157 units) was similar to the postoperative ICU dose of 789 units (95% CI: 591–986 units, p = 0.315) (Fig 2, A). Forty-three patients received FEIBA intraoperatively (median 595 units, range 125–4000 units), 28 patients received FEIBA postoperatively (POD zero-two) (median 530 units, range 125–2900 units), and 11 patients received FEIBA both intraoperatively and POD zero-to-two. The mean total dosage of FEIBA was 992 units (95% CI 821–1163 units, Fig 2, A). There was no significant difference observed between FEIBA dose for those patients with or without a 14-day thrombotic events (no event: 982 ± 782 units v composite thrombotic event: 1073 ± 784 units, p = 0.745, Fig 2, B). Univariate logistical regression did not show an association between total FEIBA units given and thrombotic events (OR: 1.00, p = 0.347). A receiver operator curve analysis for thrombotic events per unit dose of FEIBA also did not show a correlation (AUC): 0.532, p = 0.755, Fig 2, C).
Fig 2.
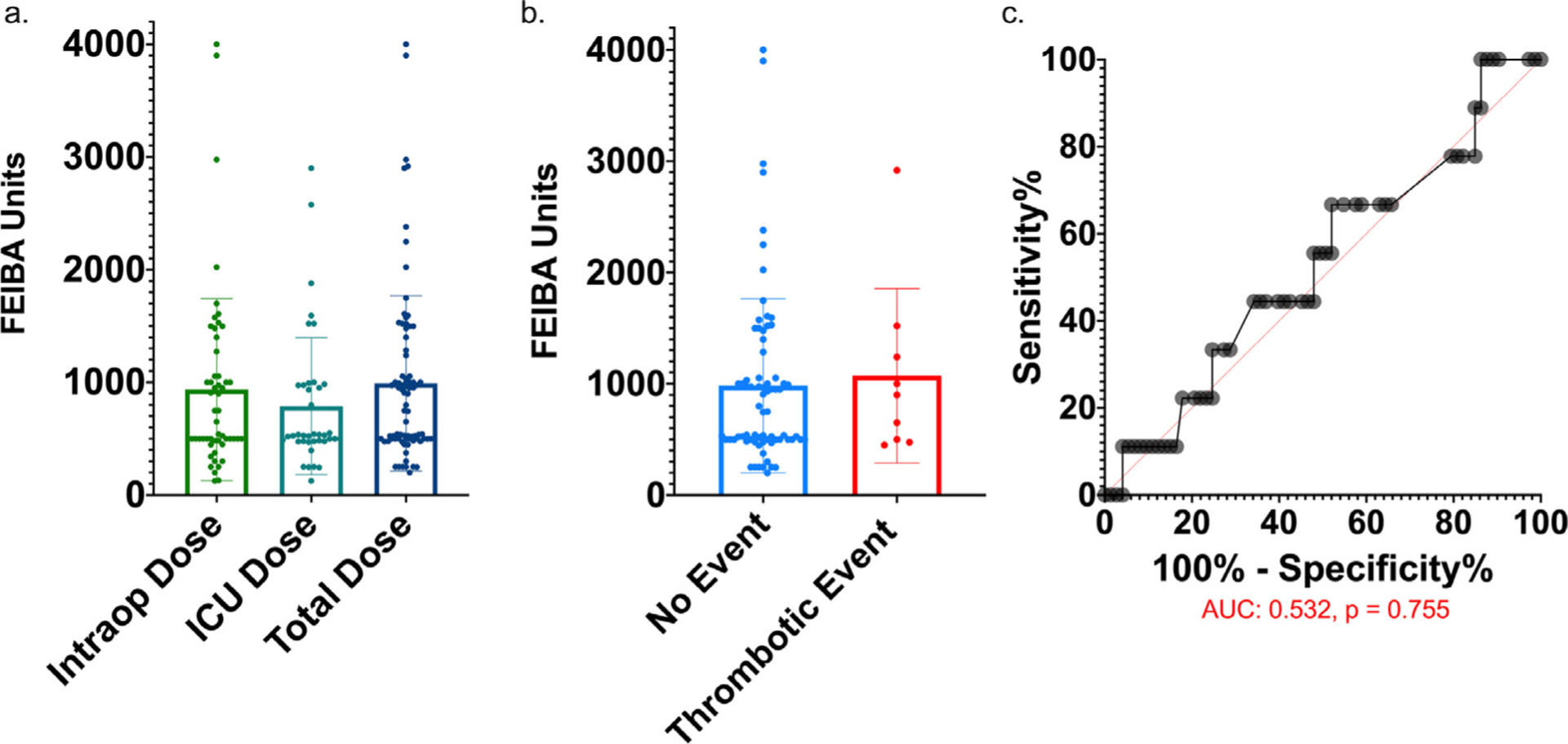
No association between FEIBA dosage and composite thrombotic events. (A) Intraop dose = units of FEIBA given intraoperatively, ICU dose = units of FEIBA given between postoperative day zero-two, total dose = total dose of FEIBA from intraoperative period to POD two. (B) No event, total units of FEIBA given to patients with no thrombotic events in 30 days postoperatively; thrombotic event, total units of FEIBA given to patients with postoperative composite thrombotic events within 30 days. No significant difference by two-sided t test. (C) Receiver operator curve (ROC) for composite thrombotic event per unit of FEIBA administered (AUC: 0.532, p = 0.755). AUC, area under the curve; FEIBA, Factor Eight Inhibitor Bypassing Activity; ICU, intensive care unit.
Multivariate Logistical Regression Model
A multivaria logistical regression model (AUC: 0.635, Akaike information criterion: 189.9) controlling for possible confounding thrombotic and surgical risk factors did not show an increase in the odds ratio for the 14-day composite outcome for treatment with FEIBA (odds ratio [OR]: 1.00, p = 0.994, Table 3). However, increases in preoperative international normalized ratio (OR: 1.30, p = 0.023, 95% CI: 1.04–1.62) and CPB time in ten-minute intervals (OR: 1.11, p = 0.015, 95% CI: 1.02–1.20) increased the odds of 14-day thrombotic events (Table 3).
Table 3.
Multivariate Logistical Regression Model for Composite Thrombotic Outcome
| 14- Day Composite Thrombotic Outcome | |||
|---|---|---|---|
| Cases (n = 302) | Odds Ratio | p Value | 95% CI |
| BMI | 1.01 | 0.853 | 0.94–1.07 |
| CPB time (10 min) | 1.11 | 0.015* | 1.02–1.20 |
| Preoperative platelets (20k) | 0.92 | 0.224 | 0.81–1.05 |
| Preoperative INR | 1.30 | 0.023* | 1.04–1.62 |
| Prior cardiac surgery | 0.72 | 0.527 | 0.26–2.00 |
| Preoperative Impella | 1.46 | 0.594 | 0.36–5.92 |
| INTERMACS profile | 1.08 | 0.700 | 0.73–1.61 |
| LVAD type | 0.82 | 0.384 | 0.52–1.29 |
| FEIBA treatment | 1.00 | 0.994 | 0.37–2.70 |
NOTE. Analyzed by multivariate logistical regression with area under the curve (AUC) for this model: 0.635 and Akaike information criterion (AIC): 189.9.
Abbreviations: BMI, body mass index; CPB Time (ten min), cardiopulmonary bypass time in ten-minute intervals; preoperative platelets (20k), change in preoperative platelet count by 20,000 platelet intervals; preoperative INR, preoperative international normalized ratio; INTERMACS profile, Interagency Registry for Mechanical Assisted Circulatory Support Profile; LVAD, left ventricular assist device (HeartWare, Heart Mate II, or Heart Mate III).
p < 0.050.
Time-to-Event Analysis
A time-to-event analysis comparing the proportion of patients free of the composite thrombotic outcome over time did not show a significant difference between the FEIBA and control cohort for timing of thrombotic events (log-rank [Mantel-Cox] test: p = 0.323, Fig 3). The timing of thromboses from surgery are listed in the Supplementary Table 3.
Fig 3.
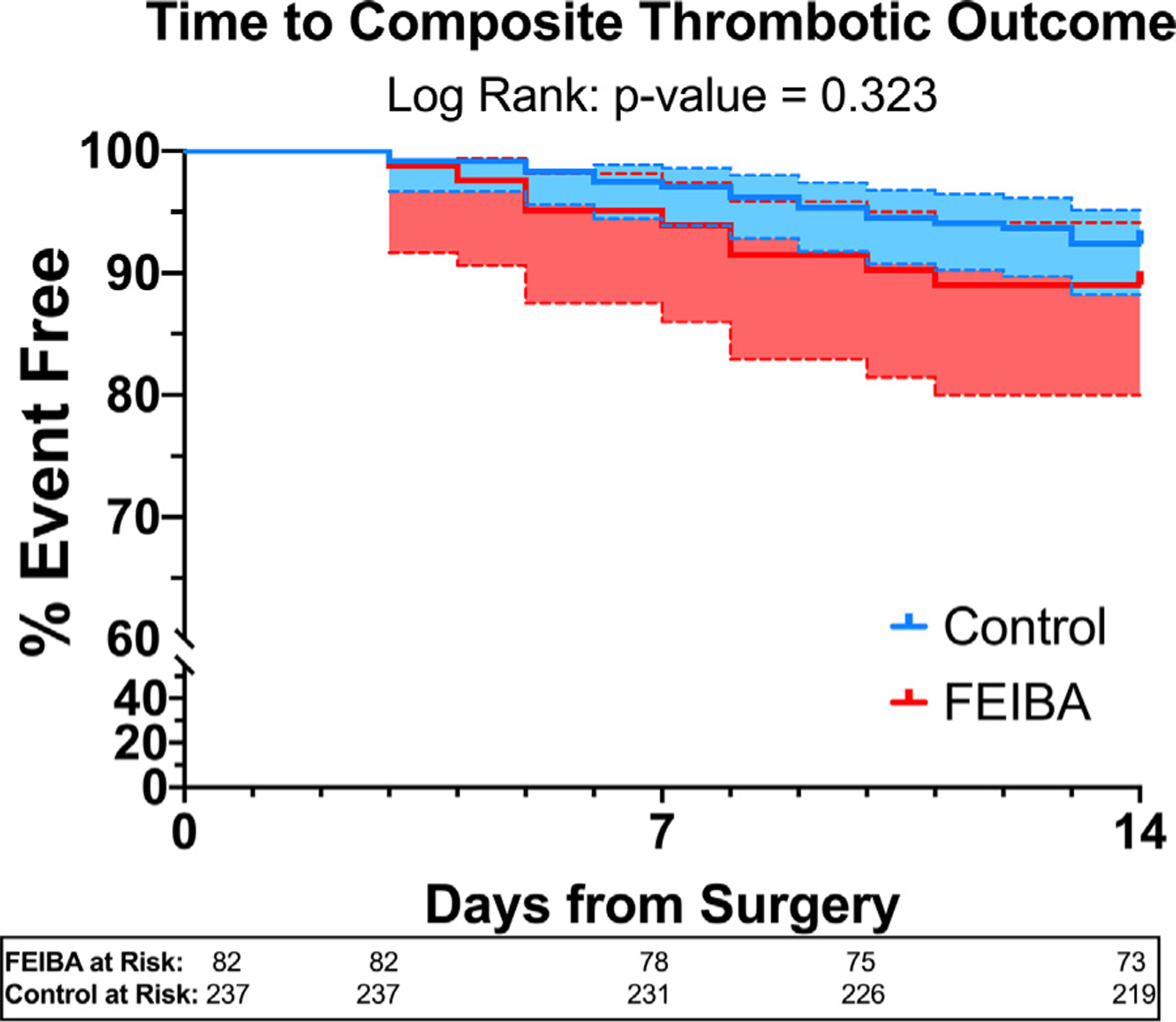
Time-to-event analysis for proportion free of composite thrombotic outcome. Control = blue (95% CI in dotted lines), FEIBA = red (95% CI in dotted lines). The proportion of patients free of the composite thrombotic outcome was analyzed over time from surgery using log-rank (Mantel-Cox) test, which did not show a difference between the FEIBA and control cohorts. CI, confidence interval; FEIBA, Factor Eight Inhibitor Bypassing Activity.
Discussion
In this study, the use of perioperative FEIBA was not associated with an increase in the 14-day primary composite thrombotic outcome when administered at a mean dose of ~1,000 units (~13 units/kg). After controlling for other known thrombotic and surgical risk factors by multivariate logistical regression, there was no association between treatment with FEIBA and thrombosis. Secondary thrombotic and hemorrhagic outcomes, such as the rates of stroke, pump thrombus, DVT, PE, ICH, and GI bleeds, were similar between the control cohort, which did not receive FEIBA, and those who received FEIBA for perioperative hemorrhage. All-cause mortality in the first 14 days postoperatively also was similar. Although the authors acknowledge that the management of perioperative hemorrhage in patients undergoing LVAD implantation remains complex, these findings supported the notion that low-dose FEIBA may be used in LVAD patients for controlling perioperative hemorrhage that has not responded to blood product administration in an attempt to correct coagulopathy.
Several studies have described the use of FEIBA in the treatment of postoperative coagulopathy in cardiac surgery, but none has focused on LVAD patients.27,28 The importance of these results is to describe the low incidence of thrombotic events the authors have seen with the use FEIBA for management of perioperative coagulopathic hemorrhage in LVAD patients who are at high risk for thromboses. The risk of iatrogenic thrombosis with FEIBA is real and should be considered before administration, but there are favorable data comparing its use to other rescue agents.29,30 In fact, Aledort et al., reported a significant increase in thrombotic complications with recombinant Factor VIIa compared with FEIBA.30 Song et. al., in 2014, studied FEIBA use in 25 patients of whom five underwent LVAD implantation and reported a reduction in blood product usage with favorable outcomes.22 A larger study by Rao et. al., in general cardiac surgery, showed similar rates of 30-day thrombotic events with FEIBA (n = 107, 12.1%) versus recombinant Factor VIIa (n = 61, 13.1%) at an average FEIBA dose of 18.6 units/kg (±12.4 units/kg).27
The favorable aspects of FEIBA for correction of coagulopathy include a rapid increase in thrombin levels within one hour of administration with a short half-life of four-seven hours have led to its expanded use for the treatment of oral anticoagulant-related bleeds and ICH.26,31–33 Dibu et al. reported using up to 50 units/kg to reverse five episodes of ICH with no iCVAs observed.31 However, a larger study by Yin et al., used a more conservative strategy, with an average of 20.3 units/kg (interquartile range, 1249–2213) in 34 patients with ICH, which resulted in a similar composite thrombotic rate to FFP alone (12% v 8%, p = 0.560).32 Engelbart et al. showed a 10% rate of thrombosis in 42 patients who received FEIBA for direct oral anticoagulant reversal, similar to this study, with the most commonly observed being DVTs.33
In this study, the average dose of FEIBA was 992 units (95% CI: 821 – 1163 units) [13.0 units/kg (95% CI: 10.5 – 15.4 units/kg)] and there was no significant association between FEIBA dose and occurrence of severe thrombotic events. Comparatively, in hemophiliacs with soft tissue hemorrhages, the pharmaceutical package insert (Baxter HealthCare Corporation) recommends a FEIBA dose of 100 units/kg. The average FEIBA dose of 13.0 units/kg in this study was much lower than the >200 units/kg/d value cited by the package insert (Baxter HealthCare Corporation), in which an increased the risk of thromboses has been reported in hemophiliacs. The goal is always to give the lowest dose of FEIBA clinically necessary to achieve hemostasis to mitigate the risk of thrombosis. The dosing strategy most commonly used at the authors’ institution for non-hemophiliacs parallels that published by Rao et al., in which, initially, 250-to-500 units are given slowly over five-ten minutes in 50-unit increments and then, if needed, additional aliquots of 250 units are dosed in a similar fashion to achieve hemostasis.27 This differs from Song et al., who reported doses administered in 1,000-unit increments, with a mean of 2,154 units.22 In this study, only six patients received >2,000 units (max 4,000 units) for uncontrolled hemorrhage.
At the authors’ institution, FEIBA treatment is not protocolized yet and is administered as a hemostatic rescue agent when standard blood product therapy has failed to correct coagulopathy and mitigate bleeding. An average of 16.6 units (95% CI: 14.3–18.9) of blood products were given prior to treatment, and the blood product requirements during POD three-seven were similar to that of the controls. However, the retrospective nature of this study made it difficult to interpret the effectiveness of FEIBA in reducing blood product administration without a similar hemorrhagic control, but provided important information to spur future studies to assess the efficacy. The goal would be a reduction in blood product administration for perioperative hemorrhage with FEIBA that, hopefully, would translate to a reduced incidence of right ventricular failure due to volume overload with the potential to improve patient outcomes.
The retrospective nature of this study lends itself to several potential limitations. As this was a single-center study, employing the authors’ institution’s use and FEIBA dosing practices, may limit the external generalizability of the findings. Additionally, the data were collected over a nine-year period when changes in practice and provider preferences could affect the outcome. To examine this possibility, the authors controlled for surgical preference using univariate logistical regression and found no association between changes in practice patterns over time with the primary outcome. Among the most notable change in practice patterns for correction of coagulopathy has been the use of PCC for those who received preoperative warfarin. As a portion of the data was obtained before the approval of PCC, it is possible that some of the patients included in the study may have been treated with PCC as opposed to FEIBA.34 Also, more controls received HeartWare devices, which could have increased the rates of pump thrombosis or thrombotic events if more had received FEIBA. Differences between the control and FEIBA cohorts in type of LVAD device were examined by univariate logistical regression and controlled for in the multivariate logistical regression model, which showed no significant effect on the composite thrombotic outcome. However, the multivariate model may be prone to overfitting due to the low number of events compare to covariates included. During POD zero-two, blood product transfusions in the FEIBA group were higher compared to controls. Although the authors interpreted this to be reflective of the reason for initial FEIBA administration in this group, the authors cannot prove causality given the retrospective nature of the study. However, although the results are suggestive of a favorable risk-benefit profile, a single-center retrospective study such as this is not capable of fully defining the safety profile of a medication. The authors hope the results spur future studies to evaluate the safety, efficacy, and appropriate dosing strategy of FEIBA for control of perioperative hemorrhage.
Conclusion
The results of this retrospective study suggested that FEIBA may be used as a rescue hemostatic agent to help control perioperative hemorrhage following LVAD implantation without associated increases in mortality or thrombotic events.
Supplementary Material
Footnotes
Declaration of Competing Interest
None.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2021.04.030.
References
- 1.Cho SM, Moazami N, Frontera JA. Stroke and intracranial hemorrhage in HeartMate II and HeartWare left ventricular assist devices: A systematic review. Neurocrit Care 2017;27:17–25. [DOI] [PubMed] [Google Scholar]
- 2.Whitson BA, Eckman P, Kamdar F, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg 2014;97:2097–103. [DOI] [PubMed] [Google Scholar]
- 3.Muslem R, Caliskan K, Van Thiel R, et al. Incidence, predictors and clinical outcome of early bleeding events in patients undergoing a left ventricular assist device implant. Eur J Cardio-thoracic Surg 2018;54:176–82. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DJ, Beauford RB. Left ventricular assist devices and bleeding: Adding insult to injury. Ann Thorac Surg 2003;75(6 Suppl):S42–7. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer JM, Arnaoutakis GJ, Allen JG, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg 2011;91:740–9. [DOI] [PubMed] [Google Scholar]
- 6.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038–47. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal JL, Starling RC. Coagulopathy in mechanical circulatory support: A fine balance. Curr Cardiol Rep 2015;17:1–9. [DOI] [PubMed] [Google Scholar]
- 8.Frontera JA, Starling R, Cho SM, et al. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant 2017;36:673–83. [DOI] [PubMed] [Google Scholar]
- 9.Bunte MC, Blackstone EH, Thuita L, et al. Major bleeding during heartmate II support. J Am Coll Cardiol 2013;62:2188–96. [DOI] [PubMed] [Google Scholar]
- 10.Despotis G, Eby C, Lublin DM. Perioperative bleeding with cardiac surgery. Transfusion 2008;48:1–30S. [DOI] [PubMed] [Google Scholar]
- 11.Ferraris VA, Brown JR, Despotis GJ, et al. Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists Blood Conservation Clinical Practice Guidelines. Ann Thorac Surg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- 12.Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med 2004;30:1873–81. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res 2010;3:618–24. [DOI] [PubMed] [Google Scholar]
- 14.Karkouti K, Beattie WS, Arellano R, et al. Comprehensive Canadian review of the off-label use of recombinant activated factor VII in cardiac surgery. Circulation 2008;118:331–8. [DOI] [PubMed] [Google Scholar]
- 15.Karkouti K, Beattie WS, Crowther MA, et al. The role of recombinant factor VIIa in on-pump cardiac surgery: Proceedings of the Canadian Census Conference. Can J Anesth 2007;54:573–82. [DOI] [PubMed] [Google Scholar]
- 16.Ponschab M, Landoni G, Biondi-Zoccai G, et al. Recombinant activated factor VII increases stroke in cardiac surgery: A meta-analysis. J Cardiothorac Vasc Anesth 2011;25:804–10. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell KA, Wood JJ, Wise RP, et al. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. J Am Med Assoc 2006;295:293–8. [DOI] [PubMed] [Google Scholar]
- 18.Romagnoli S, Bevilacqua S, Gelsomino S, et al. Small-dose recombinant activated factor VII (NovoSeven®) in cardiac surgery. Anesth Analg 2006;102:1320–6. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AJ, Blount AL, Davis AT, et al. Recombinant factor VIIa (NovoSeven RT) use in high risk cardiac surgery. Eur J Cardiothoracic Surg 2011;40:1314–9. [DOI] [PubMed] [Google Scholar]
- 20.Karkouti K, Beattie WS, Wijeysundera DN, et al. Recombinant factor VIIa for intractable blood loss after cardiac surgery: A propensity score-matched case-control analysis. Transfusion 2005;45:26–34. [DOI] [PubMed] [Google Scholar]
- 21.Von Heymann C, Redlich U, Jain U, et al. Recombinant activated factor VII for refractory bleeding after cardiac surgery – A retrospective analysis of safety and efficacy. Crit Care Med 2005;33:2241–6. [DOI] [PubMed] [Google Scholar]
- 22.Song HK, Tibayan FA, Kahl EA, et al. Safety and efficacy of prothrombin complex concentrates for the treatment of coagulopathy after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:1036–40. [DOI] [PubMed] [Google Scholar]
- 23.Hilgartner BMW, Knatterud GL. The use of factor eight inhibitor by-passing activity (FEIBA immuno) product for treatment of bleeding episodes in hemophiliacs with inhibitors. Blood 1983;61:36–40. [PubMed] [Google Scholar]
- 24.Leissinger C, Gringeri A, Antmen B, et al. Anti-inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med 2011;365:1684–92. [DOI] [PubMed] [Google Scholar]
- 25.Gritsch H, Schwarz HP, Turecek PL, et al. FEIBA Ò : mode of action. Haemophilia 2004;10(suppl 2):3–9. [DOI] [PubMed] [Google Scholar]
- 26.Varadi K, Tangada S, Loeschberger M, et al. Pro- and anticoagulant factors facilitate thrombin generation and balance the haemostatic response to FEIBA® in prophylactic therapy. Haemophilia 2016;22:615–24. [DOI] [PubMed] [Google Scholar]
- 27.Rao VK, Lobato RL, Bartlett B, et al. Factor VIII inhibitor bypass activity and recombinant activated factor vii in cardiac surgery. J Cardiothorac Vasc Anesth 2014;28:1221–6. [DOI] [PubMed] [Google Scholar]
- 28.Balsam LB, Timek TA, Pelletier MP. Factor eight inhibitor bypassing activity (FEIBA) for refractory bleeding in cardiac surgery: Review of clinical outcomes. J Card Surg 2008;23:614–21. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA®): 10-year compilation of thrombotic adverse events. Haemophilia 2002;8:83–90. [DOI] [PubMed] [Google Scholar]
- 30.Makris M, Van Veen JJ, Aledort LM, et al. Comparative thrombotic event incidence after infusion of recombinant factor VIIa versus factor VIII inhibitor bypass activity – A rebuttal. J Thromb Haemost 2005;3:818–9. [DOI] [PubMed] [Google Scholar]
- 31.Dibu JR, Weimer JM, Ahrens C, et al. The role of FEIBA in reversing novel oral anticoagulants in intracerebral hemorrhage. Neurocrit Care 2016;24:413–9. [DOI] [PubMed] [Google Scholar]
- 32.Yin EB, Tan B, Nguyen T, et al. Safety and effectiveness of Factor VIII inhibitor Bypassing Activity (FEIBA) and fresh frozen plasma in oral anticoagulant-associated intracranial hemorrhage: A retrospective analysis. Neurocrit Care 2017;27:51–9. [DOI] [PubMed] [Google Scholar]
- 33.Engelbart JM, Zepeski A, Galet C, et al. Safety and effectiveness of Factor Eight Inhibitor Bypassing Activity for direct oral anticoagulant-related hemorrhage reversal. Am J Emerg Med 2019;37:214–9. [DOI] [PubMed] [Google Scholar]
- 34.Rimsans J, Levesque A, Lyons E, et al. Four factor prothrombin complex concentrate for warfarin reversal in patients with left ventricular assist devices. J Thromb Thrombolysis 2018;46:180–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


